Abstract
Background
The mid-term respiratory sequelae in survivors of severe COVID-19 appear highly heterogeneous. In addition, factors associated with respiratory sequelae are not known. In this monocentric prospective study, we performed a multidisciplinary assessment for respiratory and muscular impairment and psychological distress 3 months after severe COVID-19. We analysed factors associated with severe persistent respiratory impairment, amongst demographic, COVID-19 severity, and 3-month assessment.
Methods
Patients with severe SARS-CoV-2 pneumonia requiring ≥ 4L/min were included for a systematic 3-month visit, including respiratory assessment (symptoms, lung function, CT scan), muscular evaluation (body composition, physical function and activity, disability), psychopathological evaluation (anxiety, depression, post-traumatic stress disorder-PTSD) and quality of life. A cluster analysis was performed to identify subgroups of patients based on objective functional measurements: DLCO, total lung capacity and 6-min walking distance (6MWD).
Results
Sixty-two patients were analysed, 39% had dyspnea on exercise (mMRC ≥ 2), 72% had DLCO < 80%, 90% had CT-scan abnormalities; 40% had sarcopenia/pre-sarcopenia and 31% had symptoms of PTSD. Cluster analysis identified a group of patients (n = 18, 30.5%) with a severe persistent (SP) respiratory impairment (DLCO 48 ± 12%, 6MWD 299 ± 141 m). This SP cluster was characterized by older age, severe respiratory symptoms, but also sarcopenia/pre-sarcopenia, symptoms of PTSD and markedly impaired quality of life. It was not associated with initial COVID-19 severity or management.
Conclusions and clinical implication
We identified a phenotype of patients with severe persistent respiratory and muscular impairment and psychological distress 3 months after severe COVID-19. Our results highlight the need for multidisciplinary assessment and management after severe SARS-CoV-2 pneumonia.
Trial registration The study was registered on ClinicalTrials.gov (May 6, 2020): NCT04376840
Similar content being viewed by others
Background
Almost 2 years after the onset of SARS-CoV-2 pandemic, identification of patients at risk of mid and long-term sequelae remains an unmet need. The first wave of SARS-CoV-2 infections hit the North-East of France in March 2020 and had a major impact in terms of hospitalizations and mortality. The severity of general and respiratory symptoms, the worrying radiological findings, the length of hospitalization, and the massive weight loss raised fears of COVID-19-induced long-term respiratory, muscular and psychologic sequelae.
Studies performed after other coronaviruses epidemics (SARS-CoV and MERS-CoV) identified mid and long-term impact on respiratory function, including altered diffusion lung capacity for carbon monoxide (DLCO) [1], and persistent radiological alterations 3 [1, 2] and 12 months after infection [3]. A major psychiatric impact has been identified in patients after SARS-CoV: 50% had anxiety, 20% had depression and/or sleep disturbance; post-traumatic stress syndrome was described [4, 5].
Little is known about mid and long-term respiratory and psychological sequelae in survivors of severe COVID-19 [6]. Recent studies including survivors of both severe and non-severe forms of COVID-19 identified impaired lung function 3–4 months after COVID-19, including a decrease in forced vital capacity (FVC), DLCO, total lung capacity (TLC), 6-min walking distance (6MWD), and impairment in respiratory muscle strength. These alterations were associated with the presence of pneumonia [7], the severity of COVID-19, the length of hospital stay [8], and the severity of CT scan score on admission [8]. Other studies observed similar dyspnea scores and pulmonary functions in intensive care unit (ICU) and non-ICU patients, although ICU patients had a higher prevalence of abnormal CT scan 3 months after discharge [9]. Therefore, mid-term clinical and functional sequelae of severe COVID-19 appear highly heterogeneous [10], and factors associated with mid-term sequelae in this population are not known.
In this monocentric prospective study, we used a multidisciplinary approach to assess both respiratory and psychological impairment 3 months after severe SARS-CoV-2 pneumonia. Given the observed impact of severe COVID-19 on weight loss and muscular weakness, we also analysed sarcopenia and performed functional assessments. Finally, we analysed factors associated with persistent respiratory impairment to identify a phenotype of “high-risk” patients to monitor closely.
