Abstract
Background
COVID-19-induced acute respiratory distress syndrome (ARDS) can result in tissue damage and multiple organ dysfunction, especially in kidney transplant recipients (KTRs) receiving immunosuppressive drugs. Presently, single-cell research on COVID-19-induced ARDS is considerably advanced, yet knowledge about ARDS in KTRs is still constrained.
Methods
Single-cell RNA sequencing (scRNA-seq) analysis was performed to construct a comprehensive single-cell immune landscape of the peripheral blood mononuclear cells (PBMCs) of eight patients with COVID-19-induced ARDS, five KTRs with COVID-19-induced ARDS, and five healthy individuals. Subsequently, we conducted a comprehensive bioinformatics analysis, including cell clustering, enrichment analysis, trajectory analysis, gene regulatory network analysis, and cell–cell interaction analysis, to investigate the heterogeneity of the immune microenvironment in KTRs with ARDS.
Result
Our study revealed that KTRs exhibit significant heterogeneity with COVID-19-induced ARDS compared with those of other individuals, with significant reductions in T cells, as well as an abnormal proliferation of B cells and monocytes. In the context of dual influences from immunosuppression and viral infection, KTRs exhibited more specific plasma cells, along with significant enrichment of dysfunctional GZMB and XAF1 double-positive effector T cells and IFI27-positive monocytes. Additionally, robust communication existed among T cells and monocytes in cytokine signaling. These effects impede the process of immune reconstitution in KTR patients.
Conclusion
Our findings suggest that KTRs with COVID-19-induced ARDS show elevated antibody levels, impaired T cell differentiation, and dysregulation of innate immunity. In summary, this study provides a theoretical foundation for a comprehensive understanding of COVID-19-induced ARDS in KTRs.
Similar content being viewed by others
Introduction
COVID-19 is primarily transmitted through droplets or direct contact. It infects the respiratory tract, causing pneumonia in most cases and acute respiratory distress syndrome (ARDS) in around 15% of cases [44]. In the early disease stages in adults, there is a notable reduction in the number of CD8+ and CD4+ T cells [42]. Decreases in these cells are accompanied by accelerated viral replication, which triggers chemotactic reactions and inflammatory infiltration [42]. ARDS typically develops within 7–10 days following disease onset [42], and is the underlying pathology of severe COVID-19, primarily resulting from elevated levels of pro-inflammatory cytokines; this is commonly known as a cytokine storm [39, 63]. The severity and mortality of COVID-19 are mainly due to sepsis, multiple organ failure caused by cytokine storms, and respiratory failure caused by acute lung injury and ARDS [21, 24, 43, 46, 63]. In a study of 204 COVID-19 patients who received systemic glucocorticoids, the mortality rate was low, with only 5 deaths recorded [21]. However, the mortality rate among COVID-19 patients who develop ARDS is usually closer to 70% [68]. Therefore, it is crucial to investigate the mechanisms of immune dysregulation in COVID-19-induced ARDS to improve the management and prognosis.
Given the potential risks of infection, complications, and immunosuppressive exposure faced by kidney transplant recipients (KTRs) [3], SARS-CoV-2 infection significantly increases patient mortality risk [46]. COVID-19 has a higher likelihood of inducing moderate to severe pneumonia in individuals who have received kidney transplants [1, 12]. Despite the significant therapeutic effects of COVID-19 vaccines in the general population, vaccinated KTRs still face the risk of breakthrough infection, with a mortality risk 8.1 times higher than that in the general population [5, 11, 23]. Hence, exploring the single-cell immune profile of KTRs with COVID-19-induced ARDS, particularly in regards to the impact of virus infection and immunosuppression, is crucial for effectively managing the disease and promoting recovery in patients.
In this study, we constructed a single-cell atlas of immune cells in the peripheral blood mononuclear cells (PBMCs) of both KTRs and non-KTRs with COVID-19-induced ARDS. Additionally, we analyzed the dynamic changes in specific subpopulations of different immune cells, as well as changes in the implicated signaling pathways, revealing the developmental trajectories of different cell subpopulations and their primary regulatory targets at the transcriptional level.
Methods
Sample groups
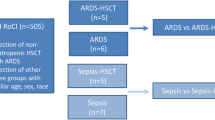
This study adhered to the principles outlined in the Declaration of Helsinki. The participants provided written informed consent prior to sample collection, following standard procedures. Eighteen peripheral blood samples were collected and analyzed in this study. The sample groups consisted of eight patients with COVID-19-induced ARDS who had not received a kidney transplant, five KTRs with COVID-19-induced ARDS, and five healthy individuals (Additional file 2: Table S1).
PBMC collection and processing
Human peripheral blood (20 mL) was collected in ethylenediaminetetraacetic acid anticoagulation tubes and centrifuged at 2000 rpm for 10 min at room temperature (27 °C) to separate the plasma. Lymphocyte separation solution (5 mL) [62] was added to each tube, samples were centrifuged, and the upper layer of plasma was aspirated [28]. An equal volume of sterile phosphate-buffered saline (PBS) was added to dilute the remaining blood cells [22]. The diluted blood cells were combined with the upper layer of the lymphocyte separation solution in a 1:1 ratio and centrifuged at 2500 rpm for 20 min. The resultant middle white film layer was collected into a new 10 mL centrifuge tube and PBS was added to a total volume of 10 mL. The mixture was then centrifuged at 2000 rpm at room temperature for 5 min, after which the lymphocyte separation solution was removed. PBS (1 mL) was added to dilute the resuspended cells to a fixed volume of 10 mL, followed by centrifugation at 1,200 rpm at room temperature for 5 min. The supernatant was discarded, and the separated PBMC precipitate was resuspended in a cell lyophilization solution.
Single-cell RNA sequencing
The DNBelab C Series Single-Cell Library Prep Set (MGI Tech, Shenzhen, China) was used to prepare single-cell RNA-seq (scRNA-seq) libraries, following an established method. Briefly, single-cell suspensions were subjected to droplet encapsulation, emulsion breakage, collection of mRNA-captured beads, reverse transcription, cDNA amplification, and purification, resulting in the generation of barcoded scRNA-seq libraries. Indexed sequencing libraries were constructed according to the manufacturer’s instructions. Sequencing libraries were quantified using the Qubit ssDNA Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). The read structure was paired-end: Read 1 covered 30 bases and encompassed two 10-bp cell barcodes and a 10-bp unique molecular identifier; Read 2 comprised 100 bases of transcript sequences along with a 10-bp sample index. Quality control was performed on the sequencing data to filter out cells that were in the highest and lowest 1% of gene expression, as well as cells with mitochondrial gene expression exceeding 10%.
