Abstract
Background
The mortality rate of acute respiratory distress syndrome (ARDS) increases with age (≥ 65 years old) in critically ill patients, and it is necessary to prevent mortality in elderly patients with ARDS in the intensive care unit (ICU). Among the potential risk factors, dynamic subphenotypes of respiratory rate (RR), heart rate (HR), and respiratory rate-oxygenation (ROX) and their associations with 28-day mortality have not been clearly explored.
Methods
Based on the eICU Collaborative Research Database (eICU-CRD), this study used a group-based trajectory model to identify longitudinal subphenotypes of RR, HR, and ROX during the first 72 h of ICU stays. A logistic model was used to evaluate the associations of trajectories with 28-day mortality considering the group with the lowest rate of mortality as a reference. Restricted cubic spline was used to quantify linear and nonlinear effects of static RR-related factors during the first 72 h of ICU stays on 28-day mortality. Receiver operating characteristic (ROC) curves were used to assess the prediction models with the Delong test.
Results
A total of 938 critically ill elderly patients with ARDS were involved with five and 5 trajectories of RR and HR, respectively. A total of 204 patients fit 4 ROX trajectories. In the subphenotypes of RR, when compared with group 4, the odds ratios (ORs) and 95% confidence intervals (CIs) of group 3 were 2.74 (1.48–5.07) (P = 0.001). Regarding the HR subphenotypes, in comparison to group 1, the ORs and 95% CIs were 2.20 (1.19–4.08) (P = 0.012) for group 2, 2.70 (1.40–5.23) (P = 0.003) for group 3, 2.16 (1.04–4.49) (P = 0.040) for group 5. Low last ROX had a higher mortality risk (P linear = 0.023, P nonlinear = 0.010). Trajectories of RR and HR improved the predictive ability for 28-day mortality (AUC increased by 2.5%, P = 0.020).
Conclusions
For RR and HR, longitudinal subphenotypes are risk factors for 28-day mortality and have additional predictive enrichment, whereas the last ROX during the first 72 h of ICU stays is associated with 28-day mortality. These findings indicate that maintaining the health dynamic subphenotypes of RR and HR in the ICU and elevating static ROX after initial critical care may have potentially beneficial effects on prognosis in critically ill elderly patients with ARDS.
Similar content being viewed by others
Introduction
Acute respiratory distress syndrome (ARDS) is a common cause of respiratory system failure in critically ill patients [1], leading to noncardiogenic pulmonary edema [1] and increased permeability of the alveolar-capillary membrane. A previous study revealed that 10% of patients in all intensive care units (ICUs) met the ARDS criteria [2]; moreover, the mortality rate of ARDS increased with age (≥ 65 years old) [3, 4], reaching as high as 43% in ICU patients ≥ 67 years [4]. It is necessary to prevent mortality in elderly patients with ARDS in the ICU.
The respiratory rate (RR) is a crucial ventilatory parameter, despite being infrequently incorporated into ventilatory protocols in preclinical and clinical studies [5,6,7]. Protective mechanical ventilation strategies employing low tidal volume often result in an elevated RR to maintain adequate alveolar ventilation [8, 9]. However, there is a lack of conclusive data regarding the safety of high respiratory rates in preventing ventilator-induced lung injury [10]. Vieillard-Baron et al. [9] observed that a high RR in ARDS patients did not improve carbon dioxide (CO2) clearance. Instead, it led to dynamic hyperinflation and impaired right ventricular ejection. In addition, recent studies have affirmed a positive association between RR and mortality [8, 11]. Moreover, other indices associated with RR also have important implicaitons. For instance, heart rate (HR), which increases with RR, has been positively linked with mortality in elderly patients [12]. Navarrete-Navarro et al. [13] demonstrated that the ICU mortality in trauma patients with ARDS was related to oxygen partial pressure (PaO2)/fractional oxygen (FiO2) on the third day. Nonetheless, there has been widespread adoption of the respiratory rate-oxygenation (ROX), calculated as the ratio of oxygen saturation (SpO2)/FiO2 to RR. It is a more comprehensive indicator for predicting disease risk and prognosis [14, 15]. The higher ROX index at 24 h after initiating ventilator support was associated with lower mortality in patients with ARDS [16].