Methods
Patients
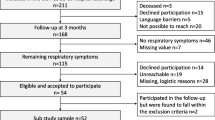
We prospectively included adult patients with severe SARS-CoV-2 pneumonia requiring hospitalization. Inclusion criteria were (1) confirmed diagnosis of SARS-CoV-2 infection via reverse transcription-polymerase chain reaction testing or suggestive computed tomography results with no alternative diagnosis and (2) patients with marked hypoxemia requiring oxygen therapy ≥ 4L/min to maintain oxygen saturation above 90%, high flow nasal oxygen or mechanical ventilation. Exclusion criteria included the inability to complete questionnaires or to perform lung function tests. Information was provided and patients were included for a systematic 3-month multidisciplinary assessment visit. Written informed consent was obtained for each patient. This study was approved by the French ethics committee (Comité de Protection des Personnes du Nord-Ouest IV, number 2020-A01260-39, 30 April 2020; NCT04376840).
Demographic data and medical history were recorded, as well as initial clinical, biological, and radiological characteristics of SARS-CoV-2 pneumonia and its therapeutic management.
3-month assessment
The respiratory assessment included respiratory symptoms, lung function, and CT scan. Briefly, dyspnea was assessed by the modified Medical Research Council (mMRC) breathlessness scale questionnaire, cough and sputum were assessed by the Cough And Sputum Assessment Questionnaire (CASA-Q). Pulmonary function tests were performed including TLC, FVC, FEV1, DLCO, 6MWD, and arterial blood gases at rest. A composite physiologic index (CPI) was calculated as previously described (CPI = 91 – (– 0.65 × DLCO %pred) – (0.53 × FVC %pred) + (0.35 × FEV1%pred)) [11].
A Thoracic CT scan was performed for all patients. The reconstructed 1 mm thick images were analysed by two independent investigators (JMP, SD) with a third investigator (GD) in case of disagreement. Final scores were established by consensus. Interstitial pneumonia was scored for each lung segment as previously described. The severity was classified into four grades (grade 0: no involvement; grade 1: 50% or less of the lung segment involved by residual ground-glass opacification with/without reticulation; grade 2: more than 50% lung segments involved by ground-glass opacification with/without reticulation; grade 3: evidence of lung fibrosis such as thick parenchymal bands, architectural distortion, traction bronchiectasis). In the statistical analysis, individual segmental scores were added together to give a summation score (maximum 54 points) [12].
The muscular evaluation included body composition measures, physical function and activity, and disability assessment. Briefly, total and regional lean, fat, and bone masses were evaluated using a whole-body Dual Energy X-absorptiometry scanner (Hologic, Horizon A). Appendicular lean mass index (ALMI) was used as a surrogate measure of total body muscle mass. Grip strength was measured using a hand dynamometer (JAMAR®) with a standardized position. SARC-F questionnaire was used as a sarcopenia screening tool [13]. Pre-sarcopenia was defined by a decrease in the ALMI or grip strength only [14]. Sarcopenia was defined by a decrease in the ALMI < 7 kg/m2 in men and < 5.5 kg/m2 in women and a decrease of grip strength < 27 kg in men and 18 kg in women according to the European Working Group on Sarcopenia in Older people (EWGSOP) [13]. Ricci and Gagnon test (RG; Montreal University, modified by Laureyns and Séné) was used for the screening of physical activity in daily life. RG score > 18 identifies active patients. Fatigue was screened by FACI-T questionnaire [15].
The psychopathological evaluations were performed using questionnaires assessing anxiety and depression (HAD) and post-traumatic stress disorder (PCLS). Scores ≥ 8 for depression (HAD-D) or anxiety (HAD-A), and ≥ 44 for PCLS were considered abnormal [16, 17]. Missing values were imputed by using the linear trend at point method (with a limit of 20% of missing values). Reliability were as follows: HAD-D, α = 0.86; HAD-A, α = 0.77; PCLS, α = 0.92.
Health-related quality of life was assessed using the 12-item Short-Form Health Survey (SF-12) and the Saint George’s Respiratory Questionnaire (SGRQ).
Statistical analysis
The primary objective was to describe respiratory, psychological, and muscular impairment 3 months after severe SARS-CoV-2 pneumonia. Secondary objectives were to identify factors associated with severe persistent respiratory impairment using cluster analysis, amongst demographic, initial COVID-19 severity, and 3-month assessment characteristics.
