Abstract
Quarrying of rock aggregates generates produced water that, if not handled properly will be a source of pollution for nearby water bodies, thus affecting the chemistry of the water. This study examined the chemistry, impact of quarrying activities on water resources and the health consequences/risks posed by ingestion of the water by humans in the Akamkpa quarry region in southeastern Nigeria. Thirty (30) water samples consisting of pond water, stream water, hand dug wells, and borehole samples were collected and analyzed for their physicochemical parameters using standard methods. The results obtained from the analyses indicated that the water was moderately acidic, fresh, and not salty, with many parameters below the recommended standards with Ca2+, and HCO3− being the dominant ions present in the water resources. Rock weathering processes including silicate weathering as illustrated by hydrochemical facies, cross plots, and Gibbs diagrams are the dominant mechanisms influencing the quality and major ions chemistry of the water resources with minor contributions from dissolution, anthropogenic activities, and ion exchange. Ca-Mg-SO4-Cl and Na–K-HCO3− are the most important water types. Although the water quality index shows that the water is suitable for human use and irrigation, the mean values of As, Cd, Pb, and Se are above the acceptable limits. Additionally, the calculated contamination factor revealed the water resources are moderate to highly contaminated by As, Cd, Cr, Mo, Pb, Sb, and Se, and are therefore unsuitable for consumption with regards to these parameters. However, the residual sodium carbonate and water hazard index (WHI) values showed that 38% to 90% of sites in the quarry area were unsuitable for cultivation, 10–30% were in the low to medium impact category, and 60% were classified as risky and are from high to very high impact category. A non-cancer study of inhabitants living in the vicinity of the quarry area indicated that 6.7% of the sites have values greater than one, indicating that it may endanger the health of the people. Therefore, constant monitoring of the water quality is recommended as long-term use of contaminated water can harm humans, plants, and soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Water is an important resource for the health of urban ecosystems for residential, construction, and industrial uses, and pollutants from different and unrelated sources have the potential to harm physical, chemical, and aquatic life [30]. In addition to natural sources of pollution, anthropogenic activities such as rock quarrying also pose challenges to sustainable water resources. The various activities of the quarry include clearing/removal of vegetation and overburden materials, excavation, blasting, transportation, and crushing of rocks [54] producing waste materials (Fig. 1a). These quarry materials include highly visible, dispersed fragments of materials such as granite, gneiss, diorite, limestone, and shale in piles and heaps around the quarry sites have become a source of contamination in the environment. Often these quarries do not have preparedness plans to combat environmental pollution or properly manage quarry waste materials, and sometimes quarries are abandoned without proper management (Fig. 1b). Some of these waste materials contain pyrite and other related minerals that can increase the acidity of water if they encountered it. The ponds (Fig. 1c) created by quarrying activities, during heavy rainfall, and high tides flood huge volumes of water which can cause the release of pollutants (Pb, Cd, Cr, Cu, Zn, Mn, Fe) into the surrounding water. Some metals such as Cu, Fe, Mn, and Ni are essential micronutrients for plants and microbes, while others such as As, Pb, Cd, are harmful at high concentrations [65]. Humans are exposed to high levels of metal(loid)s through contaminated water, the use of contaminated water to prepare food, and the irrigation of food crops. In addition, mining, and quarrying expose rock surfaces, and are prone to the weathering of contaminants into nearby water surface bodies leading to pollution and harm to water, including human health, after long periods of water consumption. It is known that heavy metals pose a threat to human health due to their toxicity, persistence, bioaccumulation in foods, and non-degradable nature in the environment [12, 51]. It is a fact that both surface and groundwater sources are dependent on each other. Many surface streams receive a major portion of their flow from groundwater. On the other hand, water coming from surface waterways is the most important source feeding groundwater. Therefore, the two sources of supply are interrelated and the use of one may affect the availability of the other [61]. Because water is scarce, irreplaceable, and essential for global health, water in the study area serves as a source of drinking water and other domestic purposes for quarry workers and neighboring communities who are subsequently exposed to health risks arising from problems because of using these contaminated water bodies.
Studies in some parts of the globe have shown that indiscriminate disposal of quarry wastes are common sources of metal(loid)s pollution in water resources [46, 55] and has the potential to affect human health through drinking water pathway. Impact of pollution from marble, sandstone, and limestone quarries of water sources by chromium and zinc in western Nigeria have been reported by Afeni et al. [4] and [9]. All these studies indicate the negative impact of quarry activities on environmental resources. Available data in the study area was limited to quality assessment of water in different seasons, spatial variation, and changes [24] while the work of [2, 3] study focused on the impact of abandoned barite mines on water, Land, and sediment. Most of the studies conducted in part of the basement area by Ekwere and Edet [27], Sikakwe and Ilaumo [68, 69] are based on the heavy metal evaluation index (HEI), and heavy metal pollution index (HPI). However, none of these studies was focused on water hazard impact and associated risk to human health in the basement and sedimentary sector of the study area where limestone is currently mined for cement production. Before quarried activities in the study area, local people were engaged in agricultural activities, including crop production, fishing, and other important sources of income. However, recently intensive agriculture in the region has been replaced by quarry activities. Indiscriminate disposal of quarry materials, including ash dumping, has negatively affected environmental quality. The relationship between water sampling, the environment, quarry operations, and other processes and humans can be affected by the movement and leaching of pollutants into surface and groundwater (Fig. 2). Runoff from quarry dumps and quarry ponds are sometimes used for cooking, drinking, bathing, and fishing by people living in the vicinity of the quarry sites. Thus, it is possible for humans and animals that depend on these water sources to accumulate these pollutants from contaminated water, ultimately posing a risk to users. However, evaluating the composition of water resources is important in terms of assessing their suitability for use, domestic use, irrigation, agricultural purposes, and the effects of consumption on human health. In this study, in addition to irrigation purposes, the geochemistry, toxicity and human health evaluation of metals and metalloids found in various water resources in the study area were also investigated. Therefore, this study is the first covering quarries in basement, and sedimentary terrains, although most of the samples were from the basement terrain. Consequently, it has become necessary to assess the environmental impact, quart siting, and quarry operations on the quality of various water bodies around quarries in parts of the south-south regions of Nigeria though.

(Modified from Geochemical Modeling- A Review of Current Capabilities and Future [15]
Conceptual model showing quarrying activities, processes and reactions
Therefore, the main purpose of this study is as follows: document the geochemical characteristics of the water resources in the Akampka quarry area, examine the quality of water, evaluate the impact and health risk of metals and metalloids, and suitability for drinking, domestic use, irrigation use, and agriculture. The results from this study will further contribute to the quality, monitoring, and management of various water resources in the study area.
Study area description
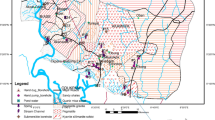
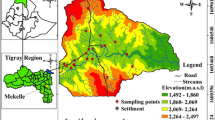
Akampka quarry area is located between Latitudes 05° 06’ and 05° 23’ North and Longitudes 08° 15’ and 08° 30’ East. It includes parts of the basement area of the Oban massif and the sedimentary terrain of the Calabar flank in southeastern Nigeria (Fig. 3). It is located in the Nigerian climatic zone, where annual rainfall varies between 180 and 200 cm and annual temperature is between 25 and 30 °C [39]. There are two main seasons in the region, the wet season (April–October) and the dry season (November-March) with a short break in August (August break). Humidity in this region is always high; it is usually over 90% in the morning, over 100% at night during the rainy season, and drops to 70% during the dry season. The surface of the area is generally curved and undulating and consists of alternating valleys. The geographical structure of the region runs in an indefinite direction and separates the low-lying areas from moderate relief landmarks. The region is heavily irrigated by the Cross River and its tributaries such as Ayipojong-Ita, and Etap-Ayip, and some long-standing rivers such as Ikpaya, Iwiri, Monayip-Netim, and others. Rivers and streams often move quickly in their headwaters with little or no bedload. Typically, the flow pattern in this region is dendritic and, in some cases, linear, indicating structural control. Most rivers in the region are seasonal: they flow heavily during the wet season and usually dry up during the dry season [1].
