Abstract
Background and objective
The definition of pre-capillary pulmonary hypertension (PH) has been modified, with lowering of the mean pulmonary arterial pressure (mPAP) threshold from 25 to 20 mmHg and addition of a mandatory criterion of pulmonary vascular resistance (PVR) ≥ 2 Wood units (WU). Our objectives were: 1/ to estimate the proportion of patients reclassified as having pre-capillary PH when using the new 2022 ESC/ERS hemodynamic criteria (i.e. mPAP 21-24 mmHg and PVR ≥ 2 WU), and to describe their clinical characteristics and outcome; and 2/ to study the relationship between PVR and survival in patients with mPAP > 20 mmHg.
Methods
We retrospectively analyzed consecutive SSc patients included in our National Reference Center for a first right-heart catheterization between 2003 and 2018. The association between survival and PVR was studied using smoothing splines.
Results
We included 126 SSc patients with mPAP > 20 mmHg. Among them, 16 (13%) had a baseline mPAP value between 21 and 24 mmHg and PVR ≥ 2 mmHg and were reclassified as pre-capillary PH; 10 of which (62%) raised their mPAP ≥ 25 mmHg during follow-up. In patients with mPAP > 20 mmHg, we observed a linear relation between PVR and mortality for values < 6 WU.
Conclusion
A significant proportion of SSc patients is reclassified as having pre-capillary PH with the new 2022 ESC/ERS hemodynamic definition. Lowering the PVR threshold from 3 to 2 WU captures patients at risk of raising their mPAP > 25 mmHg, with a possibly less severe disease.
Similar content being viewed by others
Introduction
Pulmonary hypertension (PH) is one of the most severe complications of systemic sclerosis (SSc), affecting more than 10% of patients during the course of the disease [1]. PH in SSc may result from several mechanisms: group 1 due to pulmonary microangiopathy (i.e. pulmonary arterial hypertension (PAH)), group 1’ due to pulmonary veno-occlusive disease (PVOD), group 2 due to myocardial fibrosis with left heart dysfunction, and/or group 3 due to severe interstitial lung disease (ILD). Its prognosis remains poor despite therapeutic progresses [2, 3].
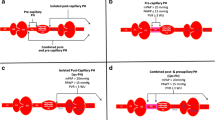
The diagnosis of PH is based on right-heart catheterization (RHC). A new hemodynamic definition of pre-capillary PH was proposed in the 2022 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines [4]. The mean pulmonary arterial pressure (mPAP) threshold was lowered from 25 to 20 mmHg in combination with pulmonary arterial wedge pressure (PAWP) ≤ 15 mmHg; and a mandatory criterion of pulmonary vascular resistance (PVR) ≥ 2 Wood units (WU) was added.
This modified definition of pre-capillary PH is debated in the field of SSc because of several unresolved issues. First, the impact of these revised criteria on the number of patients reclassified as PH is has not been extensively studied. Indeed, previous works [5,6,7] focusing on this issue have used the provisional hemodynamic definition from the 6th World Symposium on PH (WSPH) [8] that set the PVR threshold at 3 WU. Only one study used the current PVR cut-off of 2 WU and suggested a proportion of 9.85% PH-reclassified patients [6]. Second, the optimal PVR cut-off associated with increased mortality is controversial [6].
In order to add data and better decipher the impact of the new definition in SSc, we performed a retrospective monocentric study on our cohort of SSc patients with mPAP > 20 mmHg on their first-ever RHC. Our objectives were: 1/ to estimate the proportion of patients reclassified as having pre-capillary PH when using the new hemodynamic definition (i.e. mPAP between 21 and 24 mmHg and PVR ≥ 2 WU), and to describe their clinical characteristics and outcome; and 2/ to study the relationship between PVR and survival in patients with mPAP > 20 mmHg.