In summary, RR, HR, and ROX are readily monitored items in the ICU that can indicate clinical deterioration [11, 12, 16,17,18]. These parameters may serve as prognostic indicators for elderly patients with ARDS. However, there remain unresolved challenges. First, the implications of their variations have not been well studied. For ARDS patients in the ICU, their disease status could rapidly evolve within several minutes. This could be potentially signaled by simultaneous alterations in RR, HR, and ROX as a warning. Evidence supports that subclassifications of disease trajectories based on clinical biomarkers could identify typical dynamic subphenotypes in critically ill patients [10, 19,20,21, 30,31,32]. Second, linear and nonlinear effects of baseline and final readings of these indices on mortality were not investigated. The immediate responses of RR, HR, and ROX to critical care may have particular implications in mortality.
The objectives of this study are outlined as follows: (1) to identify dynamic trajectories of RR, HR, and ROX in elderly patients with ARDS during the initial 72 h of ICU admission using data from the eICU Collaborative Research Database (eICU-CRD); (2) to evaluate the relationship between these vital sign trajectories and 28-day mortality; (3) to examine both linear and nonlinear relationships of static levels of baseline and final measurements of RR, HR, and ROX during the first 72 h of ICU stays with 28-day mortality; and (4) to use a receiver operating characteristic (ROC) curve and area under the curve (AUC) to assess prediction models that incorporate significant trajectory factors and static RR-related factors.
Materials and methods
Study population and data sources
The study population was collected from the eICU-CRD 2.0 (year 2014–2015) [19] at PhysioNet [20, 21]. Patients with ICU stays > 72 h were first included, and the exclusion criteria were as follows: (1) no vital signs in the first 3 days of the ICU stay; (2) age < 65 or age ≥ 89; (3) gender unknown; (4) repeated measurements of RR and HR < 4 times during the first 72 h in the ICU; (5) no ARDS recorded; and (6) diagnosed with congestive heart failure. For screening ICU patients to fit trajectories of ROX, patients with repeated measurements of ROX < 4 times during the first 72 h in the ICU were excluded.
The international Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) were used to help define ARDS [22, 23]. We selected patients with ARDS using ICD-9-CM codes 518.51, 518.52, 518.53, and 518.82 [24, 25], ICD-10-CM code J80, and disease names including “ARDS” or “acute respiratory distress”. We included patients with ARDS diagnosis in the time range of 2 days before admission to the ICU and 1 day after admission. After screening target patients, 938 critically ill elderly patients with ARDS were collected from the eICU-CRD database.
Demographic and clinical features
Demographic and clinical features were derived from 6 components: demographic information, severity of illness, support within the first 24 h, laboratory information, Charlson comorbidity, and vital signs. The detailed demographic and clinical features were as follows: (1) demographic information included age, sex, ethnicity, and first ICU location, and other or unknown conditions of ethnicity were regarded as a separate classification; (2) severity of illness included Sequential Organ Failure Assessment (SOFA) score, Acute Physiology Score III (APS-III), and Glasgow Coma Scale (GCS) score; (3) support during the first 24 h of ICU stays involved vasopressin, ventilation, and dialysis; and (4) laboratory information included baseline levels of laboratory indicators that were collected from the initial observations during the first 72 h in the ICU or were supplemented by the values closest to the time of the ICU stays before ICU admission. Detailed laboratory indicators were hemoglobin, platelets, white blood cells (WBCs), international normalized ratio (INR), partial thromboplastin time (PTT), blood urea nitrogen (BUN), creatinine, sodium, potassium, calcium, chloride, glucose, and bicarbonate; (5) Charlson comorbidity included myocardial infarct, congestive heart failure, peripheral vascular disease, cerebrovascular disease (CVD), dementia, chronic pulmonary disease (COPD), rheumatic disease, peptic ulcer disease, mild liver disease, severe liver disease, diabetes, paraplegia, renal disease, malignant cancer, metastatic solid tumor, and aids; and (6) vital signs included RR, HR, and ROX. Vital signs from hour 1 to hour 72 were split into one-hour blocks of time. If there were multiple measurements within one block, the peak values of RR and HR were used. For ROX, we first defined the minimum SpO2, maximum FiO2, and maximum RR in each one-hour block, and the minimum ROX was then calculated as the ratio of SpO2/FiO2 to RR [14]. The first observed vital signs were considered as baseline levels, whereas the vital signs finally observed during the first 72 h in the ICU were considered as post-treatment levels.
Outcome
In the cohort, the follow-up started after 3 days in the ICU and lasted until death, loss to follow-up, or survival. Outcomes were defined as 28-day mortality, and 28 days were calculated from the fourth day of ICU admission.
Statistical analysis
The characteristics of the eICU-CRD cohort were summarized using the mean ± standard deviation (SD), median (lower quartile-upper quartile), or number (proportion, %). Continuous variables of the trajectory groups were compared using t test or ANOVA for normally distributed data, and were compared using Wilcoxon rank sum test or Kruskal Wallis test for nonnormal data. Categorical variables of trajectory groups were compared using the chi-square test.