Geologically as shown on Table 1, the basement area is made up of mainly gneisses, schists, amphibolites pegmatites, granites, and granodiorites, of varied composition. The main lithologic unit of the sedimentary terrain known as the Calabar Flank region of the study area includes sandstone, limestone, shale, and marl. The Calabar Flank holds very low prospects for groundwater because of the presence of shale that constitute thick aquitards, but where the shale is extensively fractured, it can form good groundwater reservoirs. Nevertheless, one of the potential sources of aquifer recharge in the area is surface precipitation. However, the conglomerates, sandstones, and limestone constitute water-bearing units in the area. In the basement area, the ground water occurrence is through fractures, joints fissures [26]. The water-bearing units consists of an upper highly weathered layer, a middle slightly-moderately weathered layer, and a lower fractured bedrock with the water table being highly variable and ranging between < 1.0 m in the northern part and 10.0 m in the southern part of the massif with yield in the range of 10–200 m3 d−1[53].
Quarry activities
The current study considered twelve quarry sites developed between 1976 and 2014 covering an average area of 25.5 hectares with an average production of 1154 tonnes/day. Geologically, eleven (11) of these quarries were in the basement terrain while only one (1) was within the sedimentary terrain within the Calabar Flank (Fig. 3) of the study area some of which are active and abandoned. A summary description of these quarries including the duration of the quarrying activities as well as the status and their production capacities are also outlined in Table 1. Quarrying activities have increased over the years within the quarrying district of Akamkpa. Currently, there exist more than 30 quarries in the study area. The high number of quarries is in response to the increasing demand for crushed rocks by construction industries due to the upsurge in infrastructural development in Nigeria.
A total of thirty (30) samples and two (2) control samples were collected from different locations during the rainy season. The water samples were collected in 250 ml (anions analyses) and 50 ml (cations analyses) clean plastic bottles that were thoroughly washed and rinsed with deionized water in the laboratory and then in the field to prevent contamination. Water samples were collected from quarry ponds (P), nearby streams (S), hand-dug wells (W), borewells (B) surface water control (SW) and groundwater control (GW), Fig. 3. In ponds, water bottles were placed under water to collect samples that did not contain films that could be a source of heavy metals [36]. Two samples were collected at each site and labeled to avoid mixing. One part of the sample was used to determine physical parameters and anions, and the second part was used to determine cations. These samples were passed through 0.45 μm filter paper to remove suspended solids that could dissolve and affect metal concentrations. Samples for cations analyses were acidified with 2 mL of HNO3− acid to keep metal ions in solution. Water samples for anion analysis were stored in a refrigerator at 4 °C for a week before transportation to the Laboratory for analysis to reduce the concentration of dissolved compounds. Physical parameters of water such as temperature, conductivity (EC), and pH were measured in the field using a thermometer and pH meter. Laboratory Analysis of Cations was carried out by Inductively Coupled Plasma Mass Spectrometry (ICP-MS), Perkin Elmer Sciex at the National Agency for Food and Drug Administration (NAFDAC), Lagos, Nigeria. Total hardness (TH) and anions were analyzed by titrimetric and chromatographic methods at the Institute of Oceanography (IOC), University of Calabar, Nigeria. A quality control measure was also used. Each analysis was performed in triplicate and the mean value was recorded. The control scheme used, involved two samples and in-house reference materials. Calibration standards for the Spectrophotometer was done using prepared serial solution from traceable stock and verified against in-house reference materials, and the resulted concentrations of the anions, metals, and metalloids were reported in mg/L and µg/L.
Data handling
Statistical analyses
Descriptive statistics were performed utilizing the statistical package STAISTICA [60]), and Excel spreadsheet. Mineral phases were computed using the computer program PHREEQC. It aimed to evaluate the role of mineral dissolution to reveal the potential of mineral controls on water chemistry by calculating the distribution of aqueous species and mineral saturation indices [25]). Saturation indices (SI) indicate whether a water sample is saturated or unsaturated for a particular mineral.
where Ksp = solubility product of at a given temperature.
IAP = Ionic Activity Product.
SI predicts the mineral water balance and water–rock interaction [86]. If the SI is zero, the water is saturated with certain minerals. An SI of less than zero indicates an inadequate saturation or undersaturation(dissolution) for a particular mineral and may indicate the presence of water from a rock that does not contain enough mineral to rapidly dissolve or penetrate/infiltrate and such minerals will continue to change with groundwater [86]. SI above zero indicates an oversaturated (precipitated) state for the mineral phase [64] and does not dissolve most minerals. Oversaturated water refers to groundwater resources that have sufficient mineral salts and sufficient residence time to reach equilibrium [7]. However, significant changes in alkalinity and SI values are related to time. Therefore, the SI of water is close to zero (−0. 5 and + 0. 5) will be nearly neutral, so water will not tend to dissolve or precipitate the mineral [34], which represents the equilibrium state of the mineral [86].
Principal component analysis (PCA) was used to obtain quantitative data and group the measured factors for interpretation [5]. The aim of this was to determine the source of the ions, metals, and metalloids in the water and to evaluate the correlation coefficient between them. The varimax regression method was used to determine the relationship between the data and the most important factors [52]. The data are obtained by transforming the original data set into the standard version due to variance, creating a new set of uncorrelated pseudo variables known as principal components (PC). PCs were identified by running screen plots with eigenvalues > 1 (Kaiser standard method) [48].
Hydrogeochemical evaluation
Hydrogeochemical facies of water samples were evaluated using the Piper diagram [59], Using the Piper diagram, the origin, structure, and chemical interactions between cations and anions dissolved in the waters are analyzed. Hydrochemical composition is mainly affected by lithology, residence time, and regional groundwater flow pattern [21]. Water can be classified as bicarbonate, sulfate, and chloride according to its chemical composition [11]. Gibbs diagrams [33] and cross-plots have also been used to determine the type and processes controlling water chemistry. Also, PCA, was used to determine the nature of hydrochemical interactions between water and the environment [10].
Water quality
Water quality index (WQI)
The water quality index (WQI) is a collective numerical assessment for the overall suitability of water for drinking purposes [10, 35, 63].
In this study, WQI was evaluated because people around the study area rely on these sources of water for consumption. The computation of WQI in this study was based on assigning different weights (Wi) to water quality parameters according to their influence on the overall quality of water (Table 2).
Secondly, the relative weight (Wr) for each parameter is calculated using Eq. 1. In this study the following parameters were selected: EC, pH, DO, TH, Na+, K+, Ca2+, Mg2+, Cl−, HCO32−, SO42− and NO3− Each of these parameters is assigned a weight Wi ranging from 1 to 5 [14] according to their relative importance in water quality for human consumption and its possible effects on health. In this study, the concentration of these parameters was compared to the standards for drinking water as recommended by WHO [81] and the Standard Organization of Nigeria [73] and was included for the calculation of WQI. Each parameter was calculated as follows:
W = assigned weight of each parameter.
Wi = sum of assigned weights of all the parameters and the number of parameters.
In this study the following parameters were selected: EC, pH, DO, TH, Na+, K+, Ca2+, Mg2+, Cl−, HCO32−, SO42− and NO3− Each of these parameters was assigned a weight Wi ranging from 1 to 5 (Table 2) according to their relative importance for human consumption. The water quality rating (Qi) was calculated as:
Ci represents the concentration of the parameters and Si is the water quality standard as recommended by [82] for drinking. Finally, sub-indices (SIi) and WQI are computed using Eqs. 3 and 4:
The WQI was categorized based on the classification scheme of Batabyal and Chakrabarty [14] as < 50 suitable, 50–100 good, 100–200 poor, 200–300 very poor, and > 300 unsuitable.
