Abstract
Background
Rituximab (RTX) has been previously reported as directed treatment in patients with connective-tissue disease-related interstitial lung diseases (CTD-ILD). A systematic assessment of treatment effect size on pulmonary function outcomes and related adverse effects in patients with CTD-ILD has not been previously reported.
Methods
We performed a systematic review and meta-analysis of published reports from PubMed, Embase, and Cochrane Libraries. Randomized and non-randomized controlled trials, case–control, cohort, and case series (with five or more cases) containing individual pulmonary function data and adverse effects were included. Study endpoints were pre- and post-treatment change in percent predicted forced vital capacity (FVC %) and diffusion capacity for carbon monoxide (DLCO%), along with reported drug-related adverse events.
Results
Twenty studies totaling 411 patients were identified with 14 included in the meta-analysis of pulmonary function and six in the descriptive review. Random effects meta-analysis of pre- and post-treatment pulmonary function findings demonstrated increases in FVC% (n = 296) (mean difference (MD) 4.57%, [95% CI 2.63–6.51]) and DLCO% (n = 246) (MD 5.0% [95% CI 2.71–7.29]) after RTX treatment. RTX treatment-related adverse effects were reported in 13.6% of the pooled cohort.
Conclusions
A systematic assessment of post-treatment effect size suggests a potential role for RTX in stabilizing or improving lung function in patients with CTD-ILD, with a modest but not insignificant adverse effect profile.
Similar content being viewed by others
Introduction
The connective-tissue diseases (CTD) are commonly associated with initial or subsequent interstitial lung disease, frequently portending greater morbidity than CTD without lung involvement [1]. While nearly all CTD may be associated with ILD, systemic sclerosis (SSc), the idiopathic inflammatory myopathies (IIM), and rheumatoid arthritis (RA) report the highest prevalences [2,3,4]. Radiologic patterns and clinical manifestations vary according to subtype while pulmonary function is frequently characterized by restrictive ventilatory defect and reduced diffusing capacity[5]. Therapy for the majority of CTD-ILD often involves an extension of medications already aimed at the underlying CTD, typically consisting of corticosteroids and steroid-sparing agents like cyclophosphamide (CYC), azathioprine (AZA), and mycophenolate mofetil (MMF) [3]. Except for scleroderma-ILD, management of CTD-ILD remains experiential or empiric due to lack of robust randomized controlled trials (RCT). Heterogeneity of disease subtypes, unclear and variable outcome measures, and relatively better survival compared to other progressive ILD like idiopathic pulmonary fibrosis (IPF) make large controlled studies difficult, requiring expansive RCTs with longer durations to differentiate functional or survival outcomes. Pulmonary function endpoints are often followed as reasonable markers of treatment effect.
Rituximab (RTX), a B-cell depleting chimeric monoclonal antibody against human CD20, is currently approved for the treatment of lymphoma and RA. With prior evidence suggesting aberrations in lymphocyte function may be involved in the development and evolution of CTD [6], its use for the treatment of other CTD subtypes has gained recent interest. An initial report in 2008 involving patients with SSc-ILD [7] supported its particular role in CTD with associated ILD, particularly those with clinically severe or progressive lung disease unresponsive to conventional immunosuppression [8]. Multiple case reports, case series, and one clinical trial have reported on the positive effects of rituximab in CTD-ILD though the extent of its effect on measured pulmonary function has not been summatively reported. This systematic review and meta-analysis summarizes the pooled effect size of RTX on lung function (percent predicted forced vital capacity (FVC%) and diffusion capacity for carbon monoxide (DLCO%)) and describes reported safety outcomes in the treatment of CTD-ILD.
Materials and methods
The current systematic review and meta-analysis was conducted and reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) [9] guideline and statement. The Cochrane Handbook for Systematic Reviews of Interventions [10] provided the methodology for meta-analysis, and specific scoring of the included citations. The protocol was registered with ResearchRegistry (www.researchregistry.com; identifier reviewregistry1182).
