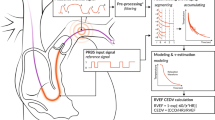
Abstract
Background
Cardiac dysfunction from pulmonary vascular disease causes characteristic findings on cardiopulmonary exercise testing (CPET). We tested the accuracy of CPET for detecting inadequate stroke volume (SV) augmentation during exercise, a pivotal manifestation of cardiac limitation in patients with pulmonary vascular disease.
Methods
We reviewed patients with suspected pulmonary vascular disease in whom CPET and right heart catheterization (RHC) measurements were taken at rest and at anaerobic threshold (AT). We correlated CPET-determined O2·pulseAT/O2·pulserest with RHC-determined SVAT/SVrest. We evaluated the sensitivity and specificity of O2·pulseAT/O2·pulserest to detect SVAT/SVrest below the lower limit of normal (LLN). For comparison, we performed similar analyses comparing echocardiographically-measured peak tricuspid regurgitant velocity (TRVpeak) with SVAT/SVrest.
Results
From July 2018 through February 2023, 83 simultaneous RHC and CPET were performed. Thirty-six studies measured O2·pulse and SV at rest and at AT. O2·pulseAT/O2·pulserest correlated highly with SVAT/SVrest (r = 0.72, 95% CI 0.52, 0.85; p < 0.0001), whereas TRVpeak did not (r = -0.09, 95% CI -0.47, 0.33; p = 0.69). The AUROC to detect SVAT/SVrest below the LLN was significantly higher for O2·pulseAT/O2·pulserest (0.92, SE 0.04; p = 0.0002) than for TRVpeak (0.69, SE 0.10; p = 0.12). O2·pulseAT/O2·pulserest of less than 2.6 was 92.6% sensitive (95% CI 76.6%, 98.7%) and 66.7% specific (95% CI 35.2%, 87.9%) for deficient SVAT/SVrest.
Conclusions
CPET detected deficient SV augmentation more accurately than echocardiography. CPET-determined O2·pulseAT/O2·pulserest may have a prominent role for noninvasive screening of patients at risk for pulmonary vascular disease, such as patients with persistent dyspnea after pulmonary embolism.
Similar content being viewed by others
Introduction
Cardiopulmonary exercise testing (CPET) had been proposed as a noninvasive method to detect pulmonary vascular disease among patients with dyspnea and exercise intolerance after acute pulmonary embolism (PE) [1, 2] Pulmonary vascular disease-associated cardiac limitation is manifested by inadequate stroke volume (SV) augmentation in response to exercise [3, 4]. CPET has disclosed evidence of inadequate SV augmentation in over half of patients with dyspnea after PE [5]. However, CPET findings suggestive of pathologically decreased SV augmentation have never been validated against the gold standard of direct measurement by right heart catheterization (RHC) in patients with pulmonary vascular disease.
The “direct Fick method” of measuring SV by RHC requires simultaneous determination of oxygen consumption rate (VO2), mixed venous O2 content, arterial O2 content and heart rate. The procedure, though highly accurate, is too invasive and too expensive to evaluate stroke volume augmentation among the vast numbers of patients with post-PE dyspnea. SV, however, is related to VO2/heart rate (O2·pulse) and the difference between arterial and mixed venous oxygen content (Ca−vO2) according to the equation.
It has been shown among patients with pulmonary vascular disease that the trajectory of O2·pulse increase during exercise does indeed reflect the pattern expected of SV increase [6]. Furthermore, since (Ca−vO2) increases predictably between rest and anaerobic threshold (AT), SV augmentation between rest and AT (SVAT/SVrest) is reflected on CPET by the relative increase in O2·pulse between AT and rest (O2·pulseAT/O2·pulserest): [7]
We retrospectively reviewed our clinical experience with combined CPET and RHC examinations to determine whether, in patients with suspected pulmonary vascular disease, O2·pulseAT/O2·pulserest, measured noninvasively by CPET predicts abnormally low SVAT/SVrest, measured invasively by RHC.
