Abstract
Background
The benefit of prompt vs delayed treatment initiation with inhaled long-acting bronchodilators in reducing exacerbations in chronic obstructive pulmonary disease (COPD) is unclear. This study aimed to investigate if long-acting bronchodilator therapy initiation within 30 days of COPD diagnosis reduces exacerbation risk in patients with COPD.
Methods
This was a retrospective cohort study of patients with COPD based on claims and electronic medical records data extracted from the Real World Data database. The index date (day 0) was the date of the first confirmed inpatient or outpatient COPD diagnosis between January 1, 2005, and December 31, 2018. Patients with COPD without an asthma diagnosis and aged ≥ 40 years at the index date were included. Patients who initiated inhaled long-acting bronchodilator therapy within the first 30 days (day 0 to day 29) were categorized into the “prompt therapy” group and the rest into the “delayed therapy” group. Time from day 30 post-diagnosis to the first exacerbation and annual exacerbation rate (AER) were evaluated for the overall population and those stratified by COPD phenotype, including chronic bronchitis (CB) and emphysema.
Results
Compared with the delayed therapy group (n = 1516), time to first exacerbation was prolonged (hazard ratio 0.78; 95% confidence interval [CI] [0.70, 0.87]) and annual rates of moderate or severe exacerbations were lower (rate ratio 0.74; 95% CI [0.65, 0.84]) in the prompt therapy group (n = 1466). Similarly, time to first exacerbation was prolonged and AERs were lower in the prompt therapy group in the subgroups of patients with CB or emphysema.
Conclusions
This is the first study to demonstrate a prolonged time to first exacerbation upon initiation of long-acting bronchodilators within 30 days of COPD diagnosis. A beneficial effect was also observed in patients with CB and emphysema. Our data support advising patients to initiate long-acting bronchodilators soon after COPD diagnosis.
Similar content being viewed by others
Background
Chronic obstructive pulmonary disease (COPD) exacerbations are associated with an accelerated decline of lung function [1, 2], resulting in poor quality of life and poor prognosis [1, 3, 4]. An increase in the financial burden of COPD on healthcare systems has also been attributed to exacerbations [5]. Exacerbations are known to become more frequent as COPD progresses [6, 7], with the most important predictor of frequent exacerbations being a history of prior exacerbations [8].
COPD is classified into chronic bronchitis (CB) and emphysema [9]. An increase in bronchial wall thickness has been shown to be associated with an increase in the annual exacerbation rate (AER), suggesting a correlation between the CB phenotype and exacerbations [10]. In the Japanese population with COPD, the emphysema phenotype is more prevalent than the CB phenotype [11] and is similarly associated with exacerbations and poor prognosis [12]. Despite a large number of patients suspected of having COPD in Japan, treatment remains suboptimal. According to a previous survey of patients with suspected COPD with CB or emphysema aged > 45 years with a smoking history in Japan, 52% of participants were not diagnosed with COPD and < 16% of those diagnosed received an inhaled bronchodilator, which is the recommended first-line treatment according to the COPD guidelines of the Japanese Respiratory Society [13].
Data from placebo-controlled trials suggest that initiation of maintenance therapy with long-acting bronchodilators in the early stages of the disease, or in younger patients, may substantially improve COPD-related parameters, such as lung function, health-related quality of life, and exacerbation, and retard disease progression [14,15,16,17,18]. However, the association between early initiation of long-acting bronchodilator therapy after COPD diagnosis and COPD outcomes compared with delayed initiation remains to be elucidated. Therefore, understanding the clinical importance of early initiation of long-acting bronchodilator therapy is essential to promote prompt therapy initiation for patients with COPD. This study aimed to explore the impact of treatment initiation with long-acting bronchodilator therapy within 30 days of COPD diagnosis on exacerbation risk in patients with COPD.
Methods
Study design and study population
This was a retrospective cohort study of patients with COPD based on data extracted from a medical record–based database (Real World Data [RWD] database, Real World Data, Co., Ltd., Kyoto, Japan). The study period was from January 1, 2004, to December 31, 2019, and the cohort enrollment period was from January 1, 2005, to December 31, 2018, which was 1 year before the end of data availability, to allow accrual of sufficient follow-up for the study outcomes to be assessed. The index date (day 0) was the date of the first inpatient or outpatient COPD diagnosis (excluding suspected COPD) between January 1, 2005, and December 31, 2018, in the electronic medical record (EMR; Fig. 1).