Literature search and study selection
PubMed, Cochrane Library, and Embase databases were searched for original full articles published in English from their inception to March 20, 2021. Search terms, combinations, and results are presented in Additional file 1: Table S1. Studies were selected from case series reporting five or more cases, case–control, retrospective or prospective cohorts, and randomized or non-randomized controlled trials. Only adults (18 to 80 years of age) with a diagnosis of CTD-ILD were included. In terms of intervention, RTX was used individually or in combination with other immunosuppressive agents for at least six months or more (one cycle). Two reviewers (YZ and YG) assessed the titles and abstracts of all search results with pre-specified inclusion and exclusion criteria. If the abstract of an article suggested relevance, the article was retrieved and independently assessed by the same reviewers for inclusion in either the descriptive review or meta-analysis of pulmonary function outcomes.
Risk of bias assessment and overall quality of evidence
Risk of bias was assessed according to study type by the following: Cochrane collaboration tool for bias assessment in randomized controlled trials; Joanna Briggs Institute (JBI) critical appraisal checklist for quasi-experimental studies for nonrandomized observational studies; JBI critical appraisal checklist for cohort studies; and the JBI critical appraisal checklist for case series [10, 11]. Publication bias was assessed by funnel plot and Egger test.
Data extraction
Pre-specified study data included design, duration of follow-up, setting, and performance dates. Participant data included number, mean age and age range, sex, and CTD-ILD subtype. Intervention data included RTX dose and number and concomitant immunosuppression. Adverse events or complications attributed to RTX were also collated and categorized. Disagreements regarding study inclusion were resolved by consensus between study reviewers (YZ and YG).
Endpoints
Primary meta-analysis outcomes were mean differences (MD) in percent predicted pre- and post-treatment reported forced vital capacity (FVC%) and diffusion capacity for carbon monoxide (DLCO%). Included articles for meta-analysis required reporting of specific pre- and post-treatment lung function findings or the mean changes in FVC% or DLCO% from baseline and their calculated standard deviations. Post-treatment FVC% and DLCO% were defined as obtained six months or greater from the first dose of RTX. All RTX dosages and infusion regimens were included, with all patients treated for at least one course of treatment (6 months). Drug-related adverse events from all studies were reviewed.
Data analysis
Meta-analysis was performed using Comprehensive Meta-Analysis software version 3.3.070 (Biostat Inc, Englewood, NJ, USA). Descriptive analysis was conducted separately for non-combined studies (n = 6). Mean and standard deviation (SD) for changes in FVC% and DLCO% were calculated using the following equations from the Cochrane Handbook for Systematic Reviews of Interventions [10]:
R in the equation above represents a correlation coefficient for which we imputed values of 0.4, 0.6, and 0.8 with sensitivity analysis for each. As the pooled MD results were similar for each value, we used a coefficient of 0.4 for r in the meta-analysis. MD for FVC% and DLCO% were reported as mean changes from baseline with 95% CI. A random-effects model with DerSimonian-Laird estimator of between-study variance was used to estimate final MD for each endpoint given baseline differences in study type and patient characteristics [12]. Magnitude of treatment effect may also vary according to sample size, disease subtype, concomitant treatment, or other unaccounted covariables, with a random effects model assuming effect size may be similar but not identical across all included studies with the intent of reporting the pooled true effects. The I2 statistic was used to describe heterogeneity among the studies (I2 > 50% or P value < 0.10 for high heterogeneity). Meta-analysis data are presented as summary Forest plots for each PFT endpoint.