Methods
Subjects
We reviewed the results of simultaneous RHC and CPET among consecutive patients suspected of having pulmonary vascular disease who were referred to the University of California, San Diego from January 2018 through February 2023. RHC-CPET was performed based on the clinical judgement of the pulmonary vascular specialist. Among patients with more than one study, we evaluated only the first study. Inclusion criteria were: (1) measurement, at rest, of heart rate (HRrest), cardiac output (Qrest) by the direct Fick method and O2·pulserest; (2) measurement, at an independently determined AT point, of O2·pulseAT; and (3) measurement, when VO2 was within 20% of the VO2 at AT, of HRAT and QAT. There were no exclusion criteria. The University of California, San Diego Institutional Review Board approved the study (IRB #171,888).
Right heart catheterization and SVAAT
RHC was performed at rest and during exercise as previously described [8]. A radial artery catheter and a pulmonary artery catheter were inserted in the cardiac catheterization laboratory while patients were in the supine position. Right atrial, right ventricular, pulmonary artery, and pulmonary artery occlusion pressure were measured in succession. Once a stable respiratory quotient was observed with the patient at rest, heart rate was recorded, and cardiac output was determined with the direct Fick method from the measured VO2 and simultaneous radial artery and pulmonary artery blood gases.
The subjects then performed incrementally increased exercise on a supine cycle ergometer (Medical Positioning, Inc.), as described below. Heart rate and cardiac output measurements were repeated in a similar fashion during exercise at point near anerobic threshold (as determined in real-time by a change in the slope VCO2 versus VCO2) and again near peak exertion.
Hemodynamic data were collected without knowledge of the CPET results. SVAT/SVrest was calculated as.
where QAT and Qrest represent cardiac output at anaerobic threshold and at rest, respectively, and HRAT and HRrest represent heart rate at anaerobic threshold and at rest, respectively. We pre-specified the lower limit of normal for SVAT/SVrest based right heart catheterization data from healthy volunteers, in whom stroke volume increased by 38.8% (SD 5.2%) between rest and AT [7]. We arbitrarily selected the mean minus two times the standard deviation from that experiment (128%) as the SVAT/SVrest lower limit of normal (LLN) for the current study.
Cardiopulmonary exercise test determination of O2·pulserest and O2·pulseAT
Simultaneously with the RHC, we performed incremental symptom-limited CPET with the patients on a recumbent bicycle, using a stepwise exercise protocol to produce a uniform increase in work rate and metabolic energy expenditure per incremental step. O2·pulserest was determined during steady state rest with a V-Max metabolic cart (CareFusion, San Diego, CA) or a Ultima Cardio2 metabolic cart (MGC Diagnostics, St Paul, MN) from VO2, measured through breath-by-breath analysis of inspired and expired gases and heart rate, measured by continuous electrocardiography (Fig. 1).
O2·pulse at rest (O2·pulserest) and at anaerobic threshold (O2·pulseAT). (A) Normal increase in O2·pulse from rest (O2·pulserest, solid vertical line) to anaerobic threshold (O2·pulseAT, dashed vertical line). O2·pulserest was 4.5 ml O2/beat and O2·pulseAT was 14.5 ml O2/beat. (B) Pathologically low increase from O2·pulserest to O2·pulseAT. O2·pulserest was 3.8 ml O2/beat and O2·pulseAT was 7.3 ml O2/beat
Anaerobic threshold was determined by a board-certified pulmonologist (TM) without knowledge of the RHC results through analysis of the slopes of VCO2 vs. VO2 (V-slope) as well as VE/VO2, VE/VCO2, PETO2 and PETCO2 vs. time, with the V-slope method as the decisive criteria. O2·pulseAT was determined retrospectively during the 20-second interval containing the anaerobic threshold point (Fig. 1).
Echocardiography
For comparison, peak tricuspid regurgitation velocity (TRVpeak) and other signs of pulmonary vascular disease [9] were recorded from echocardiograms that had been performed within six months of the combined CPET-RHC studies. In addition, the echocardiographic results were categorized as high- or intermediate-risk vs. low-risk, according to the recommendations of the European Society of Cardiology and the European Respiratory Society (ESC/ERS) Task Force for the Diagnosis and Management of Acute Pulmonary Embolism [10] and the ESC/ERS Task Force for the Diagnosis and Treatment of Pulmonary Hypertension [9].