Contamination level
To determine the contamination level of metal(loid)s in the water resources of the study area, contamination factor (Cf) and contamination index (Cd) were used to evaluate water quality by calculating the contamination level. In this study, the pollution status of metals (loids) in water was evaluated using the contamination factor (Cfi) as in [25],
where Cif = c ontamination factor; Cio–i is the concentration of the element in the sample;
Cin = background concentration/maximum allowable limits. Calculated values are classified as low (Cd < 1), medium (Cd = 1–3) and high (Cd > 3) pollution.
Irrigation water quality
There are many indicators for monitoring the quality of water for agricultural activities. Some of the parameters used in this study include electrical conductivity (EC), sodium absorption rate (SAR), percentage sodium (%Na), and residual sodium carbonate (RSC). These parameters describe the strength of water alkalinization and its potential impact on soil [57, 67]. The equations for computing these indices are presented as Eqs. 7, 8, and 9: Generally, the alkalinity risk proposed by Richards [66] is expressed as the SAR and it is calculated as:
SAR is classified as: excellent, S1 (SAR < 10), good, S2 (10–18), doubtful,
S3 (SAR, 18–26), and unsuitable, S4 (SAR > 26) [66].
Sodium Percentage (% Na) was calculated as in Eq. 8:
%Na was classified [74] as excellent (%Na < 20), good (20 < %Na < 40), permissible (40 < %Na < 60), doubtful (60 < %Na < 80) and unsuitable (%Na > 80).
The presence of more carbonate and bicarbonate than the amount of calcium and magnesium determines the appropriate irrigation system [62]. RSC was calculated using Eq. 8, where the ionic strength of all concentrations is expressed in meq/l, as suggested by [23].
RSCs were classified as safe (RSC < 1. 25), and appropriate (1.25 < 2.5) and inappropriate (RSC > 2.5), [23].
Water hazard index (WHI)
The WHI has been used to provide an assessment of the overall quality of different types of water used for various purposes. The WHI was calculated using parameters associated with quarrying activities that are known to negatively impact water quality. These include, As, Cd, Co, Cr, Fe, Ni, Pb, Sr, and Zn. It also makes it possible to compare different bodies of water. WHI was calculated as in [51, 50].
WHI was categorized as < 1 Low impact (LI), 1 < WHI < 3 moderate Impact (MI), 3 < WHI < 5 high Impact (HI), > 5 Very high Impact (VHI).
Human health risk assessment
A risk assessment was used to evaluate the non-carcinogenic risk resulting from water consumption (intake) in adults and children. Contaminant concentrations in drinking water, stream water, and groundwater were calculated from mean daily values (ADD) using Eq. 11 (USEPA 1989):
ADD in mg/L/day, C (Concentration of trace metal (mg/L), IR (Ingestion Rate), 3.3 L/day), EF (Exposure Frequency), 365 days/year [76, 77], ED(Exposure) Duration),30 years [76, 77], BW (Body Weight), 60 kg Wongassuluk et al. [84], and AT (Average Time), 52 years [76, 77] respectively.
Hazard quotient (HQ) was used to estimate the risks of drinking water contaminated with trace metal(loids) as:
RFD indicates the dose of metal that a person can be exposed to in one day of their life without causing adverse health effects [80]. The RfD values for Cd, Co, Fe, Ni, Pb, Se and Zn are 0.01, 0.0003, 0.7, 0.02, 0.0036, 0.005, and 0.3 respectively. The risk of drinking water contaminated water with more than one metal is determined by the Hazard Index (HI), given by the sum of individual HQ as in Eq. 13:
HQ/HI > 1 are indication of health risks from drinking contaminated water [44, 51].
Results and discussion
Physicochemical parameters
Table 3 contains a summary of physical and chemical water quality parameters alongside control sites. There were no significant differences among the parameters for the sites. Generally, there are significant differences in the level of physical parameters in different water bodies (pond, stream, well and borehole). Temperature, conductivity, pH, DO and TDS are significantly different in the waters except for pH and DO. This variability could be attributed to the inconsistent rates of ecological, geological, or anthropogenic change [43]. The highest temperature (29.9 °C) was recorded in pond water, while the lowest temperature (29.4 °C) was recorded in the stream water body. EC values for all water from quarrying areas, including values are below 1400 μs/cm [73, 81] guidelines for drinking water. A higher EC average value (157 μs/cm) and the lowest average EC value (89.98 μs/cm) was obtained in the pond water at the basement area, while the lowest average EC value (89.98 μs/cm) was found in the stream water. This indicates higher concentrations of dissolved solutes. Low EC values < 1400 μs/cm in the study area indicate poor/low mineralization of water in the area which can be attributed to low dilution or solubility of minerals in the area [28]. The fact that TDS values (86.5, 45.91, 65. 42, and 75. 87 mg/l) were < 1000 mg/l and were within the recommended limits showed that the water source has low salinity and is fresh. The mean pH of water from the area is an indication of the acidic nature of water and is attributed to the silicate minerals contained in the rocks and generated wastes rock materials scattered at the quarry site releasing silicic acid into the water as well as breakdown/oxidation of pyrites contained in generated dust and waste rock as shown in Eq. 14:
Additional source of acidity may be as result of the humic acids coming from organic matter [10]. Acidic water influences the release of metals into the water bodies [49]. The highest mean pH value (4.93) was obtained from hand dug-well water samples of the quarry site in the sedimentary area with the lowest value (4.59) obtained in the stream water samples of the basement area. The higher pH value from the sedimentary area could be a result of the acid-neutralizing capacity linked with the presence of the carbonates from limestone of the quarry site in the area [85].
The mean values of DO (2.54 mg/l, 2.21 mg/l, 2.42 mg/l and 2.33 mg/l) for all the waters samples from the quarry sites in the basement area and that from the Calabar flank area are all below the recommended [81] guideline of 5.0 mg/l for drinking water. Ellis [29] stated that the amount of dissolved oxygen in each water sample is determined by the balance between biological oxygen production and consumption. The low DO content in water in the study area can be attributed to the high temperature in the area, which reduces the DO content. Additionally, Ellis [29] stated that at high temperatures, water tends to release gas into the air, resulting in dissolved oxygen (DO). The mean and range of values of total hardness values in all water samples are indicative of the softness of the water in the area (Total hardness (TH) < 75 mg/L).
The main cations (Na+, K+, Ca2+ and Mg2+) and anions (HCO3−, Cl−, SO42− and NO3−) found in the waters of the study area are shown in Tables 3 and 4 with Ca2+, and HCO3− as the main cations and anions, respectively, in waters taken from all examined sites. The abundance of major anions has shown that bicarbonate (HCO3−) accounts for 60% of the total anions in all water sources in respective of the geologic controls. The amount of carbonates and bicarbonates in groundwater may be responsible for the decomposition of carbonates and the dissolution of carbonic acid due to chemical weathering [19, 37, 41, 70]. The order of abundance of anions among the water types, active and abandoned sites is HCO3− > Cl− > SO42− > NO3. Chlorides are the second most abundant anion in the water resources. Chloride ion (Cl−) recorded average concentrations of 59.09, 20.36, 36.53, and 7.64 mg/l in ponds, streams, boreholes, and well water from the quarry sites were below the allowable limit values of 250 mg/L for Cl− and do not seem to pose any environmental problem. Chloride can be used as an advance warning of the presence of other toxic contaminants [83]. The sulfate (SO42−) value of all water samples is below 250 mg/l, which is the acceptable limit in water. Besides quarry waste, other sources of chloride, and sulphate can come from rock water interaction, rainwater, industrial fertilizers such as gypsum compost, sewage, municipal waste, and leachate seepage [13] from quarry tailings and rock water interaction [79] However, when sulfates and chlorides accumulate on the water in the human body, it can cause heartburn, high blood pressure, dehydration, asthma, and osteoporosis [32].
