Abstract
Rationale
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive interstitial lung disease that has no cure. Many current research efforts center on diagnostic and therapeutic modalities for IPF while other risk factors affecting disease pathogenesis receive less attention. Emerging data support the clinical importance of weight loss in patients with IPF. However, factors associated with weight loss and the impact of weight loss on mortality remain incompletely explored.
Objectives
Explore the association between weight loss and transplant-free survival in patients with IPF and identify clinical variables associated with weight loss in this population.
Methods
Kaplan–Meier and Cox proportional hazard regression analyses were generated and stratified by weight loss or use of antifibrotic medications. Conditional logistic regression was used to evaluate for factors associated with weight loss.
Results
There was a significant increase in mortality in patients who lost ≥ 5% of their body weight loss (HR 2.21, [1.29, 4.43] p = .021). The use of supplemental oxygen (adjusted OR 13.16), and ≥ 200 mL loss of FVC over 1 year (adjusted OR 5.44) were both associated with a ≥ 5% weight loss in the year following a diagnosis of IPF. The use of antifibrotic medication did not significantly change median transplant-free survival in patients who lost more than ≥ 5% of their body mass.
Conclusions
Weight loss over the first year following a diagnosis of IPF is strongly associated with decreased transplant-free survival. More research is needed to determine the mechanisms surrounding weight loss in patients with IPF.
Similar content being viewed by others
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive idiopathic interstitial lung disease (ILD) and is the most common ILD affecting older adults in the United States [1]. The reported prevalence of IPF is as high as 494 cases per 100,000 adults over the age of 65 and appears to be increasing [2]. IPF is associated with progressive lung scarring and pulmonary function deterioration, ultimately resulting in death. While the only curative therapy is lung transplant, this option is available to relatively few patients. Anti-fibrotic therapies, pirfenidone and nintedanib, reduce the rate of lung function decline [3,4,5] and may prolong survival but unfortunately they do not cure fibrosis [6].
Many current research efforts center on diagnostic and therapeutic modalities for IPF while other risk factors affecting disease pathogenesis receive less attention. Lifestyle factors such as dietary intake and nutritional status remain severely understudied in chronic respiratory diseases. Poor nutritional status and weight loss have been identified as independent predictors of poor clinical outcomes in chronic lung diseases such as chronic obstructive pulmonary disease (COPD) [7, 8]. Other factors including depression may also contribute to weight loss in chronic lung disease [9]. More recently, weight loss, decline in muscle mass, and decreased/low body mass index (BMI) were also associated with increased mortality in chronic obstructive pulmonary disease (COPD) [10, 11], lung cancer [12,13,14], and patients with IPF [15, 16]. This is clinically relevant as dietary supplementation in malnourished patients with COPD improved 6-min walking distance (6MWD), respiratory muscle strength (maximal inspiratory and expiratory pressures), and overall health-related quality of life as measured by the St. George’s Respiratory Questionnaire [17].
Emerging data support the clinical importance of weight loss in patients with IPF. A recent cohort of IPF patients from Japan and the United Kingdom showed that significant body weight (BW) decline, defined as > 6.1% body mass loss, was an independent predictor of mortality [15]. Pugashetti et al. found that a BMI decline of > 5% was associated with two times higher risk of death in patients with IPF and undifferentiated ILD [18]. Most recently Comes et al. also showed an association between weight loss and mortality in study of combined IPF, connective tissue diseases, fibrotic hypersensitivity pneumonitis and other unclassifiable types of lung fibrosis [19]. Furthermore, multiple studies have suggested that a decline in BMI may have utility as a prognostic tool [20, 21]. These studies suggest a clinically significant relationship between disease progression and weight loss.
The use of pirfenidone or nintedanib is often associated with significant gastrointestinal side effects [3, 22, 23]. A recent study showed that 63% of patients taking pirfenidone reported anorexia and 55% reported weight loss. Anorexia and diarrhea were reported in 46% of patients taking nintedanib [24]. Studies reporting associations between weight loss and mortality were published before the use of anti-fibrotic medications became the standard of care for the treatment of IPF. As such, it remains unclear how weight loss associated with anti-fibrotic therapy affects disease progression and whether anti-fibrotic treatment is as effective in patients who experience significant weight loss.