Statistical analysis
Continuous variables are presented as mean (+/- standard deviation) or median and interquartile range [IQR]. Categorical variables are presented as number (%). The Shapiro-Wilk test was used to evaluate SVAT/SVrest values, accepting p > 0.05 as confirmation of their normal distribution. Pearson correlation was used to compare O2·pulseAT/O2·pulserest to SVAT/SVrest and to compare TRVpeak to SVAT/SVrest. O2·pulseAT/O2·pulserest and TRVpeak were also linearly regressed on SVAT/SVrest. Receiver operating characteristic curves were plotted to compare the sensitivities and specificities of O2·pulseAT/O2·pulserest and TRVpeak for detecting SVAT/SVrest below the lower limit of normal (LLN = 1.28) [7]. We pre-defined 90% as an acceptable sensitivity for a screening test to detect SVAT/SVrest below the LLN based the consensus of clinical judgment within our research team. Statistical calculations were performed with Prism version 9 (GraphPad Software, San Diego CA).
Results
Study population
During the study period, 83 simultaneous RHC and CPET tests were performed. Forty-seven tests were not included because blood was not sampled from the systemic artery (n = 8) or pulmonary artery (n = 1) for direct Fick cardiac output measurement; anaerobic threshold was not reached or was indeterminate (n = 8); or cardiac output was not measured during exercise while the VO2 was within 20% of VO2 at anaerobic threshold (n = 30). Thirty-six tests met the inclusion criteria and were included in the analysis (Fig. 2). The demographics and hemodynamics of the excluded patients were not different from the included patients (Table e1).
Included patients were 56.5 [40.25, 66] years of age and had body mass index (BMI) of 28 [24.1, 32.5] kg/m2 (Table 1). There were 11 (30.6%) men and 25 (69.4%) women. 12 patients (33.3%) had co-existing cardiopulmonary comorbidities. All patients exercised to the point of volitional exhaustion, as communicated directly to the test operators, without adverse effects. All patients reached anaerobic threshold and none demonstrated a plateau in O2·pulse, VE or VO2 over time prior to the points at which we collected data for the study. The distribution of hemodynamic measurements and CPET parameters are illustrated in Tables 2 and 3, respectively. The Shapiro–Wilk test supported that SVAT/SVrest data were normally distributed (W = 0.9539, p = 0.1388).
Relationship between O2·pulseAT/O2·pulserest and SVAAT
Figure 3A illustrates a statistically significant linear correlation between O2·pulseAT/O2·pulserest and SVAT/SVrest (r = 0.72, 95% CI 0.52, 0.85; p < 0.0001). Linear regression yielded a slope of 0.51 (95% CI 0.48, 0.55) between SVAT/SVrest and O2·pulseAT/O2·pulserest. The LLN for SVAT/SVrest (1.28) corresponded to O2·pulseAT/O2·pulserest of 2.5 (95% CI 2.3, 2.7). In contrast, Fig. 3C shows no significant correlation between TRVpeak and SVAT/SVrest (r = -0.09, 95% CI -0.47, 0.33; p = 0.69).
O2·pulseAT/O2·pulserest compared to TRVpeak to predict stroke volume augmentation. A. O2·pulseAT/O2·pulserest determined from CPET data (see text) correlated with stroke volume augmentation from rest to anaerobic threshold (SVAAT) measured by right heart catheterization (p < 0.0001). B. The ROC curve of O2·pulseAT/O2·pulserest to detect SVAAT below the lower limit of normal (LLN) had an area under the ROC curve of 0.92 (SE 0.04, p = 0.0002). C. Tricuspid regurgitant velocity peak (TRVpeak) measured by echocardiography did not correlate with SVAAT (p = 0.69). D. The ROC curve of TRVpeak to detect SVAAT below the LLN had an area of 0.69 (SE 0.10), which was not statistically significant (p = 0.12)
The area under the receiver operating characteristic curve (AUROC) of O2·pulseAT/O2·pulserest for detecting SVAT/SVrest below the LLN (Fig. 3B) was 0.92 (SE 0.04, p = 0.0002). The AUROC 95% CI was 0.832 to 1.00. O2·pulseAT/O2·pulserest of less than 2.6 was 92.6% sensitive (95% CI 76.6%, 98.7%) and 66.7% specific (95% CI 35.2%, 87.9%). O2·pulseAT/O2·pulserest of less than 2.2 was only 74% sensitive (95% CI 55.3%, 86.8%) but 100% specific (95% CI 70.1%, 100%).