Methods
Study population
Consecutive patients were recruited from our National Reference Center for SSc. They were included in the study if they met the following criteria: 1/ a definite diagnosis of SSc (diffuse cutaneous SSc (dc-SSc) or limited cutaneous SSc (lc-SSc)) according to the 2013 American College of Rheumatology/European League against Rheumatism classification criteria [9] 2/ a first-ever RHC performed between 01/01/2003 and 31/12/2018; 3/ an mPAP value > 20 mmHg; 4/ an age ≥ 18 years old.
Study measurements
Data were retrospectively collected at the baseline visit (which was defined as the visit of the first RHC) and comprised clinical characteristics (including SSc subtype, New York Heart Association (NYHA) functional class, presence of interstitial lung disease (ILD) and 6-min walking distance (6MWD)), echocardiography parameters (tricuspid regurgitation velocity (TRV), right ventricle (RV)/left ventricle (LV) ratio, right atrium (RA) area, pulmonary artery acceleration time (PAAT), tricuspid annular plane systolic excursion (TAPSE), inferior vena cava (IVC) diameter), E/A ratio, left atrium (LA) area and LV ejection fraction (LVEF)), biological results (including brain natriuretic peptide (BNP) levels), electrocardiogram (ECG), pulmonary function tests (PFT) results, RHC parameters and initial treatment strategy. Data were also collected at last follow-up and included last treatment strategy and dead-or-alive status.
RHC were performed by pulmonary hypertension specialist practitioners using a 6-French Swan-Ganz catheter in non-acute clinical conditions. The catheter was placed under fluoroscopic guidance from a right antebrachial vein or a right femoral vein as needed. The correct position of the catheter was confirmed by fluoroscopy and by the presence of characteristic pressure waveforms. The “zero-ing” transducer to atmospheric pressure was at the level of the mid-axillary line. Right atrial pressure (RAP), mPAP and PAWP were determined electronically by the mean of several beats during quiet breathing. mPAP was determined by the mean of the area under pressure curves. Cardiac Output (CO) was measured by the thermodilution technique (mean of 3–6 measures with exclusion of measurements that exceed 10% of the mean); and Cardiac Index (CI) calculated by the ratio of CO to body surface area. Mixed venous oxygen saturation (SvO2) was measured from blood drawn from the pulmonary artery. PVR were then calculated (PVR = [mPAP-PAWP]/CO). In case of mPAP > 20 mmHg and a PAWP more than 12 mmHg (13–15 mmHg), suggesting a possible post-capillary pulmonary hypertension, a fluid challenge (500 mL of saline solution over 5 min) was performed as proposed in guidelines [10].
The initial (i.e. within the first 4 months of baseline visit) and follow-up treatment strategies were defined according to the number of PAH medications prescribed (endothelin receptor antagonist, phosphodiesterase type 5 inhibitor, and prostacyclin analogue). Patients whose death was not registered were censored at the time of last clinical contact.
Statistical analysis
The characteristics of the population were described by using the mean ± standard deviation (SD), or median ± interquartile range (IQR) in case of non-normality, for quantitative variables; numbers (percentage) were used for qualitative variables. PVR were categorized in four groups: < 2, 2–3, 3–6 and > 6 WU. No imputation was performed for missing data. Characteristics of patients between these groups were compared by using a one-way analysis of variance (ANOVA), or a Kruskal–Wallis test in cases of non-normality for quantitative variables; Fisher exact test was used for qualitative variables, except for the comparison of treatments (Chi-squared test with estimated p-values by Monte Carlo simulation).
To discuss the impact of the new PH definition on our patients, we described the clinical characteristics and outcome of patients reclassified as having pre-capillary PH (i.e. patients with mPAP between 21 and 24 mmHg, PVR ≥ 2 WU and PAWP ≤ 15 mmHg) according to the new hemodynamic definition from the 2022 ESC/ERS guidelines.
Survival was right-censored at 5 years and described by using the Kaplan–Meier method, for the whole population and by PVR groups. The relationship between survival and PVR and was assessed by a smoothing spline using a penalized spline basis for predictor, without and with adjustments for age, gender, SSc subtype, date of SSc diagnosis, NYHA class, ILD and follow-up treatment strategy. We also studied the association between survival and PVR groups using Cox proportional hazards models without and with adjustments.