This study explores the relationship between change in weight over the first year following the diagnosis of IPF and lung function and transplant-free survival. We hypothesize that a decline in body weight by more than 5% within the first year of being diagnosed with IPF is associated with increased mortality and a significant decline in forced vital capacity (FVC). Furthermore, we aimed to elucidate whether the use of anti-fibrotic medications has any impact on weight loss and/or mortality in our cohort.
Materials and methods
Study population and data collection
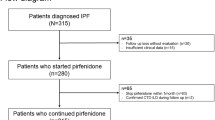
Using an existing ILD registry database at the University of Rochester (n = 498), we identified 75 patients with a diagnosis of IPF (based on URMC MDD consensus diagnosis as outlined in the ATS/ERS/EJRS/ALAT clinical practice guideline [25]). Written consent was obtained from all subjects. Data collection was started in 2008. 71 patients had at least 1 year of longitudinal pulmonary function test data and weight measurements. Data from 34 patients were collected prior to 2014 when pirfenidone and nintedanib were FDA approved for the treatment of IPF and these patients were therefore not taking an antifibrotic medication. Patients were excluded if they did not have a diagnosis of IPF, if longitudinal or incomplete pulmonary function testing was not available, and if their weight was not documented. Diagnoses were made based on clinical history, physical exam, and CT imaging.
The date of initial IPF diagnosis was established as accurately as possible based on clinical notes. For each patient, FVC and weight/BMI were documented at the time of diagnosis and over the years following the date of diagnosis. Lung function was measured at 3-month intervals, until present date or until they had a lung transplant, when they were lost to follow-up, or were deceased. Weight measurements were recorded at the time of each PFT. Additional weight measurements were documented if they were recorded in the medical record. The change in weight and FVC at the end of the first year after the diagnosis were calculated and were only considered to be significant if the changes were consistently documented on at least three measurements. Additional variables of interest were collected including: age, gender, medical co-morbidities, smoking status, and the use of anti-fibrotic medications. This study was approved by the University of Rochester institutional review board (#00000503).
Statistical analysis
Chi-square analyses and t-tests were conducted to compare differences in baseline demographics. Kaplan–Meier curves were generated and stratified by weight loss or use of antifibrotic medications. Cox proportional hazard regression analyses with hazard ratios (HRs) and 95% confidence intervals (CIs) were subsequently calculated.
Logistic regression was then used to evaluate for factors associated with significant weight loss. P values < 0.05 were considered statistically significant. To clarify which variables were independently associated with weight loss, a multivariate model was generated. All statistical analyses were performed using GraphPad prism version 9.1.0 for Windows, GraphPad Software, San Diego, California USA or SAS/ACCESS® 9.4 Interface to ADABAS, SAS Institute Inc 2013, Cary, NC.
Results
Patient characteristics
A total of 71 patients were included in the analysis (Table 1). All patients were Caucasian, all identified as non-Hispanic, 71% were male, and the average age was 71 years (SD = 9.5 years). 70% had a history of cigarette use (Table 1). There were no malignancies identified prior to or during the observation period. 51% of our cohort were on an anti-fibrotic medication. 74% percent were on an antifibrotic medication within 6 months of the diagnosis of IPF. 62% percent were on a stable dose of an antifibrotic medication for at least 2 years. None of the patients discontinued antifibrotic medication, but seven patients required a dose reduction and seven patients changed antifibrotic medications. In all cases, the changes were made after 1 year of being on an antifibrotic medication and each patient remained on a stable dose of the new antifibrotic medication for the remainder of the observation period. There were 41 deaths and 11 lung transplants over the entire period of observation. The median duration of observation in this study was 1300 days.
Weight trajectories were plotted for the entire study period, and more specifically over the first year from the date of diagnosis (Fig. 1A and B). 30% of patients experienced significant weight loss over the year following the diagnosis of IPF (Fig. 1B). Only one of the patients losing more than 5% of body weight regained weight over the following 4 years. BMI trajectories were plotted from the time of diagnosis to determine whether a low BMI at the time of diagnosis was associated with greater weight loss (Fig. 1C). There were no apparent differences in weight loss on the basis of initial BMI; however, seven patients with BMIs over 30 at the time of diagnosis lost over 10% of their body mass. Similarly, when stratified by the presence or absence of weight loss within the first year, there were no significant differences in weight or BMI at the time of diagnosis BMI (Table 2). The average FVC at the time of diagnosis was 2.6 L (70.5% of predicted) in the group who lost weight and 3.1 L (82.4% of predicted) in the group who did not lose weight (p = 0.02). This suggests that patients lower FVCs may be more likely to lose weight. The group who lost weight had a higher percentage of oxygen use (57% versus 20%, p = 0.72), reflux disease (52% versus 40% p = 0.43) and utilization of pulmonary rehabilitation (24% versus 18%, p = 0.52); however, these changes were not statistically significant (Table 2).