ScRNA-seq clustering
The Seurat package [9] was used to perform various analyses, including data integration, cell clustering, identification of clustering results, and generation of comprehensive single-cell maps. The dimensionality of the results was reduced using a consistent manifold approximation and projection (UMAP) algorithm [6]. Cell clusters were manually annotated using cell type information from cell marker databases, laboratory experiments, and previous studies [25, 45]. The Seurat package [9] was also used to identify subpopulations in the clustering analysis. The subpopulations were manually annotated by incorporating gene markers associated with the disease to obtain detailed subpopulation information.
Enrichment, trajectory, gene regulatory network, and intercellular communication analyses
The clusterProfiler package [64] was used to conduct enrichment analysis of subpopulation genes with respect to Gene Ontology and Kyoto Encyclopedia of Genes and Genomes to reveal the biological significance of these subpopulations in relation to cellular functions and disease development. The R package Monocle3 [53] was used to construct developmental trajectories for individual cell subpopulations to investigate cell development, differentiation, and transcriptional dynamics. The gene regulatory network (GRN) was analyzed using the pySCENIC module with a particular emphasis on transcription factors [4], Van [57] to investigate transcription regulation, which is a crucial aspect of gene regulation involving transcription factors and their respective binding sites. The iTALK package (https://doi.org/10.1101/507871) was used to identify high-confidence ligand-receptor interactions between subpopulations of cells, thereby uncovering significant events in intercellular communication.
Statistical analysis
Statistical analysis was performed on the bioinformatics cloud platform BioinforCloud (http://www.bioinforcloud.org.cn). Statistical significance was set at P-valve < 0.05.
Results
Immune cells in PBMCs of COVID-19-induced ARDS patients
Sequencing analysis was performed on eighteen individuals, including eight patients with COVID-19-induced ARDS (ARDS_nonKTR; n = 36,466 single cells), five KTRs with COVID-19-induced ARDS (ARDS_KTR; n = 23,980), and five healthy controls (Control; n = 50,752) (Fig. 1A). After quality control and preprocessing, we identified 106,033 high-quality single-cell transcriptomes. These transcriptomes were categorized into 33 clusters and visualized on a single-cell map (Fig. 1B, C). We examined the correlations between the expression patterns among the clusters and their respective cell markers, and seven distinct cell types were identified: CD8+ T cells, naïve T cells, B cells, innate lymphoid cells (ILCs), monocytes, monocyte-derived dendritic cells (MDDCs), and mast cells (Fig. 1D–G). Compared to the control group, ARDS patients had a higher abundance of B cells, monocytes, and mast cells and a lower abundance of naïve T cells and CD8+ T cells (Fig. 1H), consistent with a previous study [35]. Compared with the ARDS_nonKTR group, the ARDS_KTR group showed a higher abundance of B cells, CD8+ T cells, monocytes, MDDCs, ILCs, and mast cells.
Single-cell transcriptional analysis of PBMCs in ARDS patients induced by COVID-19. A Workflow of overall study. B Single-cell atlas of ARDS induced by COVID-19, with a total of 33 cell clusters of 106,033 cells captured. C The correlations among different clusters of single cells. D, E Identification of the 33 clusters with various cell types, including naïve T cells (n = 51,583 cells), B cells (n = 9,955 cells), CD8+ T cells (n = 26,652 cells), monocytes (n = 14,776 cells), monocyte-derived dendritic cells (MDDCs; n = 1,441 cells), innate lymphoid cells (ILCs; n = 837 cells), and mast cells (n = 788 cells). F Bubble chart illustrating the expression of marker genes for each individual cell type. The colors of the bubbles indicate the logFC and the sizes of the bubbles represent -log10(adj.P). G Cell clusters with marker genes mapped in the single-cell spectrum for naïve T cells (CD3D, CD3E, CD3G, and CD247), CD8+ T cells (CD8A and CD8B), B cells (MS4A1, CD79A, and CD79B), monocytes (CD14 and CSF1R), and MDDCs (CD1C). H Cellular components and changes in the control group (Control), non-kidney transplant recipients (KTRs) with ARDS group (ARDS_nonKTR), and KTRs with ARDS group (ARDS_KTRs)
Abnormal proliferation of plasma cells in KTRs with ARDS
We depicted a single-cell atlas of B cells that identified eight subpopulations with varied abundance among patients (Fig. 2A, B). By examining the changes in abundance in B cell subpopulations, we found that B_HLA-II (HLA-DRA, HLA-DQB1, HLA-DRB1, and HLA-DPB1) subpopulations were the primary B cell components of the control group (Fig. 2C–E). Several plasma cell subpopulations were prominently enriched in patients compared with the control group, especially those in the ARDS_KTR group. In comparison to the ARDS_nonKTR group, the IGHG3 high-expression subpopulation Plasma_IGHG3 exhibited a higher abundance in the ARDS_KTR group. Enrichment analysis indicated that multiple B cell subpopulations were significantly enriched in oxidative phosphorylation, antigen processing and presentation, and the B cell receptor signaling pathway (Fig. 2F). In the differentiation trajectory of the B cell subpopulations, we observed that the B_HLA-II subpopulation resided at the commencement of B cell development and gave rise to distinct subpopulations (Fig. 2G). GRN analysis revealed that these cellular subpopulations were segregated into two modules and governed by the transcription factors KLF1, FOXP1, CEBPD, and GCM1 (Fig. 2H–J).
Heterogeneity of B cell subpopulations among ARDS patients and KTRs with ARDS. A, B Composition and single-cell maps of B cells in patients with ARDS. C The dynamic changes of B cell subpopulations in patients with ARDS. D, E Expression of marker genes in B cell subpopulations. F Bubble chart illustrating the signaling pathways associated with B cell subpopulations. G Single-cell trajectory analysis of B cell subpopulations. H Motif module heatmap representing the B cell subpopulations. I Transcription factors present in B cell subpopulations. J Transcription factor activities in B_HLA-II and Plasma_IGHG3
PCNA-positive naïve T cell subpopulation was significantly enriched in KTRs with ARDS
We identified eight subpopulations of naïve T cells and aligned them with single-cell maps from the various patients (Fig. 3A, B). It was noteworthy that the naïve T cell subpopulation specifically expressing PCNA (Naive.T_PCNA) exhibited a distinctive enrichment in both ARDS groups (Fig. 3C–E). In the ARDS_KTR group, the Naive.T_PCNA subpopulation showed more enrichment compared with the ARDS_nonKTR group. Enrichment analysis demonstrated a significant enrichment of Naive.T_PCNA in pathways associated with oxidative phosphorylation, cell cycle, the p53 signaling pathway, and DNA replication (Fig. 3F). This observation implies that this subpopulation may play an important role in cellular energy metabolism and the proliferation processes. Using trajectory analysis, we elucidated the developmental trajectory of the naïve T cell subpopulation (Fig. 3G). Our findings revealed that Naive.T_PCNA was located at the final point of this trajectory, potentially playing a significant role in functional T cell differentiation. GRN analysis revealed that the clustering of markers in naïve T cell subpopulations could be categorized into two distinct modules (Fig. 3H). These modules were regulated by the transcription factors MYBL1, PRDM1, HIVEP2, and ZBTB33 (Fig. 3I–J).
