Abstract
Background
Type 2 diabetes mellitus (DM) is one of the most common comorbidities among patients with heart failure (HF) with reduced ejection fraction (HFrEF). There are limited data regarding efficacy of hybrid comprehensive telerehabilitation (HCTR) on cardiopulmonary exercise capacity in patients with HFrEF with versus those without diabetes.
Aim
The aim of the present study was to analyze effects of 9-week HCTR in comparison to usual care on parameters of cardiopulmonary exercise capacity in HF patients according to history of DM.
Methods
Clinically stable HF patients with left ventricular ejection fraction [LVEF] < 40% after a hospitalization due to worsening HF within past 6 months were enrolled in the TELEREH-HF (The TELEREHabilitation in Heart Failure Patients) trial and randomized to the HCTR or usual care (UC). Cardiopulmonary exercise tests (CPET) were performed on treadmill with an incremental workload according to the ramp protocol.
Results
CPET was performed in 385 patients assigned to HCTR group: 129 (33.5%) had DM (HCTR-DM group) and 256 patients (66.5%) did not have DM (HCTR-nonDM group). Among 397 patients assigned to UC group who had CPET: 137 (34.5%) had DM (UC-DM group) and 260 patients (65.5%) did not have DM (UC-nonDM group). Among DM patients, differences in cardiopulmonary parameters from baseline to 9 weeks remained similar among HCTR and UC patients. In contrast, among patients without DM, HCTR was associated with greater 9-week changes than UC in exercise time, which resulted in a statistically significant interaction between patients with and without DM: difference in changes in exercise time between HCTR versus UC was 12.0 s [95% CI − 15.1, 39.1 s] in DM and 43.1 s [95% CI 24.0, 63.0 s] in non-DM, interaction p-value = 0.016. Furthermore, statistically significant differences in the effect of HCTR versus UC between DM and non-DM were observed in ventilation at rest: − 0.34 l/min [95% CI − 1.60, 0.91 l/min] in DM and 0.83 l/min [95% CI − 0.06, 1.73 l/min] in non-DM, interaction p value = 0.0496 and in VE/VCO2 slope: 1.52 [95% CI − 1.55, 4.59] for DM vs. − 1.44 [95% CI − 3.64, 0.77] for non-DM, interaction p value = 0.044.
Conclusions
The benefits of hybrid comprehensive telerehabilitation versus usual care on the improvement of physical performance, ventilatory profile and gas exchange parameters were more pronounced in patients with HFrEF without DM as compared to patients with DM.
Trial registration: ClinicalTrials.gov Identifier: NCT02523560. Registered 3rd August 2015. https://clinicaltrials.gov/ct2/show/NCT02523560?term=NCT02523560&draw=2&rank=1. Other Study ID Numbers: STRATEGME1/233547/13/NCBR/2015
Similar content being viewed by others
Introduction
Heart failure patients (HF) frequently present with comorbidities, which affect their prognosis and quality of life, including everyday activities and exercise performance. Type 2 diabetes mellitus (DM) is one of the most common comorbidities among patients with HF with reduced ejection fraction (HFrEF). Diabetes is associated with poorer functional status and worse prognosis in patients with HFrEF. Patients with DM often complain of fatigue and reduced exercise capacity [1].
Recent trials have shown that incidence of hospitalization due to HF were two-fold higher in diabetic patients compared with those without DM [2, 3]. Some trials and meta-analysis supported evidence that cardiac rehabilitation might be associated with modest reduction in HF hospitalization in the short term (up to 12 months) follow-up [4, 5]. Meta-analysis of 33 trials [6] confirmed the benefit of physical training on quality of life and exercise capacity. Based on numerous publications [7] and the latest European Society of Cardiology (ESC) guidelines, comprehensive cardiac rehabilitation has a strong evidence for improving cardiopulmonary exercise capacity and exercise tolerance in patients with HF [8]. The last 2020 Sports Cardiology ESC guidelines underlined that exercise-based cardiac rehabilitation is recommended in all stable individuals [8] to improve exercise capacity, quality of life, and to reduce the frequency of hospital readmission [7]. Also, intensive lifestyle intervention with focusing on increased physical activity is recommended by American Diabetes Association for diabetic patients [9, 10].
Cardiopulmonary exercise testing (CPET) has proven to be useful for quantifying aerobic capacity and is valuable for identifying exercise tolerance in patients with cardiac diseases [11, 12], and to assess functional capacity, exercise-induced arrhythmias, and haemodynamic abnormalities [13]. According to the current ESC guidelines for the diagnosis and treatment of acute and chronic heart failure, CPET should be considered to optimize exercise training (class IIa/level of evidence C) [14]. Cardiopulmonary exercise testing allows safe, non-invasive, and reliable assessment of overall fitness which incorporates cardiovascular, pulmonary and musculoskeletal responses to exercise [15, 16]. It is a method combining continuous expired gases analysis, electrocardiography, blood pressure measurements and oxygen saturation monitoring during gradually increasing workload [17]. This method provides breath-by-breath gas exchange measures [18] of variables such as O2 uptake (VO2), carbon dioxide output (VCO2), and ventilation (VE), which are used to derive various other gas exchange patterns that reflect organ-specific maladaptive responses to exercise [16].
