Abstract
Cigarette smoke is a complex aerosol containing a large number of compounds with a variety of toxicity and carcinogenicity. Long-term exposure to cigarette smoke significantly increases the risk of a variety of diseases, including chronic obstructive pulmonary disease (COPD) and lung cancer. Epithelial–mesenchymal transition (EMT) is a unique biological process, that refers to epithelial cells losing their polarity and transforming into mobile mesenchymal cells, playing a crucial role in organ development, fibrosis, and cancer progression. Numerous recent studies have shown that EMT is an important pathophysiological process involved in airway fibrosis, airway remodeling, and malignant transformation of COPD. In this review, we summarized the effects of cigarette smoke on the development and progression of COPD and focus on the specific changes and underlying mechanisms of EMT in COPD induced by cigarette smoke. We spotlighted the signaling pathways involved in EMT induced by cigarette smoke and summarize the current research and treatment approaches for EMT in COPD, aiming to provide ideas for potential new treatment and research directions.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable, and treatable condition characterized by persistent respiratory symptoms and airflow restriction caused by respiratory and/or alveolar abnormalities, usually caused by high exposure to harmful particles or gases [1]. COPD is currently the third leading cause of death globally and is associated with significant social and economic burdens [2, 3].
The most commonly encountered risk factor for COPD is long-term direct or passive exposure to cigarette smoke (CS). Cigarette smoke contains several toxic compounds that contribute to the pathogenesis of many respiratory diseases, such as COPD and lung cancer [4]. Compared with non-smokers, smokers are more susceptible to suffering from respiratory symptoms and abnormal lung function, with a higher annual decline rate of FEV1 and a higher COPD mortality rate [5, 6].
Epithelial–mesenchymal transformation (EMT) is a unique biological process in which epithelial cells lose their polarity and transform into mobile mesenchymal cells [7]. EMT serves a crucial role in embryonic development, chronic inflammation, tissue reconstruction, cancer metastasis, and a variety of fibrotic diseases [8, 9]. Moreover, EMT is increasingly being considered a possible core pathophysiological factor in COPD and lung cancer progression [10,11,12,13,14]. In smoking-related COPD, recent studies further highlight that EMT is associated with airway remodeling, airway fibrosis, and subsequent airflow obstruction and may be associated with a higher prevalence of lung cancer [15,16,17,18]. All of these findings suggest the importance of EMT in the development of smoking-related COPD. It is important to better understand the mechanisms that lead to EMT and to develop treatments that target EMT.
Here, we provide a brief overview of the pathogenesis of cigarette smoke-induced COPD. We focus on the specific changes and underlying mechanisms of EMT in COPD induced by cigarette smoke, which may provide new ideas for innovative prevention or treatment targets for COPD. This review may contribute not only to understanding the key elements of EMT but also to developing new strategies for the treatment of COPD.
Cigarette smoke and COPD
Cigarette smoke
Cigarette smoke is a complex aerosol composed of gas phase and particle phase. It contains more than 7000 different types of chemical components with various toxic and carcinogenic properties [19]. Among these substances are nicotine, carbon monoxide, carbon dioxide, tar, ammonia, formaldehyde, acrolein, acetone, polycyclic aromatic hydrocarbons (benzo (a) pyrene), hydroxyquinones, nitrogen oxides, and heavy metals (nickel, cadmium, chromium, and arsenic) [20]. The adverse effects of cigarette smoking on human health have been well documented over the past decades. It has been suggested that cigarette smoking has irreversible effects on genetic material (DNA mutations) as well as possibly reversible effects on the epigenetic landscape (DNA methylation and chromatin modification) [21]. It is increasingly recognized that smoking not only causes health problems for smokers and passive smokers, but also environmental hazards, with consequences for ecosystems and human health [22]. Cigarette smoke is an important risk factor for several diseases, such as COPD, cardiovascular disease, and cancer [23,24,25]. Smoking is the primary cause of preventable disease globally. Public health should promote understanding of the current pathology of smoking-related diseases and encourage individuals to reduce their exposure to cigarette smoke, thereby reducing the harmful consequences of related diseases.
Effects of cigarette smoke on the development and progression of COPD
Factors that influence the development and progression of COPD include genetic factors, age and gender, and exposure to particulate matter such as cigarette smoke. Cigarette smoking is the most crucial risk factor for the development of COPD. Repeated cigarette smoke exposure can cause chronic inflammation in the lungs, which increases the number of certain inflammatory cells, as well as structural changes resulting from repeated damage and repair. These changes contribute to the clinical features of COPD, including airway remodeling, chronic bronchitis, and emphysema [1]. The following is a brief overview of the pathological changes, and cellular and molecular mechanisms underlying smoking-induced COPD.
The ciliated epithelium of the respiratory tract is the first protective line against harmful substances. It removes pathogens from the mucus layer through mucociliary clearance, establishes barriers through tight and adherent junctions, and activates and recruits immune cells in the submucosa through cytokine and chemokine production [26, 27]. Cigarette smoke contains a large number of toxic substances, including a large number of oxygen metabolite-derived or reactive oxygen species (ROS), which can directly disrupt this physical barrier, resulting in increased permeability of respiratory epithelial cells and hindering clearance of mucus cilia [28]. Importantly, cigarette smoke could induce oxidative damage to cell membrane lipids through various mechanisms, such as DNA damage, lipid peroxidation, amino acid oxidation, inorganic enzyme cofactor oxidation, etc. [29,30,31].
In the early stage of smoking, protective responses and DNA repair triggered by the lung barrier may inhibit these changes to some extent [32]. In the case of long-term smoking, these mechanisms seem to break down and lead to disease progression. Chronic inflammation and the oxidant–antioxidant balance are the main driving molecular mechanisms promoting the progression of COPD and exacerbations [33, 34]. Specifically, cigarette smoke activates damage-associated molecular patterns (DAMP) and pathogen-associated molecular patterns (PAMPS) in lung epithelial cells and alveolar macrophages, which activate Toll‑like receptors (TLRs) and NOD‑like receptors (NLRs). This process produces excess ROS and reactive nitric oxide (RNS), which may lead to an oxidation/antioxidant imbalance [35, 36]. Stimulants such as ROS in cigarette smoke attract macrophages, neutrophils, dendritic cells, natural killer cells, and T lymphocytes to migrate to the lungs by releasing cytokines and chemokines (e.g., NF-κB, IL-8, IL-1β, ROS, and TNF-α). During the chronic phase, inflammatory cells are continuously recruited and release inflammatory mediators, such as proteases (e.g., MMPs and neutrophil elastase), chemokines, cytokines, and ROS. Epithelial cells and macrophages also release fibroblast mediators, such as TGFβ, which activate fibroblasts and lead to small airway fibrosis. In addition, cigarette smoke impairs structural cell function and initiates the EMT, a process leading to dysfunction in endothelial as well as an epithelial barrier, hamper tissue repair, and eventually leading to fibrosis. Thus, chronic exposure to cigarette smoke causes persistent inflammation and oxidative stress in the lungs, leading to repeated repair and remodeling (leading to airway remodeling), stimulating mucus hypersecretion (leading to chronic bronchitis), and degrading the alveolar walls (leading to emphysema) (Fig. 1) [28, 37,38,39,40]. Cigarette smoke induces COPD-related airway remodeling phenotypes, including airway epithelial hyperplasia, myocyte hyperplasia, squamous metaplasia, EMT, ciliary alterations, loss of secretory cells that produce Scgb1a1, and reduced integrity of the apical junction barrier that controls airway epithelial permeability. Together, these phenotypes lead to airway obstruction and reduced airway epithelial barrier and host defense function [28, 41].
Overview of the effect of cigarette smoke on the development and progression of COPD. Cigarette smoke activates DAMP and PAMPS in lung epithelial cells and alveolar macrophages and produces excess ROS. Stimulants such as ROS attract macrophages, neutrophils, DC, NK cells, and T lymphocytes to migrate to the lungs by releasing cytokines and chemokines (e.g., NF-κB, IL-8, IL-1β, ROS, TNF-α). During the chronic phase, inflammatory cells are continuously recruited and release inflammatory mediators, such as proteases (e.g., MMPs and neutrophil elastase), chemokines, cytokines, and ROS. Epithelial cells and macrophages also release fibroblast mediators, such as TGFβ, which activate fibroblasts and lead to small airway fibrosis. In addition, cigarette smoke induces epithelial EMT, which leads to dysfunction of endothelial cells and epithelial barrier, hinders tissue repair, and ultimately leads to fibrosis. Hence, long-term exposure to cigarette smoke causes sustained inflammation and oxidative stress in the lungs, leading to repeated repair and remodeling (leading to airway remodeling), degrading the alveolar walls (leading to emphysema), and stimulating mucus hypersecretion (leading to chronic bronchitis)
In recent years, it has become increasingly clear that the structural integrity and functional stability of multiple organelles are important for the function and survival of cells. Various organelle dysfunction plays an important role in the pathogenesis and progression of COPD [42]. In fact, these organelles showed significant structural derangement and aberrant function under exposure to cigarette smoke. The excessive oxidative burden is considered to be one of the underlying mechanisms of COPD epithelial barrier breakdown. Chronic exposure of the lungs to cigarette smoke disrupts the mitochondrial activity and endoplasmic reticulum (ER) homeostasis that triggers an unresolvable unfolded protein response activation. Mitochondrial dysfunction induces oxidative stress through excessive production of mitochondrial ROS, thereby increasing epithelial barrier permeability. Furthermore, cigarette smoke leads to the accumulation of damaged and misfolded proteins in the endoplasmic reticulum (ER), a condition known as ER stress, accompanied by enhanced unfolded protein response (UPR). Although UPR is a compensatory cellular response that reduces protein synthesis and enhances protein folding and degradation, UPR also contributes to lung cell apoptosis and lung inflammation during excessive ER stress [43,44,45,46,47]. Therefore, quality control of multiple organelles is of great significance for maintaining cell survival and function, and maybe a potential therapeutic target for COPD.
The possible link between cigarette smoking-related COPD and lung cancer
Cigarette smoking is the principal factor driving the pathogenesis and progression of COPD and lung cancer. Several epidemiological and observational cohort studies have systematically confirmed the close relationship between COPD and lung cancer [48,49,50,51,52]. Epidemiological studies have shown a four- to sixfold increased risk of lung cancer in patients with COPD, with the onset of lung cancer associated with the severity of COPD [53, 54]. Significantly, many phenotypic and genotypic features of COPD, such as a history of smoking, chronic bronchitis, airway obstruction, and emphysema, are associated with an increased risk of lung cancer [55, 56]. COPD has been reported to be an additional burden and risk factor for the development of lung cancer, primarily squamous cell carcinoma, especially in smokers [10, 57]. Genetic susceptibility, DNA methylation changes, local chronic lung inflammation, and abnormal repair mechanisms in COPD patients are important potential factors for lung cancer development [58,59,60]. Indeed, COPD and lung cancer have many common biological mechanisms, including chronic inflammation, oxidative stress, matrix degradation, genetic susceptibility, lung barrier dysfunction, and epithelial–mesenchymal transition (EMT) [4, 59, 61]. Among these, oxidative stress, chronic inflammation, and EMT are the most studied drivers of carcinogenesis. Cigarette smoke exposure causes inflammation, oxidative stress, and lung barrier dysfunction, and leads to EMT that end up with ultimately abnormal tissue repair [4]. The protracted inflammation gave rise to EMT which ended up with aberrant tissue repair. This might help explain the link between COPD and lung cancer in smokers and may provide guidance for management and prevention strategies for COPD and lung cancer.