Latent mixture modeling (PROC TRAJ) was utilized to perform a group-based trajectory model (GBTM) [26] to identify the trajectories of RR, HR, and ROX. On the basis of prior studies [11, 27, 28], 2 to 5 trajectories were fitted using a linear and quadratic trajectory function based on a censored normal model [29] with age and sex adjustments. The average posterior probability of individuals belonging to each specific trajectory group was calculated, and the percentage of members in each trajectory group was presented. We selected the optimal model with the smallest absolute value of the Bayesian information criterion (BIC). The average posterior probability of each trajectory group was needed to be no less than 70%, and the percentage of members in each trajectory group was expected to be no less than 5%. Upon analyzing the trajectories of RR and HR, we noted that the patterns and numbers of trajectories of RR and HR were quite similar. Consequently, we calculated the Kappa statistic of the groups of RR and HR to evaluate the consistency of trajectory classification to avoid redundant analyses.
A logistic model was used to evaluate the relationships between trajectories and 28-day mortality in the univariate model. Statistically significant variables in the comparison of survivors and nonsurvivors were further adjusted in the multivariate model, and covariates with missing rates > 10% were not included. The trajectory group with lowest rate of 28-day mortality for each indicator was considered as a reference object in the logistic model.
Restricted cubic spline with 4 knots (5%, 35%, 65%, and 95%) was fitted to calculate the linear and nonlinear associations of baseline and last RR, HR, and ROX in the first 72 h of ICU stays with 28-day mortality, and statistically significant variables in the comparison of survivors and nonsurvivors were adjusted.
In the full population, ROC and AUC were used to assess the predictive ability of the general logistic model (model 1 with adjustment for statistically significant variables in the comparison of survivors and nonsurvivors) and trajectory adjusted logistic model (model 2 with adjustment for variables in model 1 plus significant trajectory risk factors). In the population with at least 4 records of ROX, model 1, model 2, and another model 3 (with adjustment for variables in model 1 plus significant static RR related risk factors) were established. The Delong test was used to compare model 1 and model 2, model 1 and model 3.
All analyses were performed with SAS 9.4 and R 4.0.2. A two-sided P value less than 0.05 was considered statistically significant.
Results
A total of 938 inpatients and 67,536 observations were involved in further analyses. Additional file 1: Fig. S1 displays the flow diagram illustrating the selection process of the population of the eICU-CRD database. The demographic information and clinical features grouped by mortality outcome are presented in Table 1. There were 748 survivors and 190 nonsurvivors (28-day mortality = 20.26%) with a median age of 74.00 [69.00, 80.00]. Compared with survivors, nonsurvivors exhibited higher rates of male sex (P = 0.014), vasopressin (P < 0.001), and ventilation (P = 0.002) and had a higher age (P < 0.001) and higher levels of SOFA (P < 0.001), APS-III (P < 0.001), GCS (P = 0.003), WBCs (P < 0.001), INR (P = 0.006), BUN (P < 0.001), creatinine (P = 0.002), and RR (P = 0.015). Among these significantly different variables, age, sex, SOFA, APS-III, vasopressin, ventilation, WBCs, BUN, creatinine, and RR which had low missing rates (≤ 10%) were adjusted in the further logistic regression models, RCS models, and models predicting 28-day mortality. Five RR trajectories, 5 HR trajectories, and 4 ROX trajectories were identified. The model fitting process of the trajectories is shown in Additional file 1: Table S1. The Kappa value equals to 0.102 (P < 0.001), which shows a poor consistency of trajectory classification of RR and HR.
Additional file 1: Tables S2, S3, and S4 present the demographic information and clinical features of the different trajectory groups. In terms of respiratory rate trajectories, outstanding observations include the following: group 1 showed higher incidence of cerebrovascular disease, group 2 displayed elevated bicarbonate level, group 3 had increased rate of myocardial infarction, group 4 exhibited older age, higher GCS score, and elevated INR level, and group 5 had increased RR and HR. Notably, groups 1 and 2 were marked by higher ROX, groups 1 and 3 received higher rate of ventilation, and groups 3 and 5 experienced a higher mortality rate, whereas group 4 had a lower mortality rate. For HR trajectories, highlighted observations include the following: group 1 demonstrated a higher rate of ventilation, group 3 had elevated WBCs, group 5 exhibited higher APS-III, and both groups 1 and 5 displayed elevated creatinine level. Groups 4 and 5 were characterized by higher RR and HR, groups 1 and 3 received a higher rate of ventilation, and group 1 had a lower mortality rate. Regarding ROX trajectories, the main differences were as follows: group 1 displayed a higher SOFA score, an increased rate of cerebrovascular disease, and a higher prevalence of malignant cancer. This group also showed higher RR and HR. Group 4 comprised a higher proportion of females, and group 5 exhibited higher levels of potassium and ROX.