Weight-loss trajectories over time. Shown are changes in weight over the entire observation period (A) or stratified by those who lost ≥ 5% weight within the first year of an IPF diagnosis (B). Red lines indicate patients losing ≥ 5% bodyweight. Panel C demonstrates initial body mass index (BMI) at diagnosis vs. time. Colored lines on this graph represent the varying stratifications of BMI
Weight loss and mortality
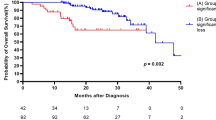
Kaplan–Meier analysis of the entire cohort showed a transplant-free median survival of 5.35 years (Fig. 2A). To examine the association between transplant-free survival and weight loss, Kaplan–Meier analyses were performed stratified by those who lost less than 5% body weight versus those who lost at least 5% body weight in the first year (Fig. 2B). The stratification clearly demonstrates there is a significant increase in mortality for those who experienced greater than 5% body weight loss (HR 2.21, [1.29, 4.43] p = 0.021). The impact of weight loss on survival remained present even after direct adjustment for age and FVC at the time of diagnosis (HR 2.03, [1.36–2.71], p = 0.038). Neither age or FVC at time of diagnosis were significantly associated with a reduction in transplant free survival in the model HRs 1.02, [0.97–1.05] (p = 0.52) and 0.87, [0.47–1.27] (p = 0.50) respectively. The median survival of patients who lost more than 5% of their body mass over the first year was 1389 days (3.8 years) while the median survival for patients who did not lose a significant amount of weight was 2142 days (5.8 years). The group of patients who lost more than 10% of the body mass (n = 4) in the first year had significantly higher mortality than the group who lost between 5–10% of their body mass, median survival 738 days (2.02 years) (Fig. 2C).
Kaplan–Meier analysis for transplant-free survival. The median survival in our cohort was 5.35 years from the time of IPF diagnosis (A). However, when patients were stratified by at least 5% weight loss vs those who lost < 5%. Those who lost weight had a significantly worse prognosis (median transplant-free survival of 3.8 years vs 5.8 years, p = 0.0102) (B). For those patients who lost more than 10% of their weight, there was an even more significant detrimental effect on transplant-free mortality (median survival of 2.02 years, p = 0.001) (C)
Weight loss and FVC decline
After demonstrating a clear relationship between weight loss over the first year after a diagnosis of IPF and reduced transplant-free survival, we next examined whether weight loss was associated with a decline in FVC. There was no significant linear association between weight loss and change in FVC in the year following a diagnosis of IPF (Fig. 3A) (p = 0.42). However, there was a statistically significant linear relationship between the total amount of weight lost over the observation period and the total change in FVC over the entire observation period (p = 0.002) (Fig. 3B). This does suggest that even early weight loss is highly associated with long-term lung function decline.
Association between weight loss and change in Forced Vital Capacity. There was no significant effect of weight change over the first year after IPF diagnosis and decline in FVC over the same time period (p = 0.46) (A). However, there was a significant association between change in weight vs change in FVC during the entire observation period (p = 0.0026) (B)
Factors associated with weight loss
We next assessed whether any demographic or clinical factors may be associated with weight loss. Univariate analysis of multiple clinical variables was performed (Table 3). Several variables including anti-fibrotic use, use of an Angiotensin Receptor Blocker and gastroesophageal reflux disease (GERD) were not associated with weight loss. Although there was no clear linear association between changes in FVC in the year following the diagnosis of IPF, weight loss was highly associated with long-term lung function decline. We hypothesized that larger FVC volume losses in the first year after diagnosis may be associated with weight loss over the first year. For purposes of these logistic regression analyses, changes in FVC were stratified to large volume losses (≥ 200 mL over the course of a year) and small volume losses (< 200 mL over the course of a year). A loss of ≥ 200 mL in the first year was associated with an increased odds of weight loss (OR 3.03 [0.985, 9.341]) but this did not reach statistical significance (p = 0.053). The use of supplemental oxygen was statistically associated with weight loss (OR 5.4 [1.86–17.18] p = 0.0031). Though not statistically significant, we observed a 90% increase in the risk of weight loss in patients on antifibrotic medications in the past year when compared to patients not taking antifibrotic medications (OR 1.907, [0.673, 5.405], p = 0.22). It is important to note that this analysis did not distinguish whether either individual medication was, by itself, associated with greater weight loss.