All statistical analyses were performed by using R software, version 3.6.2 (R Foundation for Statistical Computing), using “Hmisc” and “survival” packages. The threshold for statistical significance was set to p < 0.05.
Results
Characteristics of the whole population according to PVR values
Overall, 126 SSc patients had an mPAP > 20 mmHg on their first-ever RHC and were included in the study (Fig. 1). Patients were classified in 4 groups according to their PVR values: 12, 18, 50 and 46 patients had PVR < 2 WU, between 2–3 WU, between 3 and 6 WU, and > 6 WU, respectively (Table 1).
We did not observe statistical differences in age, gender, antibody profile and disease duration between groups. In terms of PH characteristics, we did not observe significant differences in ILD occurrence and FVC values, indicating similar proportions of group 3 PH between classes; however, PAWP and LVEF differed significantly according to PVR values, consistent with a higher frequency of post-capillary PH (either isolated or combined with pre-capillary PH) in patients with lower PVR (6, 6, 6 and 4 patients with PAWP > 15 mmHg between in the PVR < 2 WU, PVR [2, 3] WU, PVR [3,4,5,6] and PVR > 6 WU groups, respectively). Aside from BNP levels, usual markers of PH severity (NYHA class, 6MWD, DLCO, KCO, TRV, RV/LV ratio, PAAT, RA area) were significantly different among groups, with milder anomalies observed with lower PVR. Therapeutic strategies varied with PVR classes, with patients having higher values being more likely to be treated. Only 3 patients were treated by PAH-specific drugs before PAH diagnosis (bosentan prescribed because of digital ulcers in all cases), all of them from the PVR [3,4,5,6] group. Triple therapy was started a median of 2 years [1,2,3] after PH diagnosis.
Proportion of pre-capillary PH-reclassified SSc patients and population characteristics
Among the 126 included patients, we identified 16 patients (13%) that had a baseline mPAP value between 21 and 24 mmHg, PVR ≥ 2 WU and PAWP ≤ 15 mmHg, and who were thus reclassified as having pre-capillary PH according to the 2022 ESC/ERS hemodynamic definition (Table 2).
Most of them were women (14/16, 88%) with a median age of 68 (54–78) years old, a limited cutaneous SSc (13/15, 87%) diagnosed a median of 6.5 (1–6) years before and associated with anti-centromere antibodies (11/15, 73%).
Most patients had CI > 2 L/min/m2 (15/16, 94%), RAP < 8 mmHg (14/15, 93%) and SvO2 > 65% (9/13, 69%). As per definition, PAWP was normal in all cases on resting RHC; but patients #9 and #10 elevated their value above 15 mmHg after fluid challenge, suggesting a possible post-capillary component associated. Two out of 15 patients (13%) had extensive ILD based on chest CT-scan involvement, but all of them had FVC values > 70%. Four out of 9 patients (44%) had signs of PVOD, and none showed evidence of chronic thrombo-embolism on chest CT or VQ-scan (Table 2).
PH screening tests and severity markers were inconsistently informative in these patients: 6/13 (46%) had NYHA class III or IV; 12/13 (92%) had 6MWD < 440 m; 3/13 (23%) had abnormal BNP levels; none had right axis deviation on ECG; 10/14 (71%) had DLCO < 60% and FVC (%)/DLCO(%) > 1.6. Similarly, echocardiographic markers of PH were inconstantly present: 11/15 patients (73%) had TRV > 2.8 m/s; 11/15 (73%) had PAAT < 105 ms; 4/16 (25%) had RA area > 18cm2; 3/15 (20%) had RV/LV ratio > 1.0; and 1/16 (6%) had IVC diameter > 21 mm with decreased inspiratory collapse. All patients had a normal RV function (as estimated by TAPSE and RV S’ wave) (Table 2).