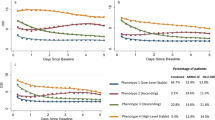
Figure 1 presents the trajectories of RR, HR, and ROX. For RR curves, group 1 (n = 157, 16.74%) had a low stable level. Group 2 (n = 280, 29.85%) had low levels at baseline and then elevated. Group 3 (n = 178, 20.47%) started with a middle level, which then increased and then declined. Group 4 (n = 192, 18.98%) started with a high level of RR, which then decreased to the middle level. Group 5 (n = 131, 13.97%) had persistent high level with a slight downward trend. Group 1 was younger, and group 3 and group 5 had higher rates of mortality. For HR curves, group 1 (n = 157, 16.74%), group 4 (n = 155, 16.52%), and group 5 (n = 125, 13.33%) had similar trends compared with those of RR. The HR in group 2 (n = 316, 33.69%) had a medium–low stable level; group 3 (n = 185, 19.72%) started with a medium level, which then increased to nearly 100 beats per minute. The ROX curves of groups 1, 2, and 3 had similar initial levels. The level of group 1 (n = 80, 39.20%) slightly increased, group 2 (n = 78, 38.20%) increased to moderate level, and the level of group 3 (n = 19, 9.30%) sharply increased and declined to middle level with an inverted U-shaped curve. Group 4 (n = 27, 13.20%) had high stable ROX.
Trajectories of RR, HR, and ROX. This figure shows the distinct trajectories of respiratory rate, heart rate, and respiratory rate-oxygenation from the first 72 h of ICU stays based on data of the eICU-CRD. Using the approach of group-based trajectory model, 5 respiratory rate trajectories were presented in (a), 5 heart rate trajectories were presented in (b), 4 respiratory rate-oxygenation trajectories were presented in (c). RR respiratory rate, HR heart rate, ROX respiratory rate-oxygenation, ICU intensive care unit, eICU-CRD eICU Collaborative Research Database
Table 2 presents the odds ratios (ORs) and 95% confidence intervals (CIs) of trajectories on the risk of 28-day mortality. In the multivariable adjustment models, compared with trajectory group 4 of RR, the ORs and 95% CIs of group 3 were 2.74 (1.48–5.07) (P = 0.001). Compared with trajectory group 1 of HR, the ORs and 95% CIs were 2.20 (1.19–4.08) (P = 0.012) for group 2, 2.70 (1.40–5.23) (P = 0.003) for group 3, and 2.16 (1.04–4.49) (P = 0.040) for group 5. There was no significant difference in mortality risk among trajectory groups of ROX.
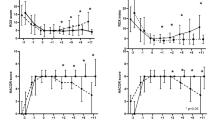
Figure 2 presents restricted cubic splines (RCS) to model relationships of the baseline and last levels of RR, HR, and ROX with 28-day mortality. The cubic spline function revealed no linear or nonlinear effect of baseline levels of RR (P for linear = 0.181, P for nonlinear = 0.612), HR (P for linear = 0.185, P for nonlinear = 0.101), or ROX (P for linear = 0.754, P for nonlinear = 0.580) on 28-day mortality. For the dimension of last levels, RR and HR were not found to be independently associated with 28-day mortality, whereas the last level of ROX was linearly (P = 0.023) and nonlinearly (P = 0.010) associated with 28-day mortality. A lower last level of ROX indicated a higher risk of mortality.
RCS of baseline, post-treatment levels of RR, HR, and ROX with 28-day mortality. Blue lines represent associations of baseline RR, heart rate, and ROX with 28-day mortality whereas red lines represent associations of post-treatment RR, heart rate, and ROX with 28-day mortality. For RR, 99.89% baseline levels were observed during 1 to 24 h in the ICU whereas 99.57% last levels were observed during 25 to 72 h in the ICU; for heart rate, 99.89% baseline levels were observed during 1 to 24 h in the ICU whereas 100.00% last levels were observed during 25 to 72 h in the ICU; for ROX, 100.00% baseline levels were observed during 1 to 24 h in the ICU whereas 97.55% levels were observed during 25 to 72 h in the ICU. RCS restricted cubic splines, ORs odds ratios, RR respiratory rate, HR heart rate, ROX respiratory rate-oxygenation, ICU intensive care unit
Figure 3 presents the ROCs of the different logistic models to predict 28-day mortality. In 938 elderly individuals, the AUC of the model with adjustment for the trajectories of RR and HR was superior to that of the model without adjustment for the trajectory factors (0.768 vs. 0.743, P = 0.020). In 204 elderly patients with at least 4 records of ROX, the AUC of model 2 was superior to that of model 1 (0.810 vs. 0.738, P = 0.015), and there was no significant difference between model 1 and model 3 (0.738 vs. 0.747, P = 0.878).