The expert group recommends assessment of the clinical impact of CPET as a high research priority [19]. Since significant proportion of HF patients presents with DM there is interest in assessing effects of cardiac rehabilitation in DM vs. non-DM patients. Our post-hoc analysis from the Telerehabilitation in Heart Failure Patients (TELEREH-HF) randomized clinical trial [20, 21] provides interesting insight into differences in response to rehabilitation in patients with and without DM.
The aim of the present subanalysis from the TELEREH-HF trial [20, 21] was to compare cardiopulmonary exercise capacity in HFrEF patients undergoing hybrid comprehensive telerehabilitation (HCTR) or usual care (UC) in patients with and without diabetes.
Methods
The design and main results of the TELEREH-HF trial have been presented already elsewhere [20, 21]. The TELEREH-HF study was a randomized, multi-center, prospective, open-label, parallel group, controlled trial, which enrolled clinically stable HF patients [New York Heart Association (NYHA) class I, II or III] with left ventricular ejection fraction (LVEF) ≤ 40% after a hospitalization due to worsening HF within 6 months prior to randomization.
All HCTR patients from both subgroups (with and without DM), underwent a 9-week HCTR program consisting of two stages: an initial stage (1 week) conducted in hospital and a basic home-based stage (8 weeks) with HCTR performed five times weekly (see Additional file 1: Table S1). Patients in UC were managed according to routine standard of care.
HCTR was conducted by a medical team (physicians, physiotherapists, nurses and psychologist). The monitoring system was composed of a remote device for tele-ECG-monitoring and supervised exercise training (the telerehabilitation set), mobile phone, and a monitoring center.
The research protocol was registered in a clinical database (ClinicalTrials.gov NCT02523560) and followed the ethical guidelines of Declaration of Helsinki. The ethics committee approved the research protocol. Informed consent was obtained from patients.
Cardiopulmonary exercise testing
In this subanalysis, data on cardiopulmonary exercise capacity in HF patients with and without DM undergoing HCTR or UC were compared. Functional capacity parameters were evaluated using symptom-limited CPET on treadmill (Schiller MTM-1500 med) with an incremental workload according to the ramp protocol equal for all patients. The initial treadmill speed and slope were 1.5 mph and 1.5%, respectively. In every 30 s, treadmill speed and slope were increased by 0.5 mph and 0.5%, respectively. Maximal exercise was defined as the respiratory exchange ratio (RER) ≥ 1 or maximum fatigue according to the Borg scale (fatigue level 14–16 on a 20-point scale). Oxygen uptake was analyzed on a continuous breath-by-breath basis. Obtained peak oxygen uptake (VO2 peak) values are presented per kilogram of mass body per minute (ml/kg/min) and as a percentage of normal for each patient called percent-predicted VO2 (pVO2% N) with gender, age, weight and height according to the criteria developed by Wasserman.
Ventilation and gas exchange parameters acquired from CPET comprised exercise duration time, carbon dioxide production (VCO2), minute ventilation (VE), breathing rate and slope of ventilatory equivalent for carbon dioxide (VE/VCO2 slope). To determine ventilatory anaerobic threshold (VAT) the V-slope method of plotting VCO2 against VO2 was applied.
The test was continued until symptoms indicating the need to discontinue the test appeared, in accordance with ESC recommendations [22].
Statistical analysis
Results are expressed as means ± SD (baseline characteristics) or means and 95% confidence limits (difference between 9-week value and baseline value) for continuous variables or counts and percentages for categorical variables. Comparisons between groups at baseline characteristics were made using chi-square test of independence or Fisher’s exact test (when the number of expected events was less than 5), Cochran Mantel–Haenszel test or Student’s t-tests (or Satterthwaite’s method), as appropriate. The differences in changes over time between the groups were compared using Two-Way Analysis of Variance with baseline measurement as covariance. Interaction was tested. A two-sided p < 0.05 was considered statistically significant. All analyses were performed using SAS statistical software version 9.4 (SAS Institute, Inc., Cary, North Carolina, USA).