The lowest concentration of major anions in the study area is nitrate (NO−3) with mean concentrations of 0.45, 0.29, 0.36, and 0.49 mg/l for pond, stream, borehole and well water samples from the quarry sites. The concentration of NO3− is generally below the maximum allowable limit [73, 81] in all the water samples. Nitrate is the product of atmospheric fixation of organic nitrogen and a byproduct of nitrogen transformation [41]. Chloride, nitrate, and sulfate are considered indicators of anthropogenic pollution.
For the major cations, calcium is the dominant ion, and accounts for over 65%, while Na ions account for over 17% and Mg and K ions account for about 10.2, and 7.8% respectively. The order of abundance is Ca2+ > Na+ > Mg2+ > K+. The mean values of the cations in the water samples were within the WHO [81] and SON [73] permissible limits of 75, 200, 100, and 12 for Ca2+, Na+, Mg2+, and K+ respectively. According to WHO [81] high concentrations of calcium and sodium above the admissible level may cause health problems such as kidney stones, abnormal nervous systems, and cardiovascular diseases such as high blood pressure. The main source of Ca2+ in water is the dissolution of carbonates from sedimentary rocks and minerals like calcite, dolomite, and limestone. Moreover, agricultural activities contribute significantly to the release of calcium and magnesium through weathering processes of silicate minerals and hydrolysis of CaCO3− and Ca-Mg-(CO3)2−, both of which are magnesium-rich minerals [11].
The interaction between calcium and magnesium is very important in the type of water in terms of hardness. Most of the different types of water in the study area contain more calcium than magnesium. Differences in water hardness in the study area may be due to the relative levels of Ca-Mg-HCO3− ions in the solution. Comparative analysis of cations and anions in ponds, streams, hand dug wells and freshwater from quarries in the Oban massif and Calabar showed that the most important ions in the study were Ca and HCO3−.
Saturation indices of mineral phases determined revealed that the water resources are oversaturated with, carbonates, goethite, and hematite with positive S.I values above zero and undersaturated with halite, anhydrite, aragonite, calcite, dolomite, gypsum, quartz, sylvite, talc having values below zero.
Associated with the major anions and cations are minor metal(loids) which are also constituents of water and usually present potential health risks to aquatic organisms and humans. High temperatures and low pH values enable this metal(loid)s to be easily released into the water [20]. Long-term exposure to polluted water for a long period can affect the normal functioning of the human body, as it interacts with biological molecules containing nitrogen, oxygen, and sulfur, triggering structural and functional changes [16].
The spatial variation of concentration of metal(loids) is presented as box and whisker plots (Fig. 4). The box plot is a powerful statistical tool that shows the distribution data across means, ranges, median, and range. Since the distributions of water quality parameters are often skewed to the right, it is preferable to consider the median as the indicator of the overall trend. The box’s centred on the horizontal line, the 25th and 75th percentiles (quartiles), on the top and bottom of the box [8]. Figure 4 shows distinct differences and variations of the metal(loid)s with no defined pattern. The data set did not fit a log-normal distribution.
Table 3 and Fig. 4 showed that the large variation and standard deviation in the parameters indicate that the geochemical properties of water resources are affected by various processes, which may be the interaction of rock weathering with anthropogenic activities [71]. The average concentration (Table 3) of the metals and metalloids decreased as As > Pb > Cr > Cd > Se > Fe > Si > Ag > Zn > Ag > Cu = Sr > Co > Mn = Ni > Sb. Mean concentration of all parameters in the active and abandoned sites are within the [73, 81] standard limits except for Se, Ni, Pb, Cd, Sb in the active sites and Se, Cr, Ni, Pb in the abandoned sites. The result of this gives indication of the enrichment of these metals in the area. In the study area there is no defined pattern of distribution of the metal(loidd)s. The trend of dominance in the composition of trace metals from the active quarry sites in the Oban Massif is in the order Sr > Cr > Zn > Se > Si > Pb > Ni > Cu > As ≥ Cd = Mn > Sb. The trend of dominance from the abandoned sites in Oban Massif is in the order Sr > Cr > Se > Zn > Si > Pb > Ni = Mn > As = Cu > Sb > Cd while the trend of dominance from the active quarry site in Calabar Flank is Sr > Ni > Se > Zn > Pb > Si > Cu > Co > Cr respectively. Similarly, comparison of metals among different water types shows that the trend of dominance of metals in water is in the order Sr > Se > Zn = Fe > Ni > Si > Pb = Co > Cu > As = Sb = Cr = Mn(pond),Sr > Se > Zn > Fe > Pb > Si > Ni > Cu = Cr > As = Co = Ag(stream),Sr > Pb > Ni > Fe > Zn > Cu > Se > Si > Cr > Cd = Mn(borehole),andSr > Zn > > Cu > Se > Fe > Si > Pb > Ni > Cr > As = Co(well).
Pond water recorded about 67% of these metals from the basement and sedimentary area. This could a result of higher anthropogenic inputs in water from these sites. The occurrences of Si may be attributed to the dissolution of silicate-bearing bedrocks of the study area. The presence of an alkaline environment and the weathering of silicate minerals in these rocks have also been mentioned as sources of silica in water by [71]. Similarly, the metal content in borehole water samples(B6) from the sedimentary area was seen to be higher than those from the well water samples of the basement area, although samples from these two sources could be comparable. The reason for this difference may be due to lithological differences in the study area. The total metal content in pond and stream water samples was higher than the samples obtained from hand-drilled wells and boreholes and this may be due to high anthropogenic contamination load to the pond and stream waters. In all cases, other than agricultural activities and mining, apparent high values can be mainly due to geogenic activities and activities associated with quarrying through waste, scrap and emissions from vehicles and machinery.
Principal Component Analysis (PCA),
A Principal component analysis (PCA) was performed for the various water resources grouped using the physicochemical parameters. The principal component analysis with varimax rotation eigenvalues > 1 is shown in Table 4 as the variance of the PCAs (Fig. 5a). PCA 1 showed a variance of 30.47% with higher weights for pH, TDS, EC, Ca2+, Mg2 + , Cl−, HCO3−, SO42−, Ni, and Sr. This relationship demonstrates the influence of environmental factors on the chemistry of the water. Positive loading on Na+ K+ HCO3−, Cl−, and SO42− has been observed and is associated to indicate the presence of both natural (geogenic) and human (anthropogenic). The natural source is weathering of rocks and minerals into the water [72] while the anthropogenic source includes atmospheric deposition with contributions from the dispersed dust during the quarry operations as well as leaching from waste rock materials scattered at the quarry sites (Fig. 1a, b) into the surface water (pond and stream). High loadings for HCO3−, Ca2+, and Mg2+ can be attributed to the natural weathering of silicate minerals from the basement and sedimentary rocks and this has been reported as the main source of these ions by [70]. Additionally, high loading for Cl−, and SO42− indicate that they were added from recharging rainwater, while the high loading on Ni, and Sr was due to minor variations in lithology. The high positive loading of HCO3− was due to atmospheric CO2 combined with soil water and carbonate dissolution [49]. PCA 2 explained 10.72% of the total variance with the highest loading for Na and Sb, and the athitectic relation with Pb suggest their different sources. The positive loading value of hardness is attributable to natural elements of silicate rocks such as pyroxene, biotite etc. PCA 3 accounts for 8.9% of total variance with high positive loadings for hardness and Si and negative loading on Se indicating their different sources. PCA 4, has a 7.27% total variance with negative loading for temperature, Cu and Zn depicting that the concentration of Cu and Zn are not influenced by temperature and are of mixed sources. PCA 5 accounts for 6.61% of variance with high loadings for Cr and Fe reflecting their geogenic sources and water–rock interactions leading to rock disintegrations. In general, the PCA did not show significant correlations between metals and metalloids in the aqueous phase. This may be due to the low mineralization of water in the area which reflects of low solubility of the minerals in the water. A graphical representation (Fig. 5b) has been used to identify the principal components that could be retained and to describe the variability of the original dataset, suggesting that the five components account for 67.97% percent of the total variance. As a result, the details of the water quality at each of the sampling locations may be calculated using only five variables.