ROCs of different logistic models to predict 28-day mortality. ROCs of different logistic models in 938 population were presented in (a), ROCs of different logistic models in 204 population with at least 4 records of ROX were presented in (b). Model 1 adjusted age, sex, SOFA, APS-III, vasopressin, ventilation, WBCs, BUN, creatinine, and RR; model 2 adjusted variables in model 1 plus trajectories of RR and HR; model 3 adjusted variables in model 1 plus last measurement of ROX. ROC receiver operating curve, SOFA Sequential Organ Failure Assessment, APS-III Acute Physiology Score III; WBCs white blood cells, BUN blood urea nitrogen, RR respiratory rate, HR heart rate, ROX respiratory rate-oxygenation. †P = 0.020, ‡P = 0.015
Discussion
We determined the shapes and numbers of the clinical featured trajectories using RR, HR, and the composite index of ROX in elderly, critically ill patients with ARDS, using data from the eICU-CRD cohort. Based on the trajectory analysis, potential subphenotypes of ARDS were identified. RR, HR, and ROX had distinct manifestations of mortality risk. For RR and HR, their longitudinal subphenotypes are risk factors for 28-day mortality and could improve the predictive ability. For the ROX index, its last levels during the initial 72 h of ICU admission are associated with 28-day mortality. To our knowledge, this is the first study to explore dynamic subphenotypes and static levels of RR, HR, and ROX, and to assess their associations with death in elderly ARDS patients who were admitted to the ICU. These findings indicate that dynamic subphenotypes of RR and HR, and static ROX after initial critical care could suggest a prognosis that may need to be managed in critically ill elderly patients with ARDS.
Compared with single time-point measurements, trajectories or average patterns of RR and HR may capture a combination of disease statuses, including dysfunctional central respiratory control, respiratory or metabolic impairments [30], infection inflammation, lung injury, and myocardial infarct. Most elderly individuals had poor RR status, among which tachypnea is usually caused by ARDS. Notably, the initial moderate level and rising stage of RR (group 3) were integral contributing factors for mortality. For HR subphenotypes, the cumulative effect of varying degrees of higher HR without a clear downward trend predicted an unfavorable prognosis. An increased HR indicates that a person has a low oxygen level, which represents a more severe condition of ARDS. Moreover, ICU patients commonly suffer from impaired physiological homeostasis and circadian rhythm disorders [31, 32], which affect neural regulation and result in HR variation [18, 33]. Furthermore, a prolonged elevated HR in critically ill, cardiac high-risk patients could result in major cardiac events [34], which may cause adverse prognosis. It needs to be noted that the lowest RR (group 1) was not independently associated with mortality which is different from that of HR. The possible reason may be that RR in group 1 was effectively controlled by clinical interventions such as mechanical ventilation, sedation, and analgesia, which dissimulate the symptoms of ventilation-induced [10] and ARDS-induced lung injury. The potential mechanism needs to be explored in the future.
The subphenotypes of ROX were not associated with mortality in elderly patients with ARDS, and interestingly, ICU inpatients could switch between two levels (low and high) of ROX. Treatment in the ICU seemed to affect ROX, as 3 subphenotypes changed to higher levels over time. Piryani et al. [16] showed that patients with a high ROX had a lower risk of mortality for ARDS, and Roca et al. [14, 15] indicated that ROX is a prognostic factor for nasal high-flow therapy. All these studies focused on the ROX at a single time point; perhaps the longitudinal measurement of ROX was not a sensitive predictor of mortality. Another possible interpretation is that the GBTM did not fit a longitudinal ROX subphenotype with a lower level, which may have a higher risk of mortality. The fitted GBTM of ROX needs to be explored in further studies.
