Abstract
Background
Red blood cell distribution width (RDW) has emerged as a prognostic factor for mortality in various diseases. Up to now, few studies have focused on the prognostic value of RDW in patients with diabetic foot ulcers (DFUs). This retrospective cohort study aimed to investigate the impact of RDW and RDW/albumin (ALB) ratio on all-cause mortality in patients with DFUs.
Methods
This study included 860 patients with DFUs in a tertiary academic hospital. The associations of RDW and RDW/ALB with all-cause mortality were assessed by multivariable cox regression analyses. The pairwise comparisons of receiver operating characteristic (ROC) curves were performed to compare the predictive performance of RDW and RDW/ALB ratio. Harrell’s concordance index, integrated discrimination improvement, and net reclassification improvement were used to estimate the improvements in risk discrimination.
Results
Patients with high RDW and RDW/ALB had lower overall survival rates (all P < 0.001). The multivariable Cox regression revealed that high RDW [adjusted hazard ratio (HR) 2.426, 95% confidence interval (CI): 1.557–3.778, P < 0.001] and high RDW/ALB (adjusted HR 2.360, 95% CI: 1.414–3.942, P = 0.001) were independent associated with high all-cause mortality. In subgroup analyses, the comparative analysis of ROC curves revealed that the discriminating ability of the RDW/ALB ratio was significantly superior to RDW in patients with no severe DFUs or no severe peripheral artery disease, or in young and middle-aged patients (all P < 0.05). Adding RDW and RDW/ALB ratio to base models improved discrimination and risk reclassification for all-cause mortality.
Conclusions
RDW and RDW/ALB ratio are robust and independent prognostic markers in patients with DFUs. The RDW/ALB ratio appears to be of more predictive value for mortality in younger and less severely ill patients with DFUs. Both RDW and RDW/ALB ratio can provide incremental predictive value for all-cause mortality over traditional risk factors. RDW and RDW/ALB ratio can be used to identify high-risk patients with DFUs.
Similar content being viewed by others
Introduction
Diabetic foot ulcers (DFUs) are a common and life-threatening complication of diabetes, leading to hospitalization, high health-care costs, and a high rate of amputation [1,2,3]. DFUs exhibit a 5—year mortality comparable to cancer [2]. Individuals with DFUs have a 2.5-fold increase in the risk for death compared with patients who have diabetes but no DFUs [4]. The excess mortality cannot be fully explained by other known comorbidities and complications of diabetes [4, 5]. It is therefore important to identify and evaluate additional risk factors that influence mortality in patients with DFUs.
The red cell distribution width (RDW) is a simple and easily-obtained parameter, representing the heterogeneity of erythrocyte volume, and is traditionally used for differential diagnosis of anemia [6]. However, in more recent years, RDW was found to be associated with multiple disease processes and prognoses [6]. RDW was associated with a higher risk of developing diabetes [7]. RDW was also associated with diabetes-related complications [8]. RDW can predict mortality and cardiovascular complications in patients with diabetes [9]. However, this observation failed to be corroborated in another population [10], which may be due to the population heterogeneity. RDW/albumin (ALB) ratio is a new combined parameter that can predict mortality in patients undergoing burn surgery, and patients with diabetic ketoacidosis [11, 12]. Diabetes-related complications were associated with an increased inflammatory burden [13, 14]. RDW seems to be a new inflammatory marker. Elevated RDW was found in inflammation-related diseases, such as Hashimoto's thyroiditis [15], thyroid cancer [16], and autoimmune liver diseases [17]. Although a definitive mechanism for the association of RDW, RDW/ALB ratio, and mortality has not yet been established, RDW and RDW/ALB seem to be nonspecific parameters that have the potential to provide effective risk stratification in patients with serious diseases [18].
To date, research related to the prognostic value of RDW with DFUs is scarce [19]. Similarly, there are no previous studies on RDW/ALB ratio in patients with DFUs. In this study, we sought to explore the impact of RDW and RDW/ALB ratio on all-cause mortality in a relatively large cohort of patients with type 2 diabetes and foot ulcers.