Data are expressed as mean ± standard deviation, median [interquartile range] depending on distribution, or number and percentages. Associations were analysed using the Student t-test, Wilcoxon test, Chi2 test, exact Fisher test, Pearson test, or Spearman test depending on the data nature and distribution. Analysis was performed using SPSS Statistics v27. p-value < 0.05 was considered significant.
A cluster analysis was performed to identify subgroups of patients according to clinical variables. We used the two-step clustering function provided by IBM-SPSS (version 27). This classification method automatically identified subgroups of patients using three variables depending on objective measurements of lung function: DLCO (% of predicted value), TLC (% of predicted value), and exercise capacity (6MWD, meters). At the first step, the log-likelihood distance was used to assign participants to the cluster leading to the largest log-likelihood. At the second step, the Bayesian Information Criterion (BIC) was used to assess multiple cluster solutions and automatically determine the optimum number of clusters. As clustering is sensitive to outliers and multicollinearity issues, we computed Mahalanobis distance for the DLCO, TLC, and 6MWD scores as well as variance inflation factors (VIFs). None of the participants were under the critical χ2 p-value (p < 0.001) on Mahalanobis distance; while VIFs were below the standard cut-off (≥ 2.5; range 1.22–1.69; [18]) indicating that there was no multicollinearity issue. Two patients did not perform DLCO measurements and 1 patient did not perform 6MWD measurement and therefore were not included in this analysis.
Results
Patients
A total of 65 patients were included. One patient was excluded (no severe pneumonia) and 2 patients did not perform a CT scan at 3 months. Sixty-two patients were analysed.
Patients were mainly men (63%), with a mean age of 63 ± 12 years. Comorbidities were frequent including cardiovascular disease (60%), hypertension (52%), obesity (34%), and diabetes mellitus (24%). The presence of previous respiratory comorbidities was uncommon (Table 1).
In March 2020 (corresponding to the local epidemic peak), no clear recommendation was available regarding drug treatment. All patients were treated with antibiotics and antithrombotic drugs, all but 2 received antiviral drugs, and 81% received systemic corticosteroids. All patients required oxygen supplementation ≥ 4L/min, 60% required hospitalization in ICU, 55% received high flow nasal oxygen therapy. Intubation and invasive mechanical ventilation were necessary for 19 patients (31%). The median hospitalization length was 19.5 days.
3-month respiratory and muscular impairment and psychological distress
Twenty-four (38.7%) patients described significant persistent dyspnea in daily living (mMRC ≥ 2), 45 (72%) had DLCO < 80%, 16 (26%) had DLCO < 60% (Table 2). Sarcopenia or pre-sarcopenia was identified in 25 patients (40.3%). The HAD questionnaire revealed significant symptoms of anxiety and depression among respectively 14.5% and 21% of the patients, 19 patients (30.6%) exhibited scores above the standard cut-off on the PCLS, suggesting potential post-traumatic-stress disorders (Table 2).
CT scan was performed with a mean of 118 ± 23 days after the onset of COVID-19 symptoms. Persistent abnormalities were identified in 90.3% of the patients, including ground glass and/or reticulations (38.7%), and fibrosis features (53.2%) characterized by thick parenchymal bands, architectural distortion, and/or traction bronchiectasis (Table 3, Fig. 1). CT scan was normal in 6 patients. When compared with the CT scan performed at admission (n = 47), the 3-month CT scan score improved in 42 cases (89.3%). The 3-month CT-score was not associated with CT-score at diagnosis (p = 0.181) (Table 3).
The severity of persistent CT scan abnormalities, as defined by the 3-month CT-score, was associated with a lower DLCO (p 0.02, r2 − 0.29) and exercise-induced oxygen desaturation (a drop of 4% or more in SpO2 or SpO2 < 90% during 6 MW test [19]; p = 0.011). CT-score tended to be associated with 6MWD (p = 0.089, r2 − 0.22) and CPI (p = 0.082, r2 − 0.25). CT-score was not associated with respiratory symptoms, FEV1, TLC, or PaO2 at rest (Additional file 1: Table S1).
Severe persistent respiratory impairment clustering
To determine factors associated with the most severe persistent respiratory impairment at 3 months, we next performed a 2-step clustering of patients depending on objective measurements of lung function and exercise capacity: TLC, DLCO, and 6MWD. This analysis resulted in 2 clusters: a “severe persistent respiratory impairment” cluster (SP, n = 18, 30.5%) and a “non severe persistent respiratory impairment” cluster (NSP, n = 41, 69.5%).