The AUROC of TRVpeak to detect SVAT/SVrest below the LLN (Fig. 3D) was 0.69 (SE 0.10), which was not statistically significant (p = 0.12). The AUROC 95% CI was 0.495 to 0.889, which includes the nondiscriminatory value of 0.5. No value of TRVpeak had a sensitivity of 90% or higher. Echocardiography meeting the ESC/ERS criteria [10] for intermediate- or high-risk (TRVpeak >2.8 and/or presence of two other pulmonary hypertension signs [9]) was only 23.0% sensitive (95% CI 11.0%, 42.0%) but 100% specific (95% CI 64.6%, 100%) for detecting SVAT/SVrest below the LLN.
Discussion
We validated the accuracy of O2·pulseAT/O2·pulserest to predict SVAT/SVrest in a consecutive series of patients receiving combined CPET and RHC for the clinical evaluation of dyspnea potentially related to pulmonary vascular disease. We derived a cutoff of 2.6 for O2·pulseAT/O2·pulserest to predict SVAT/SVrest less than the LLN. We observed a highly significant linear relationship between O2·pulseAT/O2·pulserest and SVAT/SVrest, with a slope that corresponds with previous CPET-RHC comparisons among healthy subjects and among patients with various severities of heart failure [7, 11,12,13,14,15]. Although O2·pulseAT/O2·pulserest enables merely an estimate of the RHC measurement of SVAT/SVrest, the correlation between the two is comparable to or even superior to the correlation between different invasive methods of measuring stroke volume by RHC [16,17,18,19].
SV augmentation is an important adaptation to exercise that helps increase cardiac output and maintain organ perfusion during increased oxygen utilization [12]. Increased venous pressure during exercise enhances right ventricular end-diastolic volume [20] and normally improves contractility [21]. SV rises incrementally as exercise proceeds and reaches a plateau near AT [7, 12, 22,23,24]. In healthy persons, there is an approximately 40% increase in SV by the time AT is reached [7]. The advantage to considering SVAT/SVrest, rather than SVAT alone, is that the ratio controls for demographic factors (body size, age, sex, etc.) that typically influence SV.
Pulmonary vascular disease leads to elevated right ventricular end-diastolic volume and impaired contractility at rest, which impedes the normal adaptation to exercise [21]. As a result, SV augmentation is markedly decreased [3, 4] Residual pulmonary vascular obstruction limits SV augmentation, increases pulmonary artery resistance and compromises right ventricular function [25, 26]. Since SV augmentation substantially improves among CTEPH patients after pulmonary artery thromboendarterectomy, it is reasonable to attribute the defect to pulmonary vascular obstruction itself [4]. Furthermore, insufficient SVA in response to exercise predicts mortality from pulmonary hypertension more accurately than any other exercise parameter and enhances the accuracy of mortality prediction above the six minute walking distance alone [27].
Although CTEPH is present in only a small fraction of patients with dyspnea after PE [28], less severe pulmonary vascular disease causes respiratory symptoms [29], hypoxemia [30,31,32], gas exchange deficits [31, 33, 34] and exercise intolerance [35]. Residual pulmonary vascular obstruction is associated with the risk of progression to CTEPH [36]. In our previous series of CPET for patients with long-term dyspnea after acute pulmonary embolism, low O2·pulseAT/O2·pulserest corresponded to residual pulmonary artery obstruction [5].
Among symptomatic post-pulmonary embolism patients, low O2·pulseAT/O2·pulserest measured noninvasively during CPET suggests inadequate SV augmentation because of residual pulmonary vascular occlusion [5]. Validation that O2·pulseAT/O2·pulserest accurately reflects SV augmentation enables CPET to be an informative and practical noninvasive tool to help distinguish between pulmonary vascular disease and deconditioning or anxiety (in the absence of physiological defects) among the large number of patients with dyspnea after pulmonary embolism [1].
Our results compliment the results of Held et al. and of McCabe et al., who disclosed abnormal CPET findings in a majority of patients in whom CTED or CTEPH had been confirmed by RHC [37, 38]. As was the case in our study, echocardiography (including TRVpeak) was unable to detect pulmonary vascular disease in 31% of CTEPH patients [37]. The insensitivity likely refects the fact that echocardiography is routinely performed at rest, which may not reflect defects that are manifested only during exercise. However, we recognize that echocardiography typically preceded RHC-CPET, at times by several months. It is possible that the difference between echocardiographic and CPET results were influenced by disease progression among some patients.