The study protocol was approved by the NPO-MINS Institutional Review Board (Approval No. MINS-REC-210216) in 2021. The study was conducted in accordance with the Ethical Guidelines for Biomedical Research Involving Human Subjects, the ethical principles of the Declaration of Helsinki, and all relevant regulations applicable to noninterventional studies. The requirement for informed consent was waived because the available data in the RWD database were standardized and anonymized.
The study population consisted of incident patients with COPD without a prior diagnosis of COPD identified during the study period. The full analysis set included all incident patients with COPD at the index date, excluding those with an exacerbation during the grouping periods (periods of treatment initiation). The inclusion criteria were as follows: a first-time COPD diagnosis in the inpatient or outpatient setting in the cohort enrollment period, identified by a relevant diagnosis code (International Statistical Classification of Diseases and Related Health Problems, 10th Revision [ICD-10] codes J42, J43, and J44; any position; Additional file 1: Table S1) in the EMR; availability for follow-up 365 days before and after the index date; age ≥ 40 years at the index date; and at least one dispensation of a long-acting muscarinic antagonist (LAMA; R03K2), long-acting β2-agonist (LABA; R03A3), short-acting β2-agonist (R03A4), short-acting muscarinic antagonist (R03K1), LAMA + LABA (R03L2), inhaled corticosteroid (ICS) + LAMA, ICS + LABA (R03F1), or ICS + LAMA + LABA (R03L3) after the index date (Anatomical Therapeutic Chemical Classification codes listed in Additional file 1: Table S2). The exclusion criteria included a diagnosis of asthma in the inpatient or outpatient setting (ICD-10 codes J45 and J46; any position) during the study period or reversal of COPD diagnosis within 3 months.
Incident patients with COPD meeting the eligibility criteria were categorized into the “prompt therapy” group (treatment initiation with long-acting bronchodilators [LAMA, LABA, LAMA + LABA, ICS + LABA, or ICS + LAMA + LABA] within the 30-day post-diagnosis period [day 0 to day 29]) or the “delayed therapy” group (no treatment initiation with long-acting bronchodilators within the first 30 days).
Outcomes
The primary outcome was the occurrence of an exacerbation after the grouping period. The time from the day following the grouping period to the first exacerbation and the AER of incident COPD were compared between the prompt and delayed therapy groups. Exacerbations were classified as moderate or severe. A moderate exacerbation was defined as an outpatient dispensation claim for a systemic corticosteroid (H02) at the time of COPD diagnosis in the EMR or an outpatient dispensation claim for an antibiotic (J01) commonly used to treat exacerbations at the time of upper or lower respiratory tract infection diagnosis (J00-J22) in the EMR. A severe exacerbation was defined as an inpatient admission record with a diagnosis code (any position) for a COPD exacerbation (J10, J11, J13, J14, J15, J16, J18, J20, J21, J22, J440, J441, J680, J851, C1, A481, B012, B052, or B250) and a dispensation claim for a systemic corticosteroid on the same day or within 7 days of the admission date in the EMR; a receipt of hospitalization from the Diagnosis Procedure Combination system with a diagnosis code (both primary and other) for COPD (J42, J43, or J44) as the causative disease and/or main disease for hospitalization; or an inpatient admission record with a diagnosis code (primary position) for acute respiratory failure (J80, J96, or R09.2) concomitant with a dispensation claim for a systemic corticosteroid on the same day or within 7 days of the admission date but no diagnosis with heart failure (I50), pneumothorax (J93), or myocardial infarction (I21 or I22) recorded during the same admission in the EMR. For moderate exacerbations, the date of medication dispensation was defined as the exacerbation date. For severe exacerbations, the date of admission was defined as the exacerbation date. If more than two exacerbations of any severity were identified within a 14-day period, the events were counted as the same event and the exacerbation date was recorded as the date of the first exacerbation. If the severity of these exacerbations differed, the exacerbation was classified as severe and the exacerbation date was recorded as the date of the earliest of all the events that occurred within the same 14-day window.
Statistical analysis
Patient demographics and baseline characteristics are described using mean, standard deviation, and median for continuous variables, and number and percentage for categorical variables. The proportion of missing data is reported for each variable measured in the study.