Methods
Study participants
The study participants were 907 patients diagnosed with type 2 diabetes and DFUs from 2015 to 2019 in the First Affiliated Hospital of Wenzhou Medical University, which is a tertiary academic hospital. The exclusion criteria included terminal malignancies, patients who underwent hemodialysis, or with missing data of RDW or ALB. Ultimately, a total of 860 patients constituted our study population.
The ethics committee of the First Affiliated Hospital of Wenzhou Medical University approved this study. Given the retrospective and non-intrusive nature of the study, the written consent requirement was waived.
Data collection and grouping
The medical histories and data on baseline characteristics, including demographic, anthropometric, and laboratory parameters, were retrospectively abstracted from individual medical records. For patients with multiple hospitalizations during the study period, only the data of the first hospitalization were included. All-cause mortality was considered as the endpoint. Data regarding deaths was obtained from medical records or by telephone interviews. The follow-up period started at the date of admission and ended at the date of death, or the end of the study (March 2021). The study flow is shown in Fig. 1. Peripheral artery disease (PAD) was diagnosed by ultrasonic diagnostic experts based on the duplex ultrasonography. Severe PAD was defined as the presence of stenosis ≥ 50% in any of the lower extremity arteries. DFUs were defined as the presence of foot ulcer, infection, or deep tissue damage. Severe DFUs were defined as subjects with Wagner grade score ≥ 3 according to the Wagner classification [20]. Coronary artery disease was defined as a previous history of coronary artery disease or a new coronary artery disease diagnosed by cardiologists during hospitalization. Cerebrovascular disease was defined as a history of cerebrovascular disease, or a new cerebrovascular disease diagnosed by computed tomography or magnetic resonance imaging scan during hospitalization. Diabetic retinopathy was diagnosed by digital images of the binocular fundus, if considered necessary by ophthalmologists, further checked by fluorescein fundus angiography and optical coherence tomography. Diabetic peripheral neuropathy was diagnosed by self-reported clinical signs, physical examination, perception thresholds test, and electromyography. Anti-platelet drugs defined as aspirin, clopidogrel or cilostazol. Details of the calculation of estimated glomerular filtration rate (eGFR), definition of hypertension, definition and grouping of the elderly, smoking, alcohol use, severe PAD, and severe DFUs have been described in our previous study [21, 22].
Statistical analysis
The Kolmogorov–Smirnov test was used for normality tests. All continuous variables were not normally distributed and were expressed as median and interquartile range. Categorical variables were expressed as n (%). Mann–Whitney U test (continuous variables) and Chi-squared test (categorical variables) were used to compare differences between groups. The receiver operating characteristic (ROC) analyses were used to identify the optimal cut-off values of RDW and RDW/ALB ratio for all-cause mortality. To compare the predictive performance of RDW and RDW/ALB ratio, the pairwise comparisons of ROC curves were performed [23]. Kaplan–Meier survival curves with log-rank tests were applied for overall survival (OS) analysis. Unadjusted and multivariable adjusted analyses for all-cause mortality were performed by Cox regression. Variables with P < 0.1 in the unadjusted analysis were selected for the multivariable analyses. ALB was excluded in the analyses of the RDW/ALB ratio. Harrell’s concordance index (C-index), integrated discrimination improvement (IDI), and net reclassification improvement (NRI) were used to estimate the improvements in model performance, discrimination, and risk classification after adding RDW and RDW/ALB to base models (variables with P < 0.1 in the unadjusted Cox regression analysis) [24, 25]. P values < 0.05 were considered statistically significant for all analyses. All statistical analyses were conducted using SPSS (IBM, IL, USA) version 22, MedCalc (MedCalc Software Ltd, Ostend, Belgium) version 20.019, and R version 4.1.2 (R Core Team, survival, survIDINRI, survC1).