Results
Study selection and patient characteristics
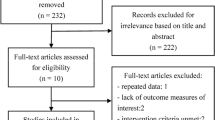
A total of 3806 individual citations were found and screened, resulting in the inclusion of 20 studies for systematic review, 14 of which were included specifically in the quantitative meta-analysis of treatment effect size on lung function [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. Study selection is presented in Fig. 1. Study exclusion on initial screening included non-English language, reporting of non-functional outcomes, reviews, editorials or commentary, incomplete manuscripts, lay press, and case reports with less than five cases. Tables 1 and 2 summarize study characteristics included in the meta-analysis (n = 14) and descriptive review (n = 6). Sample sizes ranged from seven to 56, totaling 411 patients. Study subtypes included case series (n = 13), retrospective cohorts (n = 5), one non-randomized trial, and one RCT. Six studies reported the combined outcomes of several CTD-ILD; five for SSc-ILD and antisynthetase syndrome-ILD (ASS-ILD); three for RA-ILD, and one for primary Sjögren’s-related-ILD (pSS). Summarized patient characteristics for the meta-analysis and descriptive reviews are presented in Table 3). There was female predominance for both analyses with a majority being treated for progressive ILD dominated by fibrosis unresponsive to initial immunosuppression. Thirty-three patients in one intervention study were randomized to RTX [21] as rationale for initiation with only ten patients from both analyses being provided RTX as first-line therapy [20, 33]. In most studies, RTX was administered as two 1000 mg infusions two weeks apart, or 375 mg/m2 weekly for 4 weeks. Each cycle could be repeated at six-month intervals with all included studies reporting on the outcomes of at least one treatment cycle. Steroid-sparing immunosuppressant and other therapies included AZA, MMF, CYC, methotrexate (MTX) and intravenous immunoglobulin (IVIG). Pulmonary function outcomes were measured at baseline and after at least a first RTX treatment period (six months) up to one year.
Effect size of RTX on FVC% and DLCO% endpoints
A total of 296 CTD-ILD patients from 14 studies with available pre- and post-treatment FVC% contributed to the quantitative meta-analysis (I2 = 0%, P = 0.94). There was an increase in FVC% before and after RTX treatment with a MD of 4.57% [95% CI, 2.63–6.51] (Fig. 2). Quantitative meta-analysis for DLCO% included 246 patients from ten studies (I2 = 10%, P = 0.34). There was an increase in DLCO% (MD 5.0% [95% CI 2.71–7.29]) after RTX treatment (Fig. 3).
Safety outcomes as reported for all studies
Infusion related reactions, including fever, chills, and rigors were the most reported adverse effects along with non-serious infections. Fifty six of 411 treated patients suffered some type of adverse effect (13.6%). As presented in Table 1, one study reported 12 non-serious chest infections[14] while eight studies reported zero events. There were no reported deaths as a direct result of RTX treatment.
Risk of bias and publication bias assessment
Systematic biases were scored for included studies and presented in Additional file 1: Table S2. A particular limitation for meta-analysis was the lack of RCT studies. As multiple reports with varied definitions of positive outcomes were available and our intent was to systematically review effect size, we included only those studies reporting specific baseline and post-treatment lung function. Many screened publications were also case reports with less than five patients, preemptively excluded due to smaller studies contributing greater bias. There did not appear to be publication bias for the two primary pulmonary function outcomes as represented by funnel plots (Figs. 4 and 5). The Egger’s regression asymmetry test demonstrated P values of 0.60 and 0.28 (P > 0.05 suggests no publication bias) for the outcomes of FVC% and DLCO%, respectively.
Analysis of studies included in the descriptive review
Six studies with various PFT outcomes but unreported specific pre and post treatment FVC% and DLCO% (with related standard deviation required for pooled analysis) that could not be combined were analyzed descriptively (Table 2). A cohort of 14 CTD-ILD patients who received more than one cycle of RTX found FVC% trajectory increased in eight and declined in six [23]. Another RA-ILD study found RTX treatment appeared to lower the risk of respiratory impairment (defined as a decline ≥ 5% in the predicted FVC) compared to untreated historical controls (hazard ratio, 0.51 [95% CI, 0.31–0.85]) [18]. Four case series reported RTX therapy in ASS-ILD. Andersson et al. showed that median FVC% and DLCO% increased by 24% and 17% respectively in 24 patients on RTX treatment for a median number of 2.7 cycles [20]. A case series of seven patients found that after one year of RTX treatment, median FVC% increased from 66% [range: 35–76] to 74% [range: 57–108] (P = 0.04); and median DLCO% increased from 39% [range: 20–57] to 59% [range: 49–72] (P = 0.001) [27]. Neither study was included in the quantitative meta-analysis due to the absence of reported and non-calculatable standard deviations for each PFT outcome. Allenbach et al. summarized the FVC findings of ten patients after 1.5 cycles of RTX, showing FVC improvement in four, stability in five, and worsening in one [17]. Lastly, a final study involving ASS-ILD found that after one cycle RTX, six of eleven patients showed > 10% improvement in FVC% and 3 had > 15% improvement in DLCO% [31].