Hydro-chemical facies and water types
In this study hand drilled wells and boreholes were evaluated as groundwater. All the water samples collected from the different water sources from both quarries revealed two major water types: Ca-Mg-SO4-Cl and Na–K-HCO3 (Fig. 6, Appendix 1). These water types probably result from the disintegrations of feldspars and carbonate rocks, and changes in the lithology in the study area. In addition, the presence of Na–K-HCO3 may indicate the effect of the combined effects of cations exchange and calcite/carbonate or silicate dissolution [9]. According to [47, 71], bicarbonates and calcium in groundwater samples could be due to the natural dissolution of carbonate minerals. Water chemistry is controlled by many processes including rainfall recharge, hydrological interactions, water–rock interaction, and human activities [17, 70]. Gibbs plot (Fig. 7) and cross plots (Fig. 8) of Mg2+/Na+ versus Ca2+/Na+ and HCO3−/Na+ versus Ca2+/Na+ to distinguish the sources of ions in water resources of the study area confirm rock/silicate weathering as the main processes controlling the chemistry of the water resources in the quarry areas. Weathering of alumina-silicates minerals [70] are the major contributor of Na+, K+, Ca2+, Mg2+, and HCO3−. The effect of weathering activities in the study area is that it increases the dissolution of silicates and carbonates, as well as ion exchange in water. Pyroxene, Ca-plagioclase, orthoclase, amphiboles, olivine, and biotite are the main silicate minerals found in the crystalline rocks of the region (Appendix 2). The general reaction for the weathering of silicate rocks is shown in Eq. 15:
The Na and K, Ca and Mg may be attributed to cation exchange process [42].
The incongruent dissolution of plagioclase in water can be written as:
For olivine, it is given as:
The weathering activities in this study imply the effect of dissolution of minerals such as plagioclase, amphiboles, calcite among others with the release of ions and metals into the environment.
Assessment of water quality
The assessment of water quality in this study was carried out to determine the portability of water for drinking and other domestic uses. This was done based on a comparison of the analytical results obtained from the quarry sites existing water quality guidelines [73, 81]. Based on the analyzed data for P, S, W, and B, 100% of pH and DO were not within the [73, 81], maximum admissible level. The mean concentration of As, Cd, Pb, and Se for all the water sources were above that of the WHO [81] and SON [73] admissible limits of 0.01 mg/l (As, Pb, and Se) and 0.003 mg/l(Cd) suggesting that the water resources are not suitable for drinking and irrigation for these parameters. The computed WQI varied from 11.27 to 37.26 (Table 5) showing that the water resources are suitable (WQI < 50) for drinking as classified by Batabyal and Chakrabarty [14] and are in A grade class. The mean contamination factor of the metal(loid)s (Fig. 9a and Appendix 1) has shown that 50%(Pond), 42%(Stream), 35.7%(Well) and 42.86(Borehole) were moderately and highly contaminated with As, Cd, Co, Cr, Mo, Pb and Se. These metal(loid)s are halophilic [45] in nature, have affinity for Sulphur and thus reflect the main potentially toxic elements (PTE) signature indicative of bedrock geology [50]. In respect of the geologic terrain and status of the quarry sites (Fig. 9b), the water was not polluted with Fe, Cr, Co, Cu, Zn., Ag, and Mn but were polluted with Cd, Mo, Pb, and Se. Using the classification scheme, Cd, Mo, Pb, and Se form Oban massif active sites are in the class of high contamination and Ni, and Sb are in the class of medium contamination, whereas Se and Pb from the Oban massif abandoned and Calabar flank active sites are in the class of medium contamination and thus not suitable for consumption based on these parameters. As shown on Fig. 10, Cr is the main contribution of pollution to the stream water, whereas As, Pb, and Se are associated with pond and borehole water which is explained by PCA 2 constituting 56.49% of the variance. Also, Fe is associated with the control area and is explained by PCA 1 which is 23.08% of the total variance. These associations signify areas with a significant concentration, influenced by comparable geological, geochemical, environmental, and anthropogenic sources.
Suitability for irrigation
Adequate irrigation capacity of ponds, streams, and groundwater for the agricultural needs of the population was evaluated using four parameters including electrical conductivity (EC), sodium percentage (%Na), sodium adsorption rate (SAR), and residual sodium carbonates (RSC). The EC of all the water samples ranged from 18.70 and 372.0 with an average of 122.89 μS/cm (Table 6). All the samples showed EC < 2,250 μS/cm and were considered excellent for irrigation use [66]. However, all the samples from the sedimentary area (P11, S8, and B6) were classified as Good (250 < EC < 750 μS/cm). According to SAR, all the water samples fall into the excellent category (SAR < 10). Regarding %Na (Table 6), 27, 45, and 27% of PW and 25, 50 and 25% of SW are in the excellent category (%Na < 20), good (20 < %Na < 40), and permissible water (40 < %Na < 60) respectively. For GW, 17%, 50% and 33% of the samples were in the excellent, permissible, and doubtful categories (60 < %Na < 80). Similarly, RSC values showed 9.82% and 9% of P and 50. 33% and 17% of GW were safe, marginally suitable, and unsuitable for irrigation (Table 7). For SW, 75%, and 25% were safe and marginally favorable for irrigation. Also, from the sedimentary areas (P11, S8, and B6) were not suitable for irrigation use based on RSC. According to [23], high value of RSC value in water means more sodium adsorption by water (Table 6).
Water hazard index
The WHI has been used to provide an assessment of the overall quality of different types of water used for various purposes. The WHI was computed using parameters associated with quarrying activities that are known to negatively impact water quality. WHI were classified as follows: low impact (< 1), moderate impact (1–3), high impact (3–5) and very high impact (> 5) respectively. Generally, there is no defined pattern of distribution of the impact as the various water bodies are in the class of moderate to high impact. Figure 11 showed that 10% (S2, S4, S6) of the sites were classified as low impact, 36.67%( P4, P7, P8, P10, S1, S3, W3, W4, B2, B3, B4) of the sites were classified as moderate impact whereas 26.67%(P1,P2, P3, P6, P9, W1, B1, B5) of the sites were in high impact class and, 26.67%(P5, P11, S5,S7, S8, W3, W5, B6) of the sites are considered very high impact class respectively. The low impact areas are in the NW an SE part of the study area and are characterized by few quarry sites while the moderate to high-impact zone areas are sited in the central part of the study area where most of the quarries are located and are characterized by large amount of quarry wastes. Most of the sites were from the basement terrain with few locations (B6 and P11) in very high-impact zone in the sedimentary terrain. Geologically, both terrains have areas that are highly impacted implying that the quarry waste/rock fragment randomly scattered on the ground and dust generated during blasting and crushing and weathering of the rocks material are probable major source of the pollution as the metal(loid)s contained in the waste rocks and dust are leached from these materials into the water bodies and contaminate them.