Results
Patient baseline characteristics
The clinical characteristics of patients categorized based on the cut-off values of RDW and RDW/ALB ratio are summarized in Tables 1 and 2. Patients with high RDW were older, had longer diabetic foot ulcer duration, a greater proportion of severe PAD and anti-hypertensive therapy, and lower eGFR, hemoglobin (Hb), and hemoglobin A1c (HbA1c) than those with low RDW. Patients with high RDW/ALB ratio were older, had longer diabetes duration, greater proportion of severe DFUs and using insulin, lower proportion of anti-platelet therapy, higher HbA1c, and lower body mass index (BMI), diastolic blood pressure (DBP), eGFR, ALB, Hb, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) (all P < 0.05).
Clinical outcomes
During a median follow-up of 32 months, 147 (17.1%) patients died. The Kaplan–Meier curves showed that high RDW and RDW/ALB ratio were related to lower OS rates compared to low RDW and RDW/ALB ratio (all P < 0.001, Fig. 2).
The unadjusted and multivariable-adjusted Cox regression analyses were used to evaluate the prognostic value of RDW and RDW/ALB ratio (Table 3). Variables with P < 0.1 [age, BMI, systolic blood pressure (SBP), severe DFUs, severe PAD, eGFR, ALB, Hb, cerebrovascular disease, diabetic retinopathy and diabetic peripheral neuropathy] in the unadjusted Cox regression analysis (Additional file 2: Table S1), were included as confounding variables in the multivariable cox regression analyses. In the multivariable cox regression analyses, high RDW [adjusted hazard ratio (HR) 2.426, 95% confidence interval (CI): 1.557–3.778, P < 0.001] and high RDW/ALB ratio (adjusted HR 2.360, 95% CI: 1.414–3.942, P = 0.001) were significantly associated with high all-cause mortality. Similar significant robust associations were found in subgroup analyses based on the severity of DFUs and PAD, and in the elderly (≥ 65 years). However, only RDW/ALB was associated with high all-cause mortality in young and middle-aged patients (< 65 years) (Table 3).
Comparative analysis of ROC curves
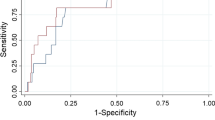
The ROC curves of RDW and RDW/ALB ratio are shown in Fig. 3. According to ROC analyses, the optimal cut-off values of RDW and RDW/ALB ratio were 14.3% and 0.3809%/(g/L), respectively. The sensitivity, specificity, positive predictive value, and negative predictive value of the optimal cut-off values of RDW and RDW/ALB ratio are shown in Additional file 3: Table S2. The comparative analysis of ROC curves revealed that the discriminating ability of the RDW/ALB was significantly superior to RDW: [area under ROC curve (AUC): 0.713, 95% CI: 0.665–0.757 vs. AUC: 0.618, 95% CI: 0.567–0.666, P = 0.025) in patients with no severe DFUs, (AUC: 0.808, 95% CI: 0.758–0.852 vs. AUC: 0.662, 95% CI: 0.604–0.716, P = 0.015) in patients with no severe PAD, and (AUC: 0.741, 95% CI: 0.688–0.790 vs. AUC: 0.551, 95% CI: 0.493–0.609, P = 0.008) in young and middle-aged patients. No significant difference was found between RDW and RDW/ALB ratio by comparative analysis of ROC curves in the overall study population, patients with severe DFUs or severe PAD, or in the elderly (Table 4, Additional file 1: Fig. S1).
ROC curves of RDW and RDW/ALB ratio for predicting the all-cause mortality in the overall study population. The optimal cut-off values of RDW and RDW/ALB ratio for the all-cause mortality were 14.3% and 0.3809%/(g/L), respectively. The AUC of RDW was 0.634, 95% CI: 0.601–0.666; the AUC of RDW/ALB ratio was 0.660, 95% CI: 0.628–0.692. ROC receiver operating characteristic, RDW red cell distribution width, ALB albumin, AUC area under ROC curve
Incremental predictive value of RDW and RDW/ALB ratio
Adding RDW and RDW/ALB ratio to base models significantly improved the predictive ability for all-cause mortality. RDW increased the C-index from 0.792 to 0.804 and RDW/ALB ratio increased the C-index from 0.783 to 0.794. IDI showed a significant improvement of 2.7% (95% CI: 0.2–6.3%, P = 0.02) and 2.3% (95% CI: 0.4–5.5%, P = 0.02) after adding RDW and RDW/ALB ratio to base model, respectively. NRI was also significant for RDW (23.4%, 95% CI: 3.0–30.8%, P = 0.03) and RDW/ALB ratio (29.6%, 95% CI: 11.0–40.0%, P = 0.02) (Table 5).