Heterogeneity of naïve T cell subpopulations among ARDS patients and KTRs with ARDS. A, B Composition and single-cell maps of naïve T cells in patients with ARDS. C The dynamic changes of naïve T cell subpopulations in patients with ARDS. D, E Expression of marker genes in naïve T cell subpopulations. F Bubble chart illustrating the signaling pathways associated with naïve T cell subpopulations. (G) Single-cell trajectory analysis of naïve T cell subpopulations. H Motif module heatmap representing the naïve T cell subpopulations. I Transcription factors present in naïve T cell subpopulations. J Transcription factor activities in Naive.T_PCNA
GZMB and XAF1 double-positive effector T cells constituted the major component of CD8+ T cells in KTRs with ARDS
A previous study has indicated that immune injury mediated by T cells could serve as a significant factor in the progression of ARDS [38]. In CD8+ T cells, nine cell subpopulations were identified, consisting of four effector T (Te) cell subpopulations, three central memory T (Tcm) cell subpopulations, and one naive CD8+ T cell subpopulations (Fig. 4A–F). Compared with the control group, there was a substantial increase in Te cells in both ARDS groups. Notably, there were differences between the ARDS_nonKTR and ARDS_KTR groups regarding the types of Te cells, which might be attributed to the immunosuppressive state of KTRs. Specifically, Te_CCL5_GZMH served as the primary component of CD8+ T cells in the ARDS_nonKTR group, whereas the Te_GZMB_XAF1 subpopulation was the most common in the ARDS_KTR group. These subpopulations were significantly enriched in natural killer cell-mediated cytotoxicity, leukocyte transendothelial migration, and cell adhesion molecules (Fig. 4F). Analysis of the CD8+ T cell developmental trajectory revealed that Te_GZMB_XAF1 was located in an intermediate state and subsequently differentiated into Te_CCL5_GZMH (Fig. 4G). This further suggests that Te_GZMB_XAF1 may be a functionally immature Te cell subpopulation. Through GRN analysis of the CD8+ T cell subpopulations, a module was identified that was regulated by many transcription factors, including FOSL2, SRF, JUNB, and KLF3 (Fig. 4H-J).
Heterogeneity of CD8+ T cell subpopulations among ARDS patients and KTRs with ARDS. A, B Composition and single-cell maps of CD8+ T cells in patients with ARDS. C The dynamic changes of CD8+ T cell subpopulations in patients with ARDS. D Single-cell maps reveal marker gene expression in CD8+ T cell subpopulations. E Identification of CD8+ T cell subpopulations based on the expression of specific biomarker genes associated with each cell subpopulation. F Expression of marker genes in CD8+ T cell subpopulations. G Bubble chart illustrating the signaling pathways associated with CD8+ T cell subpopulations. H Single-cell trajectory analysis of CD8+ T cell subpopulations. I Motif module heatmap representing the CD8+ T cell subpopulations. J Transcription factors present in CD8+ T cell subpopulations. K Transcription factor activities in Te_CCL5_GZMH and Te_GZMB_XAF1
Monocytes with IFI27 expression were significantly increased in KTRs with ARDS
We identified seven monocyte subpopulations and mapped them onto the single-cell atlas (Fig. 5A, B). In contrast to the control group, Mono_S100A12 exhibited specific enrichment in both ARDS groups, particularly dominating in the ARDS_nonKTR group (Fig. 5C–E). Compared with the control and ARDS_nonKTR groups, Mono_IFI27 specifically expressing IFI27 were significantly increased in the ARDS_KTR group. The enrichment analysis showed that Mono_S100A12 was enriched in cell adhesion molecules, glycolysis/gluconeogenesis, and the Toll-like receptor signaling pathway (Fig. 5F). Within the developmental trajectory of Mono subpopulations, Mono_IFI27 was positioned at the endpoint, and was likely derived from the differentiation of Mono_S100A12 (Fig. 5G). GRN analysis revealed that the markers of the monocyte subpopulations were clustered into two modules, and mainly regulated by JUNB, MAFG, ETV7, and KLF3 (Fig. 5H–J).
Heterogeneity of monocyte subpopulations among ARDS patients and KTRs with ARDS. A, B Composition and single-cell maps of monocytes in patients with ARDS. C The dynamic changes of monocyte subpopulations in patients with ARDS. D, E Expression of marker genes in monocyte subpopulations. F Bubble chart illustrating the signaling pathways associated with monocyte subpopulations. G Single-cell trajectory analysis of monocyte subpopulations. H Motif module heatmap representing the monocyte subpopulations. (I) Transcription factors present in monocyte subpopulations. J Transcription factor activities in Mono_IFI27 and Mono_S100A12
ANXA1-positive MDDCs constituted the major component of MDDCs in KTRs with ARDS
Nine clusters were identified from MDDCs, and the abundance of patient-specific MDDCs was estimated using UMAP (Fig. 6A, B). Compared with the control group, the MDDC_ANXA1 abundance was significantly upregulated and MDDC_AREG exhibited specific enrichment in both ARDS groups (Fig. 6C–E). Notably, MDDC_ANXA1 was the predominant MDDC component in the ARDS_KTR group. The enrichment analysis showed a significant enrichment of MDDC_ANXA1 in the adherens junction and NOD-like receptor signaling pathways, whereas MDDC_AREG was enriched in the ErbB signaling pathway (Fig. 6F). Developmental trajectory analysis revealed that both MDDC_ANXA1 and MDDC_AREG were positioned at the terminal point of the MDDC subpopulation differentiation trajectory (Fig. 6G). GRN analysis showed that markers associated with MDDC subpopulations clustered into a module, wherein ZNF143 and FOSL2 primarily regulated MDDC_ANXA1 and MDDC_AREG, respectively (Fig. 6H–J).
Heterogeneity of MDDC subpopulations among ARDS patients and KTRs with ARDS. A, B Composition and single-cell maps of MDDCs in patients with ARDS. C The dynamic changes of MDDC subpopulations in patients with ARDS. D, E Expression of marker genes in MDDC subpopulations. F Bubble chart illustrating the signaling pathways associated with MDDC subpopulations. G Single-cell trajectory analysis of MDDC subpopulations. H Motif module heatmap representing the MDDC subpopulations. I Transcription factors present in MDDC subpopulations. J Transcription factor activities in MDDC_AREG and MDDC_ANXA1
Strong cytokine communication between different immune cells
To further investigate intercellular interactions among ARDS patient groups, we analyzed receptor-ligand interactions of cell cytokines (Additional file 1: Figure S1A-C). The results indicated that CCL5 was the predominant ligand for T cells during cytokine communication. In the ARDS_KTR group, T cells had strong communication with multiple monocyte subpopulations through the CCL5-CCR1 axis.