To determine whether weight loss, ≥ 200 mL loss of FVC, and supplemental oxygen use were independently associated with a reduction in transplant free survival we generated a multivariable model using stepwise addition modelling. Additional variables included in the modelling were age, gender, and anti-fibrotic use. The final model was adjusted for age, ≥ 200 mL loss of FVC and supplemental oxygen use. The use of supplemental oxygen (adjusted OR 13.167, [3.059–56.66], p = 0.0005) and ≥ 200 mL loss of FVC over 1 year (OR 5.44, [1.28, 22.98], p = 0.02) were both associated with a ≥ 5% weight loss in the year following a diagnosis of IPF.
Anti-fibrotic use, weight loss
To better understand how anti-fibrotic use may impact weight loss on transplant-free survival, we stratified by anti-fibrotic use (Table 4). Patients were assigned to be taking pirfenidone or nintedanib if there were taking that medication following the diagnosis of IPF. There were no significant differences in baseline characteristics of patients taking antifibrotics compared to those not taking antifibrotic medications including average age (71.1 v. 71.9 p = 0.74), DLCO (10.66 v. 12.18, p = 0.17), FVC (2.83 v. 3.01, p = (0.22) or BMI (29.7 v 29.9, p = 0.81). Longitudinal weight changes were then plotted over time. In general, patients on anti-fibrotic medications exhibited similar weight trends as those patients not on anti-fibrotic medications, although there were slight differences in those taking nintedanib compared to patients on pirfenidone or patients not on an anti-fibrotic (Fig. 4A).
Effect of anti-fibrotic medications on weight loss and transplant-free survival. Patients taking anti-fibrotic medications demonstrated similar weight loss compared with those not taking anti-fibrotic medications. Red lines: pirfenidone, Green lines: nintedanib (A). There was slightly more weight loss observed in those taking nintedanib, however this did not reach statistical significance. There was no statistically significant difference in transplant-free survival between patients taking anti-fibrotic medications and those who were not (p = 0.56) (B). When patients were stratified by degree of weight loss in the first year from diagnosis, there remained no significant difference based on anti-fibrotic medications (C)
Weight loss, anti-fibrotic use and mortality
To identify how the use of antifibrotic medications may impact transplant-free survival in our cohort, we performed additional Kaplan–Meier analyses stratifying by use of antifibrotic medications. There was no significant difference in transplant-free survival between anti-fibrotic users and non-users (Fig. 4B). We next wanted to determine whether anti-fibrotic medication use was associated with increased transplant free survival in patients who lost weight as compared to patients who did not lose weight. When stratified by weight loss and anti-fibrotic medication use, the decrease in transplant-free survival was readily apparent in the groups who lost weight (Fig. 4C). However, there was no apparent difference in transplant-free survival on the basis of anti-fibrotic treatment in either the group who lost weight or in those who did not lose weight.
Discussion
Our study shows that ≥ 5% weight loss over the first year following a diagnosis of IPF is associated with decreased transplant-free survival. The results are consistent with previously reported data, reinforcing the impact of weight loss on mortality. Importantly, our study defines the timing of weight loss as being present immediately after diagnosis rather than occurring later over the disease course. Transplant-free survival in our cohort was 5.35 years but was significantly lower (3.8 years) in patients who lost more than 5% percent of their weight in the first year following the diagnosis of IPF. Critically, transplant-free survival was further decreased (2.02 years) in patients who lost more than 10% of their weight. Thus, we demonstrate an association between severity of weight loss and mortality. Interestingly the median survival of our cohort is 5.35 years which is almost a year longer than what is reported in the literature [26, 27]. It is possible a diagnosis was reached earlier in our patients or possibly by receiving medical care at a Pulmonary Fibrosis Foundation Center. It is also possible that other undocumented lifestyle factors like diet may have contributed to increased median survival in this cohort.