Discussion
In this retrospective cohort study, both high RDW and RDW/ALB ratio at the time of admission were associated with higher all-cause mortality in a cohort of 860 patients with DFUs treated in a tertiary academic hospital. The risk of mortality associated with high RDW and RDW/ALB ratio remained statistically significant even after adjustment for confounding variables. In subgroup analyses, the comparative analysis of ROC curves showed that the discriminating ability of the RDW/ALB was significantly superior to RDW in patients with no severe DFUs or no severe PAD, or young and middle-aged patients. Additionally, both RDW and RDW/ALB ratio significantly improved predictive ability for all-cause mortality over traditional risk factors. To the best of our knowledge, this is the first study to investigate the predictive value of RDW/ALB ratio, a new combined biomarker, together with RDW in patients with DFUs.
Only few studies have focused so far on the RDW in patients with DFUs. A previous study with a small sample size in Turkey reported that RDW was a predictive parameter for major amputation in patients with DFUs [26]. A recent conference abstract with little available detail indicated that RDW was a risk factor for all-cause mortality in a moderate-sized cohort of patients undergoing amputations due to DFUs [19]. No earlier studies have reported the association between RDW/ALB ratio and all-cause mortality in patients with DFUs. This study demonstrated the predictive value of RDW and RDW/ALB ratio concurrently in a relativity large cohort of patients with DFUs.
The underlying mechanisms of the associations between high RDW and mortality have not been fully elucidated. RDW may be considered as a general marker of health status, rather than disease-specific, which is associated with mortality in a variety of diseases, as well as in the general population [6]. There are several reasons leading to the higher RDW, including inflammation [27], oxidative stress [28], shortening of telomeres length [29], increased erythrocyte mechanical fragility [30], nutritional deficiencies [31], and deficiency or dysfunction of erythropoietin [6]. All of the aforementioned conditions were important prognostic factors for mortality [6]. Inflammation contributes to higher RDW by myelosuppression, promoting red cell apoptosis and erythropoietin resistance, reducing erythropoietin production and bioavailability of iron [32,33,34,35]. Oxidative stress induces increased RDW by shortening the life span of erythrocytes and increasing the migration of premature erythrocytes to the peripheral circulation [36]. Shortening of telomeres length is a telltale sign of cellular aging and is associated with age-related diseases, including diabetes [37]. Shortening of telomeres length causes cell senescence of the erythromyeloid progenitors, thus leading to an impaired capacity of replicative and maturation of erythrocytes [38]. Diabetic nephropathy is associated with erythrocyte fragmentation, and renal dysfunction is often accompanied by deficiency of erythropoietin [6, 39], thus leading to increased RDW [40]. Patients with high RDW in the present study were older, had lower eGFR, HB, BMI. However, the associations of RDW and RDW/ALB ratio with all-cause mortality remained significant after adjustment for age, eGFR, Hb, and BMI. Hence, the pathophysiological mechanisms for the predictive roles of RDW in patients with DFUs can not be fully explained by the aforementioned conditions, and need to be elucidated by further studies.
RDW is routinely measured as part of the extensively used complete blood counts. Hence, it would not require any additional cost. RDW also seems to be potentially modifiable. High RDW can be lowered by exercise training in patients with coronary artery disease [41], and by treatment with iron in hemodialysis patients [42]. However, further studies are warranted to clarify whether aggressive interventions on RDW can improve the outcomes of patients with high RDW. Moreover, due to the characteristics of patients with DFUs, with limited mobility, interventions on RDW might differ from the aforementioned patients.