Interestingly, 10/16 patients (62%) eventually had an increase of mPAP value above or equal to 25 mmHg on a follow-up RHC performed a median of 34 (20–58) months after their baseline evaluation, thus fulfilling the previous definition of precapillary PH; and 3/16 (19%) died shortly after their initial RHC. The 3 remaining patients (19%) had no PH after a 2-year follow-up (Table 2).
Survival analysis according to PVR values
Using a lower PVR threshold could increase the proportion of pre-capillary PH-reclassified patients but might not capture additional patients with increased mortality. To test the relevance of this new PVR criterion, we studied the relationship between PVR and survival in SSc patients with mPAP > 20 mmHg.
The 1-, 3- and 5-year survival rate [95% confidence interval] of the whole population was respectively 80.1% [73.4–87.4%], 63.9% [56–72.9%] and 41.1% [32.8–51.6%] (Fig. 2). In univariate analysis, spline analysis suggests a linear relation between mortality and PVR considering PVR between 0 and 6 WU, and a plateau of mortality for PVR values over 6 WU (Fig. 3). After adjusting on age, sex, SSc subtype, disease duration, presence of ILD, NYHA functional class and treatment, the relation appears similar to the univariate analysis (Fig. 4).
Spline modelling the relationship between survival and PVR values. Spline modelling the relationship between survival and PVR values. The solid line represents the spline prediction (values > 0 indicates an increased risk of mortality, values < 0 a decreased risk of mortality). The dotted curves represent the 95% confidence interval of the spline prediction. The dotted vertical lines represent the 2-WU, 3-WU and 6-WU cut-offs for PVR (from left to right)
Spline modelling the relationship between survival and PVR values with adjustments for age, gender, SSc subtype, presence of ILD, NYHA functional class and treatment. Spline modelling the relationship between survival and PVR values. The solid line represents the spline prediction (values > 0 indicates an increased risk of mortality, values < 0 a decreased risk of mortality). The dotted curves represent the 95% confidence interval of the spline prediction. The dotted vertical lines represent the 2-WU, 3-WU and 6-WU cut-offs for PVR (from left to right)
Regarding PVR groups, in univariate analysis, the hazard ratio (HR) of death of patients considering their PVR values (reference: PVR < 2WU) were 1.08 [0.2–3.9] for PVR between 2-3WU, 2.9 [0.9–9.4] for PVR between 3-6WU, and 3.7 [1.1–12] for PVR > 6WU (Fig. 5). In multivariate analysis (Additional file 1: Table S1), after adjusting for age, sex, SSc type, disease duration, presence of ILD, NYHA functional class and treatment, HR of death were respectively 0.49 [0.04–5.56], 2.56 [0.5–11.9] and 5.1 [0.97–27] for PVR between 2–3 WU, 3–6 WU and > 6 WU. Presence of ILD (2.36 [1.22–4.6], p = 0.02) and triple therapy (0.32 [0.12–0.87], p = 0.03) were independently associated with survival in our population. After excluding patients treated by triple therapy to limit survival bias, results were similar (data not shown).
Discussion
The results of our study can be summarized as follows: 1/ using the new 2022 ESC/ERS definition of PH, 13% of patients were reclassified as having precapillary PH; 2/ at least 62% of them experienced an increase of mPAP values above 25 mmHg during follow-up and eventually met the previous definition of PH; 3/ our spline analysis suggests that mortality increases linearly with PVR values in patients with mPAP > 20 mmHg and PVR < 6 WU.