Previous reports on mortality and weight loss in IPF have largely centered around cohorts of patients who were not on an anti-fibrotic medication [15, 18]. However, 52% of the patients in our cohort were taking either pirfenidone or nintedanib. The use of antifibrotic medication is therefore incorporated into the transplant-free survival reported above. Although antifibrotic medications slow the decline in FVC [3,4,5], when comparing weight loss to FVC in our cohort, unexpected trends emerge. There was no association between weight loss and FVC decline in the first year alone. However, over the entirety of the period of observation, weight loss was associated with FVC decline in a linear manner. This may indicate that weight loss could precede FVC decline in some patients. It may also suggest that weight loss is associated with more aggressive progression of IPF in another subset of patients. Regardless, a > 5% change in weight over the first year after the diagnosis of IPF is an important marker of disease progression and prognosis. Serial weight measurements may therefore have prognostic value in addition to serial FVC measurements, particularly if weight loss occurs in some patients prior to changes in more traditional objective measurements such as FVC.
Our cohort started in 2008, 6 years prior to FDA approval of nintedanib and pirfenidone, and therefore a significant portion of patients did not have these medications as therapeutic options. Weight loss therefore occurs even in the absence of antifibrotic medication use and continues to have a large impact on reducing transplant free survival. Prior reports have shown that use of anti-fibrotic medications is associated with a reduction in mortality. In our cohort, there was no difference in transplant-free survival between patients taking an anti-fibrotic and those who were not (Fig. 4B). Nor was there a difference when mortality analyses were stratified by both weight loss and anti-fibrotic medication. Weight loss appears to have a more significant impact on mortality than any potential benefit from use of antifibrotic medication. However, more research is needed to tease out the mechanisms that surround weight loss in IPF. It is not yet entirely clear whether there may be an improvement in transplant-free survival in patients who do not lose weight and who are taking an antifibrotic medication. Additional studies are required to better define whether which, if either, of the antifibrotic medication contributes to a decrease in transplant-free survival and whether either of the antifibrotic medications improves mortality in patients who do not lose weight.
This study demonstrates the importance of weight loss monitoring immediately after diagnosis in patients with IPF. There are several factors associated with weight loss in chronic disease including nutritional status, and micro- or macro-nutrient intake that were not addressed in this study. The role of proper nutrition in prevention of disease progression or maintenance of the stability is supported by data demonstrating that dietary supplementation in malnourished patients with COPD improved 6-min walking distance (6MWD), respiratory muscle strength (maximal inspiratory and expiratory pressures) and overall health-related quality of life [17]. Furthermore, preclinical data also suggests that specific micro- and macro-nutrient related metabolic pathways may be directly involved the development of pulmonary fibrosis in vivo [28, 29]. Ultimately, additional nutritional epidemiology studies will be critically important to address this knowledge gap.
There are several limitations to our data. As this is a retrospective cohort, patients lacking recorded data were unable to be included. In addition, this is a single center study with a moderate sample size. The presence of diagnosis bias may also be an additional confounder as clinicians have become more adept at recognizing IPF earlier in the course of the disease process. There may be other important confounders that affect an individual’s weight such as diet, nutritional status, concurrent metabolic disorders, amount of physical activity, medication/supplement use, and/or medical co-morbidities that were not clearly documented in each patient’s medical record. In spite of the limitations of the current study, our data support prior observations. The effect of weight loss on mortality in this cohort is striking and certainly warrants additional investigation.
In conclusion, our study shows that ≥ 5% weight loss over the year following a diagnosis of IPF is associated with a significant decrease in transplant-free survival (adjusted HR 2.02). The impact of weight loss on mortality was even more striking in patients who lost more than 10% of their body weight over the first year. Significant weight loss may precede decline in FVC but is independently associated with large volume decline in FVC. Furthermore, there was no apparent increase in transplant-free survival in patients taking antifibrotic medications, but it remains possible that there may be a mortality benefit to anti-fibrotic medications in patients who do not lose weight. Further studies are needed to evaluate the correlation between FVC and weight loss in IPF, as well as to elucidate the underlying mechanism that may put patients with weight loss at risk for IPF progression. Additionally, it remains unknown whether prevention of weight loss by dietary supplementation may mitigate weight loss and the ensuing decrease in transplant-free survival.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier J-F, Flaherty KR, Lasky JA. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824.