Epithelial–mesenchymal transformation in chronic obstructive pulmonary disease
Epithelial–mesenchymal transformation
The pulmonary epithelial cell lining forms an external protective barrier against the environmental toxins produced by smog and microbial infection. EMT is a unique biological process, that refers to epithelial cells losing their polarity and transforming into mobile mesenchymal cells. Generally, EMT can be classified into three main types based on the physiological tissue setting: type I EMT occurs in embryonic development and organogenesis, type II EMT occurs in tissue repair and fibrosis, and type III EMT occurs in epithelial malignancies associated with aggressive or metastatic phenotypes [62]. The Morphological alterations characteristic of EMT include the disruption of epithelial cell junctions, the destruction of polar complex, and the reorganization of cytoskeletal structure. Molecularly, EMT is characterized by downregulation of epithelial junction proteins (e.g., E-Cadherin and Occludins) and activation of EMT transcriptional activators (e.g., Snail, Slug, and Twist) and mesenchymal markers (e.g., S100A4, Vimentin, Fibronectin, and N-Cadherin) [63].
Alterations of epithelial–mesenchymal transformation biomarkers in COPD
The role of EMT is well documented in embryonic development, wound healing, tumor progression, and tissue fibrosis [64]. Aberrant wound repair and fibrosis are associated with many respiratory diseases. EMT has been implicated as fundamental for lung development and many respiratory diseases, particularly those characterized by increased deposition of collagen and other ECM proteins in the airways or parenchyma. These diseases include COPD, lung cancer, asthma, pulmonary fibrosis, and bronchiolitis obliterans syndrome [62, 65]. Extensive research has shown that EMT is activated in the airway tissue of smokers, particularly in those current-smoking COPD patients [66,67,68,69]. EMT is increasingly being considered a possible core pathophysiological factor in COPD and lung cancer progression [10,11,12,13,14]. Interestingly, EMT has been linked to airway fibrosis, airway remodeling, and airflow obstruction, and may even contribute to the high incidence of lung cancer in COPD patients [8, 12, 70]. In this part, we will describe in detail the alterations of EMT biomarkers in COPD.
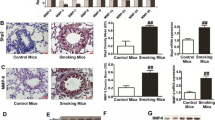
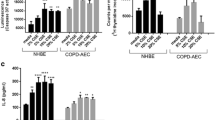
EMT transcription factors and mesenchymal markers were up-regulated and epithelial markers down-regulated in COPD, and were associated with lung function, as shown in Table 1. Specifically, the expression of EMT-related transcription factor Snail1 in α 1-antitrypsin deficient COPD was significantly higher than that in normal COPD [71]. Moreover, Mahmood et al. [17] found that transcriptional factors Snail1 and Twist were upregulation and nuclear translocation in smokers and current-smoking COPD, and their expression is closely associated with EMT activity (S100A4 expression) and the levels of airflow obstruction.
Among the expression alterations of epithelial and mesenchymal cell markers, E-cadherin is considered a prominent hallmark of EMT and serves a central role in the EMT process. E-cadherin is an adhesion molecule responsible for the organization of interepithelial junctions [72, 73]. Numerous studies have shown that E-cadherin was markedly decreased in smokers and COPD [68, 74,75,76,77,78]. Particularly, the expression of E-cadherin was positively related to FEV1/VC ratio [74]. The extracellular portion of E-cadherin can be degraded by proteases including MMPs to form circulating molecules soluble E-cadherin (sE-cadherin). Shirahata et al. [79] demonstrated that Plasma sE-cadherin levels were significantly lower in patients with COPD and symptomatic smokers than in healthy smokers and healthy non-smokers, and sE-cadherin levels were associated with the severity of airflow limitation in COPD and symptomatic smokers plasma.
In addition, mesenchymal markers (S100A4, N-cadherin, Vimentin, and α-SMA proteins) and ECM proteins (type I collagen and fibronectin) were also found increased in smokers and COPD. S100A4 (also named fibroblast specific protein 1, FSP1) is considered a canonical mesenchymal marker in EMT with biological functions of promoting cell motility, invasion, ECM remodeling, autophagy, and angiogenesis [80]. Numerous studies have demonstrated that S100A4 was upregulated in COPD and inversely associated with airflow limitation [67, 69, 81, 82]. Vimentin is expressed in all mesenchymal cells and is the core of EMT-mediated metastasis. During EMT, vimentin can induce cell migration by forming cell processes, reducing cell adhesion, and increasing cell migration ability [83]. Vimentin has also been found to be upregulated in smokers and COPD [67, 68, 74, 75, 77, 78], and the expression of vimentin in the bronchial epithelium of COPD is associated with basement membrane thickening and airflow limitation [74].
Additionally, there is an increasing number of studies identifying new biomarkers for EMT in COPD. β2-microglobulin (β2M), also known as the class I major histocompatibility complex (MHC I) light chain, is involved in the regulation of EMT processes in several diseases [84,85,86,87]. It was shown that plasma β2M concentrations were significantly higher in smoking patients with COPD and emphysema than those in normal subjects [88]. Wu et al. [89] indicated that β2M was increased in COPD patients and was correlated with lower pulmonary diffusing capacity values, increased alveolar wall/septal thickening (fibrosis changes), and higher expressions of TGF-β1, Smad4, and a-SMA. They further found that β2M expression of lung tissues was correlated with EMT and fibrosis progression in cigarette smoke-exposed COPD rats. Sphingosine-1-phosphate (S1P), a bioactive sphingolipid metabolite, plays an important role in the occurrence and development of cancer by regulating and promoting cell growth, migration, invasion, and cell survival [90]. Previous studies found that S1P drove the EMT process via the TGF-β axis and was correlated with lung function in patients with idiopathic pulmonary fibrosis and asthma-like disease [91, 92]. In vivo studies have shown that S1P expression is increased and associated with pulmonary resistance in cigarette smoke-induced COPD mice [93]. The latest finding showed that serum S1P was upregulated and inversely associated with lung function in patients with COPD. In addition, serum S1P was positively associated with mesenchymal marker S100A4 in COPD [81]. Cullin 4A (CUL4A), an E3 ubiquitin ligase, is involved in the regulation of the cell cycle, DNA replication, and DNA damage repair [94]. CUL4A has highly expressed in non-small cell lung cancer (NSCLC) tissues and can promote lung cancer progression by inducing EMT [95, 96]. Ren et al. [97] found that the expression of CUL4A in lung epithelium of smokers and smoke patients with COPD was significantly higher than that of non-smokers and CUL4A was negatively correlated with the FEV1%. Moreover, they found that silencing CUL4A inhibited CSE-induced EMT in human small airway epithelial cells.
During the EMT process in COPD, alterations in cell phenotypes contributed to the emergence of specific biomarkers. These specific biomarkers can identify levels of EMT activity level and may be used to assess airflow restriction, COPD exacerbation risk, and malignant transformation. Future studies are needed to clarify the association of specific EMT biomarkers with lung function, pulmonary fibrosis, and malignant transformation in smoking-related COPD patients.
Signalling pathways involved in EMT induced by cigarette smoke in COPD
Recently, several studies have explored the important role of cigarette smoke in the induction of EMT in COPD. We summarized these reports and suggested that cigarette smoking may induce the occurrence of EMT by using the specificity of different signaling pathways. This section provides a brief overview of the mechanisms and signaling pathways involved in EMT induced by cigarette smoke in COPD. These signaling pathways include the TGF-β/Smad signaling pathway, the Wnt/β-catenin signaling pathway, PI3K-Akt signaling pathway, and NF-κB signaling pathway, etc. (Fig. 2). A better understanding of the signaling pathways underlying cigarette smoke-induced EMT could provide new targets and potential strategies for COPD patients.
Schematic overview of EMT-related signaling pathways in cigarette smoke-induced COPD and the potential therapies targeting these signaling pathways. Cigarette smoke can induce EMT in COPD through multiple different signaling pathways. These pathways are intricate and inextricably partly crosslinked. Additionally, potential therapies based on targeting these signaling pathways were shown. Each is depicted in the text
TGF-β/Smad signaling pathway
The transforming growth factor-β (TGF-β) superfamily consists of a group of distinct polypeptides involved in regulating a wide range of cellular and physiological processes, including proliferation, differentiation, migration, adhesion, ECM synthesis and cell death [98]. TGF-β can induce EMT via both canonical TGF-β/Smad signaling pathway and non-canonical pathway (e.g. ERK, P38, MAPK, PI3K/Akt) [99, 100]. In the canonical TGF-β/Smad signaling pathway, TGF-β ligands regulate key transcription factors that promote EMT through Smads protein and synergistic kinase pathway. In general, TGF-β1 and the Smad signaling pathways are regarded as the key to the EMT-related pathogenesis of COPD [101, 102]. Particularly, TGF-β1 is a multifunctional cytokine that induces angiogenesis and regulation of extracellular matrix (ECM) components, a which is considered to be a key regulator of COPD airway pathology [16]. Studies have shown that exposure to TGF-β1 induces mesenchymal phenotype and EMT in cultured human bronchial and lung epithelial cells in vitro [74, 103,104,105]. In addition, TGF-β1 expression was significantly increased in the airway epithelium of both smokers and COPD patients, accompanied by the expression of ρ Smad2/3 [16, 106]. Likewise, cigarette smoke induces EMT in lung and bronchial epithelial cells through TGF-β1/Smad signaling pathway in vivo and in vitro [107,108,109,110,111]. Together, these studies suggest that cigarette smoke targeting the TGF-β/Smad signaling pathway induces EMT in COPD.
Wnt/β-catenin signaling pathway
The Wnt/β-catenin signaling pathway plays an important role in embryonic development, adult tissue homeostasis, and regeneration, and its abnormal regulation is closely related to various diseases [112]. After activation of the Wnt/β-catenin pathway, β-catenin is translocated to the nucleus and interacts with the transcription factor LEF/TCF to activate transcription. In addition, the Wnt/β-catenin signaling pathway has been reported to regulate EMT in several cancers [113]. Interestingly, β-catenin is a crucial regulator in the Wnt/β-catenin signaling pathway, and phosphorylation or down-regulation of β-catenin can inhibit the activation of the Wnt/β-catenin signaling pathway [114]. Numerous recent studies indicated that the Wnt/β-catenin signaling is activated in smokers and COPD, and is strongly correlated with EMT activity and airway obstruction. Carlier et al. [66] reported that Wnt/β-catenin signaling pathway-related genes and proteins were significantly upregulated in the airway epithelium of COPD smokers. They further found that activation of the Wnt/β-catenin signaling pathway in human airway epithelial cells from COPD smoking patients resulted in increased Vimentin expression, increased fibronectin release, and enhanced TGF-β1/Smad signaling. Conversely, inhibition of the Wnt/β-catenin signaling pathway increases ciliary cell count, epithelial polarity, and barrier function, while inhibiting EMT, thereby reversing COPD characteristics. This study demonstrates the relationship between Wnt/β-catenin and EMT and its important role in promoting COPD pathology. Moreover, the expression of β-catenin and Snail1 is up-regulated in the airway wall of both smokers and COPD, and their expression was strongly associated with typical EMT biomarkers (S100A4) and airway obstruction [17, 115]. Likewise, in vitro studies have shown that cigarette smoking and nicotine induce EMT by activating the Wnt/β-catenin signaling pathway in HBE cells and A549 cells [116, 117]. Therefore, regulation of Wnt/β-catenin could serve as a promising therapeutic strategy to control EMT induction by cigarette smoking in COPD.