Kaplan–Meier plots for the time to first exacerbation among incident patients with COPD are presented by treatment initiation groups (prompt therapy group and delayed therapy group). The proportion of events and censoring as well as the median time to first exacerbation were based on the Kaplan–Meier method, and hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) between groups were calculated using a Cox proportional hazards model including the treatment initiation groups. The AER was analyzed based on a negative binomial model including the treatment initiation group with the logarithmic length of the follow-up period as an offset variable. The rate ratio (RR) and 95% CIs between groups were estimated based on the negative binomial model. Adjusted HRs and RRs and corresponding 95% CIs between groups were also estimated including the treatment initiation group and covariates in the pre-index period (age, sex, number of comorbidities, systemic corticosteroid use, antibiotic use, and number of hospitalizations excluding hospitalization due to COPD exacerbation or acute respiratory failure). Kaplan–Meier plots for the time to first exacerbation are presented by treatment initiation groups and by COPD phenotypes (CB [J42], emphysema [J43], and unclassified [J44]). CB or emphysema-type COPD was identified based on the corresponding diagnosis codes assigned from January 1, 2004, to December 31, 2018 (inclusive). The unclassified COPD phenotype included patients assigned the diagnosis code J44, including the first diagnosis from January 1, 2004, to December 31, 2018 (inclusive). The time to first exacerbation between the prompt therapy and delayed therapy groups was further compared in the following subgroups: (1) index date after November 1, 2013 (the time of LAMA + LABA therapy launch in Japan), and (2) index date after April 1, 2018 (the time when COPD guidelines began recommending LAMA + LABA use for COPD in Japan). Kaplan–Meier plots for the time to first exacerbation are also presented by subgroups restricted to patients with an incident pure COPD diagnosis and by subgroups stratified by post-diagnosis treatment initiation time points as follows: day 0 (i.e., the day of diagnosis or the index date) vs day 1 and beyond; from day 0 to day 59 (i.e., within 60 days of the index date) vs day 60 and beyond (i.e., 60 days after the index date); and from day 0 to day 89 (i.e., within 90 days of the index date) vs day 90 and beyond (i.e., 90 days after the index date).
Results
Patients characteristics
A total of 1,022,447 patients diagnosed with COPD between January 2004 and December 2019 were identified from the RWD database (Fig. 2). Of the patients who met the inclusion criteria (n = 29,501), those diagnosed with asthma in the inpatient or outpatient setting (n = 18,285) and those with a reversal of COPD diagnosis within 3 months (n = 18,938) were excluded. Thus, a total of 3628 patients were included for further analysis: prompt therapy group (1466 [40.4%]), delayed therapy group (1516 [41.8%]), and patients who experienced exacerbations during the grouping period (within the first 30 days of diagnosis; 646 [17.8%]).
Analysis population. All values are n or n (%). COPD chronic obstructive pulmonary disease, EMR electronic medical record, ICD-10 International Statistical Classification of Diseases and Related Health Problems, 10th Revision, ICS inhaled corticosteroid, LABA long-acting β2-agonist, LAMA long-acting muscarinic antagonist, RWD-DB Real World Data database, SABA short-acting β2-agonist, SAMA short-acting muscarinic antagonist
The mean age of patients in the prompt therapy group was 73.4 years, with 15.1% (n = 222) of patients aged 40–64 years and 84.9% (n = 1244) aged ≥ 65 years. The mean age of patients in the delayed therapy group was 72.6 years, with 17.5% (n = 265) of patients aged 40–64 years and 82.5% (n = 1251) aged ≥ 65 years. The proportion of men was higher in both groups (prompt 83.3% [n = 1221]; delayed 82.8% [n = 1256]). Differences in the distribution of baseline covariates are summarized using the standardized mean difference (SMD; Table 1). The SMD was > 0.1 for smoking status, lung function test history, systemic corticosteroid use, antibiotic prescriptions, phenotype, comorbidities, physician’s medical specialty, and facility size. The frequency of lung function tests was higher in the prompt therapy group (prompt 45.3%; delayed 12.7%). CB (prompt 12.6%; delayed 37.7%) and unclassified (code J44) (prompt 52.8%; delayed 25.3%) were more prevalent in the prompt therapy group. The Charlson Comorbidity Index (moderate) (prompt 3.4%; delayed 1.7%) and the rates of lung cancer, malignant neoplasm of bladder, and lower respiratory tract infection were higher in the prompt therapy group. The number of facilities with ≥ 500 beds was higher in the prompt therapy group (prompt 65.0%; delayed 44.6%).