Raghu G, Chen S-Y, Yeh W-S, Maroni B, Li Q, Lee Y-C, Collard HR. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir Med. 2014;2:566–72.
King TE, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, Noble PW. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–92.
Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–82.
Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, Taguchi Y, Takahashi H, Nakata K, Sato A, Takeuchi M, Raghu G, Kudoh S, Nukiwa T, Japan tPCSGi. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–9.
Behr J, Prasse A, Wirtz H, Koschel D, Pittrow D, Held M, Klotsche J, Andreas S, Claussen M, Grohé C. Survival and course of lung function in the presence or absence of antifibrotic treatment in patients with idiopathic pulmonary fibrosis: long-term results of the INSIGHTS-IPF registry. Eur Respir J. 2020;56(2):1902279.
Wada H, Ikeda A, Maruyama K, Yamagishi K, Barnes PJ, Tanigawa T, Tamakoshi A, Iso H. Low BMI and weight loss aggravate COPD mortality in men, findings from a large prospective cohort: the JACC study. Sci Rep. 2021;11:1–9.
McDonald M-LN, Wouters EF, Rutten E, Casaburi R, Rennard SI, Lomas DA, Bamman M, Celli B, Agusti A, Tal-Singer R. It’s more than low BMI: prevalence of cachexia and associated mortality in COPD. Respir Res. 2019;20:1–9.
Corlateanu A, Covantev S, Mathioudakis AG, Botnaru V, Siafakas N. Prevalence and burden of comorbidities in chronic obstructive pulmonary disease. Respir Investig. 2016;54:387–96.
Wada H, Ikeda A, Maruyama K, Yamagishi K, Barnes PJ, Tanigawa T, Tamakoshi A, Iso H. Low BMI and weight loss aggravate COPD mortality in men, findings from a large prospective cohort: the JACC study. Sci Rep. 2021;11:1531.
McDonald MN, Wouters EFM, Rutten E, Casaburi R, Rennard SI, Lomas DA, Bamman M, Celli B, Agusti A, Tal-Singer R, Hersh CP, Dransfield M, Silverman EK. It’s more than low BMI: prevalence of cachexia and associated mortality in COPD. Respir Res. 2019;20:100.
Watte G, Nunes CHA, Sidney-Filho LA, Zanon M, Altmayer SPL, Pacini GS, Barros M, Moreira ALS, Alves RJV, Zelmanowicz AM, Matata BM, Moreira JDS. Proportional weight loss in six months as a risk factor for mortality in stage IV non-small cell lung cancer. J Bras Pneumol. 2018;44:505–9.
Chen YM, Lai CH, Lin CY, Tsai YH, Chang YC, Chen HC, Tseng CC, Chang HC, Huang KT, Chen YC, Fang WF, Wang CC, Chao TY, Lin MC. Body mass index, weight loss, and mortality risk in advanced-stage non-small cell lung cancer patients: a focus on EGFR mutation. Nutrients. 2021;13:3761.
Mytelka DS, Li L, Benoit K. Post-diagnosis weight loss as a prognostic factor in non-small cell lung cancer. J Cachexia Sarcopenia Muscle. 2018;9:86–92.
Nakatsuka Y, Handa T, Kokosi M, Tanizawa K, Puglisi S, Jacob J, Sokai A, Ikezoe K, Kanatani KT, Kubo T. The clinical significance of body weight loss in idiopathic pulmonary fibrosis patients. Respir Int Rev Thorac Dis. 2018;96:338–47.
Alakhras M, Decker PA, Nadrous HF, Collazo-Clavell M, Ryu JH. Body mass index and mortality in patients with idiopathic pulmonary fibrosis. Chest. 2007;131:1448–53.
Schols AM, Ferreira IM, Franssen FM, Gosker HR, Janssens W, Muscaritoli M, Pison C, Rutten-vanMölken M, Slinde F, Steiner MC. Nutritional assessment and therapy in COPD: a European Respiratory Society statement. Eur Respiratory Soc. 2014;44:1504.