In our cohort of SSc patients with mPAP > 20 mmHg, 16 (13%) were reclassified as having pre-capillary PH when applying the new criteria. Interestingly, in their work performed in 2 PH centres, Xanthouli et al. identified 28 SSc patients with mPAP 21–24 mmHg and PVR ≥ 2 WU, setting the proportion of reclassified patients at 9.85% when considering their total cohort and 20% when considering only patients with mPAP > 20 mmHg. Other studies found lower proportions of reclassification, ranging from 1.4 to 10.8% [5,6,7], but since they used the provisional 6th WSPH definition with a PVR cut-off at 3 WU, further comparison with our work is challenging. Coghlan et al. analyzed a 3-year follow up of patients with SSc and mPAP < 25 mmHg: 18/71 patients (25%) developed PH during follow-up including 5 with PAH, with an annual incidence of 6.1% which is higher than incidence described in previous cohorts [11]. Considering all PH causes, association between borderline PH and mortality has already been described (with a mPAP threshold of 19 mmHg) [12]. The treatment of patients with mPAP between 21 et 24 mmHg is also still on evaluation. A recent placebo-controlled trial of ambrisentan in SSc PAH patients with mPAP 21–24 failed to show a statistical difference on the primary endpoint but showed interesting results on secondary endpoints (CI and PVR) [13].
There is an ongoing discussion as to whether these new classification criteria capture more patients with decreased survival. When using PVR as a continuous variable, our spline analysis suggested a linear relationship between PVR and survival for PVR values between 0 and 6 WU, implying that patients with PVR > 2 WU may have increased mortality compared to those with lower PVR values. However, when PVR is used as a categorical variable, we did not observe significant differences in survival between PVR 2–3 and PVR 0–2 groups. This discrepancy could be explained by a modest effect size of these low PVR on mortality and by limitations due to our small sample size. In their study performed on 2 large veteran cohorts, Maron et al. [14] suggested that the optimal PVR cut-off identifying increased mortality is set at 2.2 WU. Interestingly, when categorizing PVR into 3 groups (with cut-offs at 2.2 and 3.0 WU), the hazard ratio for mortality (reference: PVR 2.2–3.0) was 0.89 (0.80–1.00) (p = 0.048) in the PVR < 2.2 group, suggesting an only mildly decreased survival for patients with PVR 2.2–3.0. Conversely, Yamamoto et al. [15] did not observe significant differences in the survival of various types of PH patients when comparing PVR < 2 WU and PVR 2–3 WU groups, although their analyses are probably limited by small sample sizes as well. Taken together, these data could suggest an increased mortality at the PVR 2WU cut-off, although it probably remains relatively modest within the 2–3 WU range.
Associations between PVR and mortality have also previously been reported in patients with idiopathic and SSc-associated PH, although with PH defined as mPAP > 25 mmHg [6, 16,17,18], with a minimum PVR threshold of 4.6 WU. PVR appears to be lower compared to patients with idiopathic PAH, but without correlation with mortality although prognosis of idiopathic PAH is better than in SSc [19, 20] In our cohort, we observed a linear relation between PVR and survival for PVR values < 6 WU, although the increase in mortality seems modest between patients with PVR < 2 WU and PVR between 2–3 WU. When patients with normal or only mildly elevated PVR were excluded, we did not observe a difference in relationship between PVR and CI in patients with SSc-PAH suggesting that increased stiffness of the pulmonary arterial bed is unlikely to be responsible for differences in outcomes. The patients with PVR between 2 and 3 also appear to be at risk of progression to PH [21], which seems to be also observed in our cohort.
Our study has several limitations. RHC was only performed in patients with a suspicion of PH, leading to selection bias with patients with milder hemodynamic alterations which may have been underdiagnosed; and RHC indications have changed during the inclusion period. Our study is also limited by its retrospective single-center design and its small sample sizes, although it includes a rather large cohort of SSc patients with hemodynamic evaluation. Our population may be heterogeneous as it mixed patients with different mechanisms of PH (groups 1 and/or 2 and/or 3) and thus possibly different prognosis; however, this remains relevant as all patients with mPAP ≥ 20 mmHg and PVR ≥ 2 WU are considered to have at least pre-capillary PH (with or without an associated post-capillary PH, depending on their PAWP value) and some degree of pulmonary microangiopathy. Moreover, in SSc patients with ILD and PH, it is often difficult to determine whether PH is exclusively due to the chronic lung disease (group 3 only) or also caused by an underlying microangiopathy (group 1 + 3). Finally, multivariate analysis must be taken with caution as the adjustment is limited by the small effectives and by missing data for several predetermined variables.