In this study, the discriminating ability of RDW/ALB for all-cause mortality was significantly superior to RDW in younger, healthier, and less severely ill patients by comparative analysis of ROC curves. The ROC curves analysis also indicated that RDW/ALB ratio had better predictive power for mortality than RDW in patients undergoing burn surgery and with acute respiratory distress syndrome [12, 43]. ALB was widely applied to assess the nutritional status and reflect the systemic inflammation [44]. Hypoalbuminemia was associated with mortality in various diseases and healthy individuals [45]. The combination of RDW and ALB may be more strongly associated with mortality than a single indicator in a particular clinical situation.
The key strengths of this study include the relatively large sample size and the use of widely available and inexpensive parameters: RDW and RDW/ALB ratio, which can be used in a variety of clinical settings, even in some economically underdeveloped areas. However, there are still several limitations that should be considered. First, our analysis is restricted to all-cause mortality, not cause-specific mortality. However, it was considered to be difficult and subjective in classifying the cause of death in patients who have multiple problems, and the all-cause mortality was an objective and clinically useful endpoint [46]. Second, RDW and RDW/ALB ratio were assessed only at one-time point at baseline, and changes over time were not accounted for in this study. Finally, this is a hospital-based study conducted in a single tertiary academic hospital with patients having greater disease burden, hence, the results might not apply to other populations. Further studies in different settings and cohorts with dynamic observations of RDW and RDW/ALB ratio are needed to clarify the predictive value of increased RDW and RDW/ALB ratio.
Conclusion
The most important conclusions of this study are the following: In patients with DFUs, RDW and RDW/ALB ratio are independent prognostic markers for all-cause mortality. The discriminating ability of the RDW/ALB ratio for all-cause mortality was significantly superior to RDW in patients with no severe DFUs or no severe PAD, or young and middle-aged patients. The combination of RDW and ALB might give a more efficacious approach for the assessment of mortality in patients with mild DFUs. All in all, RDW and RDW/ALB ratio are simple and practical parameters that may be useful in risk stratification of patients with DFUs, sequentially improving outcomes of those high-risk patients by intensive management, which needs to be confirmed by further studies.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALB:
-
Albumin
- AUC:
-
Area under ROC curve
- BMI:
-
Body mass index
- C-index:
-
Harrell’s concordance index
- CI:
-
Confidence interval
- DBA:
-
Difference between areas
- DBP:
-
Diastolic blood pressure
- DFUs:
-
Diabetic foot ulcers
- eGFR:
-
Estimated glomerular filtration rate
- HbA1c:
-
Hemoglobin A1c
- Hb:
-
Hemoglobin
- HDL:
-
High-density lipoprotein
- HR:
-
Hazard ratio
- IDI:
-
Integrated discrimination improvement
- LDL:
-
Low-density lipoprotein
- NPV:
-
Negative predictive value
- NRI:
-
Net reclassification improvement index
- OS:
-
Overall survival
- PAD:
-
Peripheral artery disease
- PPV:
-
Positive predictive value
- Ref:
-
Reference
- ROC:
-
Receiver operating characteristic
- SBP:
-
Systolic blood pressure
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
References
Epps JA, Smart NA. Remote ischaemic conditioning in the context of type 2 diabetes and neuropathy: the case for repeat application as a novel therapy for lower extremity ulceration. Cardiovasc Diabetol. 2016;15(1):130. https://doi.org/10.1186/s12933-016-0444-z.
Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1):16. https://doi.org/10.1186/s13047-020-00383-2.
Weck M, Slesaczeck T, Paetzold H, Muench D, Nanning T, von Gagern G, et al. Structured health care for subjects with diabetic foot ulcers results in a reduction of major amputation rates. Cardiovasc Diabetol. 2013;12:45. https://doi.org/10.1186/1475-2840-12-45.
Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabetic Med J Br Diabetic Assoc. 2016;33(11):1493–8. https://doi.org/10.1111/dme.13054.
Fagher K, Löndahl M. The impact of metabolic control and QTc prolongation on all-cause mortality in patients with type 2 diabetes and foot ulcers. Diabetologia. 2013;56(5):1140–7. https://doi.org/10.1007/s00125-013-2860-x.
Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52(2):86–105. https://doi.org/10.3109/10408363.2014.992064.