Results
Baseline characteristics
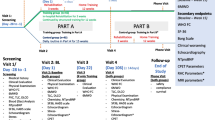
Between June 8th, 2015 and June 28th, 2017, 850 eligible patients were randomized in a 1:1 ratio to either HCTR (HCTR group) or to UC (UC group). 425 patients of either sex with HF with reduced ejection fraction (HFrEF), enrolled in the TELEREH-HF trial with no contraindication to training and able to undergo HCTR were randomized to HCTR arm (Additional file 1: Table S2). Among 850 enrolled patients, 291 (34.2%) patients had DM. Among 425 patients assigned to HCTR group, CPET was performed twice before and after telerehabilitation programme in 385 patients: 129 (33.5%) had DM (HCTR-DM group) and 256 patients (66.5%) did not have DM (HCTR-nonDM group). Among 425 patients assigned to UC group CPET was done twice in 397 patients: 137 (34.5%) had DM (UC-DM group) and 260 patients (65.5%) did not have DM (UC-nonDM group). List of the reason for lack of both CPETs and baseline characteristics of 68 patients comparing to those included in our paper are presented in the Additional file 1: Table S3. The study flow diagram is presented in Fig. 1. Study arms HCTR vs UC were not significantly different by randomization in terms of demographic data, baseline clinical parameters, and treatment. The baseline characteristics of the cohort at randomization are presented in Table 1.
Study design. HCTR-DM patients in hybrid comprehensive telerehabilitation arm with heart failure and diabetes, HCTR-nonDM patients in hybrid comprehensive telerehabilitation arm with heart failure and without diabetes, HFrEF heart failure with reduced ejection fraction, UC-DM patients in usual care arm with heart failure and diabetes, UC-nonDM patients in usual care arm with heart failure and without diabetes
Cardiopulmonary capacity
There was no difference in CPET parameters between HCTR and UC arms at the time of randomization (Table 2). But at the time of randomization the groups with and without DM differed in terms of CPET parameters. The baseline exercise time was longer in nonDM patients than DM patients in both HCTR arm (420 ± 185 vs. 338 ± 163 [s], p < 0.001) and in UC arm (407 ± 188 vs. 327 ± 163 [s], p < 0.001). At randomization VO2 peak was greater in patients without DM as compared to those with DM in HCTR group (18.1 ± 5.8 vs. 15.3 ± 4.5 [ml/min/kg], p < 0.001) and in UC group (17.7 ± 6.3 vs. 15.1 ± 5.1 [ml/min/kg], p < 0.001). Percent-predicted VO2 (%) was higher at randomization among patients without DM than in patients with DM both in HCTR arm (59.6 ± 20.7 vs. 49.4 ± 17.9 [%], p < 0.001) and UC arm (58.7 ± 22.0 vs. 47.0 ± 16.5 [%], p < 0.001). At the time of randomization ventilatory anaerobic threshold was greater in nonDM patients as compared to DM patients, either after HCTR (15.9 ± 5.7 vs. 13.6 ± 4.5 [ml/kg/min], < 0.001) and UC (15.6 ± 5.8 vs. 13.9 ± 5.5 [ml/kg/min], p = 0.029). Difference in VO2 peak was higher in HCTR-nonDM group when compared to UC-nonDM (1.33 [95% CI 0.92; 1.74] vs. 0.07 [95% CI − 0.33; 0.48] ml/min/kg, p < 0.001). Only in UC arm VE/VCO2 slope was lower in nonDM patients than in DM patients (29.3 ± 11.0 vs. 31.8 ± 10.5, p = 0.033). Other important CPET parameters did not differ between patients with and without DM at the time of randomization.
As presented in Table 3, among DM patients, cardiopulmonary parameters remained similar from baseline to 9 weeks after telerehabilitation or observation. In HCTR-DM group, VAT was achieved in 64 patients (55.2%) at baseline and in 70 patients (60.3%, p = 0.601) after telerehabilitation programme. In UC-DM group, VAT was achieved in 67 patients (55.8%) at baseline and in 64 patients (53.3%, p = 0.601) after 9-week UC.
Among patients without DM, exercise time increased significantly more in HCTR as compared to UC—change after 9 weeks from baseline: 56.7 s [95% CI 46.1, 67.3 s] vs. 13.6 s [95% CI 3.2, 24.1], p-value < 0.001. In HCTR-nonDM group patients, VAT was reached more often after 9-week telerehabilitation (in 149 patients [68.7%] vs. 129 patients [61.1%], p = 0.042, after 9 weeks vs. at baseline, respectively). In UC-nonDM group, VAT was reached in 129 patients (58.9%) at baseline and in 140 patients (63.9%, p = 0.116) after 9-week UC. But not significant changes in VAT were noticed both in patients with and without DM. All alterations from baseline to 9 weeks in parameters of cardiopulmonary capacity in patients without diabetes mellitus are shown in Table 4.
The distinct differences were noticed between patients with and without DM after HCTR vs. UC in CPET parameters such as exercise time (12.0 s [95% CI − 15.1, 39.1 s] vs. 43.1 s [95% CI 24.0, 63.0 s], respectively, interaction p-value = 0.016), ventilation at rest (− 0.34 l/min [95% CI − 1.60, 0.91 l/min] vs. 0.83 l/min [95% CI − 0.06, 1.73 l/min], respectively, interaction p value = 0.0496) and VE/VCO2 slope (1.52 [95% CI − 1.55, 4.59] vs. − 1.44 [95% CI − 3.64, 0.77], respectively, interaction p value = 0.044). All differences in CPET parameters from baseline to 9 weeks are presented on Fig. 2.