Discussion
The SARS-CoV-2 variant triggers an immune response that can cause excessive and uncontrolled release of pro-inflammatory markers. This can lead to a cytokine storm, which is associated with the development of ARDS [13, 16, 20]. Furthermore, the pathogenesis of the virus may give rise to a distinct form of ARDS, differing from “typical” ARDS [48], particularly in KTRs with COVID-19-induced ARDS.
These cytokine storms may lead to the proliferation and activation of monocytes and B cells in blood of ARDS patients. Furthermore, T cells decrease significantly in patients, indicating potential functional impairment and limited T cell proliferation under persistent inflammation [14, 69]. In contrast to the ARDS_nonKTR group, the ARDS_KTR group exhibited a higher abundance of multiple immune cells, whereas the abundance of naïve T cells was lower. KTRs who suffer from impaired immune function resulting from the prolonged administration of immunosuppressive drugs [61] may develop T cell dysfunction [33, 40]. Viral infections have the potential to intensify this effect, ultimately resulting in the depletion of naïve T cells. It is noteworthy that the abundance of CD8+ T cells was higher in the ARDS_KTR group compared to that of the ARDS_nonKTR group. This implies that in KTRs, functionally mature T cells in the bloodstream may exhibit reduced tissue migration compared with those of non-KTRs with ARDS.
In B-cell immunity, extracellular mechanisms strive to compensate for the delayed or prolonged germinal center response by intensifying activities, leading to the highest measured levels of antibodies in the most severe COVID-19 cases [56]. Importantly, B_HLA-II, the dominant element in the B-plasma cell lineage of the control group, significantly decreased in both ARDS groups. This observation aligns with the previously described characteristics of SARS-CoV-2 infection, resulting in the dysregulation of intercellular crosstalk among adaptive immune cells [60]. When compared to the ARDS_nonKTR group, the diminished abundance of B_HLA-II in the ARDS_KTR group implied a greater degree of compromised B cell activation, indicating a potential link to the increased vulnerability of KTRs to COVID-19 [46]. We found that the ARDS_KTR group exhibit an enhanced plasma cell response compared with the ARDS_nonKTR group, leading to a greater production of antibodies for combating COVID-19. Proliferating and hyperactive plasma cells participate in the inflammatory responses and immune processes [7]. Nevertheless, this alteration may also result in the excessive release of inflammatory mediators, which, in turn, worsens tissue damage and exacerbates ARDS. Notably, unlike the ARDS_nonKTR group, the ARDS_KTR group exhibited a higher abundance of Plasma_IGHG3. In COVID-19 cases, somatic hypermutation (SHM) and class-switch recombination (CSR) events of IGHG3 are more frequent than those of other IgG subclasses [26]. High frequencies of SHM and CSR can specifically alter the hinge length polymorphism of IGHG3, diminishing the neutralizing ability of antibodies against the virus [34]. Hence, the elevated abundance of Plasma_IGHG3 in ARDS_KTR group could potentially exacerbate the risk of disease progression and mortality in these patients.
We observed that naïve T cells significantly decreased in both ARDS groups, which increases the likelihood of cytokine storms [47]. Terminally differentiated Naive.T_PCNA was found to be specifically enriched in both ARDS groups, likely resulting from DNA damage and genomic instability in host cells caused by viral infection [27, 36]. Previous studies indicate that the M protein of SARS-CoV-2 is capable of regulating the translocation of PCNA from the cell nucleus to the cytoplasm, and potentially sustains cell viability via PCNA ubiquitination during viral infection [52, 67]. Thus, viral infection can induce Naive.T_PCNA to engage in signaling pathways or ubiquitination, thereby contributing to DNA repair in host cells and facilitating viral replication. In the ARDS_KTR group, there was a significant increase in the abundance of PCNA-positive naïve T cells compared with the ARDS_nonKTR group. We postulate that the abundance of Naive.T_PCNA could indicate the proliferative potential of T cells in patients after viral infection under post-kidney transplantation immunosuppressive conditions [41, 50].
The marked reduction in CD8+ T cells in patients detrimentally affects the antiviral immune response [59], amplifying the severity of ARDS. It is noteworthy that both Te_GZMB_XAF1 and Te_CCL5_GZMH demonstrated elevated expression levels of GZMB and PRF1. GZMB and PRF1 act as crucial effector molecules in cytotoxic T lymphocytes, leading to acute and chronic rejection of solid organ transplants (Corrales-Tellez et al., 2013). It has been reported that PRF1 and GZMB expression in CD8+ T cells increases during COVID-19 progression, potentially causing host cell damage during viral clearance (Jiang et al., 2020). In this study, we observed that the T cells in the ARDS_KTR group were primarily composed of the Te_GZMB_XAF1 subpopulation. XAF1 enhances immune responses against RNA viruses by modulating chromatin accessibility [30]. Although Te_GZMB_XAF1 plays a vital role in antiviral responses, its elevated abundance may result in worsening the condition of KTRs. Furthermore, Te_GZMB_XAF1 had the potential to differentiate into Te_CCL5_GZMH, which formed a significant portion of CD8+ T cells in the ARDS_nonKTR group. Dysregulated CCL5 expression leads to chemotactic alterations and sustained inflammatory cascades mediated by cytokines, resulting in the development of ARDS [2]. Hence, Te_CCL5_GZMH could display a heightened effector phenotype, resulting in increased levels of chemokines and pro-inflammatory cytokines, potentially contributing to a more severe cytokine storm. In summary, we suggest that in KTRs with ARDS under immunosuppressive conditions and virus infection, impaired differentiation of Te_GZMB_XAF1 leads to its abnormal accumulation in the ARDS_KTR group. This could reduce the body's efficiency to clear the virus, hamper normal immune reconstitution, promote chronic inflammation formation, and ultimately worsen ARDS progression in KTRs.
Following SARS-CoV-2 infection, monocytes undergo metabolic reprogramming, manifesting bioenergetic changes and mitochondrial dysfunction [19]. We found that Mono_S100A12 constituted a major component in the monocytes of the ARDS_nonKTR group. Previous studies have demonstrated that S100A12 enhances the expression of adhesion factors and induces the activation of human monocytes via Toll-like receptor 4, thereby acting as an enhancer of innate immunity in the early stages of inflammation and sepsis [17, 29, 31, 37]. We observed that Mono_S100A12 was significantly enriched in cell adhesion molecules and the Toll-like receptor signaling pathway, suggesting its potential role in mediating immune dysregulation in the non-KTRs with ARDS through these pathways. Additionally, the interferon-inducible gene IFI27 is highly expressed in the respiratory tract and PBMCs of patients with COVID-19, and it may also affect the molecular mechanisms of the innate immune response through negative feedback regulation, thus facilitating viral replication [49, 58]. Our study revealed a significant enrichment of the Mono_IFI27 subpopulation in the ARDS_KTR cohort. The specific expression of IFI27 in this subpopulation may adversely modulate the immune response in KTRs, impairing viral clearance and the regulation of inflammatory processes. Consequently, Mono_IFI27 serves as an indicator of viral infection and immune activity in KTRs with ARDS and potentially plays a critical role in the pathogenesis of ARDS. Overall, the aberrant changes in monocyte subpopulations may further exacerbate the innate immune dysregulation in patients.