Engström G, Smith JG, Persson M, Nilsson PM, Melander O, Hedblad B. Red cell distribution width, haemoglobin A1c and incidence of diabetes mellitus. J Intern Med. 2014;276(2):174–83. https://doi.org/10.1111/joim.12188.
Malandrino N, Wu WC, Taveira TH, Whitlatch HB, Smith RJ. Association between red blood cell distribution width and macrovascular and microvascular complications in diabetes. Diabetologia. 2012;55(1):226–35. https://doi.org/10.1007/s00125-011-2331-1.
Jie Chee Y, Seneviratna A, Joo Lim C, Chiong CX, Peh DS, Hawkins R, Chew DE, Dalan R. Red cell distribution width is associated with mortality and cardiovascular complications in diabetes mellitus in Singapore. Eur J Prev Cardiol. 2020;27(2):216–9. https://doi.org/10.1177/2047487319836854.
Cardoso CRL, Leite NC, Salles GF. Importance of hematological parameters for micro- and macrovascular outcomes in patients with type 2 diabetes: the Rio de Janeiro type 2 diabetes cohort study. Cardiovasc Diabetol. 2021;20(1):133. https://doi.org/10.1186/s12933-021-01324-4.
Zhou D, Wang J, Li X. The red blood cell distribution width-albumin ratio was a potential prognostic biomarker for diabetic ketoacidosis. Int J Gen Med. 2021;14:5375–80. https://doi.org/10.2147/ijgm.S327733.
Seo YJ, Yu J, Park JY, Lee N, Lee J, Park JH, Kim HY, Kong YG, Kim YK. Red cell distribution width/albumin ratio and 90-day mortality after burn surgery. Burns Trauma. 2022;10:50. https://doi.org/10.1093/burnst/tkab050.
Bilgin S, Kurtkulagi O, Atak Tel BM, Duman TT, Kahveci G, Khalid A, Aktas G. Does C-reactive protein to serum Albumin Ratio correlate with diabEtic nephropathy in patients with Type 2 dIabetes MEllitus? The CARE TIME study. Prim Care Diabetes. 2021;15(6):1071–4. https://doi.org/10.1016/j.pcd.2021.08.015.
Kocak MZ, Aktas G, Atak BM, Duman TT, Yis OM, Erkus E, Savli H. Is Neuregulin-4 a predictive marker of microvascular complications in type 2 diabetes mellitus? Eur J Clin Investig. 2020;50(3):e13206. https://doi.org/10.1111/eci.13206.
Aktas G, Sit M, Dikbas O, Tekce BK, Savli H, Tekce H, Alcelik A. Could red cell distribution width be a marker in Hashimoto’s thyroiditis? Exp Clin Endocrinol Diabetes. 2014;122(10):572–4. https://doi.org/10.1055/s-0034-1383564.
Aktas G, Sit M, Karagoz I, Erkus E, Ozer B, Kocak MZ, et al. Could red cell distribution width be a marker of thyroid cancer? J Coll Phys Surg Pak JCPSP. 2017;27(9):556–8.
Ustaoglu M, Aktas G, Avcioglu U, Bas B, Bahceci BK. Elevated platelet distribution width and red cell distribution width are associated with autoimmune liver diseases. Eur J Gastroenterol Hepatol. 2021;33(1S Suppl 1):e905–8. https://doi.org/10.1097/meg.0000000000002296.
Foy BH, Carlson JCT, Reinertsen E, Padros IVR, Pallares Lopez R, Palanques-Tost E, et al. Association of red blood cell distribution width with mortality risk in hospitalized adults with SARS-CoV-2 infection. JAMA Netw Open. 2020;3(9): e2022058. https://doi.org/10.1001/jamanetworkopen.2020.22058.
Park JH, Park KH, Yoon YK, Han SH, Lee JW. Red blood cell distribution width: a novel predictor of mortality following amputation in diabetic foot. Foot Ankle Orthop. 2022;7(1):2473. https://doi.org/10.1177/2473011421s00388.
Bravo-Molina A, Linares-Palomino JP, Vera-Arroyo B, Salmerón-Febres LM, Ros-Díe E. Inter-observer agreement of the Wagner, University of Texas and PEDIS classification systems for the diabetic foot syndrome. Foot Ankle Surg. 2018;24(1):60–4. https://doi.org/10.1016/j.fas.2016.10.009.