Notably, CPET data from CTED and CTEPH patients reported by Held et al. and McCabe et al. reflected ventilatory inefficiency, presumably based on ventilation/perfusion mismatching [37, 38]. We observed similar ventilatory inefficiency in our patients, which we are investigating in a separate study. The current study focuses on the ability of O2·pulseAT/O2·pulserest, to reflect stroke volume augmentation itself. However, we anticipate that both factors are likely implicated in pulmonary vascular disease after acute PE [5, 39].
Since acute pulmonary embolism occurs in about 63/100,000 persons per year [40], up to half of whom report chronic dyspnea [41,42,43], the method we validated could detect SV augmentation limitation due to pulmonary vascular disease in a large number of at-risk patients [28]. It is more practical than RHC and more sensitive than echocardiography. Since our patients ranged from normal to very poor cardiopulmonary reserve during exercise, our results suggest that O2·pulseAT/O2·pulserest would reflect SV augmentation across a wide spectrum of dysfunction.
Besides acute pulmonary embolism, there are numerous risk factors associated with pulmonary vascular disease, including scleroderma and other connective tissue diseases. In these at-risk patients, symptoms begin with dyspnea on exertion, but pulmonary hypertension may not be present at rest. Non-invasive CPET to screen for decreased exercise-related stroke volume augmentation by detecting impaired O2·pulseAT/O2·pulserest has the potential to identify these patients as well, earlier in their disease course.
ESC/ERS guidelines recommend transthoracic echocardiography as an initial test to evaluate dyspnea on exertion after pulmonary embolism [10]. However, while TTE can be useful as a screen for chronic thromboembolic pulmonary hypertension (CTEPH), it may not be the best approach for evaluating patients who are limited by persistent perfusion defects that cause exercise-induced pulmonary hypertension. Our present research has shown that tricuspid regurgitant velocity peak is less sensitive than non-invasively measured O2·pulseAT/O2·pulserest in detecting directly measured SVA AT below the lower limit of normal. Therefore, we recommend non-invasive cardiopulmonary exercise testing (CPET) as the first step in evaluating dyspnea after PE.
Like other studies of CPET and RHC for pulmonary vascular disease [37, 38], our study is limited by its relatively small size and its retrospective nature. In addition, because O2·pulseAT/O2·pulserest, reflects SV augmentation at AT, our study included only RHC tests that measured both SVrest and SV near AT. Estimation of SV augmentation from O2·pulse ratios at other times would have been erroneous due to changes in oxygen extraction and heart rate during exercise above AT [7, 12, 22,23,24]. Nevertheless, with careful attention to technique, SVAT/SVrest could serve as a standard by which to evaluate SV augmentation during exercise.
A limitation of our study is that we selected the LLN for SVAT/SVrest based on RHC-CPET studies performed on young, healthy subjects during upright cycling. Supine position increases venous return at rest and may lower the relative increase in diastolic volume during exercise that contributes to the SV response [20]. Further studies are needed to determine if the predicted and LLN for SVAT/SVrest should be different between upright and recumbent CPETs.
Chronotropic incompetence may confound the clinical implication of stroke volume augmentation, since slow heart rates during exercise would allow more time for diastolic filling and potentially dampen the effect of cardiac dysfunction on stroke volume during exercise. For example, among the five patients in our study who were taking beta blockers, pharmacological slowing of the heart rate, rather than inherent recovery of the ventricles, might have lead to increased stroke volume during exercise. Nevertheless, since beta blockers are unlikely to change Ca−vO2_AT/Ca−vO2_rest, we reasoned that it was still informative to include those patients in the validation that stroke volume augmentation is reflected by the O2·pulseAT/O2·pulserest.
However, it is unlikely that chronotropic incompetence played a substantial role in the current study, since heart rates were similar between subjects with normal SVA (110.3 +/- 19.5) and those with low SVA (107.2 +/- 17.4).
The CPET-based estimation of SVAT/SVrest that we validated during right heart catheterization may be useful as a stand-alone test in other settings. For example, the method may be used for the noninvasive screening of ambulatory patients with a variety of cardiac and pulmonary disorders for exercise-related heart dysfunction. The method would help quantify cardiac adaptation to exercise in patients with known or suspected heart failure.