PI3K/AKT signaling pathway
The PI3K/AKT signaling pathway is a key regulator of important biological functions, including metabolism, cell proliferation, epithelial–mesenchymal transition, survival, and apoptosis [118]. Rac1 is involved in several dynamic cell biological processes, such as cell survival, cell–cell contact, cell motility, EMT, and cell invasion [119]. A previous study showed CS-induced EMT via Rac1/PI3K/Akt and Rac1/Smad2 signaling pathways. Pharmacological inhibition of Rac1 could alleviate TGF-β1 production and prevent alterations in the expression of EMT markers in CS-exposed mice. In addition, knockdown or inhibition of Rac1 ameliorated CSE-induced TGF-β1 release and CSE-induced EMT and inhibited CSE-induced Akt and Smad2 activation in A549 pulmonary epithelial cells. Furthermore, inhibition of PI3K, Akt, or Smad2 could suppress CSE-induced alterations in epithelial and mesenchymal marker expression [107]. Another study has found that Rac1 could regulate cigarette smoke-induced pulmonary inflammation in the lung through the STAT3 and Erk1/2 MAPK signaling pathways [120]. According to Milara et al. [121], in primary human bronchial epithelial cells, CSE induces EMT partially through the activation of Rac1, PI3K/Akt/β-catenin pathways, and the generation of ROS. Particularly, EMT can be induced by TGF-β through the activation of PI3K/AKT and MAPK in the Smad-independent pathway [122,123,124]. mTOR is a member of the PI3K family and an effector protein downstream of the PI3K/AKT signaling pathway [125]. A study by Jiang et al. [126] found that CS exposure in mice and CSE exposure in bronchial epithelial cells could induce EMT by activating the Akt signaling pathway. They further demonstrated inhibition of Akt activity can inhibit the progression of smoke-induced EMT by down-regulating TGF-β1/Akt/Smad/mTOR and Akt/P38 MAPK signaling pathway. Collectively, these results suggest that PI3K/AKT signaling pathway activation may be involved in the pathogenesis of CS-induced pulmonary EMT and has potential therapeutic significance in COPD, lung cancer, and other smoking-related diseases.
NF-κB signaling pathways
The transcription factor nuclear factor-kappa B (NF-kB) is an essential stressor in the cellular environment and regulates a series of genes involved in survival, oxidative stress, inflammation, and immunity [127]. EMT is regarded as an intersection of inflammation, oxidative stress, fibrotic diseases, and cancer. Moreover, NF-kB is a major pro-inflammatory transcription factor activated by inflammatory cytokines and ROS, which is one of the key roles in the formation of EMT [128, 129]. Zhao et al. [130] reported that the NF-κB signaling pathway is involved in CSE-induced EMT in HBE cells, suggesting that NF-κB activation acts as a bridge between CSE-induced chronic inflammation, EMT, and lung cancer. The data showed that inhibition of NF-κB activation could block CSE-induced upregulation expression of E-cadherin, and reverse the downregulation of IL-6 and N-cadherin in HBE cells. In addition, the study found that silencing of NF-κB decreased CSE-induced colony formation and the invasion and migration capacities in HBE cells [131]. Li et al. [132] further demonstrated that CSE can induce EMT through activation of the IL17R/NF-κB signaling pathway in murine bronchial epithelial cells. Interestingly, Hong et al. [133] used N-acetylcysteine (NAC), an inhibitor of the oxidative stress signaling pathway, to prove that the oxidative stress signaling pathway is involved in the cigarette smoke-induced EMT process. Taken together, the NF-κB signaling pathway is a suitable therapeutic target for CSE-induced EMT, inflammation, oxidative stress, and malignant transformation in COPD.
Other signaling pathways
EMT is an extremely complex pathological process, which is often not the activation of a single signaling pathway. The protein tyrosine phosphatase Shp2 is thought to be involved in chronic pneumonia and fibrosis [134]. Shp2 plays a key role in acute cigarette smoke-induced lung inflammation, in which pulmonary epithelial knockout of Shp2 reduced IL-8 release and lung inflammation in CS-exposed mice [135]. Furthermore, Liu et al. [136] found that Shp2 inhibition reduced BMP-9 production, EMT progression, and phosphorylation of ERK1/2, JNK, and SMAD2/3 in CS and CSE exposure mouse lungs and pulmonary epithelial cells. MAPK, an intracellular Ser/Thr protein kinase, is involved in a variety of signaling pathways and plays an important role in cell cycle regulation [137]. Studies have reported that NAC can improve COPD-related pulmonary fibrosis by activating immune response and suppressing the EMT process through VWF/P38 MAPK signaling pathway in vivo and in vitro experiments [15]. In addition, TACE/TGF-α/EGFR signaling pathway is found activated in CSE-exposed human airway epithelial cells, and blocking the TACE/TGF-α/EGFR signaling pathway can inhibit the CSE-induced EMT process [138].
P53 (TP53), as a tumor suppressor, is thought to be the most frequently mutated gene in cancer cells. There is increasing evidence that mutated p53 enhances tumor metastasis and affects EMT processes [139]. Moreover, a large number of recent studies have shown that p53 signaling plays a role in regulating the EMT process in lung cancer [140,141,142,143]. Notably, chronic exposure to cigarette smoke has been linked to the development of p53 mutations and may contribute to p53 mutations in lung cancer [144,145,146,147]. A bioinformatics study has found that the P53 pathway may play an important role in promoting the progression of COPD to lung squamous cell carcinoma [148]. In addition, studies have shown that p53 gene polymorphisms are associated with apoptotic signaling and smoking-related emphysema in smokers [149]. Additionally, previous studies have shown that p53 gene polymorphism was significantly related to the incidence of smoking-related COPD, and p53 protein was markedly increased in COPD smokers [150, 151]. Given the key role of p53 in EMT and lung cancer, cigarette smoke-induced EMT may involve p53; thus, the relationship between P53, EMT, and cancer transformation in cigarette smoking-related COPD requires further investigation.
In fact, EMT induced by cigarette smoke in COPD is a complex network involving the regulation of multiple signaling pathways. These pathways are intricate, inevitably partially cross-linked and require further exploration. Therefore, cigarette smoking induces EMT through the above signaling pathways, and whether there are other signaling pathways affecting EMT deserves further study.
Potential therapies for EMT in COPD
At present, bronchodilators (short-acting Beta2-agonists (SABA) and long-acting Beta2-agonists (LABA)), antimuscarinic drugs (short-acting antimuscarinics (SAMAs), and Long-acting muscarinic antagonists (LAMAs)), methylxanthines (Theophylline), anti-inflammatory agents, Inhaled corticosteroids (ICS), antibiotics, mucolytic (mucokinetics and dmucoregulators), antioxidant agents (N-acetylcysteine, carbocysteine, and erdosteine) and Phosphodiesterase-4 (PDE4) inhibitors are commonly used drugs for the treatment of COPD [1]. However, many patients are unable to manage their daily symptoms even with standard care. Therefore, there is a critical need to explore new drug targets and develop more effective drugs. EMT plays a critical role in the development of smoking-related COPD airway remodeling disease and related lung cancer, especially in smoking-related COPD, where it may lead to airway remodeling, obstruction/occlusion, and tumor development. Therefore, drugs that inhibit the EMT process or kill EMT-type cells will be a novel therapeutic target for COPD. However, there are few studies on the anti-EMT effects of different drugs and even fewer data from human clinical studies in vivo. Drugs already used to target COPD may be able to retarget their long-term anti-EMT prophylaxis.
EMT is considered to be part of the pathophysiology of fibrosis, remodeling, and malignant consequences associated with COPD, and thus clinical trials targeting EMT have been conducted. The following sections, summarized in Table 2, will give an overview of EMT-targeting therapies and an understanding of how these may specifically or non-specifically target the EMT process. These therapies are Inhaled corticosteroids (ICS), N-acetylcysteine (NAC), Phosphodiesterase-4 (PDE4) inhibitors and statins, urokinase-type plasminogen activators and urokinase-type plasminogen activator receptor (uPA and uPAR), Adipose-derived stem cell-conditioned medium (ADSC-CM) and Ginsenoside Rg1. Partial potential therapies based on these signaling pathways are shown in Fig. 2. These therapies will require further mechanism exploration, and preclinical and clinical trials to establish their efficacy as a single or combined treatment of EMT in COPD.
Inhaled corticosteroids (ICS)
Inhaled corticosteroids (ICS) have become a standard treatment in more severe COPD, based on empirical results from large multicenter studies. A “proof of concept” randomized controlled trial concluded that the use of inhaled corticosteroids for more than 6 months inhibited EMT-related changes in COPD patients. Results showed that epithelial activation (EGFR expression), “clefts/fragmentation” in the Rbm, and EMT biomarkers (S100A4 and MMP-9) were significantly regressed after treatment in the ICS group compared to the placebo group [152]. According to some cohort studies, inhaled corticosteroids may reduce the incidence of lung cancer [153,154,155]. Interestingly, EMT activity in epithelial cells may be prone to malignant transformation, and EMT in the airway of COPD patients is likely to be an important bridge between airway fibrosis and cancer development [8]. An analysis of nine prospective cohorts suggests that inhaled corticosteroids have a protective effect against lung cancer in COPD patients. Results showed that inhaled corticosteroids were correlated with a reduced risk of lung cancer in COPD patients, providing clinicians with guidance for lung cancer prevention in COPD patients [156]. However, further study is needed to determine whether inhaled corticosteroids have a protective effect against lung cancer in COPD through anti-EMT. These may suggest that EMT may be a potential pathway through which the therapeutic effects of inhaled corticosteroids may occur in COPD patients. This has important implications for treatment and public health policy. Because it recommends that inhaled corticosteroids or other drugs with similar effects be administered early in the natural course of COPD, this not only inhibits airway inflammation, but may also inhibit epithelial activation, EMT, and associated fibrosis and malignant consequences. Larger sample sizes are needed in the future to verify whether inhaled corticosteroids are treated with EMT in COPD by acting directly on the EMT.
N-acetylcysteine (NAC)
The antioxidant N-acetylcysteine (NAC) is a mucolytic agent that is involved in the treatment of COPD as a mucolytic agent and has been shown to prevent exacerbation of COPD [157, 158]. COPD rat models demonstrated that N-acetylcysteine significantly reduced α-SMA level, collagen volume fraction, wall thickness/bronchiole diameter, and wall area/total bronchiole area (MA%) in COPD rats. Moreover, NAC inhibits the EMT process by inhibiting VWF/P38 MAPK signaling pathway. This suggested antioxidant N-acetylcysteine may contribute to EMT inhibition, thereby alleviating COPD pulmonary fibrosis. NAC could ameliorate COPD-induced pulmonary fibrosis by promoting immune response and inhibiting the EMT process via the VWF/p38 MAPK axis [15].
Phosphodiesterase-4 (PDE4) inhibitors and statins
Phosphodiesterase 4 (PDE4) inactivates adenosine cyclophosphamide and guanosine cyclophosphamide and is the main PDE isoenzyme in cells involved in inflammatory airway diseases such as COPD. Roflumilast, an oral PDE4 inhibitor, has proven to reduce the rate of acute exacerbation rates and help improve mortality and quality of life in COPD patients [159, 160]. Studies have found that PDE4 inhibitors could affect lung architectural remodeling. In vivo studies indicate that roflumilast mitigated cigarette smoke-induced airspace enlargement and alleviated bleomycin-induced lung fibrotic and vascular remodeling in mice [161, 162]. Likewise, Roflumilast N-oxide (RNO) protected CSE-induced EMT in human bronchial epithelial cells (HBECs). It was shown that RNO inhibited the upregulation of mesenchymal cell markers (α-SMA, vimentin, and collagen type I) induced by CSE in HBECs. RNO also reversed the downregulation of epithelial markers (E-cadherin, ZO-1, and KRT5) in HBECs. Moreover, RNO reduced mesenchymal markers while increasing epithelial markers in primary human bronchial epithelial cells isolated from smokers and COPD patients’ small bronchi. In addition, RNO reduced the CSE-induced increase in TGF-β1 release and Smad3 and ERK1/2 phosphorylation [163].