We applied restricted cubic spline to model associations of baseline and last levels of RR-related indicators during the first 72 h of stays in the ICU with 28-day mortality, and only the last ROX was linearly and nonlinearly associated with mortality. Strauß et al. [35] indicated that compared with an RR on hospital admission of 12–20/min, an RR of 27–33/min and above 33/min were associated with high mortality in patients with community-acquired pneumonia. Our different results could be attributed to population heterogeneity and unstable changes in RR in ICU patients with ARDS. In addition, the RR may be improved by ventilation and other therapeutic interventions which are relatively homogenous behaviors in the ICU. Therefore, using single time-point measurements may not effectively differentiate between varying mortality risks. Laskey et al. [36] showed that HR at discharge in patients with heart failure is associated with mortality. Wang et al. [37] indicated that a low minimum HR under 60 bpm may be associated with a higher risk for 30-day mortality in critically ill myocardial infarction patients. The association between HR and mortality in ARDS patients is rarely explored; based on our results, the original and last HR is not related to mortality. Guo et al. [18] demonstrated a U-shaped curve relating HR fluctuation (maximum HR minus the minimum HR in the initial 24 h) to mortality in critically ill ICU patients, highlighting the implication of longitudinal HR. Our results could explain the adverse prognosis of low HR fluctuations [18], for example, sustained moderate and sustained high heart rates (groups 2 and 5) had low heart rate fluctuations, which resulted in a higher mortality risk. What is interesting is the linear and nonlinear effects of last ROX. ROX could reflect dyspnea and the severity of respiratory failure [16]. Leszek et al. [38] indicated that early measurement of the ROX index in the intermediary care unit is associated with mortality in intubated COVID-19 patients. Lee et al. [39] showed that the ROX index could be used as a prognostic marker in sepsis. We specifically investigated the last ROXs, which were measured mostly (97.55%) from 25 to 72 h in the ICU. The reinvention ability of ROX through ICU care had a specific effect on mortality. The work of Piryani et al. [16] was consistent with our findings to some extent. The possible reason for the lack of a significant risk of the longitudinal ROX subphenotype may be that high variation in ROX and transient low ROX (such as values < 5) were difficult to quantify by the trajectory model.
Dynamic subphenotypes of RR and HR had additional predictive value of 28-day mortality (AUC increased by 2.5%, P = 0.020), whereas last ROX during the first 72 h of ICU stays did not. This phenomenon indicats that pieces of information on longitudinal RR and HR could supplement the cause of death in addition to the portion explained by other adjusted variables. First, in critically ill elderly patients with ARDS, the dynamic RR and HR may be sensitive and early altered bioindicators of a combination of disease statuses. Second, the suppressed respiratory central and insufficient blood supply caused by persistent deterioration of RR and HR may directly result in death. Further research is needed to explore the mechanism.
There were several limitations in our study. First, trajectory analysis is a data-driven method and may not be applicable for emergencies, in which the patient dies in a shorter time. Second, the results may not apply to ICUs elsewhere with different resources that have heterogeneity in populations, environments, and treatment methods.
Conclusion
Longitudinal dynamic subphenotypes of RR and HR and last static levels of ROX during the first 72 h of ICU stays play specific roles in 28-day mortality. These findings indicate that the dynamic subphenotypes of RR and HR, and static ROX after initial critical care could suggest a prognosis that may need to be controlled in critically ill elderly patients with ARDS.
Availability of data and materials
The data from the eICU-CRD can be obtained after approval of proposal with a signed data access agreement by checking physionet (https://physionet.org/).
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- ICUs:
-
Intensive care units
- RR:
-
Respiratory rate
- CO2 :
-
Carbon dioxide
- HR:
-
Heart rate
- PaO2 :
-
Oxygen partial pressure
- FiO2 :
-
Fractional oxygen
- ROX:
-
Respiratory rate-oxygenation
- SpO2 :
-
Pulse oxygen saturation
- eICU-CRD:
-
eICU Collaborative Research Database
- ROC:
-
Receiver operating curve
- AUC:
-
Area under the curve
- ICD-9-CM:
-
International Classification of Diseases, Ninth Revision, Clinical Modification
- ICD-10-CM:
-
International Classification of Diseases, Tenth Revision, Clinical Modification
- SOFA:
-
Sequential Organ Failure Assessment
- APS-III:
-
Acute Physiology Score III
- GCS:
-
Glasgow Coma Scale
- WBCs:
-
White blood cells
- INR:
-
International normalized ratio
- PTT:
-
Partial thromboplastin time
- BUN:
-
Blood urea nitrogen
- CVD:
-
Cerebrovascular disease
- COPD:
-
Chronic pulmonary disease
- SD:
-
Standard deviation
- GBTM:
-
Group-based trajectory model
- BIC:
-
Bayesian information criterion
- ORs:
-
Odds ratios
- CIs:
-
Confidence intervals
- RCS:
-
Restricted cubic splines
References
Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5(1):18.