Health risk assessment
The fact that, the mean concentration of As, Cd, Pb, and Se are above the WHO [81] and SON [73] permissible limits for the different water resources is an indication that the population in the study area may be exposed to these potentially toxic metals. Long-term exposure to inorganic arsenic, especially through drinking water and food, can cause chronic arsenic poisoning. Many studies have shown the negative effects of arsenic on cognitive development, intelligence, and memory [75]. The International Agency for Research on Cancer (IARC) has classified arsenic and arsenic compounds as carcinogenic to humans. Arsenic is also associated with increased mortality in young adults due to many types of cancer, lung disease, disease, and kidney failure [31]. Exposure to elevated levels of concentration of Cd and Pb through drinking water pathways can lead to serious challenges for both humans and animals. Exposure to high concentrations of Cd, and Pb through drinking water can caused kidney disease, lung damage, high blood pressure, fragile bones and nervous disorder [6]. Also, exposure of infants and children to Pb concentrations above acceptable levels can delay physical and cognitive development and cause deficits in cognitive and learning abilities [6] (Environmental Protection Agency 2005). Health risk assessment of metal(loid)s in all the water samples from the study area were obtained by calculating the hazard quotients (HQ) and health risk index (HRI). HQ/HI < 1 is considered to have low health risk, HQ/HI > 1 are considered to have high health risk [58].
As, Cd, Cr, Cu, Ni, Pb, and Zn were considered in this study via drinking water pathways for the calculations of the ADD and HQ values (Figs. 12a and b). It was observed that the average daily dose (ADD) < 1 for all the locations (Fig. 12a) suggested low intake. However, HQ values for Zn (1.87) at S5 and Pb (1.14) via drinking water pathway at W2 is > 1 as well as the HRI (Fig. 13) of S1 (1.01), S5 (2.46) and W2 (1.67) all in the southeastern part of the study area are considered risky for ingestion. HQ and HRI values less than 1 indicate that there is no negative effect. However, areas with HQ value > 1 showed that the health of people living in these areas may be affected [38, 40, 80, 84].
Although HRI values were below 1 in some areas during the study period, long-term consumption of contaminated water from these sources may cause health problems in the future if water purification measures are not considered [38]. Lead exposure can cause diarrhea, anemia, gastrointestinal disturbances, sensitization, and progressive muscle paralysis [18]. Therefore, treatment is recommended.
Conclusion
Investigation of the water resources near quarry sites in Akamkpa quarry district of southeastern Nigeria was carried out using hydrogeochemical, statistical, water hazard index (WHI), and health risk index. Many of the physicochemical parameters reflect differences in the composition of the water resources basically of natural, environmental, and anthropogenic origin. pH, DO values of P, S, W, and G were not within the acceptable limits of WHO [81] and SON [73]. The order of abundance of major anions in the water resources is HCO3—> Cl− > SO42− > NO3 – and cations Ca2+ > Na+ > Mg2+ > K+.
The chemical composition due to major cations and anions showed that water resources were largely controlled by silicate weathering through ion exchange, dissolution, and some anthropogenic activities, mainly from nearby agricultural and quarrying activities. Principal component analysis and positive correlation with R > 0.7 between Ca2+ with HCO3−, Na+ with HCO3− confirm their natural geogenic sources with silicate mineral dissolution as the main processes controlling the chemistry of the water resources. In addition, cross plots and saturation indices revealed that rock weathering including the dissolution of carbonates, and silicate minerals are significant rock-water interactions affecting the chemistry of the water resources.
However, except for some places, the water is classified as good and suitable for drinking and irrigation. Water samples in the study area were moderately and highly contaminated by As, Cd, Cr, Co, Mo, Pb, and Se probably from leaching of quarry waste materials and dust particles. Contamination of the pond water and boreholes is associated with As, Pb and Se while stream water is associated with Cr. The calculated water hazard index suggested that the water resources are mostly moderately to highly impacted. While a few places with HRI > 1 are considered risky. To reduce the health risks caused by drinking polluted water, local people should be informed by the operating companies as part of their social corporate responsibility on the importance of treating water to minimize health hazards associated with drinking polluted water.
Access to some of the quarry sites was not granted especially during operations like blasting to avoid injury, thus, it was not possible to collect quarry dust. There was no defined pattern of collection of the water samples and due to lack of funds, only 12 of the quarry sites were considered even though there were over thirty quarries.
Monitoring the water quality of the study area as well as proper location of the quarry sites is encouraged to reduce the risk of pollution. Additionally, environmentally friendly operation methods should be applied to properly manage the environment.
Hence further studies would be conducted by analyzing the quarry dust to establish the potential contribution of the quarry dust from quarrying operations to surface water alongside human activity.
Availability of data and materials
Data generated has been used for this study and is available on demand from the corresponding author.
References
Abuah M, Eyo E (2013) Assessment of soils around quarry terrain in Akamai Local Government Area, of Cross River State, Nigeria. Mer Res J of Agar Soil Sci 1(1):001–005
Adamu CI, Nganje TN, Edet A (2013) Hydrochemical assessment of pond and stream water near abandoned barite mine sites in parts of Oban Massif and Mamfe Embayment, Southeastern Nigeria. Environ Ear Sci 71:3793–3811
Adamu CI, Nganje TN, Edet A (2015) Major and trace element pollution of sediments associated with Abandoned Barite Mines in parts of Oban Massif and Mamfe Embayment, Southeastern Nigeria. J Geochem Expl 151:17–33
Afeni TB, Jimo BO, Adeogun AA (2021) Impact of limestone quarrying operations on quality standards of soil and water at Ewekoro (Nigeria). J Appl Sci Technol 17:1–2
Agbor E, Besong E, Ebai P et al (2024) Baseline assessment of the health risk of potentially toxic heavy metals in commonly consumed vegetables in parts of Mamfe, Southwest Region, Cameroon. J Trace Elem Miner 8:100115
Agency for toxic substances and Disease Registry (ASTDR) (2018) Toxicological Profile for hazardous substances. US Department of Health and Human Service, Atlanta
Aghazadeh N, Mogaddam AA (2011) Investigation of hydrochemical characteristics of groundwater in the Harzandat aquifer Northwest of Iran. Environ Monit Assess 176(1–4):183–195. https://doi.org/10.1007/s10661-010-1575-4
Ahmed N, Bodrud-Doza M, Islam SMDU, Choudhry MA, Muhib MI, Zahid A, Hossain S, Moniruzzaman M, Deb N, Bhuiyan MAQ (2019) Hydrogeochemical evaluation and statistical analysis of groundwater of Sylhet, north-eastern Bangladesh. Acta Geochimica 38(3):440–455
Akande JM, Lawani AI, Adeoye WA (2013) Evaluation of the environmental effect of the abandoned Strabag Quarry at Ibadan, Nigeria and RCC Quarry at Wasinmi, Ikere, Nigeria. J Environ Ear Sci 3(4):129–134
Alam W, Singh KS, Gyanendra L, Nesa N (2020) Hydrogeochemical assessment of groundwater quality for few habitations of Chandel District, Manipur (India). Appl Wat Sci 10:123. https://doi.org/10.1007/s13201-020-01208-0
Alsuhaimi AO, Almohaimidi KM, Momani KA (2019) Preliminary assessment for physicochemical quality parameters of groundwater in Oqdus Area, Saudi Arabia. J Saudi Soc Agric Sci 18(1):22–31. https://doi.org/10.1016/j.jssas.2016.12.00
Asare-Donkor NK, Kwaansa-Ansah EE, Opoku F, Adimado AA (2015) Concentrations, hydrochemistry and risk evaluation of selected heavy metals along the Jimi River and its tributaries at Obuasi a mining enclave in Ghana. Environ Syst Res 4:1
Aweda AK, Jatau BS, Goki NG (2023) Groundwater geochemistry and hydrochemical processes in the Egbako aquifer, Northern Bida Basin, Nigeria. Brazilian J Wat Res 28(31):1–12
Batabyal AK, Chakrabarty S (2015) Hydrogeochemistry and water quality index for drinking use. Wat Env Res 87(7):607–617
Crawford J (1999) Geochemical modeling-a review of current capabilities and future directions.