Conclusion
A significant proportion of SSc patients is reclassified as having pre-capillary PH with the new 2022 ESC/ERS hemodynamic definition. Lowering the PVR threshold from 3 to 2 WU captures patients at risk of raising their mPAP > 25 mmHg, with a possibly less severe disease. Prospective studies on larger cohorts are warranted to draw firm conclusions on the impact of this new definition.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request from any qualified researcher.
References
Sobanski V, Launay D, Hachulla E, Humbert M. Current approaches to the treatment of systemic-sclerosis-associated pulmonary arterial hypertension (SSc-PAH). Curr Rheumatol Rep. 2016;18(2):10.
Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67–119.
Tyndall AJ, Bannert B, Vonk M, Airò P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69(10):1809–15.
Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. European Heart J. 2022;89:7.
Jaafar S, Visovatti S, Young A, Huang S, Cronin P, Vummidi D, et al. Impact of the revised haemodynamic definition on the diagnosis of pulmonary hypertension in patients with systemic sclerosis. Eur Respir J. 2019;54(2):1900586.
Xanthouli P, Jordan S, Milde N, Marra A, Blank N, Egenlauf B, et al. Haemodynamic phenotypes and survival in patients with systemic sclerosis: the impact of the new definition of pulmonary arterial hypertension. Ann Rheum Dis. 2020;79(3):370–8.
Sarı A, Şener YZ, Armağan B, Kılıç L, Kaya EB, Hekimsoy V, et al. How did the updated hemodynamic definitions affect the frequency of pulmonary hypertension in patients with systemic sclerosis. Anatol J Cardiol. 2021;25(1):30–5.
Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1.
van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. Classification Criteria for Systemic Sclerosis: An ACR-EULAR Collaborative Initiative. Arthritis Rheum. 2013;65(11):2737–47.
Vachiéry J, Tedford RJ, Rosenkranz S, Palazzini M, Lang I, Guazzi M, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53(1):1801897.
Coghlan JG, Wolf M, Distler O, Denton CP, Doelberg M, Harutyunova S, et al. Incidence of pulmonary hypertension and determining factors in patients with systemic sclerosis. Eur Respir J. 2018;51(4):1701197.
Maron BA, Hess E, Maddox TM, Opotowsky AR, Tedford RJ, Lahm T, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation. 2016;133(13):1240–8.
Pan Z, Marra AM, Benjamin N, et al. Early treatment with ambrisentan of mildly elevated mean pulmonary arterial pressure associated with systemic sclerosis: a randomized, controlled, double-blind, parallel group study (EDITA study). Arthritis Res Ther. 2019;21:217.
Maron BA, Brittain EL, Hess E, Waldo SW, Barón AE, Huang S, et al. Pulmonary vascular resistance and clinical outcomes in patients with pulmonary hypertension: a retrospective cohort study. Lancet. 2022;67:34.
Yamamoto K, Tanabe N, Takahashi Y, Naito A, Sekine A, Suda R, et al. Characteristics of patients meeting the new definition of pre-capillary pulmonary hypertension (Nice 2018) in a single Japanese pulmonary hypertension center. BMC Pulm Med. 2021;21:1.
Corte TJ, Wort SJ, Gatzoulis MA, Macdonald P, Hansell DM, Wells AU. Pulmonary vascular resistance predicts early mortality in patients with diffuse fibrotic lung disease and suspected pulmonary hypertension. Thorax. 2009;64(10):883–8.
Chung L, Domsic RT, Lingala B, Alkassab F, Bolster M, Csuka ME, et al. Survival and predictors of mortality in systemic sclerosis-associated pulmonary arterial hypertension: outcomes from the pulmonary hypertension assessment and recognition of outcomes in scleroderma registry. Arthritis Care Res (Hoboken). 2014;66(3):489–95.