Hendrickson KW, Peltan ID, Brown SM. The epidemiology of acute respiratory distress syndrome before and after coronavirus disease 2019. Crit Care Clin. 2021;37(4):703–16.
Killien EY, Mills B, Vavilala MS, Watson RS, O’Keefe GE, Rivara FP. Association between age and acute respiratory distress syndrome development and mortality following trauma. J Trauma Acute Care Surg. 2019;86(5):844–52.
Schouten LRA, Bos LDJ, Serpa Neto A, van Vught LA, Wiewel MA, Hoogendijk AJ, et al. Increased mortality in elderly patients with acute respiratory distress syndrome is not explained by host response. Intensive Care Med Exp. 2019;7(1):58.
Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–8.
Marini JJ, Jaber S. Dynamic predictors of VILI risk: beyond the driving pressure. Intensive Care Med. 2016;42(10):1597–600.
Retamal J, Damiani LF, Basoalto R, Benites MH, Bruhn A, Larsson A, et al. Physiological and inflammatory consequences of high and low respiratory rate in acute respiratory distress syndrome. Acta Anaesthesiol Scand. 2021;65(8):1013–22.
Costa ELV, Slutsky AS, Brochard LJ, Brower R, Serpa-Neto A, Cavalcanti AB, et al. Ventilatory variables and mechanical power in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2021;204(3):303–11.
Vieillard-Baron A, Prin S, Augarde R, Desfonds P, Page B, Beauchet A, et al. Increasing respiratory rate to improve CO2 clearance during mechanical ventilation is not a panacea in acute respiratory failure. Crit Care Med. 2002;30(7):1407–12.
Xavier PH, Fonseca ACF, Gonçalves LA, de Sousa GC, Silva MCD, Sacramento RFM, et al. Lung injury is induced by abrupt increase in respiratory rate but prevented by recruitment maneuver in mild acute respiratory distress syndrome in rats. Anesthesiology. 2023;138(4):420–35.
Bhavani SV, Semler M, Qian ET, Verhoef PA, Robichaux C, Churpek MM, et al. Development and validation of novel sepsis subphenotypes using trajectories of vital signs. Intensive Care Med. 2022;48(11):1582–92.
Zhou D, Li Z, Shi G, Zhou J. Effect of heart rate on hospital mortality in critically ill patients may be modified by age: a retrospective observational study from large database. Aging Clin Exp Res. 2021;33(5):1325–35.
Luecke T, Muench E, Roth H, Friess U, Paul T, Kleinhuber K, et al. Predictors of mortality in ARDS patients referred to a tertiary care centre: a pilot study. Eur J Anaesthesiol. 2006;23(5):403–10.
Roca O, Caralt B, Messika J, Samper M, Sztrymf B, Hernández G, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019;199(11):1368–76.
Roca O, Messika J, Caralt B, García-de-Acilu M, Sztrymf B, Ricard JD, et al. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: the utility of the ROX index. J Crit Care. 2016;35:200–5.
Nishiyama K, Ichikado K, Anan K, Nakamura K, Kawamura K, Suga M, et al. The ROX index (index combining the respiratory rate with oxygenation) is a prognostic factor for acute respiratory distress syndrome. PLoS ONE. 2023;18(2):e0282241.
Gutierrez G, Das A, Ballarino G, Beyzaei-Arani A, Türkan H, Wulf-Gutierrez M, et al. Decreased respiratory rate variability during mechanical ventilation is associated with increased mortality. Intensive Care Med. 2013;39(8):1359–67.
Guo Q, Xiao Z, Lin M, Yuan G, Qiu Q, Yang Y, et al. Heart rate fluctuation predicts mortality in critically ill patients in the intensive care unit: a retrospective cohort study. Ann Transl Med. 2021;9(4):334.
Pollard TJ, Johnson AEW, Raffa JD, Celi LA, Mark RG, Badawi O. The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Sci Data. 2018. https://doi.org/10.1038/sdata.2018.178.
Pollard T, Johnson A, Raffa J, Celi LA, Badawi O, Mark R. eICU Collaborative Research Database (version 2.0). 2019. PhysioNet. https://doi.org/10.13026/C2WM1R.
Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101(23):e215–20.
International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). 2021. https://www.cdc.gov/nchs/icd/icd9cm.htm.
International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). 2023. https://www.cdc.gov/nchs/icd/icd-10-cm.htm.
Reynolds HN, McCunn M, Borg U, Habashi N, Cottingham C, Bar-Lavi Y. Acute respiratory distress syndrome: estimated incidence and mortality rate in a 5 million-person population base. Crit Care. 1998;2(1):29–34.