Creta C, Horga C, Vlad M, Panescu VA, Bocos, Bintintan V, Coman MV, Herghelegiu MC, Berg V, Lyche JL, Beldean-Galea MS (2024) Water Quality and associated human health risk assessment related to some ions and trace elements in a series of rural roma communities in Transylvania, Romania. Foods 13:496. https://doi.org/10.3390/foods13030496
Datta PS, Tyagi SK (1996) Major ion chemistry of groundwater of Delhi area: chemical weathering processes and groundwater flow regime. J Geol Soc India 47:179–188
DeZuane J (1997) Drinking water quality, 2nd edn. John Wiley & Son, New York
Dev R, Bali M (2018) Evaluation of groundwater quality and its suitability for drinking and agricultural use in district Kangra of Himachal Pradesh India. J Saudi Soc Agric Sci 17:350–358. https://doi.org/10.1016/j.jssas
Dippong T, Mihali C, Marian M, Mare Rosca O, Resz MA (2023) Correlations between chemical, hydrological and biotic factors in rivers from the protected area of Tisa Superioară, Romania. Process Saf Environ Prot 176:40–55
Domenico PA (1972) Concepts and models in groundwater hydrology. McGraw-Hill, New York
EPA (2005) Guidelines For carcinogen risk assessment. EPA/630/P-03?001F. US Environmental Protection Agency Risk Assessment Forum, Washington, DC
Eaton FM (1950) Significance of carbonates in irrigation waters. Soil Sci 39:123–133
Edet A (2018) Seasonal and spatio-temporal patterns, evolution and quality of groundwater in Cross River State, Nigeria: implications for groundwater management. Sustain Wat Res Manage. https://doi.org/10.1007/s40899-018-0236-6
Edet AE, Offiong OE (2002) Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo-Odukpani area, Lower Cross River Basin (southeastern Nigeria). GeoJournal 57:295–304. https://doi.org/10.1023/B:GEJO.0000007250.92458.de
Edet AE, Teme SC, Okereke CS, Esu EO (1994) Lineament analysis for groundwater exploration in Precambrian Oban Massif and Obudu Plateau, SE Nigeria. J Min Geol 30(1):87–95
Ekwere AS, Edet BB (2021) Temporal variations of heavy metals in sediments, soils and dust particulates across the rock quarrying districts of the Oban Massif, southeastern Nigeria. Environ Nanotech Monit Manage 15:100431
Ekwere AS, Edet AE, Ekwere SJ (2012) Ground water chemistry of Oban Massif, Southeastern Nigeria. An Inter J Appl Sci 7:51–66
Ellis KU (1993) Surface water pollution and its control. Macmillan press, London
Etikala B, Madhav S, Somagouni SG (2022) Urban water systems: an overview. Curr Dir Water Scarcity Res 6:1–19. https://doi.org/10.1016/B978-0-323-91838-1.00016-6
Farzan SF, Karagas MR, Chen Y (2013) In utero and early life arsenic exposure in relation to long-term health and disease. Toxicol Appl Pharmacol 272(2):384–390
Ghalib HB (2017) Groundwater chemistry evaluation for drinking and irrigation utilities in east Wasit province, Central Iraq. Appl Wat Sci 7(7):3447–3467. https://doi.org/10.1007/s13201-017-0575-8
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088–1090. https://doi.org/10.1126/science.170.3962.1088
Haile-Meskale M (2017) An overview of saturation state of groundwater with respect to some common minerals in South Central Ontario. Intern J Sci Basic Appl Res 36(5):32–47
Hameed A, Alobaidyb MJ, Abid MS, Mauloom B (2010) Application of water quality index for assessment of Dodkan lake ecosystem, Kurdistan region, Iraq. J Wat Resou Protec 2:792–798
Hem JD (1985) Study and interpretation of the chemical characteristics of natural waters. US Geological Survey Water Supply Paper 1473, 3rd edn. Harvard University Press, Massachusetts
Humbarde SV, Panaskar DB, Pawar RS (2014) Evaluation of the seasonal variation in the hydro geochemical parameters and quality assessment of the groundwater in the proximity of Parli Thermal power plant, Beed, Maharashtra India. Adv Appl Sci Res 5:24–33
Ibe CF, Opara AI, Amaobi CE, Ibe O (2021) Environmental risk assessment of the intake of contaminants in aquifers in the vicinity of a reclaimed waste dumpsite in Owerri municipal, Southeastern Nigeria. Appl Wat Sci 11:24. https://doi.org/10.1007/s13201-020-01355-4
Iloeje PN (2001) A New geography of Nigeria. New Revised Edition. Longman, Harlow
Issa HM, Alshatteri AH (2021) Impacts of waste water discharge from Kalar city on Diyala-Sirwan river water quality, Iraq: pollution evaluation, health risks of heavy metals contamination. Appl Wat Sci 11:73. https://doi.org/10.1007/s13201-021-01397-2
Krishna KS, Rammohan V, Dajkumar SJ, Jeevanandam M (2009) Assessment of groundwater quality and Hydro-geochemistry of Manimuktha River basin, Tamil Nadu, India. Environ Monit Assess 159:341–351
Lavitt N, Acworth R, Jankowski J (1997) Vertical hydro geochemical zonation in a coastal section of the Botany sands aquifer, Sydney, Australia. J Hydrol 5:64–74
Lee JS, Chon HT, Jung MC (2005) Toxic risk assessment and environmental contamination of heavy metals around abandoned metal mine sites in Korea. Key Eng Mater 277:542–547
Li SY, Zhang QF (2010) Spatial characterization of dissolved trace elements and heavy metals in the upper Han River (China) using multivariate statistical techniques. J Hazard Mater 176(1–3):579
Mason B, Moore C (1982) Principles of geochemistry. Wiley, New York.