Mean time to treatment initiation was 2.9 days (median 0.0 days) and 45.9 months (median 35.0 months) in the prompt and delayed therapy groups, respectively (Additional file 2: Fig. S1 and Additional file 3: Fig. S2). Approximately 70% of patients initiated inhaled therapy on the day of COPD diagnosis (day 0) in the prompt therapy group, whereas more than 20% of patients initiated inhaled therapy after 60 months in the delayed therapy group. LAMA monotherapy was the most common initial therapy in both groups (prompt 61.9%; delayed 38.2%), and LAMA + LABA combination use was similar between the groups (prompt 17.2%; delayed 17.9%; Additional file 4: Fig. S3).
In the delayed therapy group, approximately 50% of patients had received long-acting bronchodilators and/or short-acting bronchodilators before the first exacerbation (Additional file 1: Table S3).
Time to first exacerbation and AERs in the overall population
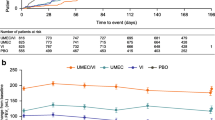
Time to first exacerbation was longer in the prompt therapy group than in the delayed therapy group (Fig. 3). The median time to first exacerbation was 96.1 months in the prompt therapy group vs 66.0 months in the delayed therapy group (unadjusted HR 0.78 [95% CI 0.70, 0.87]; adjusted HR 0.72 [95% CI 0.65, 0.81]; Table 2).
Unadjusted and adjusted RRs for the prompt therapy group compared with the delayed therapy group were 0.74 (95% CI [0.65, 0.84]) and 0.71 (95% CI [0.62, 0.80]), respectively (Table 3).
Time to first exacerbation and AERs by phenotype
In the CB and emphysema phenotype subgroups, similar to the results of the overall population, the time to first exacerbation was longer in the prompt therapy group than in the delayed therapy group (unadjusted HRs for CB and emphysema 0.72 [95% CI 0.56, 0.92] and 0.81 [95% CI 0.69, 0.96], respectively; Table 2). The adjusted HRs for the CB and emphysema phenotypes were consistent with the unadjusted HRs. Unadjusted and adjusted HRs for the prompt therapy group in the unclassified subgroup were 0.91 (95% CI [0.76, 1.09]) and 0.82 (95% CI [0.68, 0.99]), respectively (Table 2).
Unadjusted RRs for the annual estimated rates of moderate or severe exacerbations for CB, emphysema, and unclassified were 0.78 (95% CI [0.60, 1.02]), 0.69 (95% CI [0.57, 0.84]), and 0.92 (95% CI [0.73, 1.15]), respectively (Table 3). The annual rates of moderate or severe exacerbation were higher in the CB group than in the overall population (prompt 0.26; delayed 0.33).
Time to first exacerbation by time periods
Time to first exacerbation in patients with an index date after the launch of LAMA + LABA in Japan (after November 1, 2013) was increased in the prompt therapy group, with an unadjusted HR of 0.65 (95% CI [0.53, 0.80]) (Fig. 4). Moreover, in the subgroup with an index date after LAMA + LABA recommendation according to COPD guidelines in Japan, the unadjusted HR for the prompt therapy group compared with the delayed therapy group was 0.56 (95% CI [0.23, 1.39]) (Additional file 5: Fig. S4 and Table 4). Among patients with an index date after the launch of LAMA + LABA, the proportion of patients who initiated LAMA monotherapy (38.4%) and LAMA + LABA (34.3%) was similar in the prompt therapy group. In the delayed therapy group also, the proportion was similar between LAMA monotherapy (24.9%) and LAMA + LABA (27.5%), while the proportion of patients receiving no treatment was 30.1% (Additional file 6: Fig. S5).
Time to first exacerbation by grouping periods
As patients who initiated inhaled therapy within 30 days of diagnosis had a prolonged time to first exacerbation, we evaluated whether these results may have been driven by the selected time periods. Therefore, we expanded our analysis to include longer time windows. The results of the analysis with different grouping periods were similar to those seen with the 30-day grouping period. The time to first exacerbation was longer in the prompt therapy group than in the delayed therapy group (HR 0.79 [95% CI 0.71, 0.88] for the grouping period of 0 days, 0.77 [95% CI 0.69, 0.86] for the grouping period of 60 days, and 0.75 [95% CI 0.67, 0.84] for the grouping period of 90 days) (Additional file 1: Table S4).