We first analysed patients’ respiratory phenotypes for each cluster (Table 4). Compared with the NSP cluster, patients in the SP cluster had more severe dyspnea in daily living and more frequent and severe cough. As expected, lung function (FEV1, FVC, TLC, DLCO) and CPI were significantly impaired as well as the 6MWD in the SP cluster. Compared to the NSP cluster, patients in the SP cluster had a lower PaO2 at rest and higher mean CT-score. Of notes, patients with the highest CT-scores at 3 months were mainly, but not exclusively, in the SP cluster (Fig. 2). The SP cluster also exhibited higher blood inflammation markers a 3 months and impaired quality of life (Table 4, Additional file 1: Table S2).
3-month CT-score (a) and CT-score change from admission (b) in patients in the severe persistent respiratory impairment (SP) cluster (red bars) and the non severe persistent respiratory impairment (NSP) cluster (green bars). Three patients were not included in the clustering analysis: DLCO was not available for 2 patients, 6MWD was not available for one patient (white bars; NA: not applicable)
The SP cluster was associated with more frequent sarcopenia or pre-sarcopenia, a lower median grip test, and decreased ALMI in men. No difference was observed in fat mass percentage, fatigue, or reported physical activity. Regarding psychological distress, patients in the SP cluster had more frequent post-traumatic stress symptoms (33.3%) when compared with the NSP group (10.3%, p = 0.042). Anxiety tended to be more frequent in the SP group (27.8% vs 10.0% in the NSP group, p = 0.084), while depression scores did not significantly differ between groups.
We next compared clusters depending on demographic data and COVID-19 initial features, severity, and management (Table 4, Additional file 1: Table S3). Compared with the NSP cluster, the SP cluster was characterized by older patients and a trend for more frequent diabetes mellitus. The initial clinical presentation did not differ between groups, except for a shorter delay between the first symptoms and admission in the SP group and more frequent diarrhea at diagnosis in the NSP group. The total length for the hospital stay tended to be longer in the SP group, but ICU hospitalization requirement and length as well as high flow nasal oxygen or invasive mechanical ventilation did not differ between groups. Patients in the SP group needed more frequently a post-acute hospitalization in a department of physical medicine and rehabilitation before discharge (p = 0.055).
Discussion
Using a multidisciplinary approach to assess respiratory, muscular, and psychological sequelae 3 months after severe SARS-CoV-2 pneumonia, we identified that patients frequently suffered from persistent respiratory symptoms, functional alteration, and CT-scan abnormalities. Impaired DLCO has been previously reported after severe COVID-19. In line with our results, DLCO < 80% was identified in 52% of patients requiring hospitalization [6, 20] and 76–82% of patients requiring ICU [20, 21], 1 to 4 months after COVID-19. DLCO < 60% was reported in 15.5% of patients in a population with less severe initial features of COVID-19 [6]. A very recent study analysing lung function 12 months after severe COVID-19 in patients that did not require mechanical ventilation showed improvement in DLCO over time. However, 33% of the patients in this study had persistent impaired DLCO < 80%. No associated factor was identified except female gender and initial CT peak score [10]. In our study, persistent CT-scan abnormalities were very frequent (90%); however, the majority of patients had an improved CT-score at 3 months. In a previous series of 62 patients requiring ICU hospitalization, 70% of patients had abnormal CT-scan at 3 months [21]. Fibrotic patterns were described in 21% of those patients [21]. In a recent review, around 50% of hospitalised survivors of COVID-19 had CT abnormalities 3 months after acute infection, including ground-glass opacity, parenchymal or subpleural bands, reticulation, fibrotic abnormality and air trapping [22]. In our study, we used a CT-scan score validated in the SARS-CoV epidemic [12], allowing a quantification and follow-up of CT-scan changes overtime, demonstrating a dramatic improvement of CT-scan at 3-month compared to admission. Of note, the term of post-COVID-19 lung fibrosis should be reserved for those with clear evidence of traction bronchiectasis, honeycombing or architectural distortion [23]. We cannot exclude in our study that some CT were classified as “fibrosis” because of thick parenchymal bands only which may overestimate the prevalence of fibrosis. A standardisation of post-COVID-19 CT-scan including a sub-classification of CT appearances such as predominantly ground glass, predominantly fibrotic or mixed ground glass and fibrotic [24] may help to better describe post-COVID lung complications. Whether those interstitial lung features including lung fibrosis will persist in the long term is currently not known and remains to be elucidated.