Previous studies have indicated that SARS-CoV-2 infection is not efficient in MDDCs [15]. The MDDC_AREG subpopulation was specifically enriched in both ARDS groups, and high expression of the pro-fibrotic gene AREG acts as a distinct marker for monocytes in severe COVID-19 cases [54, 55]. In diverse inflammatory and pathological contexts, AREG is expressed by a variety of activated immune cells, which coordinate mechanisms for tolerance and host resistance [51]. Our findings revealed enrichment of the MDDC_AREG subpopulation in the ErbB signaling pathway, which regulates cell survival, growth, and movement [66]. Consequently, MDDC_AREG may play a regulatory role in immune responses, tissue repair, and the modulation of inflammation in ARDS patients. Notably, MDDC_ANXA1 constituted a major component in the MDDC of the ARDS_KTR group. The expression of ANXA1 is markedly increased in the sera of critically ill COVID-19 patients [8]. Specific drugs, including dexamethasone, hydrocortisone, and prednisolone, have been used to manage severe cases of COVID-19 that target ANXA1 [32]. This indicates that the increased presence of MDDC_ANXA1 may contribute to mitigating the severity of COVID-19 and its related complications in immunosuppressed individuals.
In our study, we found that the receptor-ligand interaction pair CCL5-CCR1 was significantly activated in the ARDS_KTR group, potentially facilitating the migration and recruitment of immune cells. CCL5 has the ability to recruit lymphocytes to the sites of inflammation and allograft rejection, with CCR1 being one of its well-characterized ligands in various inflammatory diseases [10]. Previous studies have demonstrated the inhibitory effects of blocking CCL5-CCR1 on the development of acute and chronic cardiac allograft rejection [18, 65]. Thus, it is hypothesized that T cells expressing high levels of CCL5 facilitate monocyte infiltration in KTRs by interacting with the receptor CCR1 on the surface of monocytes, thereby promoting cellular rejection and contributing to the progression of ARDS.
Despite these findings, our study has some limitations. Our analysis is limited to PBMC samples from patients, and it is possible that distinct cellular changes occurred at the organ and tissue levels that were missed. Additionally, the clinical phenotypes of COVID-19-induced ARDS in patients who have undergone different types of organ transplantation can vary significantly. Finally, due to the unique nature of our sample types, we currently lack sufficient samples to conduct experimental validation at the mechanistic level.
Conclusion
The key findings suggest that KTRs with ARDS exhibit a stronger delayed humoral response and elevated antibody levels attributed to abnormal changes in B cells. In the ARDS_KTR group, there may be an impaired differentiation function of Te_GZMB_XAF1, resulting in its abnormal enrichment in KTRs and thereby unfavorably affecting immune reconstitution in the body. Furthermore, the abnormal proliferation of monocytes leads to dysregulation of the innate immune system in KTRs with ARDS.
Availability of data and materials
The datasets presented in this study can be found online at the National Genomics Data Center under the accession numbers HRA004752 and HRA005498.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- GRN:
-
Gene regulatory network
- KTRs:
-
Kidney transplant recipients
- MDDCs:
-
Monocyte-derived dendritic cells
- PBS:
-
Phosphate-buffered saline
References
Abolghasemi S, Mardani M, Sali S, Honarvar N, Baziboroun M. COVID-19 and kidney transplant recipients. Transpl Infect Dis. 2020;22: e13413.
Agresti N, Lalezari JP, Amodeo PP, Mody K, Mosher SF, Seethamraju H, Kelly SA, Pourhassan NZ, Sudduth CD, Bovinet C, Elsharkawi AE, Patterson BK, Stephen R, Sacha JB, Wu HL, Gross SA, Dhody K. Disruption of CCR5 signaling to treat COVID-19-associated cytokine storm: Case series of four critically ill patients treated with leronlimab. J Transl Autoimmun. 2021;4: 100083.
Ahmadian E, Zununi Vahed S, Mammadova S, Abediazar S. Immunosuppressant management in renal transplant patients with COVID-19. Biomed Res Int. 2021;2021:9318725.
Aibar S, Gonzalez-Blas CB, Moerman T, Huynh-Thu VA, Imrichova H, Hulselmans G, Rambow F, Marine JC, Geurts P, Aerts J, van den Oord J, Atak ZK, Wouters J, Aerts S. SCENIC: single-cell regulatory network inference and clustering. Nat Methods. 2017;14:1083–6.
Basic-Jukic N, Furic-Cunko V, Jelakovic B. COVID-19 vaccine hesitancy in renal transplant recipients. Ther Apher Dial. 2022;26:482–3.
Becht E, McInnes L, Healy J, Dutertre CA, Kwok IW, Ng LG, Ginhoux F, Newell EW. Dimensionality reduction for visualizing single-cell data using UMAP. Nat Biotechnol. 2018;37:38.
Bernardes JP, Mishra N, Tran F, Bahmer T, Best L, Blase JI, Bordoni D, Franzenburg J, Geisen U, Josephs-Spaulding J, Kohler P, Kunstner A, Rosati E, Aschenbrenner AC, Bacher P, Baran N, Boysen T, Brandt B, Bruse N, Dorr J, Drager A, Elke G, Ellinghaus D, Fischer J, Forster M, Franke A, Franzenburg S, Frey N, Friedrichs A, Fuss J, Gluck A, Hamm J, Hinrichsen F, Hoeppner MP, Imm S, Junker R, Kaiser S, Kan YH, Knoll R, Lange C, Laue G, Lier C, Lindner M, Marinos G, Markewitz R, Nattermann J, Noth R, Pickkers P, Rabe KF, Renz A, Rocken C, Rupp J, Schaffarzyk A, Scheffold A, Schulte-Schrepping J, Schunk D, Skowasch D, Ulas T, Wandinger KP, Wittig M, Zimmermann J, Busch H, Hoyer BF, Kaleta C, Heyckendorf J, Kox M, Rybniker J, Schreiber S, Schultze JL, Rosenstiel P, Network HCALB & Deutsche C-OI. Longitudinal multi-omics analyses identify responses of megakaryocytes, erythroid cells, and plasmablasts as hallmarks of severe COVID-19. Immunity. 2020;53(1296–1314): e9.