Discussion
We assessed real-world evidence to elucidate the benefit of early initiation of inhaled long-acting bronchodilators. Patients with COPD who initiated long-acting bronchodilators within 30 days of diagnosis had a prolonged time to first exacerbation and decreased AER compared with those with delayed initiation of therapy. Prompt therapy similarly prolonged the time to first exacerbation in patients with CB and emphysema. Among patients stratified by the index date following the launch of LAMA + LABA therapy in Japan, the time to first exacerbation was prolonged in the prompt therapy group after diagnosis. Our findings indicate a beneficial effect of prompt therapy, and thus lend credence to the recommendation that patients initiate timely inhaled long-acting bronchodilator treatment upon COPD diagnosis.
The use of long-acting anticholinergic bronchodilator therapies in patients with mild and moderate COPD can reduce the risk and severity of exacerbations compared with placebo [17, 19]. Prompt diagnosis in patients with COPD is associated with a longer time to first exacerbation, as well as a decreased exacerbation rate [20, 21], and prompt initiation of triple therapy after two moderate exacerbations or one severe exacerbation is associated with decreased morbidity and economic burden [22]. The findings in the current study are consistent with these previous observations. As exacerbations can irreversibly reduce lung function [23] and accelerate disease progression [24], prompt initiation of bronchodilator therapy may further contribute to better disease outcomes by reducing exacerbations.
The baseline sociodemographic characteristics of the patients included in the present cohort were consistent with those in previous studies [25, 26], although the prevalence of each comorbidity was lower than that described previously in the Japanese population [27]. Since the current database only includes registered facilities, information regarding comorbidities may not be available for patients if they were diagnosed or received treatment at non-registered facilities. Although the SMD was > 0.1 for age, systemic corticosteroid use, antibiotic prescription, Charlson Comorbidity Index, lung cancer, and lower respiratory tract infection, the overall SMD suggests adequate matching between the prompt therapy group and the delayed therapy group. The prevalence of emphysema was similar between the current study and a previous study (36–44%) [28]. In the delayed therapy group, some patients initiated long-acting bronchodilators following an episode of the first exacerbation, suggesting that a lack of obvious clinical signs could delay treatment initiation in COPD. Lung function testing was more commonly performed in the prompt therapy group (45.3%) than in the delayed therapy group (12.7%). Moreover, COPD diagnosis was more commonly made by pulmonologists in the prompt therapy group (49.0%) than in the delayed therapy group (31.1%). Healthcare professionals rely on spirometry to confirm a diagnosis of COPD [29]; therefore, the timing of therapy initiation may be affected by the specialty of the treating physician. The rates of moderate or severe exacerbations among patients analyzed in our cohort were consistent with those described previously in the Japanese population [25, 26, 30]. The incidence of COPD exacerbations may be lower in Japan than in other countries because Japanese physicians often use asthma-COPD overlap as a disease label, resulting in an underreporting of the incidence of COPD exacerbations [30].
Initiation of inhaled bronchodilators within 30 days of diagnosis was associated with suppression of future exacerbations in CB. The relatively higher annual rates of moderate or severe exacerbations among patients with CB were consistent with the results of a previous study that described an association between bronchial wall thickness and increased AER [10]. The time to first exacerbation was also prolonged in the prompt therapy group in the emphysema subgroup. There was a tendency toward reduction in exacerbations, with an unadjusted HR of 0.91 and an unadjusted RR of 0.92, in the unclassified group. In line with a previous study suggesting an increased risk of exacerbations with CB or emphysema [10], our findings indicate the benefit of timely initiation of inhaled therapy for patients with COPD irrespective of disease phenotype.
The prolonged time to first exacerbation was more clearly observed approximately 36 months after diagnosis in the overall population. Unintentional exclusion of high-risk patients may have minimized the difference between the two groups up to 36 months as patients who experienced exacerbations within 30 days of the index date were excluded from the study. Interestingly, the time to first exacerbation was also prolonged in selected patients with the index date after the launch of LAMA + LABA therapy, with a clear prolongation of approximately 12 months after diagnosis. A greater proportion of patients in the prompt therapy group initiated LAMA + LABA therapy in the subgroup with an index date after the launch of LAMA + LABA (Additional file 6: Fig. S5). LAMA + LABA combinations are more effective than the individual components because of their distinct mechanisms [31,32,33]. Use of LAMA + LABA may have been responsible for the difference in the prolonged time to first exacerbation between the overall population and those with an index date after LAMA + LABA launch.