Forest plots of changes in parameters of main cardiopulmonary capacity in patients with and without diabetes. delta alteration in CPET parameters between baseline and 9-week based on formula: HCTR-UC for DM minus HCTR-UC for non-DM, DM patients with diabetes, nonDM patients without diabetes, VO2 peak peak oxygen uptake, VAT ventilatory anaerobic threshold, VE/VCO2 slope slope of ventilatory equivalent for carbon dioxide
Our study also provided evidence that both HCTR and UC are similarly safe in DM as in nonDM without significant adverse events, therefore participation in rehabilitation of HF patients with diabetes should be encouraged.
Discussion
Our study is the first to present the characteristics of cardiac performance parameters in large group of HF patients with vs. without diabetes undergoing cardiac rehabilitation emplying innovative HCTR vs. UC. We have to emphasize that the randomization in the TELEREH-HF trial was done to compare HFrEF patients undergoing HCTR vs. UC and analyses of DM vs. nonDM groups is a secondary post-hoc analysis. The patients with and without DM were initially different in their clinical characteristics (Additional file 1: Table S4) and the CPET dissimilarities (Additional file 1: Table S5) were therefore consequences of its metabolic disparities. Till now, there were no large available data on the CPET in patients with HFrEF and DM. Therefore, it seems that our study is the first to present the characteristics of cardiac performance parameters in this specific but numerous group of patients.
In this substudy of the TELEREH-HF clinical trial, we analyzed effects of 9-week HCTR in comparison to usual care on parameters of cardiopulmonary exercise capacity in HF patients according to history of DM. Patients with DM accounted for about one-third of studied patients. Among DM patients, differences in cardiopulmonary parameters from baseline to 9 weeks remained similar among HCTR and UC patients. In contrast, among patients without DM, HCTR was associated with greater 9-week changes than UC in exercise time, in ventilation at rest and in VE/VCO2 slope. The benefits of HCTR versus UC on the improvement of physical performance, ventilatory profile and gas exchange parameters were found to be more pronounced in patients with HFrEF without DM as compared to patients with DM.
Measured VO2 during a maximal symptom-limited CPET is the most objective method to assess functional capacity and consists of the following components such as maximal heart rhythm, stroke volume, the net oxygen extraction of the peripheral tissues. Peak oxygen consumption is an important predictor of prognosis in HF patients [23]. In patients with HF, the important prognostic value of a reduced peak VO2 has been studied in detail to identify patients at higher risk.
In one recent trial, among many CPET variables assessed in patients with HF, VO2 peak, percent predicted VO2, and exercise duration had the strongest ability to predict mortality in HFrEF [24]. In line with that, improvements of cardiopulmonary capacity determined by VO2 peak, percent predicted VO2 and distance, was observed in our study in majority of patients after HCTR. An aerobic exercise training has been recommended as non-pharmacological treatment for patients with HFrEF. As we described in the previous article of TELEREH-HF trial, the HCTR intervention was effective at 9 weeks, significantly improving VO2 peak (0.95 [95% CI 0.65–1.26] ml/kg/min vs. 0.00 [95% CI − 0.31 to 0.30] ml/kg/min; p < 0.001) [20]. EMPA-TROPISM Trial with 84 HFrEF patients demonstrated that empagliflozin was associated with significant improvements in peak VO2 (1.1 ± 2.6 ml/min/kg vs. − 0.5 ± 1.9 ml/min/kg for empagliflozin vs. placebo; p = 0.017) [25].
Some studies have found decline in VO2 peak of 20–30% in population of diabetic patients without cardiovascular disorders. Authors of the recent paper tried to explain impaired cardiopulmonary capacity in diabetic patients by two underlying mechanism [26, 27]. It is the first one, called myocardiogenic determinants, an insufficient cardiac function reduces muscle perfusion, and thus determines insufficient muscle energy production and strength. In the hypothesis with skeletal myogenic determinants, reduced VO2 peak and peripheral oxygen extraction are consequence of slower muscle blood flow adjustment and early stimulation of the muscle metaboreflex [28]. In study by Ishihara et al. performed on group of 69 HF patients (with preserved or moderate EF) VO2 peak was lower in 14 DM patients when compared to 55 patients without DM (13.0 ± 2.2 vs. 14.9 ± 4.4 ml/kg/min, p < 0.05, respectively) [29].
In the recent study, a multivariate analysis showed that the history of DM was an independent predictor of lower VO2 peak in HF patients, but the impact on exercise capacity was dependent on the systolic dysfunction [30]. This is in line with the results of our study, where VO2 peak was lower in DM patients with HFrEF. Our baseline data of cardiopulmonary exercise profile are consistent with recent data from the study concerning exercise capacity in patients with HFrEF and metabolic syndrome [31] as well as from the relatively small study with HFrEF and DM [25].