Statins are commonly prescribed clinically to lower serum cholesterol. Retrospective studies [164,165,166] described statins improves survival in patients with lung cancer. However, whether statins’ effectiveness in lung cancer remains controversial [167, 168]. Nishikawa et al. [169] described statins suppress EMT and improve the prognosis of lung adenocarcinoma patients in a p53 mutation-dependent manner. Recently, population pharmaco-epidemiological evidence suggests that statin use reduces the risk of lung cancer in patients with COPD [170, 171]. Milara et al. [121] proved that RNO partly alleviated the CSE-induced EMT and simvastatin increases the ability of RNO to Inhibit CSE-induced EMT in HBEC in vitro. Moreover, PDE4 inhibitor and statin may act on different pathways involved in CSE-induced EMT, such as ROS, PI3K/Akt, GTP-Rac1, and nuclear β-catenin. Further preclinical and clinical trials of PDE4 inhibitors and statins will be required to determine their efficacy as a single or combined treatment of EMT in COPD.
Urokinase-type plasminogen activator and urokinase-type plasminogen activator receptor (uPA and uPAR)
The binding of urokinase-type plasminogen activator (uPA) with urokinase-type plasminogen activator receptor (uPAR) is involved in the proteolytic activation of plasmin, which degrades fibrin and other ECM components, activating matrix metalloproteinases and promoting cell migration [172]. A retrospective study found that uPA and uPAR expression was significantly increased in pulmonary macrophages and alveolar wall cells from patients with COPD compared to the control, and that uPA expression was positively correlated with collagen levels [173]. Similarly, UPA and uPAR expression were increased in the airway epithelium of smokers and COPD patients compared with non-smokers. Moreover, in human small airway epithelial cell lines (HSAEpiCs), uPA and uPAR inhibition can block CSE-induced EMT by reversing the expression of E-cadherin and α-catenin and delaying the induction of N-cadherin and Vimentin [174, 175].
Adipose-derived stem cell
Adipose-derived stem cells (ADSCs) are the most abundant stem cell type in adults. The transplantation of ADSCs by intravenous injection could reduce inflammatory cell infiltration, airway enlargement, and lung cell death in CS-exposure mice [176, 177]. In vitro studies, ADSC-conditioned medium culture effectively reversed CSE-induced decreased E-cadherin expression, increased Vimentin expression, and accelerated cell migration in A549 cells [178]. This suggests that ADSCs might be a potential target for EMT in CSE-induced COPD or lung cancer. However, future animal studies and clinical trials are needed to verify these findings.
Ginsenoside Rg1
Ginsenoside Rg1 is the main active ingredient of Panax ginseng, having anti-inflammatory, antioxidant, and neuroprotective actions [179]. In CS-exposed COPD rats and CSE-exposed human bronchial epithelial (HBE) cells, Ginsenoside Rg1 alleviated CS or CSE-induced EMT via blocking the regulation of α-SMA and E-cadherin expression induced by CS or CSE. Additionally, ginsenoside Rg1 inhibited CSE-induced EMT through the TGF-β1/Smad pathway in HBE [108].
Other EMT-targeted therapies in clinical applications
EMT has been increasingly recognized as an interesting target for the development of new therapeutic strategies. Particularly, multiple EMT-targeted therapies have emerged in oncology over the last decade. So far, reasonable strategies such as inhibition of EMT induction, reversal of EMT process, and strategic killing of cells undergoing EMT seem to be promising in controlling the occurrence of EMT [180].
Galunisertib (LY2157299), a TGFβ receptor 1 inhibitor, has been studied in clinical trials in various solid tumors [181,182,183,184]. Notably, Galunisertib has been reported to be significantly sensitive to enzalutamide treatment in prostate cancer by inhibiting TGF-β-mediated EMT process [185]. This suggests that the anticancer effect of Galunisertib may be partly attributable to its ability to inhibit EMT. Additionally, other target inhibitors such as COX 2 inhibitors and AXL inhibitors have been found to block the EMT induction. Celecoxib, a selective cyclooxygenase‑2 inhibitor, has synergistic anti-cancer effects in different cancer types and has been found to inhibit the EMT process in oral squamous cell carcinoma, hypopharyngeal ca, cancer and bladder cancer, etc. [186,187,188,189]. AXL is a tyrosine kinase receptor that has been reported as an oncogene in a range of cancers, including NSCLC [190]. Cabozantinib, a tyrosine kinase inhibitor that includes AXL, has been reported to reverse EMT-associated osimertinib resistance in NSCLC [191]. In addition, cabozantinib suppressed EMT-associated sunitinib resistance in renal cell carcinoma [192]. All-trans retinoic acid (ATRA) is a front-line treatment of acute promyelocytic leukemia and neuroblastoma [193, 194]. ATRA is now widely used in preclinical and clinical studies and has shown great anticancer potential in phase II and III clinical trials in patients with various cancers [180]. Specifically, ATRA has been shown to reverse the EMT process in breast cancer cells and hepatocarcinoma cells [195,196,197]. Compared with the prevention of EMT induction, a promising alternative strategy is to selectively target EMT-induced mesenchymal‐like cancer cells by therapeutically inhibiting the functions of EMT-specific markers. N-cadherin antagonist ADH-1 is the synthetic cyclic peptide (also known as CHAVC and Exherin). The biological effects of ADH-1 on tumors have been extensively investigated in various preclinical animal models and clinical trials [198]. It was found that ADH-1 treatment significantly enhanced the anti-tumor effects of chemotherapy in melanoma [199]. In addition to anti-tumorigenic drugs, anti-fibrosis drugs also have potential EMT targeting effects. Notably, the anti-fibrosis drugs pirfenidone and nintedanib, which are approved for the treatment of idiopathic pulmonary fibrosis (IPF), appear to work by inhibiting the TGF-β pathway [200, 201]. Pirfenidone has been reported to inhibit EMT in pulmonary fibrosis by regulating the Wnt/GSK-3β/β-catenin and TGF-β1/SMad2/3 signaling pathways [202]. Furthermore, nintedanib inhibited EMT by mediating the TGF-β/Smad pathway in A549 alveolar epithelial cells [203]. Collectively, all of these EMT-targeted therapies hold great promise in terms of translating into inhibiting the development of COPD and lung cancer. However, further preclinical experiments and clinical trials are required to validate the clinical benefits of EMT-targeted therapies in COPD.
Conclusions
In recent years, EMT has become one of the research hotspots in the pathogenetic mechanism research of COPD. EMT is considered to be part of the pathophysiology of COPD-related fibrosis, remodeling, and malignant consequences. In this review, we summarized the effects of cigarette smoke on the pathogenesis of COPD, and focus on the cigarette smoke-induced EMT in COPD that occurs in the development of the latest clinical evidence. We reviewed the current research and treatment approaches for EMT in COPD. Therapies such as Inhaled corticosteroids (ICS) may offer EMT-targeting treatments that suppress airway inflammation, epithelial activation, EMT, and related fibrotic, and malignant consequences, offering a new idea for the use of ICS. Novel approaches to suppressing EMT formation or the associated inflammation are in development and represent an important therapeutic target. In conclusion, this review highlights the importance of understanding the molecular mechanisms of EMT in smoke-induced COPD, which is critical for identifying innovative therapies targeting EMT in COPD.
Availability of data and materials
Not applicable.
Abbreviations
- COPD:
-
Chronic obstructive pulmonary disease
- EMT:
-
Epithelial–mesenchymal transition
- TGF-β:
-
Transforming growth factor-β
- CS:
-
Cigarette smoke
- CSE:
-
Cigarette smoke extract
- DAMP:
-
Damage-associated molecular patterns
- PAMPS:
-
Pathogen-associated molecular patterns
- ROS:
-
Reactive oxygen species
- TLRs:
-
Toll‑like receptors
- NLRs:
-
NOD‑like receptors
- RNS:
-
Reactive nitric oxide
- ER:
-
Endoplasmic reticulum
- UPR:
-
Unfolded protein response
- FSP1:
-
Fibroblast specific protein 1
- β2M:
-
β2-Microglobulin
- MHC I:
-
Major histocompatibility complex
- TGF-β:
-
Transforming growth factor-β
- NF-kB:
-
Nuclear factor-kappa B
- NAC:
-
N-Acetylcysteine
- SABA:
-
Short-acting Beta2-agonists
- LABA:
-
Long-acting Beta2-agonists
- SAMAs:
-
Short-acting antimuscarinics
- LAMAs:
-
Long-acting muscarinic antagonists
- ICS:
-
Inhaled corticosteroids
- PDE4:
-
Phosphodiesterase-4
- uPA:
-
Urokinase-type plasminogen activators
- uPAR:
-
Urokinase-type plasminogen activator receptor
- ADSC:
-
Adipose-derived stem cell
- ADSC-CM:
-
Adipose-derived stem cell-conditioned medium
References
Global Strategy for Prevention, Diagnosis and Management of COPD: 2022 report. In: Global Initiative for Chronic Obstructive Lung Disease—GOLD. https://goldcopd.org/2022-gold-reports-2/.
Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. https://doi.org/10.1016/S0140-6736(12)61728-0.
Halpin DMG, Celli BR, Criner GJ, et al. The GOLD Summit on chronic obstructive pulmonary disease in low- and middle-income countries. Int J Tuberc Lung Dis. 2019;23:1131–41. https://doi.org/10.5588/ijtld.19.0397.
Hou W, Hu S, Li C, et al. Cigarette smoke induced lung barrier dysfunction, EMT, and tissue remodeling: a possible link between COPD and lung cancer. Biomed Res Int. 2019;2019:2025636. https://doi.org/10.1155/2019/2025636.
Woodruff PG, Barr RG, Bleecker E, et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374:1811–21. https://doi.org/10.1056/NEJMoa1505971.
Kohansal R, Martinez-Camblor P, Agustí A, et al. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med. 2009;180:3–10. https://doi.org/10.1164/rccm.200901-0047OC.
Yang J, Antin P, Berx G, et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2020;21:341–52. https://doi.org/10.1038/s41580-020-0237-9.
Mahmood MQ, Shukla SD, Ward C, Walters EH. The underappreciated role of epithelial mesenchymal transition in chronic obstructive pulmonary disease and its strong link to lung cancer. Biomolecules. 2021;11:1394. https://doi.org/10.3390/biom11091394.
Pain M, Bermudez O, Lacoste P, et al. Tissue remodelling in chronic bronchial diseases: from the epithelial to mesenchymal phenotype. Eur Respir Rev. 2014;23:118–30. https://doi.org/10.1183/09059180.00004413.
Szalontai K, Gémes N, Furák J, et al. Chronic obstructive pulmonary disease: epidemiology, biomarkers, and paving the way to lung cancer. J Clin Med. 2021;10:2889. https://doi.org/10.3390/jcm10132889.
Lee H-W, Jose CC, Cuddapah S. Epithelial-mesenchymal transition: insights into nickel-induced lung diseases. Semin Cancer Biol. 2021;76:99–109. https://doi.org/10.1016/j.semcancer.2021.05.020.
Nowrin K, Sohal SS, Peterson G, et al. Epithelial-mesenchymal transition as a fundamental underlying pathogenic process in COPD airways: fibrosis, remodeling and cancer. Expert Rev Respir Med. 2014;8:547–59. https://doi.org/10.1586/17476348.2014.948853.
Vu T, Jin L, Datta PK. Effect of cigarette smoking on epithelial to mesenchymal transition (EMT) in lung cancer. J Clin Med. 2016;5:44. https://doi.org/10.3390/jcm5040044.
Eapen MS, Sharma P, Thompson IE, et al. Heparin-binding epidermal growth factor (HB-EGF) drives EMT in patients with COPD: implications for disease pathogenesis and novel therapies. Lab Invest. 2019;99:150–7. https://doi.org/10.1038/s41374-018-0146-0.