To analyse factors associated with the most severe respiratory abnormalities at 3 months, we performed clustering analysis based on lung function and exercise capacity and identified a cluster of patients, that we named severe persistent respiratory impairment. This cluster was characterized by marked respiratory functional impairment, decreased exercise capacity, and more pronounced CT-scan abnormalities. When analysing clinical features, we identified that this SP cluster was characterized by a clinical phenotype including older age, respiratory symptoms, frequent sarcopenia/pre-sarcopenia and symptoms of post-traumatic stress disorders, and markedly impaired quality of life. This SP cluster was not associated with initial COVID-19 features, except for less frequent diarrhea at diagnosis and a shorter delay from symptom onset to admission. It must be pointed out that all the patients in our study had a severe form of COVID-19.
We found that the patients in the SP cluster were significantly older than those in the NSP cluster. Older age is a recognised risk factor for severe Covid-19 [25]. However, associations between older age and mid-term respiratory impairment are not clear. Previous studies performed 3–4 months after severe forms of Covid-19 found that age was not associated with functional impairment, DLCO decrease [6], or CT score [21], but with tolerance to exercise, a younger age being associated with a perception of reduced tolerance to physical exercise [6]. Digestive symptoms appear common in the acute forms of Covid-19. In a recent study performed on 300 hospitalised patients, up to 83% of patients described diarrhea [26]. In this study, patients with digestive symptoms presented a longer delay from symptom onset to admission, similar to patients of the NSP group in our analysis. The presence of gastrointestinal symptoms was not associated with mortality in a meta-analysis including more than 55.000 patients with Covid-19 [27]. Associations between initial digestive symptoms and respiratory persistent impairment at 3 months were not described in previous studies.
In our study, sarcopenia/pre-sarcopenia was diagnosed in 67% of the SP group and 30% of the NSP group, much more frequent than in the general population of 60–70 years old (5 to 24%) [14, 28]. Sarcopenia is known to be associated with a poorer prognosis in chronic respiratory diseases [29]. In this population, sarcopenia is associated with manual strength [30, 31], which is a predictor of disability, morbidity, and mortality [32]. In our study, the manual strength was significantly impaired in the SP group when compared with the NSP group. The interpretation of those results may be limited by the unknown pre-COVID-19 sarcopenia/pre-sarcopenia status. However, our results are supported by those from a recent study performed in patients hospitalized for COVID-19, showing that a decrease in grip strength was associated with a severe form of infection [33]. Interestingly, we did not find any difference between fat mass and obesity status between the SP and the NSP groups. Furthermore, the differences observed in the body composition were not associated with physical activity, or the need for ICU care or intubation. Our results suggest that systematic strength assessment using the SARC-F questionnaire and grip test at SARS-COV-2 infection diagnosis and during follow-up may be helpful to identify patients at risk of an impaired outcome at mid-term after a severe COVID-19.
The SP cluster was further characterized by higher levels of psychological distress. More specifically, we found that these patients were more prone to display anxiety (28%) and post-traumatic stress symptoms (PTSS, 33%). A recent meta-analysis focusing on severe COVID-19 survivors found a pooled prevalence of PTSS of 16%, 4–16 weeks after COVID-19 diagnosis [34]. A possible PTSS deterioration between 3 and 6 months after COVID-19 has been suggested [35]. Our results suggest that the patients with severe persistent respiratory impairment may benefit from a close monitoring of mental wellness. Our results also suggest that severe persistent respiratory impairment could be a risk factor of psychological distress.
The persistence of this phenotype over time is a crucial yet unanswered question that requires further investigation. Given the design of our study, the patients were not systematically rescheduled for long-term reassessment, which represents a major limitation. Interestingly, two recent studies decribed 1-year follow-up features of severe hospitalized COVID-19 patients, showing frequent persistent fatigue, dyspnea, and quality of life impairment [36] and also DLCO alteration and persistent interstitial lung abnormalities especially in older patients requiring ventilatory support [37]. Our study is also limited by its single-center design, the potential selection bias of patients who consented to a 3-month assessment, the relatively low number of patients, the absence of pre-COVID-19 data regarding respiratory symptoms, pulmonary function, CT scan, sarcopenia and psychological symptoms, and the absence of systematic assessment of pulmonary arterial hypertension by echography. Despite these limitations, we think that the prospective design, the quantification of CT-scan using a validated CT-score and the cluster analysis provide original data in this field.