The definition of “prompt” was assessed by comparing the time to first exacerbation between the prompt therapy and delayed therapy groups stratified by different grouping periods (0 days, 60 days, and 90 days). The time to first exacerbation was prolonged in the prompt therapy group in all three subgroups. Similar results between those classified by different grouping periods further support the beneficial effect of early initiation of inhaled therapy following COPD diagnosis. There was a proportionally small difference in prompt therapy patients between the grouping periods of 0 days and 90 days; thus, further studies are required to determine the most appropriate timing of prompt treatment initiation.
Some limitations of this study should also be acknowledged. First, there are no clear guidelines for the optimal timing to initiate inhaled therapy for COPD following diagnosis. Although the current study defined “within 30 days of diagnosis (including the day of diagnosis)” as “prompt” based on expert opinion, evidence-based guidance on the right timing for patients with COPD to initiate therapy requires further evaluation. The time to first exacerbation was prolonged in the prompt therapy group, including the alternative grouping period subgroups, thereby confirming the robustness of the results of the 30-day grouping period. Second, in the current study, only facilities registered in the RWD database were analyzed. Thus, the AERs may have been underestimated if the enrolled patients had received treatment in non-registered facilities. Third, the algorithm for identifying moderate or severe exacerbations was not validated in this study and exacerbation was defined based on previous literature [34]; however, the AERs in this study were consistent with those reported previously. Fourth, the effect of disease severity was not considered in the analysis because data on forced expiratory volume in 1 s were not available in the RWD database. The frequency of exacerbations tends to be higher at more advanced stages of the disease, with recurrence of exacerbations observed even in milder COPD [35]. Finally, ICS use in combination with long-acting bronchodilators may provide a positive clinical effect [36]. The current study did not consider the role of ICS, and the impact of ICS on time to first exacerbation needs to be investigated further.
Conclusions
This is the first study to demonstrate an association between early initiation of bronchodilators and favorable clinical outcomes in patients with COPD. Initiation of bronchodilator therapy within 30 days of diagnosis positively affected the clinical outcomes in patients with CB or emphysema. Timing of initiation of inhaled bronchodilator therapy is a critical factor for future exacerbations; hence, patients should be advised to initiate bronchodilators in a timely manner.
Availability of data and materials
The analyses were conducted on medical records data provided under a commercial license, which the authors are unable to share.
Change history
07 November 2022
A Correction to this paper has been published: https://doi.org/10.1186/s12931-022-02219-y
Abbreviations
- AER:
-
Annual exacerbation rate
- CB:
-
Chronic bronchitis
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- EMR:
-
Electronic medical record
- HR:
-
Hazard ratio
- ICD-10:
-
International Statistical Classification of Diseases and Related Health Problems, 10th Revision
- ICS:
-
Inhaled corticosteroid
- LABA:
-
Long-acting β2-agonist
- LAMA:
-
Long-acting muscarinic antagonist
- RR:
-
Rate ratio
- RWD:
-
Real World Data
- SMD:
-
Standardized mean difference
References
Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–52.
Spencer S, Calverley PM, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J. 2004;23(5):698–702.
Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–22.
Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–31.
Wouters EF. The burden of COPD in the Netherlands: results from the Confronting COPD survey. Respir Med. 2003;97(Suppl C):S51–9.
Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320(7245):1297–303.
Paggiaro PL, Dahle R, Bakran I, Frith L, Hollingworth K, Efthimiou J. Multicentre randomised placebo-controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. International COPD Study Group. Lancet. 1998;351(9105):773–80.
Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–38.
Miravitlles M, Soler-Cataluña JJ, Calle M, Molina J, Almagro P, Quintano JA, et al. Spanish guidelines for management of chronic obstructive pulmonary disease (GesEPOC) 2017. Pharmacological treatment of stable phase. Arch Bronconeumol. 2017;53(6):324–35.
Han MK, Kazerooni EA, Lynch DA, Liu LX, Murray S, Curtis JL, COPDGene Investigators, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261(1):274–82.