Busch MH, Timmermans SA, Aendekerk JP, Ysermans R, Amiral J, Damoiseaux JG, Reutelingsperger CP, Paassen PV. Annexin A1 is associated with adverse clinical outcomes in patients with COVID-19. J Clin Med. 2022;11:7486.
Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36:411–20.
Choi SW, Hildebrandt GC, Olkiewicz KM, Hanauer DA, Chaudhary MN, Silva IA, Rogers CE, Deurloo DT, Fisher JM, Liu C, Adams D, Chensue SW, Cooke KR. CCR1/CCL5 (RANTES) receptor-ligand interactions modulate allogeneic T-cell responses and graft-versus-host disease following stem-cell transplantation. Blood. 2007;110:3447–55.
Demir E, Dheir H, Safak S, Artan AS, Sipahi S, Turkmen A. Differences in clinical outcomes of COVID-19 among vaccinated and unvaccinated kidney transplant recipients. Vaccine. 2022;40:3313–9.
Demir E, Uyar M, Parmaksiz E, Sinangil A, Yelken B, Dirim AB, Merhametsiz O, Yadigar S, Atan Ucar Z, Ucar AR, Demir ME. COVID-19 in kidney transplant recipients: a multicenter experience in Istanbul. Transpl Infect Dis. 2020;22:e13371.
Dey A, Vaishak K, Deka D, Radhakrishnan AK, Paul S, Shanmugam P, Daniel AP, Pathak S, Duttaroy AK, Banerjee A. Epigenetic perspectives associated with COVID-19 infection and related cytokine storm: an updated review. Infection. 2023;12:1–16.
Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, Chen L, Li M, Liu Y, Wang G, Yuan Z, Feng Z, Zhang Y, Wu Y, Chen Y. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. 2020;11:827.
Falck-Jones S, Osterberg B, Smed-Sorensen A. Respiratory and systemic monocytes, dendritic cells, and myeloid-derived suppressor cells in COVID-19: implications for disease severity. J Intern Med. 2023;293:130–43.
Fara A, Mitrev Z, Rosalia RA, Assas BM. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol. 2020;10: 200160.
Foell D, Wittkowski H, Kessel C, Luken A, Weinhage T, Varga G, Vogl T, Wirth T, Viemann D, Bjork P, van Zoelen MA, Gohar F, Srikrishna G, Kraft M, Roth J. Proinflammatory S100A12 can activate human monocytes via Toll-like receptor 4. Am J Respir Crit Care Med. 2013;187:1324–34.
Gao W, Topham PS, King JA, Smiley ST, Csizmadia V, Lu B, Gerard CJ, Hancock WW. Targeting of the chemokine receptor CCR1 suppresses development of acute and chronic cardiac allograft rejection. J Clin Invest. 2000;105:35–44.
Gibellini L, De Biasi S, Paolini A, Borella R, Boraldi F, Mattioli M, Lo Tartaro D, Fidanza L, Caro-Maldonado A, Meschiari M, Iadisernia V. Altered bioenergetics and mitochondrial dysfunction of monocytes in patients with COVID-19 pneumonia. EMBO Mol Med. 2020;12:e13001.
Grasselli G, Tonetti T, Filippini C, Slutsky AS, Pesenti A, Ranieri VM. Pathophysiology of COVID-19-associated acute respiratory distress syndrome—authors’ reply. Lancet Respir Med. 2021;9:e5–6.
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China medical treatment expert group for C. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20.
Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, Pere H, Charbit B, Bondet V, Chenevier-Gobeaux C, Breillat P, Carlier N, Gauzit R, Morbieu C, Pene F, Marin N, Roche N, Szwebel TA, Merkling SH, Treluyer JM, Veyer D, Mouthon L, Blanc C, Tharaux PL, Rozenberg F, Fischer A, Duffy D, Rieux-Laucat F, Kerneis S, Terrier B. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–24.
Hippisley-Cox J, Coupland CA, Mehta N, Keogh RH, Diaz-Ordaz K, Khunti K, Lyons RA, Kee F, Sheikh A, Rahman S, Valabhji J, Harrison EM, Sellen P, Haq N, Semple MG, Johnson PWM, Hayward A, Nguyen-Van-tam JS. Risk prediction of covid-19 related death and hospital admission in adults after covid-19 vaccination: national prospective cohort study. BMJ. 2021;374: n2244.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.
Huang L, Shi Y, Gong B, Jiang L, Zhang Z, Liu X, Yang J, He Y, Jiang Z, Zhong L, Tang J, You C, Jiang Q, Long B, Zeng T, Luo M, Zeng F, Zeng F, Wang S, Yang X, Yang Z. Dynamic blood single-cell immune responses in patients with COVID-19. Signal Transduct Target Ther. 2021;6:110.
Jin X, Zhou W, Luo M, Wang P, Xu Z, Ma K, Cao H, Xu C, Huang Y, Cheng R, Xiao L. Global characterization of B cell receptor repertoire in COVID-19 patients by single-cell V(D)J sequencing. Brief Bioinform. 2021;22:bbab192.
Kannouche PL, Wing J, Lehmann AR. Interaction of human DNA polymerase eta with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol Cell. 2004;14:491–500.
Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–7.
Krishnamachary B, Cook C, Kumar A, Spikes L, Chalise P, Dhillon NK. Extracellular vesicle-mediated endothelial apoptosis and EV-associated proteins correlate with COVID-19 disease severity. J Extracell Vesicles. 2021;10: e12117.
Kuang M, Zhao Y, Yu H, Li S, Liu T, Chen L, Chen J, Luo Y, Guo X, Wei X, Li Y. XAF1 promotes anti-RNA virus immune responses by regulating chromatin accessibility. Sci Adv. 2023;9:eadg5211.
Lei H. A two-gene marker for the two-tiered innate immune response in COVID-19 patients. PLoS One. 2023;18:e0280392.
Li Y, Hou G, Zhou H, Wang Y, Tun HM, Zhu A, Zhao J, Xiao F, Lin S, Liu D, Zhou D, Mai L, Zhang L, Zhang Z, Kuang L, Guan J, Chen Q, Wen L, Zhang Y, Zhuo J, Li F, Zhuang Z, Chen Z, Luo L, Liu D, Chen C, Gan M, Zhong N, Zhao J, Ren Y, Xu Y. Multi-platform omics analysis reveals molecular signature for COVID-19 pathogenesis, prognosis and drug target discovery. Signal Transduct Target Ther. 2021;6:155.
Liu Y, Liu X, Zhou S, Xu R, Hu J, Liao G, Liao J, Guo Z, Li Y, Yang S, Li S, Chen H, Guo Y, Li M, Fan L, Li L, Zhao M, Liu D. Single-cell profiling of kidney transplant recipients with immunosuppressive treatment reveals the dynamic immune characteristics. Front Immunol. 2021;12: 639942.