Misra AK (2013) Influence of stone quarries on groundwater quality and health in Fatehpur Sikri, India. Inter J Sust Built Env 2:73–88
Modibo SA, Lin X, Koné S (2019) Assessing groundwater mineralization process, quality, and isotopic recharge origin in the Sahel region in Africa. Water 11:789. https://doi.org/10.3390/w11040789
Nayak A, Matta G, Uniyal DP (2023) Hydrochemical characterization of groundwater quality using chemometric analysis and water quality indices in the foothills of Himalayas. Environ Dev Sustain 25:14229–14260. https://doi.org/10.1007/s10668-022-02661-4
Nganje TN, Admu CI, Ugbaja AN, Amah AE (2010) Evaluation of hydrogeochemical characteristics of groundwater in parts of the lower Benue Trough, Nigeria. Chin J Geochem 29:398–406. https://doi.org/10.1007/s11631-0472-43
Nganje TN, Agbor EE, Adamu CI, Ukpong AS, Katte BF, Edet AE, Hursthouse AS (2020) Public health challenges as a result of contaminated water sources in Kumba, Cameroon. Environ Geochem Health 42(4):1167–1195
Nganje TN, Edet A, Cuthbert S, Adamu CI, Hursthouse AS (2020) The concentration, distribution and health risk from potentially toxic elements in the soil - plant - water system developed on black shales in SE Nigeria. J Afri Ear Sci 165:103806. https://doi.org/10.1016/j.jafrearsci.2020.103806
Noori R, Khakpour A, Omidvar B (2010) Comparison of principal component analysis multivariate linear regression models for predicting the river flow based on developed discrepancy ratiostatistic. Expert Syst Appl 37:5856–5862
Okereke CS, Esu EO, Edet AE (1998) Determination of potential groundwater sites using geological and geophysical techniques in the Cross-River State, Southeastern Nigeria. J Afri Ear Sci 27(1):149–163
Orinya FO, Akubugwo TE, Edeogu CO (2013) Evaluation of the concentration of selected heavy metals and effects on soils enzymatic activities in an abandoned cement factory, Niger cement, Ngalagu and its Environs. Inter J Biochem Res Rev 41:16–17
Ozcelik M (2016) Environmental pollution and its effect on water sources from marble quarries in western Turkey. Env Ear Sci 75:796. https://doi.org/10.1007/s12665-016-5627-0
Pais I, Jones JB (1997) The handbook of trace elements. St Lucie Press, Boca Raton Florida
Panpan X, Wenwen F, Hui Q, Qiying Z (2019) Hydrogeochemical characterization and irrigation quality assessment of shallow groundwater in the Central-Western Guanzhong basin, China. Inter J Env Res Publ Heal. https://doi.org/10.3390/ijerph16091492
Paustenbach DJ (2002) Human and ecological risk assessment: theory and practice. Wiley, New York
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Eos Trans Amer Geophy Union 25(6):914–928. https://doi.org/10.1029/TR025i006p00914
Pliz J (1993) Statistics for windows. Statsoft Inc, Tulsa
Rahman MM, Mondal MA (2015) Assessment of groundwater and its impact on soil properties along with plant growth. Bangladesh J Agric Sci 34(1):39–42
Rajesh R, Brindha K, Elango L (2015) Groundwater quality and its hydrochemical characteristics in a shallow weathered rock aquifer of Southern India. Water Qual Expo Health 7(515):524
Ramakrishnaiah ZR, Sadashivaiah C, Ranganna (2009) Assessment of water quality index for groundwater in Tumkur Taluk, Karnataka State, India. E-J Chem 6(2):523–530. https://doi.org/10.1155/2009/757424
Rao NS (2017) Controlling factors of fluoride in groundwater in a part of South India. Arab Jour of Geosci 10(23):524. https://doi.org/10.1007/s12517-017-3291-7
ReddyY-S SV (2023) Assessment of Heavy metal pollution and its health implications in groundwater for drinking purpose around inactive mines, SW region of Cuddapah Basin, South India. Total Environ Res Themes 8:100069
Richards LA (1954) Diagnosis and improvement of saline alkali soils. US Department of Agriculture, Handbook. 1954; 60.
Rouabhia AEK, Djabri L (2010) Irrigation and the risk of saline pollution. Example of the groundwater of the Miocene aquifer of the El Ma El Abiod plain. Larhyss J 8:55–67
Sikakwe GU, Ilaumo BU (2021) Contamination of water sources by potentially toxic elements around agricultural and quarry land uses of Oban Massif and environs southeastern Nigeria. Arab J Geosci. https://doi.org/10.1007/s12517-021-07647-6
Sikakwe GU, Ilaumo BU (2021) Geochemical characteristics and evaluation of the potentially toxic metals in surface and groundwater in Akamkpa-Biase, southeastern Nigeria. Arab J Geosci. https://doi.org/10.1007/s12517-021-07162-8
Singh AK, Raj B, Tiwari AK, Mahato MK (2013) Evaluation of hydrogeochemical processes and groundwater quality in the Jhansi district of Bundelkhand region, India. Env Ear Sci. https://doi.org/10.1007/s12665-012-2209-7
Srinivasamoorthy K, Gopinath M, Chidambaram S, Vasanthavigar M, Sarma VS (2014) Hydrochemical characterization and quality appraisal of groundwater from Pungar sub basin, Tamilnadu India. J King Saud Univ Sci 26(1):37–52
Srivastava SK, Ramanathan AL (2008) Geochemical assessment of groundwater quality in vicinity of Bhalswa landfill, Delhi, India, using graphical and multivariate statistical methods. Environ Geol 53:1509–1528
Standard Organisation of Nigeria (SON 2007) Nigerian Industrial Standard (NIS); Abuja, Nigeria
Todd DK (1980) Groundwater hydrology, 2nd edn. Wiley, New York
Tolins M, Ruchirawat M, Landrigan P (2014) The developmental neurotoxicity of arsenic: cognitive and behavioral consequences of early life exposure. Ann Glob Health 80(4):303–314
USEPA (1997) Risk assessment guidance for superfund, vol 1, human health evaluation manual (part A). Report EPA/540/1-89/002, US Environ Prot Agency Washington DC
USEPA (1997) Exposure Factor Hand Book (EPA/600/p-95/002Fa) (Update to Exposure Factors Handbook EPA/600/8–89/043). Environmental Protection Agency Region 1, Washington DC
USEPA (1989) Risk assessment: guidance for superfund. In: Human health evaluation manual (Part A), Interim Final, vol 1. Office of Emergency and Remedial Response, U.S. Environmental Protection Agency, Washington DC
Vengosh A, Gill J, Lee DM, Bryant HG (2002) A multi-isotope (B, Sr, O, H, and C) and age dating (3H–3He and 14C) study of groundwater from Salinas Valley, California: hydrochemistry, dynamics, and contamination processes. Wat Resour Res 38(1):1–9
Vetrimurugan E, Brindha K, Elango L (2017) Human exposure risk to heavy metals through groundwater used for drinking in an intensively irrigated river delta. Appl Wat Sci 7:3267–3280. https://doi.org/10.1007/s13201-016-0472-6
WHO (2017) Guidelines for drinking-water quality, 4th edn. World Health Organization, Geneva
WHO (2011) World Health Organisation Guidelines for drinking water quality. In: Health criteria and other supporting information, 4th edn. WHO, Geneva, 2011 http://www.whqlibdoc.who.int/publications/2011/978924151_eng.pdf Accessed 15 Jan 2011
Walton, R., Samuel, V. P. & Keith, H. (2012). The sources, Distribution and trends of chloride in waters of Illinois at Urbana-Champaign, Champaign, Illinois 67
Wongsasuluk P, Chotpantarat S, Siriwong W, Robson M (2014) Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province, Thailand. Environ Geochem Heal 36:169–182
Yidana SM (2010) Groundwater classification using multivariate statistical methods, Southern Ghana. Environmentalist 57(5):445–469
Zhang B, Zhao D, Zhou P, Qu S, Liao F, Wang G (2020) Hydrochemical characteristics of groundwater and dominant water-rock interactions in the Delingha Area, Qaidam Basin, Northwest China. Water 12(3):836. https://doi.org/10.3390/w12030836
Acknowledgements
This study is part of the PhD dissertation of the first author. The authors wish to thank Mr. Adeniyi, A of National Agency for Food and Drug Administration Control (NAFDAC), Lagos, Nigeria for granting access to the first author for the use of ICP-MS in the analyses of the water samples. Also, the authors thank the owners of the various quarry sites for access to their facilities.
Funding
No funding was provided for this study.
Author information
Authors and Affiliations
Contributions
G I—PhD thesis, field work TN- Draft of manuscript AE- Conceptualization and design CA- Field work, GE- Fieldwork The authors have seen and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work has not been published elsewhere and the sources of materials used have been duly acknowledged.
Consent for publication
All authors have given their consent for publication of this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ikpi, G.E., Nganje, T.N., Edet, A. et al. Investigation of hydrochemical characteristic, water quality and associated health risks of metals and metalloids in water resources in the vicinity of Akamkpa quarry district, southeastern, Nigeria. Geochem Trans 25, 7 (2024). https://doi.org/10.1186/s12932-024-00090-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12932-024-00090-y