Discussion
To our knowledge this is the first systematic review and meta-analysis to assess RTX effect size on FVC% (MD of 4.57%) and DLCO% (MD of 5.0%) in patients with CTD-ILD, reporting a low but not insignificant level of drug-related adverse effects (13.6% of the pooled cohort). Over 240 pooled patient observations were included for each functional endpoint suggesting RTX may modestly improve or stabilize lung function as an adjunct to traditional immunosuppression.
While the combined effect size on PFT outcomes for CTD-ILD was reported here, it is worth reviewing the individual characteristics and responses to RTX in reported specific diseases. SSc has the highest mortality associated with ILD [34] along with the highest ILD prevalence [35]. Incidentally, studies involving SSc-ILD patients were also the largest represented subtype in our meta-analysis. FVC% appeared to improve after RTX therapy in the combined meta-analysis [13, 15, 21, 22, 30] inclusive of the only RCT in our systematic review [19]. Divergent findings though were reported in 23 patients of a subgroup in the study by Lepri et al. [15]. Similarly, DLCO in SSc-ILD appeared to stabilize or improve with RTX treatment. The second largest group of represented CTD-ILD in our meta-analysis was ASS-ILD, whose disease-defining manifestations often include inflammatory myopathy, ILD, arthritis, and various hand manifestations [36, 37]. ILD prevalence ranges from 67 to 100% based on antibody type and the diagnostic criteria used [38, 39]. Our meta-analysis and descriptive review included five studies demonstrating FVC improvement or stability with RTX [17, 20, 26, 27, 31]. ILD is also an important comorbidity of RA often associated with similar outcomes to IPF, prompting novel approaches to treatment to improve or extend survival [40]. RTX has already been approved for the treatment of joint symptoms while there is less data on the treatment of related ILD. RTX demonstrated stabilization and in some cases, improvement of ILD in patients with RA [41]. All included studies in our meta-analysis suggested stabilization or improvement of FVC and DLCO [14, 18, 28]. ILD associated with primary Sjögren’s syndrome (pSS) occurs less commonly compared to other CTD though contributes to significant morbidity and mortality [42, 43]. RTX may be a promising treatment in this setting given the suggested role of B cell hyperactivity in the immunopathogenesis of pSS [44]. The study by Chen et al. in included in this meta-analysis suggests RTX may stabilize pulmonary function in patients with pSS [29].
Data for treatment of the other CTD-ILD with RTX remains limited. ILD prevalence in the idiopathic inflammatory myopathies (IIMs) is about 30–40% and contributes to an estimated mortality of 40% [45]. A recent systematic review suggested immunosuppressive therapies were associated with significant functional improvement for most patients with IIMs and chronic ILD, though the mortality of rapidly progressive disease remains high [46]. A case report of four patients on RTX therapy for rapidly progressive lung disease related to anti-MDA5 antibody-positive amyopathic dermatomyositis showed clinically significant improvement in lung function, though post-treatment infection risk was increased [47]. ILD is less common in systemic lupus erythematosus (SLE). A large multicenter observational cohort of 147 patients suggested RTX may be a possible maintenance option [48], though little data was provided regarding response of ILD findings to directed treatment. In contrast, there are reports of rituximab-induced interstitial pneumonitis seen in SLE patients [49].