Oxygen consumption at the ventilatory threshold (VAT) is another measurement of O2 uptake that provides valuable information at submaximal exercise. It represents upper border of workload which can be sustained for a prolonged period of exercise [32]. But, in the most advanced stages of HF a clear VAT is often not identifiable, which is indices for poor prognosis [33]. Not only lower VO2 peak but also reduced VAT was noticed in the diabetic patients with both HFrEF and HF with preserved ejection fraction [30]. In our work, only in HCTR-nonDM, VAT was reached more often. It cannot be interpreted as a weak cardiac telerehabilitation effect in the group of patients with DM, but this result might be a consequence of too short telerehabilitation duration. Maybe to gain better results in subpopulation in patients with HFrEF and DM, there is a need for prolongation of cardiac rehabilitation.
Other ventilatory parameters, such as increased ventilatory efficiency (expressed as ventilation to carbon dioxide production, VE/VCO2 slope) during exercise have been observed and associated with poor prognosis in HF [34, 35]. During exercise, efficient pulmonary gas exchange is characterized by uniform matching of lung ventilation with perfusion. By contrast, mismatching is marked by inefficient pulmonary gas exchange, requiring increased ventilation for a given CO2 production [36]. Inefficient ventilatory response to exercise in heart disease is multifactorial. Previous investigations found ventilation/perfusion inequalities during exercise in HF patients [37]. In fact, this finding suggests pathologically high ventilation/perfusion mismatching defined as a reduced or absent perfusion in well ventilated lung.
Notably, substantial evidence demonstrated that ventilatory inefficiency is an independent powerful prognostic marker for cardiac mortality or hospitalization in HF patients. VE/VCO2 slope broadly reflects disease severity in population of HF patients. VE/VCO2 slope as a continuous variable may be useful as a predictor for major cardiac events in patients with HF. VE/VCO2 slope can provide prognostic information beyond VO2 peak [38]. The inappropriate ratio of hyperventilation over CO2 production may reflect autonomic dysfunction and altered central control of breathing, but there are a few physiological mechanism of that phenomenon in DM. Steeper VE/VCO2 slope was observed in diabetic patients with cardiac autonomic dysfunction [39]. In recent study, there was present only a trend toward improvement in the VE/VCO2 in the empagliflozin versus placebo group (− 1.2 ± 3.4 vs. 0.5 ± 3.9, respectively; p = 0.09) [25].
Special focus was dedicated to the role of aerobic exercise training in improving the indices of ventilatory efficiency among patients with HFrEF, as well as to the underlying mechanisms involved. We observed improvement in ventilatory drive after HCTR. In literature, there is scarce data regarding ventilatory and capacity profile of CPET parameters among diabetic patients. Albeit a specific derangement of the alveolar capillary membrane in diabetic patients has been suggested [40].
In contrary to our work, in population without HF, DM was associated with greater improvements in response to exercise programme, when compared to the non-diabetic controls undergoing the same training [41]. There is literature bias, if exercise training may be effective in increasing VO2 peak in patients with DM. The physiological explanation for that observation is that the muscle deconditioning is not the only one mechanism of altered cardiopulmonary capacity, as mentioned previously. As it is well known effort has a positive effect on the course of diabetes in terms of its control.
As we described in previous substudy of TELEREH-HF trial, HCTR might have had beneficial effects on cardiopulmonary exercise test time after 9 weeks in patients with ischaemic HF, but it not met statistical significance from that observed among non-ischaemic HF patients [42]. Although, in our study there were higher percentage of ischaemic etiology among DM patients (Additional file 1: Table S4), we did not observed improvement in profile of cardiopulmonary capacity in patients with HFrEF and DM.
Less effective 9-week comprehensive telerehabilitation program in patients with DM may indicate the need to diversify training in these groups. This hypothesis fits nicely with the fashionable and right concept of ‘tailored training’ and ‘personalized therapy’ for some patients’ party, in this case DM patients. Initial conclusions may lead to statement consistent with European recommendations for exercise programme in DM populations. According to the 2020 ESC Guidelines on sports cardiology [8] and exercise in patients with CVD the ideal exercise programme in subjects with diabetes is daily exercise of at least moderate intensity. Among recommended activity there should we brisk walking, for at least 30 min, resistance training for 15 min on most days and lighter-intensity activities for at least 30 min. In case of microvascular complications due to diabetes those activities might be supplemented by flexibility and balance exercise. As we know, rehabilitation programme combining aerobic and resistance training [43] has been shown to be superior in diabetic population, whereas the effects on the outcomes are unproven [44]. Patients with DM are more demanding and need longer protocols and more intensive aerobic exercise training programme with health life counselling [45].