We speculate that deficient stroke volume augmentation from various types of heart failure (right side or left side, systolic or diastolic) will have similar increases in Ca−vO2 between rest and AT and therefore similar effects of SVAT/SVrest on O2·pulseAT/O2·pulserest [7]. However, we would not expect O2·pulseAT/O2·pulserest to reflect SVAT/SVrest accurately among patients with myopathies that cause poor O2 extraction (e.g. mitochondrial enzyme defects), since the Ca−vO2 might not change in a predictable fashion at AT. Although the current study did not include such patients, it is possible that myopathies could be differentiated from stroke volume augmentation defects by differences in O2·pulse trajectories subsequent to AT. Degani-Costa et al. reported flattening of the O2·pulse trajectory (and upward deflection of the heart rate vs. VO2 plot) during the later portions of exercise among subjects with pulmonary hypertension but not among those with mitochondrial myopathies [6].
We hope that our results will open a line of investigation about the role of this technique in the management of patients at risk of right ventricular dysfunction from pulmonary vascular disease. The technique may be particularly helpful in patients in whom dysfunction occurs during exercise but is not apparent at rest. Also, because SVAT/SVrest reflects overall cardiac adaptation to exercise, it is a potential predictor of poor outcomes that may supplement the roles of peak VO2, VO2 at AT and VE/VCO2. Future research would be needed to ascertain the clinical utility of using O2·pulseAT/O2·pulserest to evaluate patients with dyspnea of unknown origin.
Summary
We validated the accuracy of O2·pulseAT/O2·pulserest, measured noninvasively by CPET, compared to invasive measurement of SVAT/SVrest during RHC. We derived a cutoff value of 2.6, which should be validated in future studies. Our results suggest that CPET can be used to evaluate SV augmentation in symptomatic patients at risk for pulmonary vascular disease, such as those with dyspnea after acute pulmonary embolism, to detect early compromise of the right ventricle.
Data availability
Deidentified data relevant to this study is available upon request to the corresponding author.
Abbreviations
- AT:
-
Anaerobic threshold
- CPET:
-
Cardiopulmonary exercise test
- HRAT :
-
Heart rate at anaerobic threshold
- HRrest :
-
Heart rate at rest
- QAT :
-
Cardiac output at anaerobic threshold
- Qrest :
-
Cardiac output at rest
- RHC:
-
Right heart catheterization
- SVA:
-
Stroke volume augmentation
- SVAAT :
-
Stroke volume augmentation at anaerobic threshold measured by RHC
- O2·pulse:
-
Oxygen consumption rate divided by heart rate
- O2·pulserest :
-
O2·pulse at rest
- O2·pulseAT :
-
O2·pulse at anaerobic threshold
References
Morris TA, Fernandes TM, Channick R. How we do it: evaluation of dyspnea and exercise intolerance after acute pulmonary embolism. Chest.
Farmakis IT, Valerio L, Barco S et al. Cardiopulmonary exercise testing in the follow-up after acute pulmonary embolism. Eur Respiratory Journal: Official J Eur Soc Clin Respiratory Physiol 2023.
Holverda S, Gan CT, Marcus JT, Postmus PE, Boonstra A, Vonk-Noordegraaf A. Impaired stroke volume response to exercise in pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47(8):1732–3.
Surie S, van der Plas MN, Marcus JT, et al. Effect of pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension on stroke volume response to exercise. Am J Cardiol. 2014;114(1):136–40.
Fernandes TM, Alotaibi M, Strozza DM, et al. Dyspnea Postpulmonary Embolism from physiological Dead Space Proportion and Stroke volume defects during Exercise. Chest. 2020;157(4):936–44.
Degani-Costa LH, Nery LE, Rodrigues MT et al. Does oxygen pulse trajectory during incremental exercise discriminate impaired oxygen delivery from poor muscle oxygen utilisation? ERJ Open Res 2019;5(2).
Stringer WW, Hansen JE, Wasserman K. Cardiac output estimated noninvasively from oxygen uptake during exercise. J Appl Physiol (1985). 1997;82(3):908–12.