Lopez-Martinez R, Albaiceta GM, Amado-Rodriguez L, Gomez J, Cuesta-Llavona E, Garcia-Clemente M, Hermida-Valverde T, Enriquez-Rodriguez AI, Hernandez-Gonzalez C, Martinez-Borra J, Lopez-Larrea C, Gil-Pena H, Alvarez V, Coto E. IGHG3 hinge length variation was associated with the risk of critical disease and death in a Spanish COVID-19 cohort. Genes Immun. 2022;23:205–8.
Lu J, Zeng X, Lu W, Feng J, Yang Y, Wei Y, Chen Y, Zhang J, Pinhu L. Documenting the immune response in patients with COVID-19-induced acute respiratory distress syndrome. Front Cell Dev Biol. 2023;11:1207960.
Luftig MA. Viruses and the DNA damage response: activation and antagonism. Annu Rev Virol. 2014;1:605–25.
Ma Y, Qiu F, Deng C, Li J, Huang Y, Wu Z, Zhou Y, Zhang Y, Xiong Y, Yao Y, Zhong Y, Qu J, Su J. Integrating single-cell sequencing data with GWAS summary statistics reveals CD16+monocytes and memory CD8+T cells involved in severe COVID-19. Genome Med. 2022;14:16.
Mo J, Yang Y, Feng J, Lei Y, Huang S, Cen W, Wei S, Huang H, Lu J, Zhang J. Single-cell analysis reveals dysregulated inflammatory response in peripheral blood immunity in patients with acute respiratory distress syndrome. Front Cell Dev Biol. 2023;11:1199122.
Montazersaheb S, Hosseiniyan Khatibi SM, Hejazi MS, Tarhriz V, Farjami A, Ghasemian Sorbeni F, Farahzadi R, Ghasemnejad T. COVID-19 infection: an overview on cytokine storm and related interventions. Virol J. 2022;19:92.
Niu Q, Mendoza Rojas A, Dieterich M, Roelen DL, Clahsen-van Groningen MC, Wang L, Van Gelder T, Hesselink DA, van Besouw NM, Baan CC. Immunosuppression has long-lasting effects on circulating follicular regulatory T cells in kidney transplant recipients. Front Immunol. 2020;11:1972.
Niwa M, Miwa Y, Kuzuya T, Iwasaki K, Haneda M, Ueki T, Katayama A, Hiramitsu T, Goto N, Nagasaka T, Watarai Y, Uchida K, Nakao A, Kobayashi T. Stimulation index for PCNA mRNA in peripheral blood as immune function monitoring after renal transplantation. Transplantation. 2009;87:1411–4.
Panahi Y, Gorabi AM, Talaei S, Beiraghdar F, Akbarzadeh A, Tarhriz V, Mellatyar H. An overview on the treatments and prevention against COVID-19. Virol J. 2023;20:23.
Puelles VG, Lutgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schroder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383:590–2.
Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446.
Ren X, Wen W, Fan X, Hou W, Su B, Cai P, Li J, Liu Y, Tang F, Zhang F, Yang Y, He J, Ma W, He J, Wang P, Cao Q, Chen F, Chen Y, Cheng X, Deng G, Deng X, Ding W, Feng Y, Gan R, Guo C, Guo W, He S, Jiang C, Liang J, Li YM, Lin J, Ling Y, Liu H, Liu J, Liu N, Liu SQ, Luo M, Ma Q, Song Q, Sun W, Wang G, Wang F, Wang Y, Wen X, Wu Q, Xu G, Xie X, Xiong X, Xing X, Xu H, Yin C, Yu D, Yu K, Yuan J, Zhang B, Zhang P, Zhang T, Zhao J, Zhao P, Zhou J, Zhou W, Zhong S, Zhong X, Zhang S, Zhu L, Zhu P, Zou B, Zou J, Zuo Z, Bai F, Huang X, Zhou P, Jiang Q, Huang Z, Bei JX, Wei L, Bian XW, Liu X, Cheng T, Li X, Zhao P, Wang FS, Wang H, Su B, Zhang Z, Qu K, Wang X, Chen J, Jin R, Zhang Z. COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas. Cell. 2021;184(1895–1913): e19.
Sagnelli C, Sica A, Gallo M, Peluso G, Varlese F, D’Alessandro V, Ciccozzi M, Crocetto F, Garofalo C, Fiorelli A, Iannuzzo G, Reginelli A, Schonauer F, Santangelo M, Sagnelli E, Creta M, Calogero A. Renal involvement in COVID-19: focus on kidney transplant sector. Infection. 2021;49:1265–75.
Schwartz MD, Emerson SG, Punt J, Goff WD. Decreased naive T-cell production leading to cytokine storm as cause of increased COVID-19 severity with comorbidities. Aging Dis. 2020;11:742–5.
Selickman J, Vrettou CS, Mentzelopoulos SD, Marini JJ. COVID-19-related ARDS: key mechanistic features and treatments. J Clin Med. 2022;11:4896.
Shojaei M, Shamshirian A, Monkman J, Grice L, Tran M, Tan CW, Teo SM, Rodrigues Rossi G, Mcculloch TR, Nalos M, Raei M, Razavi A, Ghasemian R, Gheibi M, Roozbeh F, Sly PD, Spann KM, Chew KY, Zhu Y, Xia Y, Wells TJ, Senegaglia AC, Kuniyoshi CL, Franck CL, Dos Santos AFR, De Noronha L, Motamen S, Valadan R, Amjadi O, Gogna R, Madan E, Alizadeh-Navaei R, Lamperti L, Zuniga F, Nova-Lamperti E, Labarca G, Knippenberg B, Herwanto V, Wang Y, Phu A, Chew T, Kwan T, Kim K, Teoh S, Pelaia TM, Kuan WS, Jee Y, Iredell J, O’Byrne K, Fraser JF, Davis MJ, Belz GT, Warkiani ME, Gallo CS, Souza-Fonseca-Guimaraes F, Nguyen Q, Mclean A, Kulasinghe A, Short KR, Tang B. IFI27 transcription is an early predictor for COVID-19 outcomes, a multi-cohort observational study. Front Immunol. 2022;13:1060438.
Simic-Ogrizovic S, Starcevic-Bozovic A, Blagojevic R, Radivojevic D, Djkanovic L. Evaluation of the rate of progression of chronic renal transplant rejection using the proliferating cell nuclear antigen. Srp Arh Celok Lek. 2002;130:159–64.
Singh SS, Chauhan SB, Kumar A, Kumar S, Engwerda CR, Sundar S, Kumar R. Amphiregulin in cellular physiology, health, and disease: potential use as a biomarker and therapeutic target. J Cell Physiol. 2022;237:1143–56.