Zhu L, Xu F, Kang X, et al. The antioxidant N-acetylcysteine promotes immune response and inhibits epithelial-mesenchymal transition to alleviate pulmonary fibrosis in chronic obstructive pulmonary disease by suppressing the VWF/p38 MAPK axis. Mol Med. 2021;27:97. https://doi.org/10.1186/s10020-021-00342-y.
Mahmood MQ, Reid D, Ward C, et al. Transforming growth factor (TGF) β1 and Smad signalling pathways: a likely key to EMT-associated COPD pathogenesis. Respirology. 2017;22:133–40. https://doi.org/10.1111/resp.12882.
Mahmood MQ, Walters EH, Shukla SD, et al. β-catenin, Twist and Snail: transcriptional regulation of EMT in smokers and COPD, and relation to airflow obstruction. Sci Rep. 2017;7:10832. https://doi.org/10.1038/s41598-017-11375-x.
Mahmood MQ, Ward C, Muller HK, et al. Epithelial mesenchymal transition (EMT) and non-small cell lung cancer (NSCLC): a mutual association with airway disease. Med Oncol. 2017;34:45. https://doi.org/10.1007/s12032-017-0900-y.
Rodgman A, Smith CJ, Perfetti TA. The composition of cigarette smoke: a retrospective, with emphasis on polycyclic components. Hum Exp Toxicol. 2000;19:573–95. https://doi.org/10.1191/096032700701546514.
Horiyama S, Kunitomo M, Yoshikawa N, Nakamura K. Mass spectrometric approaches to the identification of potential ingredients in cigarette smoke causing cytotoxicity. Biol Pharm Bull. 2016;39:903–8. https://doi.org/10.1248/bpb.b16-00032.
Talikka M, Sierro N, Ivanov NV, et al. Genomic impact of cigarette smoke, with application to three smoking-related diseases. Crit Rev Toxicol. 2012;42:877–89. https://doi.org/10.3109/10408444.2012.725244.
Soleimani F, Dobaradaran S, De-la-Torre GE, et al. Content of toxic components of cigarette, cigarette smoke vs cigarette butts: a comprehensive systematic review. Sci Total Environ. 2022;813:152667. https://doi.org/10.1016/j.scitotenv.2021.152667.
Aghapour M, Raee P, Moghaddam SJ, et al. Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: role of cigarette smoke exposure. Am J Respir Cell Mol Biol. 2018;58:157–69. https://doi.org/10.1165/rcmb.2017-0200TR.
Hecht SS, Hatsukami DK. Smokeless tobacco and cigarette smoking: chemical mechanisms and cancer prevention. Nat Rev Cancer. 2022;22:143–55. https://doi.org/10.1038/s41568-021-00423-4.
Benowitz NL, Liakoni E. Tobacco use disorder and cardiovascular health. Addiction. 2021. https://doi.org/10.1111/add.15703.
Vareille M, Kieninger E, Edwards MR, Regamey N. The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev. 2011;24:210–29. https://doi.org/10.1128/CMR.00014-10.
Nawroth JC, van der Does AM, Ryan Firth A, Kanso E. Multiscale mechanics of mucociliary clearance in the lung. Philos Trans R Soc Lond B Biol Sci. 2020;375:20190160. https://doi.org/10.1098/rstb.2019.0160.
Hikichi M, Mizumura K, Maruoka S, Gon Y. Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke. J Thorac Dis. 2019;11:S2129–40. https://doi.org/10.21037/jtd.2019.10.43.
Ma Y, Long Y, Chen Y. Roles of inflammasome in cigarette smoke-related diseases and physiopathological disorders: mechanisms and therapeutic opportunities. Front Immunol. 2021;12:720049. https://doi.org/10.3389/fimmu.2021.720049.
Brajer-Luftmann B, Nowicka A, Kaczmarek M, et al. Damage-associated molecular patterns and myeloid-derived suppressor cells in bronchoalveolar lavage fluid in chronic obstructive pulmonary disease patients. J Immunol Res. 2019;2019:9708769. https://doi.org/10.1155/2019/9708769.
Kopa PN, Pawliczak R. Effect of smoking on gene expression profile—overall mechanism, impact on respiratory system function, and reference to electronic cigarettes. Toxicol Mech Methods. 2018;28:397–409. https://doi.org/10.1080/15376516.2018.1461289.
Hung RJ, Hall J, Brennan P, Boffetta P. Genetic polymorphisms in the base excision repair pathway and cancer risk: a HuGE review. Am J Epidemiol. 2005;162:925–42. https://doi.org/10.1093/aje/kwi318.
Rennard SI. Inflammation and repair processes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:S12-16. https://doi.org/10.1164/ajrccm.160.supplement_1.5.
Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138:16–27. https://doi.org/10.1016/j.jaci.2016.05.011.
McGuinness AJA, Sapey E. Oxidative stress in COPD: sources, markers, and potential mechanisms. J Clin Med. 2017;6:E21. https://doi.org/10.3390/jcm6020021.
Taniguchi A, Tsuge M, Miyahara N, Tsukahara H. Reactive oxygen species and antioxidative defense in chronic obstructive pulmonary disease. Antioxidants (Basel). 2021;10:1537. https://doi.org/10.3390/antiox10101537.
Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9:377–84. https://doi.org/10.1038/nri2530.
Barnes PJ. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat Rev Drug Discov. 2013;12:543–59. https://doi.org/10.1038/nrd4025.
Zuo L, He F, Sergakis GG, et al. Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am J Physiol Lung Cell Mol Physiol. 2014;307:L205-218. https://doi.org/10.1152/ajplung.00330.2013.
Nasri A, Foisset F, Ahmed E, et al. Roles of mesenchymal cells in the lung: from lung development to chronic obstructive pulmonary disease. Cells. 2021;10:3467. https://doi.org/10.3390/cells10123467.
Shaykhiev R, Crystal RG. Early events in the pathogenesis of chronic obstructive pulmonary disease. Smoking-induced reprogramming of airway epithelial basal progenitor cells. Ann Am Thorac Soc. 2014;11 Suppl 5:S252-258. https://doi.org/10.1513/AnnalsATS.201402-049AW.
Yao R-Q, Ren C, Xia Z-F, Yao Y-M. Organelle-specific autophagy in inflammatory diseases: a potential therapeutic target underlying the quality control of multiple organelles. Autophagy. 2021;17:385–401. https://doi.org/10.1080/15548627.2020.1725377.
Aghapour M, Remels AHV, Pouwels SD, et al. Mitochondria: at the crossroads of regulating lung epithelial cell function in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2020;318:L149–64. https://doi.org/10.1152/ajplung.00329.2019.
Kanithi M, Junapudi S, Shah SI, et al. Alterations of mitochondrial network by cigarette smoking and E-cigarette vaping. Cells. 2022;11:1688. https://doi.org/10.3390/cells11101688.
Naiel S, Tat V, Padwal M, et al. Protein misfolding and endoplasmic reticulum stress in chronic lung disease: will cell-specific targeting be the key to the cure? Chest. 2020;157:1207–20. https://doi.org/10.1016/j.chest.2019.11.009.
Kelsen SG. The unfolded protein response in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13:S138–45. https://doi.org/10.1513/AnnalsATS.201506-320KV.
Bradley KL, Stokes CA, Marciniak SJ, et al. Role of unfolded proteins in lung disease. Thorax. 2021;76:92–9. https://doi.org/10.1136/thoraxjnl-2019-213738.
Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:155–61. https://doi.org/10.1164/rccm.201201-0034OC.
de-Torres JP, Wilson DO, Sanchez-Salcedo P, et al. Lung cancer in patients with chronic obstructive pulmonary disease. Development and validation of the COPD lung cancer screening score. Am J Respir Crit Care Med. 2015;191:285–91. https://doi.org/10.1164/rccm.201407-1210OC.
Goffin JR, Pond GR, Puksa S, et al. Chronic obstructive pulmonary disease prevalence and prediction in a high-risk lung cancer screening population. BMC Pulm Med. 2020;20:300. https://doi.org/10.1186/s12890-020-01344-y.
Ruparel M, Quaife SL, Dickson JL, et al. Prevalence, symptom burden, and underdiagnosis of chronic obstructive pulmonary disease in a lung cancer screening cohort. Ann Am Thorac Soc. 2020;17:869–78. https://doi.org/10.1513/AnnalsATS.201911-857OC.
Zheng Y, Huang Y, Zheng X, et al. Deaths from COPD in patients with cancer: a population-based study. Aging. 2021;13:12641–59. https://doi.org/10.18632/aging.202939.
Hopkins RJ, Duan F, Chiles C, et al. Reduced expiratory flow rate among heavy smokers increases lung cancer risk. Results from the National Lung Screening Trial-American College of Radiology Imaging Network Cohort. Ann Am Thorac Soc. 2017;14:392–402. https://doi.org/10.1513/AnnalsATS.201609-741OC.
Punturieri A, Szabo E, Croxton TL, et al. Lung cancer and chronic obstructive pulmonary disease: needs and opportunities for integrated research. J Natl Cancer Inst. 2009;101:554–9. https://doi.org/10.1093/jnci/djp023.
Vermaelen K, Brusselle G. Exposing a deadly alliance: novel insights into the biological links between COPD and lung cancer. Pulm Pharmacol Ther. 2013;26:544–54. https://doi.org/10.1016/j.pupt.2013.05.003.
Lee K, Cohn D, Liu Z, et al. Phenotypic and endotypic features of COPD associated with lung cancer development. JCO. 2022;40:e13563–e13563. https://doi.org/10.1200/JCO.2022.40.16_suppl.e13563.
Papi A, Casoni G, Caramori G, et al. COPD increases the risk of squamous histological subtype in smokers who develop non-small cell lung carcinoma. Thorax. 2004;59:679–81. https://doi.org/10.1136/thx.2003.018291.
Sohal SS, Mahmood MQ, Walters EH. Clinical significance of epithelial mesenchymal transition (EMT) in chronic obstructive pulmonary disease (COPD): potential target for prevention of airway fibrosis and lung cancer. Clin Transl Med. 2014;3:33. https://doi.org/10.1186/s40169-014-0033-2.
Caramori G, Ruggeri P, Mumby S, et al. Molecular links between COPD and lung cancer: new targets for drug discovery? Expert Opin Ther Targets. 2019;23:539–53. https://doi.org/10.1080/14728222.2019.1615884.
Eapen MS, Hansbro PM, Larsson-Callerfelt A-K, et al. Chronic obstructive pulmonary disease and lung cancer: underlying pathophysiology and new therapeutic modalities. Drugs. 2018;78:1717–40. https://doi.org/10.1007/s40265-018-1001-8.
Durham AL, Adcock IM. The relationship between COPD and lung cancer. Lung Cancer. 2015;90:121–7. https://doi.org/10.1016/j.lungcan.2015.08.017.
Jolly MK, Ward C, Eapen MS, et al. Epithelial–mesenchymal transition, a spectrum of states: role in lung development, homeostasis, and disease. Dev Dyn. 2018;247:346–58. https://doi.org/10.1002/dvdy.24541.
Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84. https://doi.org/10.1038/s41580-018-0080-4.
Kim DH, Xing T, Yang Z, et al. Epithelial mesenchymal transition in embryonic development, tissue repair and cancer: a comprehensive overview. J Clin Med. 2017;7:1. https://doi.org/10.3390/jcm7010001.
Knight DA, Grainge CL, Stick SM, et al. Epithelial mesenchymal transition in respiratory disease: fact or fiction. Chest. 2020;157:1591–6. https://doi.org/10.1016/j.chest.2019.12.014.
Carlier FM, Dupasquier S, Ambroise J, et al. Canonical WNT pathway is activated in the airway epithelium in chronic obstructive pulmonary disease. EBioMedicine. 2020;61:103034. https://doi.org/10.1016/j.ebiom.2020.103034.