Hong J, Liu WY, Hu X, Jiang FF, Xu ZR, Li F, Shen FX, Zhu H. Association between heart rate-corrected QT interval and severe peripheral arterial disease in patients with type 2 diabetes and foot ulcers. Endocr Connect. 2021;10(8):845–51. https://doi.org/10.1530/ec-21-0140.
Hong J, Liu WY, Hu X, Chen WW, Jiang FF, Xu ZR, Shen FX, Zhu H. Free triiodothyronine and free triiodothyronine to free thyroxine ratio predict all-cause mortality in patients with diabetic foot ulcers. Diabetes Metab Syndr Obes Targets Ther. 2022;15:467–76. https://doi.org/10.2147/dmso.S354754.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45.
Sze S, Pellicori P, Kazmi S, Rigby A, Cleland JGF, Wong K, Clark AL. Prevalence and prognostic significance of malnutrition using 3 scoring systems among outpatients with heart failure: a comparison with body mass index. JACC Heart Fail. 2018;6(6):476–86. https://doi.org/10.1016/j.jchf.2018.02.018.
Oikonomou EK, Marwan M, Desai MY, Mancio J, Alashi A, Hutt Centeno E, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet (London, England). 2018;392(10151):929–39. https://doi.org/10.1016/s0140-6736(18)31114-0.
Arıcan G, Kahraman H, Özmeriç A, İltar S, Alemdaroğlu KB. Monitoring the prognosis of diabetic foot ulcers: predictive value of neutrophil-to-lymphocyte ratio and red blood cell distribution width. Int J Low Extremity Wounds. 2020;19(4):369–76. https://doi.org/10.1177/1534734620904819.
Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133(4):628–32. https://doi.org/10.5858/133.4.628.
Semba RD, Patel KV, Ferrucci L, Sun K, Roy CN, Guralnik JM, Fried LP. Serum antioxidants and inflammation predict red cell distribution width in older women: the Women’s Health and Aging Study I. Clin Nutr (Edinburgh, Scotland). 2010;29(5):600–4. https://doi.org/10.1016/j.clnu.2010.03.001.
Kozlitina J, Garcia CK. Red blood cell size is inversely associated with leukocyte telomere length in a large multi-ethnic population. PLoS ONE. 2012;7(12): e51046. https://doi.org/10.1371/journal.pone.0051046.
Lippi G, Mercadanti M, Aloe R, Targher G. Erythrocyte mechanical fragility is increased in patients with type 2 diabetes. Eur J Intern Med. 2012;23(2):150–3. https://doi.org/10.1016/j.ejim.2011.11.004.
Sun H, Weaver CM. Decreased iron intake parallels rising iron deficiency anemia and related mortality rates in the US population. J Nutr. 2021;151(7):1947–55. https://doi.org/10.1093/jn/nxab064.
Pierce CN, Larson DF. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion. 2005;20(2):83–90. https://doi.org/10.1191/0267659105pf793oa.
Laftah AH, Sharma N, Brookes MJ, McKie AT, Simpson RJ, Iqbal TH, Tselepis C. Tumour necrosis factor alpha causes hypoferraemia and reduced intestinal iron absorption in mice. Biochem J. 2006;397(1):61–7. https://doi.org/10.1042/bj20060215.
Jelkmann W. Proinflammatory cytokines lowering erythropoietin production. J Interf Cytokine Res. 1998;18(8):555–9. https://doi.org/10.1089/jir.1998.18.555.
Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–23. https://doi.org/10.1056/NEJMra041809.
Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal. 2008;10(11):1923–40. https://doi.org/10.1089/ars.2008.2142.
Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, Buxton JL, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45(4):422–7, 7e1–2. https://doi.org/10.1038/ng.2528
Xi H, Li C, Ren F, Zhang H, Zhang L. Telomere, aging and age-related diseases. Aging Clin Exp Res. 2013;25(2):139–46. https://doi.org/10.1007/s40520-013-0021-1.