McGuire WC, Alotaibi M, Morris TA, Kim NH, Fernandes TM. Chronic Thromboembolic Disease: Epidemiology, Assessment with Invasive Cardiopulmonary Exercise Testing, and options for management. Struct Heart. 2021;5(2):120–7.
Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46(4):903–75.
Konstantinides SV, Meyer G, Becattini C et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J 2019;54(3).
Detry JM, Rousseau M, Vandenbroucke G, Kusumi F, Brasseur LA, Bruce RA. Increased arteriovenous oxygen difference after physical training in coronary heart disease. Circulation. 1971;44(1):109–18.
Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58(2):281–91.
Chatterjee NA, Murphy RM, Malhotra R, et al. Prolonged mean VO2 response time in systolic heart failure: an indicator of impaired right ventricular-pulmonary vascular function. Circ Heart Fail. 2013;6(3):499–507.
Dhakal BP, Malhotra R, Murphy RM, et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015;8(2):286–94.
Agostoni PG, Wasserman K, Perego GB, et al. Non-invasive measurement of stroke volume during exercise in heart failure patients. Clin Sci (Lond). 2000;98(5):545–51.
Yung GL, Fedullo PF, Kinninger K, Johnson W, Channick RN. Comparison of impedance cardiography to direct Fick and Thermodilution cardiac output determination in pulmonary arterial hypertension. Congest Heart Fail. 2004;10(2 Suppl 2):7–10.
Opotowsky AR, Hess E, Maron BA, et al. Thermodilution vs estimated Fick Cardiac output measurement in clinical practice: an analysis of Mortality from the Veterans Affairs Clinical Assessment, Reporting, and Tracking (VA CART) Program and Vanderbilt University. JAMA Cardiol. 2017;2(10):1090–9.
Desole S, Obst A, Habedank D, et al. Comparison between thermodilution and Fick methods for resting and exercise-induced cardiac output measurement in patients with chronic dyspnea. Pulm Circ. 2022;12(3):e12128.
Fares WH, Blanchard SK, Stouffer GA, et al. Thermodilution and Fick cardiac outputs differ: impact on pulmonary hypertension evaluation. Can Respir J. 2012;19(4):261–6.
Steingart RM, Wexler J, Slagle S, Scheuer J. Radionuclide ventriculographic responses to graded supine and upright exercise: critical role of the Frank-Starling mechanism at submaximal exercise. Am J Cardiol. 1984;53(11):1671–7.
Spruijt OA, de Man FS, Groepenhoff H, et al. The effects of exercise on right ventricular contractility and right ventricular-arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med. 2015;191(9):1050–7.
Stickland MK, Welsh RC, Petersen SR, et al. Does fitness level modulate the cardiovascular hemodynamic response to exercise? J Appl Physiol (1985). 2006;100(6):1895–901.
Zhou B, Conlee RK, Jensen R, Fellingham GW, George JD, Fisher AG. Stroke volume does not plateau during graded exercise in elite male distance runners. Med Sci Sports Exerc. 2001;33(11):1849–54.
Vieira SS, Lemes B, de TCdCP, et al. Does Stroke volume increase during an Incremental Exercise? A systematic review. Open Cardiovasc Med J. 2016;10:57–63.
Ribeiro A, Lindmarker P, Johnsson H, Juhlin-Dannfelt A, Jorfeldt L. Pulmonary embolism: one-year follow-up with echocardiography doppler and five-year survival analysis. Circulation. 1999;99(10):1325–30.
Ribeiro A, Lindmarker P, Juhlin-Dannfelt A, Johnsson H, Jorfeldt L. Echocardiography Doppler in pulmonary embolism: right ventricular dysfunction as a predictor of mortality rate. Am Heart J JID – 0370465. 1997;134(3):479–87.
Groepenhoff H, Vonk-Noordegraaf A, Boonstra A, Spreeuwenberg MD, Postmus PE, Bogaard HJ. Exercise testing to estimate survival in pulmonary hypertension. Med Sci Sports Exerc. 2008;40(10):1725–32.
Fernandes T, Planquette B, Sanchez O, Morris T. From Acute to Chronic Thromboembolic Disease. Ann Am Thorac Soc. 2016;13(Suppl 3):207–14.
Phear D. Pulmonary embolism. A study of late prognosis. Lancet. 1960;2:832–5.