Mahmood MQ, Sohal SS, Shukla SD, et al. Epithelial mesenchymal transition in smokers: large versus small airways and relation to airflow obstruction. Int J Chron Obstruct Pulmon Dis. 2015;10:1515–24. https://doi.org/10.2147/COPD.S81032.
Milara J, Peiró T, Serrano A, Cortijo J. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax. 2013;68:410–20. https://doi.org/10.1136/thoraxjnl-2012-201761.
Sohal SS, Reid D, Soltani A, et al. Reticular basement membrane fragmentation and potential epithelial mesenchymal transition is exaggerated in the airways of smokers with chronic obstructive pulmonary disease. Respirology. 2010;15:930–8. https://doi.org/10.1111/j.1440-1843.2010.01808.x.
Courtney J-M, Spafford PL. The role of epithelial–mesenchymal transition in chronic obstructive pulmonary disease. Cells Tissues Organs. 2017;203:99–104. https://doi.org/10.1159/000450919.
Koczulla A-R, Jonigk D, Wolf T, et al. Krüppel-like zinc finger proteins in end-stage COPD lungs with and without severe alpha1-antitrypsin deficiency. Orphanet J Rare Dis. 2012;7:29. https://doi.org/10.1186/1750-1172-7-29.
Wong SHM, Fang CM, Chuah L-H, et al. E-cadherin: its dysregulation in carcinogenesis and clinical implications. Crit Rev Oncol Hematol. 2018;121:11–22. https://doi.org/10.1016/j.critrevonc.2017.11.010.
Loh C-Y, Chai JY, Tang TF, et al. The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic implications, and challenges. Cells. 2019;8:E1118. https://doi.org/10.3390/cells8101118.
Gohy ST, Hupin C, Fregimilicka C, et al. Imprinting of the COPD airway epithelium for dedifferentiation and mesenchymal transition. Eur Respir J. 2015;45:1258–72. https://doi.org/10.1183/09031936.00135814.
Zhu J, Wang F, Feng X, et al. Family with sequence similarity 13 member A mediates TGF-β1-induced EMT in small airway epithelium of patients with chronic obstructive pulmonary disease. Respir Res. 2021;22:192. https://doi.org/10.1186/s12931-021-01783-z.
Ye R, Wang C, Sun P, et al. AGR3 regulates airway epithelial junctions in patients with frequent exacerbations of COPD. Front Pharmacol. 2021;12:669403. https://doi.org/10.3389/fphar.2021.669403.
Xu F, Liu X-C, Li L, et al. Effects of TRPC1 on epithelial mesenchymal transition in human airway in chronic obstructive pulmonary disease. Medicine (Baltimore). 2017;96:e8166. https://doi.org/10.1097/MD.0000000000008166.
Zheng L, Jiang Y-L, Fei J, et al. Circulatory cadmium positively correlates with epithelial-mesenchymal transition in patients with chronic obstructive pulmonary disease. Ecotoxicol Environ Saf. 2021;215:112164. https://doi.org/10.1016/j.ecoenv.2021.112164.
Shirahata T, Nakamura H, Nakajima T, et al. Plasma sE-cadherin and the plasma sE-cadherin/sVE-cadherin ratio are potential biomarkers for chronic obstructive pulmonary disease. Biomarkers. 2018;23:414–21. https://doi.org/10.1080/1354750X.2018.1434682.
Li Z, Li Y, Liu S, Qin Z. Extracellular S100A4 as a key player in fibrotic diseases. J Cell Mol Med. 2020;24:5973–83. https://doi.org/10.1111/jcmm.15259.
Qin H-Y, Li M-D, Xie G-F, et al. Associations among S100A4, Sphingosine-1-phosphate, and pulmonary function in patients with chronic obstructive pulmonary disease. Oxid Med Cell Longev. 2022;2022:6041471. https://doi.org/10.1155/2022/6041471.
Sohal SS, Reid D, Soltani A, et al. Evaluation of epithelial mesenchymal transition in patients with chronic obstructive pulmonary disease. Respir Res. 2011;12:130. https://doi.org/10.1186/1465-9921-12-130.
Usman S, Waseem NH, Nguyen TKN, et al. Vimentin is at the heart of epithelial mesenchymal transition (EMT) mediated metastasis. Cancers (Basel). 2021;13:4985. https://doi.org/10.3390/cancers13194985.
Nomura T, Huang W-C, Zhau HE, et al. β2-Microglobulin-mediated signaling as a target for cancer therapy. Anticancer Agents Med Chem. 2014;14:343–52. https://doi.org/10.2174/18715206113139990092.
Josson S, Nomura T, Lin J-T, et al. β2-microglobulin induces epithelial to mesenchymal transition and confers cancer lethality and bone metastasis in human cancer cells. Cancer Res. 2011;71:2600–10. https://doi.org/10.1158/0008-5472.CAN-10-3382.
Zhang A, Wang B, Yang M, et al. β2-microglobulin induces epithelial-mesenchymal transition in human renal proximal tubule epithelial cells in vitro. BMC Nephrol. 2015;16:60. https://doi.org/10.1186/s12882-015-0057-x.
Wang J, Yang W, Wang T, et al. Mesenchymal stromal cells-derived β2-microglobulin promotes epithelial-mesenchymal transition of esophageal squamous cell carcinoma cells. Sci Rep. 2018;8:5422. https://doi.org/10.1038/s41598-018-23651-5.
Gao N, Wang Y, Zheng C-M, et al. β2-Microglobulin participates in development of lung emphysema by inducing lung epithelial cell senescence. Am J Physiol Lung Cell Mol Physiol. 2017;312:L669–77. https://doi.org/10.1152/ajplung.00516.2016.
Wu Z, Yan M, Zhang M, et al. β2-microglobulin as a biomarker of pulmonary fibrosis development in COPD patients. Aging (Albany NY). 2020;13:1251–63. https://doi.org/10.18632/aging.202266.
Zheng X, Li W, Ren L, et al. The sphingosine kinase-1/sphingosine-1-phosphate axis in cancer: potential target for anticancer therapy. Pharmacol Ther. 2019;195:85–99. https://doi.org/10.1016/j.pharmthera.2018.10.011.
Riemma MA, Cerqua I, Romano B, et al. Sphingosine-1-phosphate/TGF-β axis drives epithelial mesenchymal transition in asthma-like disease. Br J Pharmacol. 2022;179:1753–68. https://doi.org/10.1111/bph.15754.
Milara J, Navarro R, Juan G, et al. Sphingosine-1-phosphate is increased in patients with idiopathic pulmonary fibrosis and mediates epithelial to mesenchymal transition. Thorax. 2012;67:147–56. https://doi.org/10.1136/thoraxjnl-2011-200026.
De Cunto G, Brancaleone V, Riemma MA, et al. Functional contribution of sphingosine-1-phosphate to airway pathology in cigarette smoke-exposed mice. Br J Pharmacol. 2020;177:267–81. https://doi.org/10.1111/bph.14861.
Sharma P, Nag A. CUL4A ubiquitin ligase: a promising drug target for cancer and other human diseases. Open Biol. 2014;4:130217. https://doi.org/10.1098/rsob.130217.
Wang Y, Wen M, Kwon Y, et al. CUL4A induces epithelial-mesenchymal transition and promotes cancer metastasis by regulating ZEB1 expression. Cancer Res. 2014;74:520–31. https://doi.org/10.1158/0008-5472.CAN-13-2182.
Cao P-P, Chen W-J, Pang H-L, et al. Effect of CUL4A on the metastatic potential of lung adenocarcinoma to the bone. Oncol Rep. 2020;43:662–70. https://doi.org/10.3892/or.2019.7448.
Ren Y, Zhang Y, Fan L, et al. The cullin4A is up-regulated in chronic obstructive pulmonary disease patient and contributes to epithelial-mesenchymal transition in small airway epithelium. Respir Res. 2019. https://doi.org/10.1186/s12931-019-1048-4.
Aschner Y, Downey GP. Transforming growth factor-β: master regulator of the respiratory system in health and disease. Am J Respir Cell Mol Biol. 2016;54:647–55. https://doi.org/10.1165/rcmb.2015-0391TR.
Ahmadi A, Najafi M, Farhood B, Mortezaee K. Transforming growth factor-β signaling: tumorigenesis and targeting for cancer therapy. J Cell Physiol. 2019;234:12173–87. https://doi.org/10.1002/jcp.27955.
Zhang YE. Non-Smad pathways in TGF-β signaling. Cell Res. 2009;19:128–39. https://doi.org/10.1038/cr.2008.328.
Katsuno Y, Derynck R. Epithelial plasticity, epithelial–mesenchymal transition, and the TGF-β family. Dev Cell. 2021;56:726–46. https://doi.org/10.1016/j.devcel.2021.02.028.
Kahata K, Dadras MS, Moustakas A. TGF-β family signaling in epithelial differentiation and epithelial-mesenchymal transition. Cold Spring Harb Perspect Biol. 2018;10:a022194. https://doi.org/10.1101/cshperspect.a022194.
Kasai H, Allen JT, Mason RM, et al. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respir Res. 2005;6:56. https://doi.org/10.1186/1465-9921-6-56.
Hackett T-L, Warner SM, Stefanowicz D, et al. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am J Respir Crit Care Med. 2009;180:122–33. https://doi.org/10.1164/rccm.200811-1730OC.
Xie L, Zhou D, Xiong J, et al. Paraquat induce pulmonary epithelial-mesenchymal transition through transforming growth factor-β1-dependent mechanism. Exp Toxicol Pathol. 2016;68:69–76. https://doi.org/10.1016/j.etp.2015.09.010.
Soltani A, Sohal SS, Reid D, et al. Vessel-associated transforming growth factor-beta1 (TGF-β1) is increased in the bronchial reticular basement membrane in COPD and normal smokers. PLoS ONE. 2012;7:e39736. https://doi.org/10.1371/journal.pone.0039736.
Shen H, Sun Y, Zhang S, et al. Cigarette smoke-induced alveolar epithelial-mesenchymal transition is mediated by Rac1 activation. Biochim Biophys Acta. 2014;1840:1838–49. https://doi.org/10.1016/j.bbagen.2014.01.033.
Guan S, Xu W, Han F, et al. Ginsenoside Rg1 attenuates cigarette smoke-induced pulmonary epithelial-mesenchymal transition via inhibition of the TGF-β1/Smad pathway. Biomed Res Int. 2017;2017:7171404. https://doi.org/10.1155/2017/7171404.
Lin L, Hou G, Han D, et al. Ursolic acid alleviates airway-vessel remodeling and muscle consumption in cigarette smoke-induced emphysema rats. BMC Pulm Med. 2019;19:103. https://doi.org/10.1186/s12890-019-0826-6.
Wu N, Wu Z, Sun J, et al. Small airway remodeling in diabetic and smoking chronic obstructive pulmonary disease patients. Aging (Albany NY). 2020;12:7927–44. https://doi.org/10.18632/aging.103112.
Pan K, Lu J, Song Y. Artesunate ameliorates cigarette smoke-induced airway remodelling via PPAR-γ/TGF-β1/Smad2/3 signalling pathway. Respir Res. 2021;22:91. https://doi.org/10.1186/s12931-021-01687-y.
Huang P, Yan R, Zhang X, et al. Activating Wnt/β-catenin signaling pathway for disease therapy: challenges and opportunities. Pharmacol Ther. 2019;196:79–90. https://doi.org/10.1016/j.pharmthera.2018.11.008.
Gonzalez DM, Medici D. Signaling mechanisms of the epithelial–mesenchymal transition. Sci Signal. 2014;7:re8. https://doi.org/10.1126/scisignal.2005189.
Wang Z, Li Z, Ji H. Direct targeting of β-catenin in the Wnt signaling pathway: current progress and perspectives. Med Res Rev. 2021;41:2109–29. https://doi.org/10.1002/med.21787.