Rhee J, Dominici F, Zanobetti A, Schwartz J, Wang Y, Di Q, et al. Risk of acute respiratory distress syndrome among older adults living near construction and manufacturing sites. Epidemiology. 2020;31(4):468–77.
Nagin DS. Group-based trajectory modeling: an overview. Ann Nutr Metab. 2014;65(2–3):205–10.
Bhavani SV, Carey KA, Gilbert ER, Afshar M, Verhoef PA, Churpek MM. Identifying novel sepsis subphenotypes using temperature trajectories. Am J Respir Crit Care Med. 2019;200(3):327–35.
Chen J, Gao X, Shen S, Xu J, Sun Z, Lin R, et al. Association of longitudinal platelet count trajectory with ICU mortality: a multi-cohort study. Front Immunol. 2022;13:936662.
Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Method Res. 2016;29(3):374–93.
Baumert M, Linz D, Stone K, McEvoy RD, Cummings S, Redline S, et al. Mean nocturnal respiratory rate predicts cardiovascular and all-cause mortality in community-dwelling older men and women. Eur Respir J. 2019;54(1):1802175.
Dessap AM, Roche-Campo F, Launay JM, Charles-Nelson A, Katsahian S, Brun-Buisson C, et al. Delirium and circadian rhythm of melatonin during weaning from mechanical ventilation: an ancillary study of a weaning trial. Chest. 2015;148(5):1231–41.
Oldham M, Pisani MA. Sedation in critically ill patients. Crit Care Clin. 2015;31(3):563–87.
Guyton AC. Blood pressure control–special role of the kidneys and body fluids. Science. 1991;252(5014):1813–6.
Sander O, Welters ID, Foëx P, Sear JW. Impact of prolonged elevated heart rate on incidence of major cardiac events in critically ill patients with a high risk of cardiac complications. Crit Care Med. 2005;33(1):81–8.
Strauß R, Ewig S, Richter K, König T, Heller G, Bauer TT. The prognostic significance of respiratory rate in patients with pneumonia: a retrospective analysis of data from 705,928 hospitalized patients in Germany from 2010–2012. Dtsch Arztebl Int. 2014;111(29–30):503–8.
Laskey WK, Alomari I, Cox M, Schulte PJ, Zhao X, Hernandez AF, et al. Heart rate at hospital discharge in patients with heart failure is associated with mortality and rehospitalization. J Am Heart Assoc. 2015;4(4):e001626.
Wang J, Zhou L, Zhang Y, Zhang H, Xie Y, Chen Z, et al. Minimum heart rate and mortality in critically ill myocardial infarction patients: an analysis of the MIMIC-III database. Ann Transl Med. 2021;9(6):496.
Leszek A, Wozniak H, Giudicelli-Bailly A, Suh N, Boroli F, Pugin J, et al. Early measurement of ROX index in intermediary care unit is associated with mortality in intubated COVID-19 patients: a retrospective study. J Clin Med. 2022;11(2):365.
Lee CU, Jo YH, Lee JH, Kim J, Park SM, Hwang JE, et al. The index of oxygenation to respiratory rate as a prognostic factor for mortality in Sepsis. Am J Emerg Med. 2021;45:426–32.
Acknowledgements
None.
Funding
This study was funded by National Natural Science Foundation of China (Grant No. 71804093) and Natural Science Foundation of Shandong Province (ZR2023MG005). The funding body played no role in the design of the study, collection, analyses, and interpretation of data and in writing the article.
Author information
Authors and Affiliations
Contributions
FT and ML conceived the study. ML and FT drafted the manuscript. YY, JL, CY, YW, ZY, and YW helped manage the data. ML and FT performed the statistical analysis and helped interpret the results. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The eICU database has received ethical approval from the Institutional Review Boards at Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology. Due to the retrospective design, public available database, lack of direct patient intervention, and de-identified personal information, a waiver of informed consent was included in the approval from the Institutional Review Boards at Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1
: Fig. S1. Flow diagram used for selection of the population. Table S1. The model fitting process of trajectories of respiratory rate, heart rate, and respiratory rate-oxygenation. Table S2. Description of baseline variables for ICU patients grouped by respiratory rate trajectories. Table S3. Description of baseline variables for ICU patients grouped by heart rate trajectories. Table S4. Description of baseline variables for ICU patients grouped by respiratory rate-oxygenation trajectories.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, M., Liu, F., Yang, Y. et al. Identifying vital sign trajectories to predict 28-day mortality of critically ill elderly patients with acute respiratory distress syndrome. Respir Res 25, 8 (2024). https://doi.org/10.1186/s12931-023-02643-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-023-02643-8