Shih HM, Wu CJ, Lin SL. Physiology and pathophysiology of renal erythropoietin-producing cells. J Formos Med Assoc Taiwan yi zhi. 2018;117(11):955–63. https://doi.org/10.1016/j.jfma.2018.03.017.
Ujszaszi A, Molnar MZ, Czira ME, Novak M, Mucsi I. Renal function is independently associated with red cell distribution width in kidney transplant recipients: a potential new auxiliary parameter for the clinical evaluation of patients with chronic kidney disease. Br J Haematol. 2013;161(5):715–25. https://doi.org/10.1111/bjh.12315.
Nishiyama Y, Niiyama H, Harada H, Katou A, Yoshida N, Ikeda H. Effect of exercise training on red blood cell distribution width as a marker of impaired exercise tolerance in patients with coronary artery disease. Int Heart J. 2016;57(5):553–7. https://doi.org/10.1536/ihj.16-015.
Vashistha T, Streja E, Molnar MZ, Rhee CM, Moradi H, Soohoo M, Kovesdy CP, Kalantar-Zadeh K. Red cell distribution width and mortality in hemodialysis patients. Am J Kidney Dis. 2016;68(1):110–21. https://doi.org/10.1053/j.ajkd.2015.11.020.
Yoo JW, Ju S, Lee SJ, Cho YJ, Lee JD, Kim HC. Red cell distribution width/albumin ratio is associated with 60-day mortality in patients with acute respiratory distress syndrome. Infect Dis (London, England). 2020;52(4):266–70. https://doi.org/10.1080/23744235.2020.1717599.
Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. 2019;43(2):181–93. https://doi.org/10.1002/jpen.1451.
Gatta A, Verardo A, Bolognesi M. Hypoalbuminemia. Intern Emerg Med. 2012;7(Suppl 3):S193–9. https://doi.org/10.1007/s11739-012-0802-0.
Gottlieb SS. Dead is dead—artificial definitions are no substitute. Lancet (London, England). 1997;349(9053):662–3. https://doi.org/10.1016/s0140-6736(97)22010-6.
Acknowledgements
The authors like to acknowledge all the patients who participated in the study. The authors are also grateful to Dr. Xiaobin Gao and Jiahong Jiang for their contributions to additional data collection.
Funding
National Natural Science Foundation of China (81900737), the Basic Scientific Research Program of Wenzhou Medical University, China (KYYW202015).
Author information
Authors and Affiliations
Contributions
JH and HZ contributed to the conception and design of the study. JH, W-YL, X–HQ, F-FJ, and Z-RX contributed to the acquisition, analysis, and interpretation of the data. JH drafted the manuscript. JH, XH, W-YL, F-XS and HZ revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the ethics committee of the First Affiliated Hospital of Wenzhou Medical University. Due to the retrospective nature of the study, the informed consent was exempted.
Consent for publication
Not applicable.
Competing interests
All authors: no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1
. ROC curves of RDW and RDW/ALB ratio for predicting the all-cause mortality in: (A) patients with no severe DFUs, (B) patients with severe DFUs, (C) patients with no severe PAD, (D) patients with severe PAD, (E) young and middle-aged patients, and (F) the elderly. The discriminating ability of the RDW/ALB ratio was superior to RDW in patients with (A) no severe DFUs, (C) no severe PAD, or in (E) young and middle-aged patients (all P < 0.05). ROC: receiver operating characteristic; RDW: red cell distribution width; ALB: albumin; DFUs: diabetic foot ulcers; PAD: peripheral artery disease.
Additional file 2: Table S1
. Unadjusted Cox regression analyses for all-cause mortality.
Additional file 3: Table S2
. Diagnostic performances of optimal cut-off values of RDW and RDW/ALB ratio.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hong, J., Hu, X., Liu, W. et al. Impact of red cell distribution width and red cell distribution width/albumin ratio on all-cause mortality in patients with type 2 diabetes and foot ulcers: a retrospective cohort study. Cardiovasc Diabetol 21, 91 (2022). https://doi.org/10.1186/s12933-022-01534-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01534-4