Donnamaria V, Palla A, Petruzzelli S, Carrozzi L, Pugliesi O, Giuntini C. Early and late follow-up of pulmonary embolism. Respiration. 1993;60(1):15–20.
Prediletto R, Paoletti P, Fornai E, et al. Natural course of treated pulmonary embolism. Evaluation by perfusion lung scintigraphy, gas exchange, and chest roentgenogram. Chest. 1990;97(3):554–61.
Paraskos JA, Adelstein SJ, Smith RE, et al. Late prognosis of acute pulmonary embolism. N Engl J Med. 1973;289(2):55–8.
Bass H, Banas JS Jr., Dalen JE. Pulmonary function studies. Aid to diagnosis of pulmonary embolism. Arch Intern Med. 1970;126(2):266–8.
Sharma GV, Burleson VA, Sasahara AA. Effect of thrombolytic therapy on pulmonary-capillary blood volume in patients with pulmonary embolism. N Engl J Med. 1980;303(15):842–5.
Helmers RA, Zavala DC. Serial exercise testing in pulmonary embolism. Chest. 1988;94(3):517–20.
Sanchez O, Helley D, Couchon S, et al. Perfusion defects after pulmonary embolism: risk factors and clinical significance. J Thromb Haemost. 2010;8(6):1248–55.
Held M, Grun M, Holl R, et al. Cardiopulmonary exercise testing to detect chronic thromboembolic pulmonary hypertension in patients with normal echocardiography. Respiration. 2014;87(5):379–87.
McCabe C, Deboeck G, Harvey I, et al. Inefficient exercise gas exchange identifies pulmonary hypertension in chronic thromboembolic obstruction following pulmonary embolism. Thromb Res. 2013;132(6):659–65.
Morris TA, Fernandes TM, Channick RN. Evaluation of Dyspnea and Exercise Intolerance after Acute Pulmonary Embolism. Chest. 2023;163(4):933–41.
DeMonaco NA, Dang Q, Kapoor WN, Ragni MV. Pulmonary embolism incidence is increasing with use of spiral computed tomography. Am J Med. 2008;121(7):611–7.
Klok FA, Cohn DM, Middeldorp S, et al. Quality of life after pulmonary embolism: validation of the PEmb-QoL questionnaire. J Thromb Haemost. 2010;8(3):523–32.
Klok FA, Tijmensen JE, Haeck ML, van Kralingen KW, Huisman MV. Persistent dyspnea complaints at long-term follow-up after an episode of acute pulmonary embolism: results of a questionnaire. Eur J Intern Med. 2008;19(8):625–9.
Kahn SR, Hirsch AM, Akaberi A, et al. Functional and Exercise limitations after a First Episode of Pulmonary Embolism: results of the ELOPE prospective cohort study. Chest. 2017;151(5):1058–68.
Acknowledgements
The authors thank, Brenda G. Money, Alicia E. Salcido, Sandee Lombardi and Cynthia Rodriguez for their performance of the technical aspects of the invasive CPETs and their expertise in processing the CPET data. We thank Khadizhat Dakaeva for her careful attention to detail in organizing the data for publication. The authors also thank Drs. William Stringer and Atul Malhotra for critical review of the manuscript and helpful comments prior to submission.
Funding
There was no external funding for this study.
Author information
Authors and Affiliations
Contributions
TAM and TMF contributed equally to the conception, design and execution of the research. Material preparation and data collection performed by JZY, TMF, DGP, MA, and WCM. Data analysis performed by TAM and MA. All authors contributed to writing, editing, and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Summary of conflict-of-interest statements: The authors report no conflicts of interest and no financial support relevant to this work.
Ethical approval
The University of California, San Diego Institutional Review Board approved the study (IRB #171888). A waiver of the requirement for consent to participate was granted for this retrospective review in accordance with the requirements of the US Code of Federal Regulations on the Protection of Human Subjects (45 CFR 46.116(d)). Consent to publish is not relevant because no identifying images or other personal or clinical details of participants were shared in the manuscript or outside the investigator group.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alotaibi, M., Yang, J.Z., Papamatheakis, D.G. et al. Cardiopulmonary exercise test to detect cardiac dysfunction from pulmonary vascular disease. Respir Res 25, 121 (2024). https://doi.org/10.1186/s12931-024-02746-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-024-02746-w