Pugashetti J, Graham J, Boctor N, Mendez C, Foster E, Juarez M, Harper R, Morrissey B, Kadoch M, Oldham JM. Weight loss as a predictor of mortality in patients with interstitial lung disease. Eur Respir J. 2018;52:1801289.
Comes A, Wong AW, Fisher JH, Morisset J, Johannson KA, Farrand E, Fell CD, Kolb M, Manganas H, Cox G, Gershon AS, Halayko AJ, Hambly N, Khalil N, Sadatsafavi M, Shapera S, To T, Wilcox PG, Collard HR, Ryerson CJ. Association of BMI and change in weight with mortality in patients with fibrotic interstitial lung disease. Chest. 2022;161:1320–9.
Awano N, Jo T, Yasunaga H, Inomata M, Kuse N, Tone M, Morita K, Matsui H, Fushimi K, Nagase T, Izumo T. Body mass index and in-hospital mortality in patients with acute exacerbation of idiopathic pulmonary fibrosis. ERJ Open Res. 2021;7:00037–2021.
Gao J, Kalafatis D, Carlson L, Pesonen IHA, Li CX, Wheelock Å, Magnusson JM, Sköld CM. Baseline characteristics and survival of patients of idiopathic pulmonary fibrosis: a longitudinal analysis of the Swedish IPF Registry. Respir Res. 2021;22:40.
Vancheri C, Kreuter M, Richeldi L, Ryerson CJ, Valeyre D, Grutters JC, Wiebe S, Stansen W, Quaresma M, Stowasser S, Wuyts WA. Nintedanib with add-on pirfenidone in idiopathic pulmonary fibrosis. Results of the INJOURNEY Trial. Am J Respir Crit Care Med. 2018;197:356–63.
Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, Richeldi L, Kolb M, Tetzlaff K, Stowasser S, Coeck C, Clerisme-Beaty E, Rosenstock B, Quaresma M, Haeufel T, Goeldner RG, Schlenker-Herceg R, Brown KK. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381:1718–27.
Ikeda S, Sekine A, Baba T, Katano T, Tabata E, Shintani R, Sadoyama S, Yamakawa H, Oda T, Okuda R. Negative impact of anorexia and weight loss during prior pirfenidone administration on subsequent nintedanib treatment in patients with idiopathic pulmonary fibrosis. BMC Pulm Med. 2019;19:1–11.
Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, Behr J, Cottin V, Danoff SK, Morell F, Flaherty KR, Wells A, Martinez FJ, Azuma A, Bice TJ, Bouros D, Brown KK, Collard HR, Duggal A, Galvin L, Inoue Y, Jenkins RG, Johkoh T, Kazerooni EA, Kitaichi M, Knight SL, Mansour G, Nicholson AG, Pipavath SNJ, Buendía-Roldán I, Selman M, Travis WD, Walsh S, Wilson KC. Diagnosis of idiopathic pulmonary fibrosis. An Official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e44–68.
American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. 2000;161:646–64.
American Thoracic S, European Respiratory S. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002; 165:277–304.
Dolivo DM, Larson SA, Dominko T. Tryptophan metabolites kynurenine and serotonin regulate fibroblast activation and fibrosis. Cell Mol Life Sci. 2018;75:3663–81.
Kennedy JI, Chandler DB, Fulmer JD, Wert MB, Grizzle WE. Dietary fish oil inhibits bleomycin-induced pulmonary fibrosis in the rat. Exp Lung Res. 1989;15:315–29.
Acknowledgements
Not applicable.
Funding
University of Rochester, Department of Medicine. The funding body had no impact on the design of the study and data collection, data analysis, or interpretation of data and in the manuscript.
Author information
Authors and Affiliations
Contributions
AK—conceptualization, data curation, analysis, writing, investigation, methodology; ARR—conceptualization, editing; DN—conceptualization, editing and review; DC and HMM—statistical analysis, editing and review; RMK—funding acquisition, conceptualization, data curation, analysis, writing investigation, editing and review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the University of Rochester institutional review board (#00000503).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kalininskiy, A., Rackow, A.R., Nagel, D. et al. Association between weight loss and mortality in idiopathic pulmonary fibrosis. Respir Res 23, 377 (2022). https://doi.org/10.1186/s12931-022-02277-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-022-02277-2