The optimal duration, volume and intensity of comprehensive cardiac rehabilitation programme in patients with cardiac comorbidities and DM vary with respect to the training goals. It should be definitely personalized. Patients severely detrained and with HF should start exercising at low intensity, and then with incremental workload. High-volume and moderate intensity training are recommended for improving body composition and CV risk factors. High intensity interval programme can be helpful for improvements in exercise capacity and glycaemic control, with goal of better prognosis. If cardiorespiratory fitness is a target for rehabilitation programme in patients with DM, combined aerobic and resistance training is preferred [45]. Lifestyle education and dietary counselling are crucial during comprehensive cardiac rehabilitation programme. In order to improve adherence, patient with DM should be engaged in home-based rehabilitation programme [46]. Nevertheless, rehabilitation programmes based on exercise in population with HFrEF and DM are associated with the higher risk of post-exercise hypotension, arrhythmias and HF decompensation.
Because of the study design with randomization of eligible patients in a 1:1 ratio (block size of 2, stratified by site) to HCTR or UC group, we decided to present and discuss reliable data in four subgroups (HCTR-DM, HCTR-nonDM, UC-DM, UC-nonDM). This study was not randomizing patients with vs. without diabetes and the analyses presented are post-hoc analyses, nevertheless, based on large well-balanced patient subgroups with and without diabetes.
Conclusions
The beneficial effect of hybrid comprehensive telerehabilitation versus usual care on the improvement of physical performance, ventilatory profile and gas exchange parameters in cardiopulmonary exercise test was observed only in patients with HFrEF without DM.
Availability of data and materials
An independent data safety monitoring board reviewed patient data, and a clinical end point committee, blinded to treatment allocation, was appointed to adjudicate deaths and hospitalizations. The study followed the Consolidated Standards of Reporting Trials guidelines.
Abbreviations
- CPET:
-
Cardiopulmonary exercise test
- DM:
-
Diabetes mellitus
- HCTR:
-
Hybrid comprehensive telerehabilitation
- HCTR-nonDM:
-
Hybrid comprehensive telerehabilitation group without diabetes
- HCTR-DM:
-
Hybrid comprehensive telerehabilitation group with diabetes
- HF:
-
Heart failure
- HFrEF:
-
Heart failure with reduced ejection fraction
- VAT:
-
Ventilatory threshold
- VCO2 :
-
Carbon dioxide production
- VE:
-
Minute ventilation
- VE/VCO2slope:
-
Slope of ventilatory equivalent for carbon dioxide or “ventilatory efficiency”
- VO2 :
-
Oxygen uptake
- VO2peak:
-
Peak oxygen uptake
- UC:
-
Usual care
- UC-nonDM:
-
Usual care group with diabetes
- UC-DM:
-
Usual care group without diabetes
References
Fang ZY, Sharman J, Prins JB, Marwick TH. Determinants of exercise capacity in patients with type 2 diabetes. Diabetes Care. 2005;28:1643–8.
McAllister DA, Read SH, Kerssens J, et al. Incidence of hospitalization for heart failure and case-fatality among 3.25 million people with and without diabetes mellitus. Circulation. 2018;138:2774–86.
Cavender MA, Steg PG, Smith SC Jr, REACH Registry Investigators, et al. Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: outcomes at 4 years from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation. 2015;132:923–31.
Taylor RS, Walker S, Smart NA, et al. Impact of exercise-based cardiac rehabilitation in patients with heart failure (ExTraMATCH II) on mortality and hospitalisation: an individual patient data meta-analysis of randomised trials. Eur J Heart Fail. 2018;20:1735–43.
O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–50.
Taylor RS, Walker S, Smart NA, et al. Impact of exercise rehabilitation on exercise capacity and quality-of-life in heart failure: individual participant meta-analysis. J Am Coll Cardiol. 2019;73:1430–43.
Long L, Mordi IR, Bridges C, et al. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev. 2019;1:CD003331.
Pelliccia A, Sharma S, Gati S, et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease The Task Force on sports cardiology and exercise in patients with cardiovascular disease of the European Society of Cardiology (ESC). Eur Heart J. 2020. https://doi.org/10.1093/eurheartj/ehaa605.
Disease C, Management R. Standards of medical care in diabetes—2021. Am Diabetes Assoc Diabetes Care. 2021;44(Supplement 1):S125–50. https://doi.org/10.2337/dc21-S010.
Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065–79. https://doi.org/10.2337/dc16-1728.
Arena R, Myers J, Guazzi M. The clinical and research applications of aerobic capacity and ventilatory efficiency in heart failure: an evidence-based review. Heart Fail Rev. 2008;13:245–69. https://doi.org/10.1007/s10741-007-9067-5.
Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128:873–934. https://doi.org/10.1161/CIR.0b013e31829b5b44.