Tatsumi K, Kasahara Y, Kurosu K, Tanabe N, Takiguchi Y, Kuriyama T, Respiratory Failure Research Group in Japan. Clinical phenotypes of COPD: results of a Japanese epidemiological survey. Respirology. 2004;9(3):331–6.
Celli BR, Locantore N, Tal-Singer R, Riley J, Miller B, Vestbo J, ECLIPSE Study Investigators, et al. Emphysema and extrapulmonary tissue loss in COPD: a multi-organ loss of tissue phenotype. Eur Respir J. 2018;51(2):1702146.
The Japanese Respiratory Society. The Japanese Respiratory Society guidelines for the management of chronic obstructive pulmonary disease. 5th ed. Tokyo: Medical Review; 2018.
Welte T, Vogelmeier C, Papi A. COPD: early diagnosis and treatment to slow disease progression. Int J Clin Pract. 2015;69(3):336–49.
Tashkin DP, Celli BR, Decramer M, Lystig T, Liu D, Kesten S. Efficacy of tiotropium in COPD patients with FEV1 ≥60% participating in the UPLIFT® trial. COPD. 2012;9(3):289–96.
Jenkins CR, Jones PW, Calverley PM, Celli B, Anderson JA, Ferguson GT, et al. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH study. Respir Res. 2009;10(1):59.
Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP, UPLIFT Investigators. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009;374(9696):1171–8.
Morice AH, Celli B, Kesten S, Lystig T, Tashkin D, Decramer M. COPD in young patients: a pre-specified analysis of the four-year trial of tiotropium (UPLIFT). Respir Med. 2010;104(11):1659–67.
Zhou Y, Zhong NS, Li X, Chen S, Zheng J, Zhao D, et al. Tiotropium in early-stage chronic obstructive pulmonary disease. N Engl J Med. 2017;377(10):923–35.
Kostikas K, Price D, Gutzwiller FS, Jones B, Loefroth E, Clemens A, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD Database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729–38.
Larsson K, Janson C, Ställberg B, Lisspers K, Olsson P, Kostikas K, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995–1008.
Tkacz J, Evans KA, Touchette DR, Portillo E, Strange C, Staresinic A, et al. PRIMUS—Prompt Initiation of Maintenance Therapy in the US: a real-world analysis of clinical and economic outcomes among patients initiating triple therapy following a COPD exacerbation. Int J Chron Obstruct Pulmon Dis. 2022;17:329–42.
Watz H, Tetzlaff K, Magnussen H, Mueller A, Rodriguez-Roisin R, Wouters EFM, et al. Spirometric changes during exacerbations of COPD: a post hoc analysis of the WISDOM trial. Respir Res. 2018;19(1):251.
Dransfield MT, Kunisaki KM, Strand MJ, Anzueto A, Bhatt SP, Bowler RP, COPDGene Investigators, et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(3):324–30.
Suzuki M, Makita H, Ito YM, Nagai K, Konno S, Nishimura M, Hokkaido COPD Cohort Study Investigators. Clinical features and determinants of COPD exacerbation in the Hokkaido COPD cohort study. Eur Respir J. 2014;43(5):1289–97.
Muro S, Suzuki M, Nakamura S, Wang JR, Garry EM, Sakamoto W, et al. Real-world effectiveness of early intervention with fixed-dose tiotropium/olodaterol vs tiotropium in Japanese patients with COPD: a high-dimensional propensity score-matched cohort analysis. Respir Res. 2021;22(1):180.
Chubachi S, Sato M, Kameyama N, Tsutsumi A, Sasaki M, Tateno H, Keio COPD Comorbidity Research (K-CCR) Group, et al. Identification of five clusters of comorbidities in a longitudinal Japanese chronic obstructive pulmonary disease cohort. Respir Med. 2016;117:272–9.
Tanabe N, Shimizu K, Terada K, Sato S, Suzuki M, Shima H, et al. Central airway and peripheral lung structures in airway disease-dominant COPD. ERJ Open Res. 2021;7(1):00672–2020.
Dales RE, Vandemheen KL, Clinch J, Aaron SD. Spirometry in the primary care setting: influence on clinical diagnosis and management of airflow obstruction. Chest. 2005;128(4):2443–7.
Ishii T, Nishimura M, Akimoto A, James MH, Jones P. Understanding low COPD exacerbation rates in Japan: a review and comparison with other countries. Int J Chron Obstruct Pulmon Dis. 2018;13:3459–71.