Stukalov A, Girault V, Grass V, Karayel O, Bergant V, Urban C, Haas DA, Huang Y, Oubraham L, Wang A, Hamad MS, Piras A, Hansen FM, Tanzer MC, Paron I, Zinzula L, Engleitner T, Reinecke M, Lavacca TM, Ehmann R, Wolfel R, Jores J, Kuster B, Protzer U, Rad R, Ziebuhr J, Thiel V, Scaturro P, Mann M, Pichlmair A. Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV. Nature. 2021;594:246–52.
Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, Morse M, Lennon NJ, Livak KJ, Mikkelsen TS, Rinn JL. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol. 2014;32:381–6.
Unterman A, Sumida TS, Nouri N, Yan X, Zhao AY, Gasque V, Schupp JC, Asashima H, Liu Y, Cosme C, Deng JRW, Chen M, Raredon MSB, Hoehn KB, Wang G, Wang Z, Deiuliis G, Ravindra NG, Li N, Castaldi C, Wong P, Fournier J, Bermejo S, Sharma L, Casanovas-Massana A, Vogels CBF, Wyllie AL, Grubaugh ND, Melillo A, Meng H, Stein Y, Minasyan M, Mohanty S, Ruff WE, Cohen I, Raddassi K, Yale IRT, Niklason LE, Ko AI, Montgomery RR, Farhadian SF, Iwasaki A, Shaw AC, van Dijk D, Zhao H, Kleinstein SH, Hafler DA, Kaminski N, Dela-Cruz CS. Single-cell multi-omics reveals dyssynchrony of the innate and adaptive immune system in progressive COVID-19. Nat Commun. 2022;13:440.
Utrero-Rico A, Gonzalez-Cuadrado C, Chivite-Lacaba M, Cabrera-Marante O, Laguna-Goya R, Almendro-Vazquez P, Diaz-Pedroche C, Ruiz-Ruigomez M, Lalueza A, Folgueira MD, Vazquez E, Quintas A, Berges-Buxeda MJ, Martin-Rodriguez M, Dopazo A, Serrano-Hernandez A, Aguado JM, Paz-Artal E. Alterations in circulating monocytes predict COVID-19 severity and include chromatin modifications still detectable six months after recovery. Biomedicines. 2021;9:1253.
Valyi-Nagy I, Uher F, Rakoczi E, Szekanecz Z. Adaptive immunity to viruses: what did we learn from SARS-CoV-2 infection? Int J Mol Sci. 2022;23:13951.
van de Sande B, Flerin C, Davie K, de Waegeneer M, Hulselmans G, Aibar S, Seurinck R, Saelens W, Cannoodt R, Rouchon Q, Verbeiren T, de Maeyer D, Reumers J, Saeys Y, Aerts S. A scalable SCENIC workflow for single-cell gene regulatory network analysis. Nat Protoc. 2020;15:2247–76.
Villamayor L, Lopez-Garcia D, Rivero V, Martinez-Sobrido L, Nogales A, Dediego ML. The IFN-stimulated gene IFI27 counteracts innate immune responses after viral infections by interfering with RIG-I signaling. Front Microbiol. 2023;14:1176177.
Warren JA, Clutton G, Goonetilleke N. Harnessing CD8(+) T cells under HIV antiretroviral therapy. Front Immunol. 2019;10:291.
Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martinez-Colon GJ, McKechnie JL, Ivison GT, Ranganath T, Vergara R, Hollis T, Simpson LJ, Grant P, Subramanian A, Rogers AJ, Blish CA. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. 2020;26:1070–6.
Wojciechowski D, Wiseman A. Long-term immunosuppression management: opportunities and uncertainties. Clin J Am Soc Nephrol. 2021;16:1264–71.
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–42.
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2.
Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–7.
Yun JJ, Whiting D, Fischbein MP, Banerji A, Irie Y, Stein D, Fishbein MC, Proudfoot AE, Laks H, Berliner JA, Ardehali A. Combined blockade of the chemokine receptors CCR1 and CCR5 attenuates chronic rejection. Circulation. 2004;109:932–7.
Zaiss DMW, Gause WC, Osborne LC, Artis D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity. 2015;42:216–26.
Zambalde EP, Pavan ICB, Mancini MCS, Severino MB, Scudero OB, Morelli AP, Amorim MR, Bispo-Dos-santos K, Gois MM, Toledo-Teixeira DA, Parise PL, Mauad T, Dolhnikoff M, Saldiva PHN, Marques-Souza H, Proenca-Modena JL, Ventura AM, Simabuco FM. Characterization of the interaction between SARS-CoV-2 membrane protein (M) and proliferating cell nuclear antigen (PCNA) as a potential therapeutic target. Front Cell Infect Microbiol. 2022;12: 849017.
Zhang B, Zhou X, Qiu Y, Song Y, Feng F, Feng J, Song Q, Jia Q, Wang J. Clinical characteristics of 82 cases of death from COVID-19. PLoS ONE. 2020;15: e0235458.
Zheng HY, Zhang M, Yang CX, Zhang N, Wang XC, Yang XP, Dong XQ, Zheng YT. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020;17:541–3.
Acknowledgements
Not applicable.
Funding
This study was supported by the Key Research and Development Program of Guangxi (GuiKe AB22080088), the Joint Project on Regional High-Incidence Diseases Research of the Guangxi Natural Science Foundation (2023GXNSFAA026318 and 2023GXNSFDA026023), the National Natural Science Foundation of China (8236037 and 82360374), the High-level Medical Expert Training Program of Guangxi “139” Plan Funding (G202003010 and G201903027), and the Guangxi Medical and health key discipline construction project.
Author information
Authors and Affiliations
Contributions
YL, XZ and KQ conceived the study and designed the study. YC, KZ, YL, QQ, WL, LQ, C.M and JZ performed data collection and data analysis. JL, YC, KZ and XZ drafted the manuscript. K.Q supervised manuscript preparation. All authors read and approved the final manuscript for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board and Ethical Committee of the Second Affiliated Hospital of Guangxi Medical University. The studies involving human participants complied with the Declaration of Helsinki and were reviewed and approved by the Ethics Committee of the Ethics Committee of the Second Affiliated Hospital of Guangxi Medical University, and samples were obtained from all patients which informed consent were obtained from all participants and /or their legal guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
The intercellular communication of cytokines. cirPlot visualizing the intercellular communication events within cell subpopulations in patients with (A) COVID-19-induced ARDS and (B) COVID-19-induced ARDS after kidney transplantation. (C) CCL5 expression in naïve T cells and CD8+ T cells.
Additional file 2: Table S1.
Clinical information of the samples.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lu, J., Chen, Y., Zhou, K. et al. Immune characteristics of kidney transplant recipients with acute respiratory distress syndrome induced by COVID-19 at single-cell resolution. Respir Res 25, 34 (2024). https://doi.org/10.1186/s12931-024-02682-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-024-02682-9