Corra U, Agostoni PG, Anker SD, et al. Role of cardiopulmonary exercise testing in clinical stratification in heart failure. A position paper from the Committee on exercise physiology and training of the heart failure association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:3–15.
Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975.
Albouaini K, Egred M, Alahmar A, Wright DJ. Cardiopulmonary exercise testing and its application. Postgrad Med J. 2007;83(985):675–82. https://doi.org/10.1136/hrt.2007.121558.
Malhotra R, Bakken K, D’Elia E, Lewis GD. Cardiopulmonary exercise testing in heart failure. JACC Heart Fail. 2016;4(8):607–16. https://doi.org/10.1016/j.jchf.2016.03.022.
Guazzi M, Bandera F, Ozemek C, Systrom D, Arena R. Cardiopulmonary exercise testing: what is its value? J Am Coll Cardiol. 2017;70(13):1618–36. https://doi.org/10.1016/j.jacc.2017.08.012.
Balady GJ, Arena R, Sietsema K, et al. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225.
Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. 2018;39:1144–61.
Piotrowicz E, Pencina MJ, Opolski G, et al. Effects of a 9-week hybrid comprehensive telerehabilitation program on long-term outcomes in patients with heart failure: the telerehabilitation in heart failure patients (TELEREH-HF) randomized clinical trial. JAMA Cardiol. 2020;5(3):300–8. https://doi.org/10.1001/jamacardio.2019.5006.
Piotrowicz E, Piotrowicz R, Opolski G, Pencina M, Banach M, Zaręba W. Hybrid comprehensive telerehabilitation in heart failure patients (TELEREH-HF): a randomized, multicenter, prospective, open-label, parallel group controlled trial-study design and description of the intervention. Am Heart J. 2019;217:148–58. https://doi.org/10.1016/j.ahj.2019.08.015.
Piepoli MF, Corrà U, Agostoni PG, et al. Task Force of the Italian Working Group on Cardiac Rehabilitation and Prevention (Gruppo Italiano di Cardiologia Riabilitativa e Prevenzione, GICR); Working Group on Cardiac Rehabilitation and Exercise Physiology of the European Society of Cardiology. Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction: recommendations for performance and interpretation Part II: how to perform cardiopulmonary exercise testing in chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2006;13(3):300–11.
Corra U, Piepoli MF, Adamopoulos S, et al. Cardiopulmonary exercise testing in systolic heart failure in 2014: the evolving prognostic role: a position paper from the committee on exercise physiology and training of the heart failure association of the ESC. Eur J Heart Fail. 2014;16:929–41.
Keteyian SJ, Patel M, Kraus WE, et al. Variables measured during cardiopulmonary exercise testing as predictors of mortality in chronic systolic heart failure. J Am Coll Cardiol. 2016;67:780–9.
Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021;77(3):243–55. https://doi.org/10.1016/j.jacc.2020.11.008.
Guazzi M, Belletti S, Bianco E, Lenatti L, Guazzi MD. Endothelial dysfunction and exercise performance in lone atrial fibrillation or associated with hypertension or diabetes: different results with cardioversion. Am J Physiol Heart Circ Physiol. 2006;291(2):H921–8.
Gurdal A, Kasikcioglu E, Yakal S, Bugra Z. Impact of diabetes and diastolic dysfunction on exercise capacity in normotensive patients without coronary artery disease. Diabetes Vasc Dis Res. 2015;12(3):181–8.
Nesti L, Pugliese NR, Sciuto P, Natali A. Type 2 diabetes and reduced exercise tolerance: a review of the literaturę through an integrated physiology approach. Cardiovasc Diabetol. 2020;19:134. https://doi.org/10.1186/s12933-020-01109-1.
Ishihara K, Morisawa T, Kawada J, et al. Influence of complications of diabetes mellitus on exercise tolerance of patients with heart failure: focusing on autonomic nervous activity and heart rate response during cardiopulmonary exercise tests. Phys Ther Res. 2019;22:81–9.
Abe T, Yokota T, Fukushima A, et al. Type 2 diabetes is an independent predictor of lowered peak aerobic capacity in heart failure patients with non-reduced or reduced left ventricular ejection fraction. Cardiovasc Diabetol. 2020;19:142. https://doi.org/10.1186/s12933-020-01114-4.
Vest AR, Young JB, Cho L. The metabolic syndrome, cardiovascular fitness and survival in patients with advanced systolic heart failure. Am J Cardiol. 2018;122(9):1513–9. https://doi.org/10.1016/j.amjcard.2018.07.024.
Guazzi M, Adams V, Conraads V, et al. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–74.
Piepoli MF, Corra U, Agostoni PG, et al. Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction: recommendations for performance and interpretation. Part I: definition of cardiopulmonary exercise testing parameters for appropriate use in chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2006;13:150–64.
Corra U, Pistono M, Mezzani A, et al. Sleep and exertional periodic breathing in chronic heart failure: prognostic importance and interdependence. Circulation. 2006;113:44–50.