Cazzola M, Matera MG. The effective treatment of COPD: anticholinergics and what else? Drug Discov Today Ther Strateg. 2006;3(3):277–86.
Barisione G, Baroffio M, Crimi E, Brusasco V. Beta-adrenergic agonists. Pharmaceuticals (Basel). 2010;3(4):1016–44.
Yamada H, Hida N, Hizawa N. Effects of a single long-acting muscarinic antagonist agent and a long-acting muscarinic antagonist/long-acting β2-adrenoceptor agonist combination on lung function and symptoms in untreated COPD patients in Japan. Int J Chron Obstruct Pulmon Dis. 2018;13:3141–7.
Sato M, Chubachi S, Sasaki M, Haraguchi M, Kameyama N, Tsutsumi A, et al. Impact of mild exacerbation on COPD symptoms in a Japanese cohort. Int J Chron Obstruct Pulmon Dis. 2016;11:1269–78.
O’Reilly JF, Williams AE, Holt K, Rice L. Defining COPD exacerbations: impact on estimation of incidence and burden in primary care. Prim Care Respir J. 2006;15(6):346–53.
Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, ETHOS Investigators, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48.
Acknowledgements
Not applicable.
Funding
This study was funded by AstraZeneca K.K. IM, NM, YA, NH, KM, and NT are employees of AstraZeneca K.K and were involved in study design, data interpretation, analysis, and preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
HY, NHi, IM, NM, YA, NH, KM, and NT designed the study, interpreted the data, and critically reviewed and approved the manuscript. IM, NM, YA, NH, KM, and NT developed the analysis plan. All authors read and approved the final manuscript and take responsibility for the content of the manuscript, including the data and analysis.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the NPO-MINS Institutional Review Board (Approval No. MINS-REC-210216) in 2021. The study was conducted in accordance with the Ethical Guidelines for Biomedical Research Involving Human Subjects, the ethical principles of the Declaration of Helsinki, and all relevant regulations applicable to noninterventional studies. The requirement for informed consent was waived because the available data in the RWD database were standardized and anonymized.
Consent for publication
Not applicable.
Competing interests
HY has received honoraria from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Sanofi, and Novartis. IM, NM, YA, NH, KM, and NT are employees of AstraZeneca K.K. NHi has received honoraria from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, KYORIN Pharmaceutical, Novartis, and Sanofi.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: due to some scientific content has been updated in Table, References, and Additional file 1. Revised file has been updated.
Supplementary Information
Additional file 1: Table S1.
ICD-10 codes. ICD-10, International Statistical Classification of Diseases and Related Health Problems, 10th Revision. Table S2. ATC classification codes. ATC, Anatomical Therapeutic Chemical; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; SABA, short-acting β2-agonist; SAMA, short-acting muscarinic antagonist. Table S3. Proportion of delayed therapy patients who used long-/short-acting bronchodilators before exacerbation. Table S4. Hazard ratios for exacerbations in subgroups stratified by periods of grouping. COPD, chronic obstructive pulmonary disease; CI, confidence interval. aCalculated by Cox proportional hazards model. Covariates in pre-index period include age, sex, number of comorbidities, use of systemic corticosteroids, use of antibiotics, and number of hospitalizations excluding hospitalization due to COPD exacerbation or acute respiratory failure.
Additional file 2: Figure S1.
Distribution of time between diagnosis and inhaler therapy initiation in the prompt therapy group
Additional file 3: Figure S2.
Distribution of time between diagnosis and inhaler therapy initiation in the delayed therapy group
Additional file 4: Figure S3.
Class-wise distribution of initial therapy in the prompt and delayed therapy groups. ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist.
Additional file 5: Figure S4.
Moderate or severe exacerbations in the prompt and delayed therapy populations with index dates after April 1, 2018. CI, confidence interval.
Additional file 6: Figure S5.
Class-wise distribution of initial therapy in the prompt therapy and delayed therapy populations with index dates after November 1, 2013 (LAMA + LABA product launch). ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yamada, H., Matsumoto, I., Makita, N. et al. Effect of timing of bronchodilator therapy initiation on exacerbations in patients with chronic obstructive pulmonary disease: a retrospective cohort study. Respir Res 23, 255 (2022). https://doi.org/10.1186/s12931-022-02184-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-022-02184-6