Eapen MS, Sohal SS. WNT/β-catenin pathway: a novel therapeutic target for attenuating airway remodelling and EMT in COPD. EBioMedicine. 2020;62:103095. https://doi.org/10.1016/j.ebiom.2020.103095.
Zou W, Zou Y, Zhao Z, et al. Nicotine-induced epithelial-mesenchymal transition via Wnt/β-catenin signaling in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2013;304:L199–209. https://doi.org/10.1152/ajplung.00094.2012.
Su X, Chen J, Lin X, et al. FERMT3 mediates cigarette smoke-induced epithelial–mesenchymal transition through Wnt/β-catenin signaling. Respir Res. 2021;22:286. https://doi.org/10.1186/s12931-021-01881-y.
Deng S, Leong HC, Datta A, et al. PI3K/AKT signaling tips the balance of cytoskeletal forces for cancer progression. Cancers (Basel). 2022;14:1652. https://doi.org/10.3390/cancers14071652.
Kotelevets L, Chastre E. Rac1 signaling: from intestinal homeostasis to colorectal cancer metastasis. Cancers (Basel). 2020;12:E665. https://doi.org/10.3390/cancers12030665.
Jiang J-X, Zhang S-J, Shen H-J, et al. Rac1 signaling regulates cigarette smoke-induced inflammation in the lung via the Erk1/2 MAPK and STAT3 pathways. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1778–88. https://doi.org/10.1016/j.bbadis.2017.04.013.
Milara J, Peiró T, Serrano A, et al. Simvastatin increases the ability of roflumilast N-oxide to inhibit cigarette smoke-induced epithelial to mesenchymal transition in well-differentiated human bronchial epithelial cells in vitro. COPD. 2015;12:320–31. https://doi.org/10.3109/15412555.2014.948995.
Cho HJ, Baek KE, Saika S, et al. Snail is required for transforming growth factor-beta-induced epithelial–mesenchymal transition by activating PI3 kinase/Akt signal pathway. Biochem Biophys Res Commun. 2007;353:337–43. https://doi.org/10.1016/j.bbrc.2006.12.035.
Gui T, Sun Y, Shimokado A, Muragaki Y. The roles of mitogen-activated protein kinase pathways in TGF-β-induced epithelial-mesenchymal transition. J Signal Transduct. 2012;2012:289243. https://doi.org/10.1155/2012/289243.
Li NY, Weber CE, Wai PY, et al. An MAPK-dependent pathway induces epithelial-mesenchymal transition via Twist activation in human breast cancer cell lines. Surgery. 2013;154:404–10. https://doi.org/10.1016/j.surg.2013.05.012.
Weichhart T, Säemann MD. The PI3K/Akt/mTOR pathway in innate immune cells: emerging therapeutic applications. Ann Rheum Dis. 2008;67(Suppl 3):iii70-74. https://doi.org/10.1136/ard.2008.098459.
Jiang B, Guan Y, Shen H-J, et al. Akt/PKB signaling regulates cigarette smoke-induced pulmonary epithelial-mesenchymal transition. Lung Cancer. 2018;122:44–53. https://doi.org/10.1016/j.lungcan.2018.05.019.
Zinatizadeh MR, Schock B, Chalbatani GM, et al. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. 2021;8:287–97. https://doi.org/10.1016/j.gendis.2020.06.005.
Markopoulos GS, Roupakia E, Marcu KB, Kolettas E. Epigenetic regulation of inflammatory cytokine-induced epithelial-to-mesenchymal cell transition and cancer stem cell generation. Cells. 2019;8:E1143. https://doi.org/10.3390/cells8101143.
Budovsky A, Fraifeld VE, Aronov S. Linking cell polarity, aging and rejuvenation. Biogerontology. 2011;12:167–75. https://doi.org/10.1007/s10522-010-9305-4.
Zhao Y, Xu Y, Li Y, et al. NF-κB-mediated inflammation leading to EMT via miR-200c is involved in cell transformation induced by cigarette smoke extract. Toxicol Sci. 2013;135:265–76. https://doi.org/10.1093/toxsci/kft150.
Lu L, Xu H, Yang P, et al. Involvement of HIF-1α-regulated miR-21, acting via the Akt/NF-κB pathway, in malignant transformation of HBE cells induced by cigarette smoke extract. Toxicol Lett. 2018;289:14–21. https://doi.org/10.1016/j.toxlet.2018.02.027.
Ma L, Jiang M, Zhao X, et al. Cigarette and IL-17A synergistically induce bronchial epithelial-mesenchymal transition via activating IL-17R/NF-κB signaling. BMC Pulm Med. 2020;20:26. https://doi.org/10.1186/s12890-020-1057-6.
Zhou H, Liu Y, Wang Z, et al. CD147 promoted epithelial mesenchymal transition in airway epithelial cells induced by cigarette smoke via oxidative stress signaling pathway. COPD. 2020;17:269–79. https://doi.org/10.1080/15412555.2020.1758051.
Chang C-J, Lin C-F, Chen B-C, et al. SHP2: The protein tyrosine phosphatase involved in chronic pulmonary inflammation and fibrosis. IUBMB Life. 2022;74:131–42. https://doi.org/10.1002/iub.2559.
Li F-F, Shen J, Shen H-J, et al. Shp2 plays an important role in acute cigarette smoke-mediated lung inflammation. J Immunol. 2012;189:3159–67. https://doi.org/10.4049/jimmunol.1200197.
Liu Y-N, Guan Y, Shen J, et al. Shp2 positively regulates cigarette smoke-induced epithelial mesenchymal transition by mediating MMP-9 production. Respir Res. 2020;21:161. https://doi.org/10.1186/s12931-020-01426-9.
Whitaker RH, Cook JG. Stress relief techniques: p38 MAPK determines the balance of cell cycle and apoptosis pathways. Biomolecules. 2021;11:1444. https://doi.org/10.3390/biom11101444.
Jiang Z, Zhang Y, Zhu Y, et al. Cathelicidin induces epithelial-mesenchymal transition to promote airway remodeling in smoking-related chronic obstructive pulmonary disease. Ann Transl Med. 2021;9:223. https://doi.org/10.21037/atm-20-2196.
Babaei G, Aliarab A, Asghari Vostakolaei M, et al. Crosslink between p53 and metastasis: focus on epithelial-mesenchymal transition, cancer stem cell, angiogenesis, autophagy, and anoikis. Mol Biol Rep. 2021;48:7545–57. https://doi.org/10.1007/s11033-021-06706-1.
Wang W, Xiao L, Pan D, Hu L. ASF1B enhances migration and invasion of lung cancers cell via regulating the P53-mediated epithelial-mesenchymal transformation (EMT) signaling pathway. Neoplasma. 2022;69:361–9. https://doi.org/10.4149/neo_2021_210818N1181.
Liu X, Wang Z, Yang Q, et al. RNA demethylase ALKBH5 prevents lung cancer progression by regulating EMT and stemness via regulating p53. Front Oncol. 2022;12:858694. https://doi.org/10.3389/fonc.2022.858694.
Kawami M, Takenaka S, Akai M, et al. Characterization of miR-34a-induced epithelial-mesenchymal transition in non-small lung cancer cells focusing on p53. Biomolecules. 2021;11:1853. https://doi.org/10.3390/biom11121853.
Jung S, Kim DH, Choi YJ, et al. Contribution of p53 in sensitivity to EGFR tyrosine kinase inhibitors in non-small cell lung cancer. Sci Rep. 2021;11:19667. https://doi.org/10.1038/s41598-021-99267-z.
Smith LE, Denissenko MF, Bennett WP, et al. Targeting of lung cancer mutational hotspots by polycyclic aromatic hydrocarbons. J Natl Cancer Inst. 2000;92:803–11. https://doi.org/10.1093/jnci/92.10.803.
Bein K, Leikauf GD. Acrolein—a pulmonary hazard. Mol Nutr Food Res. 2011;55:1342–60. https://doi.org/10.1002/mnfr.201100279.
Le Calvez F, Mukeria A, Hunt JD, et al. TP53 and KRAS mutation load and types in lung cancers in relation to tobacco smoke: distinct patterns in never, former, and current smokers. Cancer Res. 2005;65:5076–83. https://doi.org/10.1158/0008-5472.CAN-05-0551.
Toyooka S, Tsuda T, Gazdar AF. The TP53 gene, tobacco exposure, and lung cancer. Hum Mutat. 2003;21:229–39. https://doi.org/10.1002/humu.10177.
Sun X, Shang J, Wu A, et al. Identification of dynamic signatures associated with smoking-related squamous cell lung cancer and chronic obstructive pulmonary disease. J Cell Mol Med. 2020;24:1614–25. https://doi.org/10.1111/jcmm.14852.
Mizuno S, Ishizaki T, Kadowaki M, et al. p53 signaling pathway polymorphisms associated with emphysematous changes in patients with COPD. Chest. 2017;152:58–69. https://doi.org/10.1016/j.chest.2017.03.012.
Arif E, Vibhuti A, Deepak D, et al. COX2 and p53 risk-alleles coexist in COPD. Clin Chim Acta. 2008;397:48–50. https://doi.org/10.1016/j.cca.2008.07.010.
Siganaki M, Koutsopoulos AV, Neofytou E, et al. Deregulation of apoptosis mediators’ p53 and bcl2 in lung tissue of COPD patients. Respir Res. 2010;11:46. https://doi.org/10.1186/1465-9921-11-46.
Sohal SS, Soltani A, Reid D, et al. A randomized controlled trial of inhaled corticosteroids (ICS) on markers of epithelial-mesenchymal transition (EMT) in large airway samples in COPD: an exploratory proof of concept study. Int J Chron Obstruct Pulmon Dis. 2014;9:533–42. https://doi.org/10.2147/COPD.S63911.
Raymakers AJN, Sadatsafavi M, Sin DD, et al. Inhaled corticosteroids and the risk of lung cancer in COPD: a population-based cohort study. Eur Respir J. 2019;53:1801257. https://doi.org/10.1183/13993003.01257-2018.
Kiri VA, Fabbri LM, Davis KJ, Soriano JB. Inhaled corticosteroids and risk of lung cancer among COPD patients who quit smoking. Respir Med. 2009;103:85–90. https://doi.org/10.1016/j.rmed.2008.07.024.
Parimon T, Chien JW, Bryson CL, et al. Inhaled corticosteroids and risk of lung cancer among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:712–9. https://doi.org/10.1164/rccm.200608-1125OC.
Ge F, Feng Y, Huo Z, et al. Inhaled corticosteroids and risk of lung cancer among chronic obstructive pulmonary disease patients: a comprehensive analysis of nine prospective cohorts. Transl Lung Cancer Res. 2021;10:1266–76. https://doi.org/10.21037/tlcr-20-1126.
Zheng J-P, Wen F-Q, Bai C-X, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2014;2:187–94. https://doi.org/10.1016/S2213-2600(13)70286-8.
Barnes PJ. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020;33:101544. https://doi.org/10.1016/j.redox.2020.101544.
Rennard SI, Calverley PMA, Goehring UM, et al. Reduction of exacerbations by the PDE4 inhibitor roflumilast–the importance of defining different subsets of patients with COPD. Respir Res. 2011;12:18. https://doi.org/10.1186/1465-9921-12-18.
Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol. 2011;163:53–67. https://doi.org/10.1111/j.1476-5381.2011.01218.x.
Cortijo J, Iranzo A, Milara X, et al. Roflumilast, a phosphodiesterase 4 inhibitor, alleviates bleomycin-induced lung injury. Br J Pharmacol. 2009;156:534–44. https://doi.org/10.1111/j.1476-5381.2008.00041.x.