Lefèvre G, Dauchet L, Hachulla E, Montani D, Sobanski V, Lambert M, et al. Survival and prognostic factors in systemic sclerosis-associated pulmonary hypertension: a systematic review and meta-analysis. Arthritis Rheum. 2013;65(9):2412–23.
Fisher MR, Mathai SC, Champion HC, Girgis RE, Housten-Harris T, Hummers L, et al. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum. 2006;54(9):3043–50.
Ramjug S, Hussain N, Hurdman J, Billings C, Charalampopoulos A, Elliot CA, et al. Idiopathic and systemic sclerosis-associated pulmonary arterial hypertension: a comparison of demographic, hemodynamic, and mri characteristics and outcomes. Chest. 2017;152(1):92–102.
Ratwatte S, Anderson J, Strange G, Corrigan C, Collins N, Celermajer DS, et al. Pulmonary arterial hypertension with below threshold pulmonary vascular resistance. Eur Respir J. 2020;56(1):1901654.
Acknowledgements
The authors wish to thank Aissa Boughaba, Quentin Ducrocq, Lucie Delbarre and Céline Podevin for their help in elaborating the database.
Funding
No specific funding was obtained for this work.
Author information
Authors and Affiliations
Contributions
All individuals listed as authors met the ICMJE guidelines for determining authorship. SP, JG, EH and SS conceived and designed the study. SP and SS screened patients and collected clinical data. NL, PDG and MF extracted RHC and TTE data. JLE extracted PFT data. AL constituted and managed the database. JG performed statistical analyses and created the figures. SP wrote the first draft of the manuscript. SS made major revisions to the manuscript. JG provided his methodological expertise. NL, PDG and MF provided their cardiological expertise. JLE, JFB and MHB provided his pneumological expertise. VS, DL and EH provided their SSc and CTD-related PH expertise. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study complied with the recommendations of the Helsinki declaration. French legislation on noninterventional studies does not require ethics committee approval for the use of de-identified data collected during patient care. The data were de-identified and complied with the requirements of the “Commission Nationale de l’Informatique et des Libertés” (CNIL), the organization responsible for ensuring the ethical use of data collected for scientific purposes in France. The CNIL approved the methods used to collect and analyze data from our patient database (approval #DEC18-445).
French legislation on noninterventional studies requires collecting the non-opposition of patients but does not require written consent. As such, non-opposition was obtained from each patient included in the study for the use of their de-identified medical record data.
Consent for publication
Not applicable.
Competing interests
SS reports travel grants from Shire, Sanofi-Genzyme, SOBI and Novartis; consulting fees from Novartis. DL reports research support from Actelion, GSK, and Pfizer; Octapharma, Takeda, Biocryst, CSL Behring. NL reported personal fees from Actelion (Janssen), Akcea, Alnylam, Amicus therapeutics, Bayer, Novartis, MSD, Pfizer, Sanofi-Aventis, and travel grants from Amgen and Bristol-Myers Squibb. PDG reports fees from Novartis, Servier, Astra-Zeneca, Abbott, Vifor, Janssen, Boehringer Ingelheim, Bayer, MSD. JFB reports travel grants from Pfizer, MSD, Janssen, AstraZeneca, Novartis Pharma France, and Chiesi; and board participation fees from Novartis Pharma France. EH reports consulting fees from Actelion, Boehringer Ingelheim, GSK, Roche-Chugai, Sanofi-Genzyme; speaking fees from Actelion, GSK, Roche-Chugai; and research funding from CSL Behring, GSK, Roche-Chugai.
SP, JG, MF, AL, JLE, MHB and VS did not report any conflict of interest related to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Multivariate analysis after adjusting for age, sex, SSc type, disease duration, presence of ILD, NYHA functional class and treatment.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Puigrenier, S., Giovannelli, J., Lamblin, N. et al. Mild pulmonary hemodynamic alterations in patients with systemic sclerosis: relevance of the new 2022 ESC/ERS definition of pulmonary hypertension and impact on mortality. Respir Res 23, 284 (2022). https://doi.org/10.1186/s12931-022-02205-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-022-02205-4