Conclusion
In our study, one-third of the patients exhibited severe persistent respiratory impairment 3-months after a severe SARS-CoV-2 pneumonia. These patients were characterized by a clinical phenotype associating impaired lung function and exercise capacity, respiratory symptoms, sarcopenia/pre-sarcopenia, post-traumatic stress disorders, and markedly impaired quality of life, which was not associated with initial COVID-19 features. Our results highlight the need for multidisciplinary assessment and management after the most severe cases of COVID-19. The persistence of respiratory and muscular impairment and psychological distress over time in those patients is currently under investigation.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Xie L, Liu Y, Fan B, Xiao Y, Tian Q, Chen L, et al. Dynamic changes of serum SARS-coronavirus IgG, pulmonary function and radiography in patients recovering from SARS after hospital discharge. Respir Res. 2005;6:5.
Das KM, Lee EY, Singh R, Enani MA, Al Dossari K, Van Gorkom K, et al. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imaging. 2017;27(3):342–9.
Hui DS, Wong KT, Ko FW, Tam LS, Chan DP, Woo J, et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128(4):2247–61.
Tsang HW, Scudds RJ, Chan EY. Psychosocial impact of SARS. Emerg Infect Dis. 2004;10(7):1326–7.
Sim K, Huak Chan Y, Chong PN, Chua HC, Wen SS. Psychosocial and coping responses within the community health care setting towards a national outbreak of an infectious disease. J Psychosom Res. 2010;68(2):195–202.
Bellan M, Soddu D, Balbo PE, Baricich A, Zeppegno P, Avanzi GC, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4(1):e2036142.
Anastasio F, Barbuto S, Scarnecchia E, Cosma P, Fugagnoli A, Rossi G, et al. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur Respir J. 2021;58(3):2004015.
Qin W, Chen S, Zhang Y, Dong F, Zhang Z, Hu B, et al. Diffusion capacity abnormalities for carbon monoxide in patients with COVID-19 at 3-month follow-up. Eur Respir J. 2021;58(1):200317.
Lerum TV, Aaløkken TM, Brønstad E, Aarli B, Ikdahl E, Lund KMA, et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J. 2021;57(4):20034418.
Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9(7):747–54.
Wells AU, Desai SR, Rubens MB, Goh NS, Cramer D, Nicholson AG, et al. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med. 2003;167(7):962–9.
Ng CK, Chan JW, Kwan TL, To TS, Chan YH, Ng FY, et al. Six month radiological and physiological outcomes in severe acute respiratory syndrome (SARS) survivors. Thorax. 2004;59(10):889–91.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23.
Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70.
Yao SN, Cottraux J, Note I, De Mey-Guillard C, Mollard E, Ventureyra V. Evaluation of Post-traumatic Stress Disorder: validation of a measure, the PCLS. Encephale. 2003;29(3 Pt 1):232–8.
Johnston R, Jones K, Manley D. Confounding and collinearity in regression analysis: a cautionary tale and an alternative procedure, illustrated by studies of British voting behaviour. Qual Quant. 2018;52(4):1957–76.
Waatevik M, Frisk B, Real FG, Hardie JA, Bakke P, Nilsen RM, et al. One Year Change in 6-Minute Walk Test Outcomes is Associated with COPD Prognosis. COPD. 2020;17(6):662–71.
Huang Y, Tan C, Wu J, Chen M, Wang Z, Luo L, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21(1):163.
González J, Benítez ID, Carmona P, Santisteve S, Monge A, Moncusí-Moix A, et al. Pulmonary Function and Radiologic Features in Survivors of Critical COVID-19: A 3-Month Prospective Cohort. Chest. 2021;8:67.
Solomon JJ, Heyman B, Ko JP, Condos R, Lynch DA. CT of post-acute lung complications of COVID-19. Radiology. 2021;301(2):E383–95.
Garg M, Maralakunte M, Dhooria S. Sequelae of COVID-19 pneumonia: Is it correct to label everything as post-COVID lung fibrosis? J Postgrad Med. 2021;67(4):224–7.