Martorana PA, Beume R, Lucattelli M, et al. Roflumilast fully prevents emphysema in mice chronically exposed to cigarette smoke. Am J Respir Crit Care Med. 2005;172:848–53. https://doi.org/10.1164/rccm.200411-1549OC.
Milara J, Peiró T, Serrano A, et al. Roflumilast N-oxide inhibits bronchial epithelial to mesenchymal transition induced by cigarette smoke in smokers with COPD. Pulm Pharmacol Ther. 2014;28:138–48. https://doi.org/10.1016/j.pupt.2014.02.001.
Cardwell CR, Mc Menamin Ú, Hughes CM, Murray LJ. Statin use and survival from lung cancer: a population-based cohort study. Cancer Epidemiol Biomarkers Prev. 2015;24:833–41. https://doi.org/10.1158/1055-9965.EPI-15-0052.
Lin JJ, Ezer N, Sigel K, et al. The effect of statins on survival in patients with stage IV lung cancer. Lung Cancer. 2016;99:137–42. https://doi.org/10.1016/j.lungcan.2016.07.006.
Ms H, Ic C, Cp L, et al. Statin improves survival in patients with EGFR-TKI lung cancer: a nationwide population-based study. PLoS ONE. 2017;12:e0171137. https://doi.org/10.1371/journal.pone.0171137.
Seckl MJ, Ottensmeier CH, Cullen M, et al. Multicenter, phase III, randomized, double-blind, placebo-controlled trial of pravastatin added to first-line standard chemotherapy in small-cell lung cancer (LUNGSTAR). J Clin Oncol. 2017;35:1506–14. https://doi.org/10.1200/JCO.2016.69.7391.
Lee Y, Lee KH, Lee GK, et al. Randomized phase II study of afatinib plus simvastatin versus afatinib alone in previously treated patients with advanced nonadenocarcinomatous non-small cell lung cancer. Cancer Res Treat. 2017;49:1001–11. https://doi.org/10.4143/crt.2016.546.
Nishikawa S, Menju T, Takahashi K, et al. Statins may have double-edged effects in patients with lung adenocarcinoma after lung resection. Cancer Manag Res. 2019;11:3419–32. https://doi.org/10.2147/CMAR.S200819.
Raymakers A, Sin DD, Sadatsafavi M, et al. Statin use and lung cancer risk in chronic obstructive pulmonary disease patients: a population-based cohort study. Respir Res. 2020;21:118. https://doi.org/10.1186/s12931-020-01344-w.
Liu J-C, Yang T-Y, Hsu Y-P, et al. Statins dose-dependently exert a chemopreventive effect against lung cancer in COPD patients: a population-based cohort study. Oncotarget. 2016;7:59618–29. https://doi.org/10.18632/oncotarget.11162.
Mahmood N, Mihalcioiu C, Rabbani SA. Multifaceted role of the urokinase-type plasminogen activator (uPA) and its receptor (uPAR): diagnostic, prognostic, and therapeutic applications. Front Oncol. 2018;8:24. https://doi.org/10.3389/fonc.2018.00024.
Zhang Y, Xiao W, Jiang Y, et al. Levels of components of the urokinase-type plasminogen activator system are related to chronic obstructive pulmonary disease parenchymal destruction and airway remodelling. J Int Med Res. 2012;40:976–85. https://doi.org/10.1177/147323001204000316.
Wang Q, Wang Y, Zhang Y, et al. Involvement of urokinase in cigarette smoke extract-induced epithelial-mesenchymal transition in human small airway epithelial cells. Lab Invest. 2015;95:469–79. https://doi.org/10.1038/labinvest.2015.33.
Wang Q, Wang Y, Zhang Y, et al. The role of uPAR in epithelial-mesenchymal transition in small airway epithelium of patients with chronic obstructive pulmonary disease. Respir Res. 2013;14:67. https://doi.org/10.1186/1465-9921-14-67.
Schweitzer KS, Johnstone BH, Garrison J, et al. Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking. Am J Respir Crit Care Med. 2011;183:215–25. https://doi.org/10.1164/rccm.201001-0126OC.
Li X, Michaeloudes C, Zhang Y, et al. Mesenchymal stem cells alleviate oxidative stress-induced mitochondrial dysfunction in the airways. J Allergy Clin Immunol. 2018;141:1634-1645.e5. https://doi.org/10.1016/j.jaci.2017.08.017.
Chen T-Y, Liu C-H, Chen T-H, et al. Conditioned media of adipose-derived stem cells suppresses sidestream cigarette smoke extract induced cell death and epithelial–mesenchymal transition in lung epithelial cells. Int J Mol Sci. 2021;22:12069. https://doi.org/10.3390/ijms222112069.
Gao Y, Chu S, Zhang Z, Chen N. Hepataprotective effects of ginsenoside Rg1—a review. J Ethnopharmacol. 2017;206:178–83. https://doi.org/10.1016/j.jep.2017.04.012.
Huang Z, Zhang Z, Zhou C, et al. Epithelial–mesenchymal transition: the history, regulatory mechanism, and cancer therapeutic opportunities. MedComm. 2022;3:e144. https://doi.org/10.1002/mco2.144.
Herbertz S, Sawyer JS, Stauber AJ, et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Dev Ther. 2015;9:4479–99. https://doi.org/10.2147/DDDT.S86621.
Gunderson CC, Walker JL, Mannel RS, et al. A phase IB trial of paclitaxel and carboplatin + galunisertib in patients with uterine and ovarian carcinosarcoma. Gynecol Oncol. 2020;159:23. https://doi.org/10.1016/j.ygyno.2020.06.048.
Gueorguieva I, Tabernero J, Melisi D, et al. Population pharmacokinetics and exposure-overall survival analysis of the transforming growth factor-β inhibitor galunisertib in patients with pancreatic cancer. Cancer Chemother Pharmacol. 2019;84:1003–15. https://doi.org/10.1007/s00280-019-03931-1.
Giannelli G, Santoro A, Kelley RK, et al. Biomarkers and overall survival in patients with advanced hepatocellular carcinoma treated with TGF-βRI inhibitor galunisertib. PLoS ONE. 2020;15:e0222259. https://doi.org/10.1371/journal.pone.0222259.
Paller C, Pu H, Begemann DE, et al. TGF-β receptor I inhibitor enhances response to enzalutamide in a pre-clinical model of advanced prostate cancer. Prostate. 2019;79:31–43. https://doi.org/10.1002/pros.23708.
Chiang S-L, Velmurugan BK, Chung C-M, et al. Preventive effect of celecoxib use against cancer progression and occurrence of oral squamous cell carcinoma. Sci Rep. 2017;7:6235. https://doi.org/10.1038/s41598-017-06673-3.
Liu X, Wu Y, Zhou Z, et al. Celecoxib inhibits the epithelial-to-mesenchymal transition in bladder cancer via the miRNA-145/TGFBR2/Smad3 axis. Int J Mol Med. 2019;44:683–93. https://doi.org/10.3892/ijmm.2019.4241.
Watanabe Y, Imanishi Y, Ozawa H, et al. Selective EP2 and Cox-2 inhibition suppresses cell migration by reversing epithelial-to-mesenchymal transition and Cox-2 overexpression and E-cadherin downregulation are implicated in neck metastasis of hypopharyngeal cancer. Am J Transl Res. 2020;12:1096–113.
Xiong S, Huang W, Liu X, et al. Celecoxib synergistically enhances MLN4924-induced cytotoxicity and EMT inhibition via AKT and ERK pathways in human urothelial carcinoma. Cell Transplant. 2022;31:9636897221077920. https://doi.org/10.1177/09636897221077921.
Zaman A, Bivona TG. Targeting AXL in NSCLC. Lung Cancer (Auckl). 2021;12:67–79. https://doi.org/10.2147/LCTT.S305484.
Namba K, Shien K, Takahashi Y, et al. Activation of AXL as a preclinical acquired resistance mechanism against osimertinib treatment in EGFR-mutant non-small cell lung cancer cells. Mol Cancer Res. 2019;17:499–507. https://doi.org/10.1158/1541-7786.MCR-18-0628.
Zhou L, Liu X-D, Sun M, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. 2016;35:2687–97. https://doi.org/10.1038/onc.2015.343.
Chlapek P, Slavikova V, Mazanek P, et al. Why differentiation therapy sometimes fails: molecular mechanisms of resistance to retinoids. Int J Mol Sci. 2018;19:E132. https://doi.org/10.3390/ijms19010132.
Liang C, Qiao G, Liu Y, et al. Overview of all-trans-retinoic acid (ATRA) and its analogues: structures, activities, and mechanisms in acute promyelocytic leukaemia. Eur J Med Chem. 2021;220:113451. https://doi.org/10.1016/j.ejmech.2021.113451.
Bobal P, Lastovickova M, Bobalova J. The role of ATRA, natural ligand of retinoic acid receptors, on EMT-related proteins in breast cancer: minireview. Int J Mol Sci. 2021;22:13345. https://doi.org/10.3390/ijms222413345.
Zanetti A, Affatato R, Centritto F, et al. All-trans-retinoic acid modulates the plasticity and inhibits the motility of breast cancer cells: ROLE OF NOTCH1 AND TRANSFORMING GROWTH FACTOR (TGFβ). J Biol Chem. 2015;290:17690–709. https://doi.org/10.1074/jbc.M115.638510.
Cui J, Gong M, Fang S, et al. All-trans retinoic acid reverses malignant biological behavior of hepatocarcinoma cells by regulating miR-200 family members. Genes Dis. 2021;8:509–20. https://doi.org/10.1016/j.gendis.2019.12.012.
Blaschuk OW. Potential therapeutic applications of N-cadherin antagonists and agonists. Front Cell Dev Biol. 2022;10:866200. https://doi.org/10.3389/fcell.2022.866200.
Augustine CK, Yoshimoto Y, Gupta M, et al. Targeting N-cadherin enhances antitumor activity of cytotoxic therapies in melanoma treatment. Cancer Res. 2008;68:3777–84. https://doi.org/10.1158/0008-5472.CAN-07-5949.
Inui N, Sakai S, Kitagawa M. Molecular pathogenesis of pulmonary fibrosis, with focus on pathways related to TGF-β and the ubiquitin-proteasome pathway. Int J Mol Sci. 2021;22:6107. https://doi.org/10.3390/ijms22116107.
Skibba M, Drelich A, Poellmann M, et al. Nanoapproaches to modifying epigenetics of epithelial mesenchymal transition for treatment of pulmonary fibrosis. Front Pharmacol. 2020;11:607689. https://doi.org/10.3389/fphar.2020.607689.
Lv Q, Wang J, Xu C, et al. Pirfenidone alleviates pulmonary fibrosis in vitro and in vivo through regulating Wnt/GSK-3β/β-catenin and TGF-β1/Smad2/3 signaling pathways. Mol Med. 2020;26:49. https://doi.org/10.1186/s10020-020-00173-3.
Ihara H, Mitsuishi Y, Kato M, et al. Nintedanib inhibits epithelial–mesenchymal transition in A549 alveolar epithelial cells through regulation of the TGF-β/Smad pathway. Respir Investig. 2020;58:275–84. https://doi.org/10.1016/j.resinv.2020.01.003.
Acknowledgements
Not applicable.
Funding
This work was sponsored by the National Key Research and Development Program of China (Grant number 2016YFC1304003) and the Startup Fund for scientific research of Fujian Medical University (Grant number 2019QH2038).
Author information
Authors and Affiliations
Contributions
XS and WW conducted the literature review and wrote the draft. XS and ZZ created the figures and tables. WW, XL, and YZ edited the paper. YZ conceived the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Su, X., Wu, W., Zhu, Z. et al. The effects of epithelial–mesenchymal transitions in COPD induced by cigarette smoke: an update. Respir Res 23, 225 (2022). https://doi.org/10.1186/s12931-022-02153-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-022-02153-z