Piepoli MF, Corra U, Agostoni P. Cardiopulmonary exercise testing in patients with heart failure with specific comorbidities. Ann Am Thorac Soc. 2017;14(Supplement_1):S110–5. https://doi.org/10.1513/AnnalsATS.201610-803FR.
Wasserman K, Zhang YY, Gitt A, et al. Lung function and exercise gas exchange in chronic heart failure. Circulation. 1997;96:2221–7. https://doi.org/10.1161/01.CIR.96.7.2221.
Arena R, Myers J, Abella J, et al. Development of a ventilatory classification system in patients with heart failure. Circulation. 2007;115:2410–7.
Ponikowski P, Francis DP, Piepoli MF, et al. Enhanced ventilatory response to exercise in patients with chronic heart failure and preserved exercise tolerance: marker of abnormal cardiorespiratory reflex control and predictor of poor prognosis. Circulation. 2001;103:967–72. https://doi.org/10.1161/01.CIR.103.7.967.
Francisco CO, Catai AM, Moura-Tonello SCG, Lopes SLB, Benze BG, Del Vale AM, Leal AMO. Cardiorespiratory fitness, pulmonary function and C-reactive protein levels in nonsmoking individuals with diabetes. Braz J Med Biol Res. 2014;47(5):426–31.
Guazzi M, Agostoni PG. Monitoring gas exchange during a constant work rate exercise in patients with left ventricular dysfunction treated with carvedilol. Am J Cardiol 2000;85:660–664, A10.
Brandenburg SL, Reusch JE, Bauer TA, Jeffers BW, Hiatt WR, Regensteiner JG. Effects of exercise training on oxygen uptake kinetic responses in women with type 2 diabetes. Diabetes Care. 1999;22(10):1640–6.
Szalewska D, Główczyńska R, Piotrowicz R, et al. An aetiology-based subanalysis of the telerehabilitation in heart failure patients (TELEREH-HF) trial. ESC Heart Fail. 2021. https://doi.org/10.1002/ehf2.13189.
Sigal RJ, Kenny GP, Boule NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:357–69.
Pan B, Ge L, Xun Y-Q, et al. Exercise training modalities in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Int J Behav Nutr Phys Act. 2018;15:72.
Kemps H, Kränkel N, Dörr M, et al. Exercise training for patients with type 2 diabetes and cardiovascular disease: what to pursue and how to do it. A position paper of the European Association of Preventive Cardiology (EAPC). Eur J Prev Cardiol. 2019;26(7):709–27. https://doi.org/10.1177/2047487318820420.
Thomas RJ, Beatty AL, Beckie TM, et al. Home-based cardiac rehabilitation. JACC. 2019;74:133–53. https://doi.org/10.1016/j.jacc.2019.03.008.
Acknowledgements
The authors thank all the medical and technology members of the Telerehabilitation in Heart Failure Patients team.
Funding
The study was supported by the National Centre for Research and Development, Warsaw, Poland (Grant Number: STRATEGMED1/233547/13/NCBR/2015). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Concept and design: EP, MJP, GO, WZ, MB, PO, RG, ZK, RP. Acquisition, analysis, or interpretation of data: EP, MJP, GO, WZ, MB, IK, PO, DS, SP, RG, RI. Drafting of the manuscript: RG, EP. Critical revision of the manuscript for important intellectual content: RG, EP, MJP, WZ, GO, MB, IK, PO, DS, SP, RI, RP. Statistical analysis: IK, MJP. Obtained funding: EP, RG. Supervision: EP, MJP, GO, WZ, MB, RP. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The main investigator and steering committee designed the trial and wrote the study protocol. The trial was approved by the local ethics committee (Terenowa Komisja Bioetyczna przy Instytucie Kardiologii im. Prymasa Tysiąclecia Kardynała StefanaWyszyńskiego). Each patient provided written informed consent.
Consent for publication
All the authors gave their consent to publication.
Competing interests
The authors were supported by the National Centre for Research and Development, Warsaw, Poland (Grant Number: STRATEGMED1/233547/13/ NCBR/2015).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. TELEREH-HF Exercise Training Model [21]. Table S2. Inclusion and Exclusion Criteria for TELEREH-HF trial [20, 21]. Table S3. Baseline characteristics of excluded vs studied patients. Table S4. Baseline characteristics of studied patients with and without diabetes. Table S5. Baseline parameters of cardiopulmonary capacity.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Główczyńska, R., Piotrowicz, E., Szalewska, D. et al. Effects of hybrid comprehensive telerehabilitation on cardiopulmonary capacity in heart failure patients depending on diabetes mellitus: subanalysis of the TELEREH-HF randomized clinical trial. Cardiovasc Diabetol 20, 106 (2021). https://doi.org/10.1186/s12933-021-01292-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-021-01292-9