Mylvaganam RJ, Bailey JI, Sznajder JI, Sala MA, Consortium NCCC. Recovering from a pandemic: pulmonary fibrosis after SARS-CoV-2 infection. Eur Respir Rev. 2021;30(162):210194.
Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol. 2020;35(12):1123–38.
Hashem WM, Abdelaziz H, Sallam DE, Ismail MA, Ahmed AE. Impact of COVID-19 on digestive system: prevalence, clinical characteristics, outcome, and relation to the severity of COVID-19. Egypt J Intern Med. 2022;34(1):45.
Wang Y, Li Y, Zhang Y, Liu Y, Liu Y. Are gastrointestinal symptoms associated with higher risk of Mortality in COVID-19 patients? A systematic review and meta-analysis. BMC Gastroenterol. 2022;22(1):106.
Tournadre A, Vial G, Capel F, Soubrier M, Boirie Y. Sarcopenia. Joint Bone Spine. 2019;86(3):309–14.
Bone AE, Hepgul N, Kon S, Maddocks M. Sarcopenia and frailty in chronic respiratory disease. Chron Respir Dis. 2017;14(1):85–99.
Maddocks M, Kon SS, Canavan JL, Jones SE, Nolan CM, Labey A, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71(11):988–95.
Jones SE, Maddocks M, Kon SS, Canavan JL, Nolan CM, Clark AL, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax. 2015;70(3):213–8.
Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A, Orlandini A, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386(9990):266–73.
Tuzun S, Keles A, Okutan D, Yildiran T, Palamar D. Assessment of musculoskeletal pain, fatigue and grip strength in hospitalized patients with COVID-19. Eur J Phys Rehabil Med. 2021;57(4):653–62.
Nagarajan R, Krishnamoorthy Y, Basavarachar V, Dakshinamoorthy R. Prevalence of post-traumatic stress disorder among survivors of severe COVID-19 infections: A systematic review and meta-analysis. J Affect Disord. 2022;299:52–9.
Tu Y, Zhang Y, Li Y, Zhao Q, Bi Y, Lu X, et al. Post-traumatic stress symptoms in COVID-19 survivors: a self-report and brain imaging follow-up study. Mol Psychiatry. 2021;26:7475.
O’Brien K, Townsend L, Dowds J, Bannan C, Nadarajan P, Kent B, et al. 1-year quality of life and health-outcomes in patients hospitalised with COVID-19: a longitudinal cohort study. Respir Res. 2022;23(1):115.
Faverio P, Luppi F, Rebora P, D’Andrea G, Stainer A, Busnelli S, et al. One-year pulmonary impairment after severe COVID-19: a prospective, multicenter follow-up study. Respir Res. 2022;23(1):65.
Acknowledgements
The authors wish to thank Florent Paroche (psychologist) for his help in collecting psychopathological data among patients.
Funding
The study was supported by the University Hospital of Reims, France (Grant AOL 2020-COVID-19).
Author information
Authors and Affiliations
Contributions
Substantial contributions to the conception or design of the work: JMP, FG, LB, AK, JHS, GD. Acquisition, analysis or interpretation of the data: JMP, FG, LB, SD, SB, CL, MD, SC, EB, DP, ET, YNG, BM, AS, AR, CM, FL, AK, JHS, GD. Drafting the manuscript or revising it critically for important intellectual content: JMP, FG, LB, SD, SB, CL, MD, SC, EB, DP, ET, YNG, BM, AS, AR, CM, FL, AK, JHS, GD. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the French ethics committee (Comité de Protection des Personnes du Nord-Ouest IV, number 2020-A01260-39, 30 April 2020; NCT04376840). Written informed consent was obtained for each patient. All methods have been performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Associations between 3-month CT-score and 3-month respiratory assessment results. Table S2. 3-month patient’s characteristics in the severe persistent (SP) respiratory impairment cluster and in the non-severe persistent (NSP) respiratory impairment cluster. Table S3. Patient’s demographic characteristics and COVID-19 features at admission in the severe persistent (SP) respiratory impairment cluster and in the non-severe persistent (NSP) respiratory impairment cluster.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Perotin, JM., Gierski, F., Bolko, L. et al. Cluster analysis unveils a severe persistent respiratory impairment phenotype 3-months after severe COVID-19. Respir Res 23, 199 (2022). https://doi.org/10.1186/s12931-022-02111-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-022-02111-9