Pooled analysis across a spectrum of CTD-ILD suggested a modest 4–5% increase in both FVC% and DLCO% after treatment with RTX compared to stabilization or slowing of prior decline. Similar effect in improved PFT findings were seen in prior observational and RCTs assessing CYC and MMF in patients with scleroderma-ILD [50,51,52,53], as well as azathioprine in one series of CTD patients with fibrotic ILD [54]. Specific effect sizes ranged from 1.5% to 15% in terms of FVC% change. In the majority of included studies for this meta-analysis, patients were considered non-responsive or refractory to typical immunosuppression, suggesting a separate role for the targeting of other immune-mediated or inflammatory processes for RTX. Current approval of anti-fibrotic therapy for progressive fibrotic lung disease including CTD-ILD warrants consideration as preferred secondary or tertiary therapies for lung fibrosis over RTX [55, 56]. Whether anti-fibrotics are preferred over RTX remains debatable but might be more justified by available controlled studies in progressive ILD over currently uncontrolled and mostly descriptive data for RTX [57]. Lack of controlled data though does not necessarily suggest evidence of inefficacy with future controlled studies needed to support or refute the role of RTX. Relevant decision making for clinicians and patients therefore may be geared more towards balancing quality of life vs. adverse effects. It is unclear from our meta-analyses if increases in FVC% or DLCO% of 4 to 5% compared to pre-treatment baseline is clinically impactful (in terms of symptomatic or radiologic improvement) or sustained with subsequent treatment. These remain important caveats to real-world management as RTX immunosuppression is often more prolonged and less immediately reversible. One study reported the direct effects of immunosuppressant treatment (not RTX) on patient-reported outcomes (PRO) and health-related quality of life in scleroderma-ILD patients, noting improvements in PRO scores meeting minimal clinically important differences, but little correlation with baseline or subsequent FVC change [58].
Additional concerns include cost and risk of serious adverse effects which may limit immediate or first-line use of RTX in the treatment of CTD-ILD. Our systematic review suggests RTX was overall well-tolerated and safe in the majority of treated CTD-ILD patients [59], including those on long-term therapy [60]. RTX-associated interstitial lung disease (RTX-ILD) or lung injury may be particularly concerning in those with already present lung disease. However, RTX-related lung injury was previously reported more commonly in combination with other chemotherapeutic agents for the treatment of lymphoma, which may confound accurate assessments of causation [61]. No direct RTX-related ILD or lung injury was reported in our review, highlighted by only a few serious adverse events due to infection with no therapy-related deaths.
There are several limitations to our systematic review and meta-analysis. First, variation in disease subtype and patient characteristics likely increased pooled heterogeneity and limits a true assessment of treatment effect size. We accounted for this with use of a random effects model and estimated the degree of heterogeneity for each endpoint, though still found I2 for example in the quantitative meta-analysis of FVC (I2 = 0%) was low and suggestive of little heterogeneity. It is known though that I2 does not necessarily describe how much an effect size varies but more what proportion of the observed variance would remain if all sampling error could be eliminated. When I2 is near zero dispersion in a forest plot may be minimal but does not suggest the absence of any heterogeneity, particularly when sample sizes in included studies were small with wider standard variations [62]. Additional limitations to our meta-analysis include the inability to account for duration of drug exposure, variation in timing of PFT follow-up, and the balance of CTD-ILD subtypes, of which pooled analyses may be weighed by one disease type over another. As presented in Tables 1 and 3, patients treated with RTX were often treated after or concomitantly with other immunosuppressive agents. CYC has previously demonstrated short-term improvement in FVC in SSc-ILD patients, though with a higher incidence of adverse effects [50, 63]. AZA as maintenance therapy after six months of CYC did not demonstrate significant FVC improvement in this same disease subtype [64]. MMF is thought to be safer and equally effective in the management of CTD-ILD when compared to CYC and AZA [50, 54]. We could not completely account for the role of concomitant therapy which may have also contributed to measured effect sizes. Lastly, pulmonary hypertension is common and well-described in CTD-ILD [65, 66]. Unfortunately, no included studies in our meta-analysis provided descriptions or assessments of pulmonary hypertension as assessed either by echocardiography or right heart catheterization. Its presence particularly in more severe disease may confound DLCO% measurements and degree of suggested response to treatment.
Conclusion
The present systematic review and meta-analysis suggests a modest stabilization or improvement in pulmonary function (FVC% and DLCO%) in CTD-ILD patients treated with RTX. There also appears to be a relatively safe adverse effects profile alone or in combination with other immunosuppressant agents. However, the lack of RCTs and other controlled quantitative studies along with heterogeneity of underlying diseases when data is pooled may pose important limitations for the confident use of RTX in real-world practice, with treatment initiation considered on a case-by-case basis.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- ASS:
-
Antisynthetase syndrome
- AZA:
-
Azathioprine
- CI:
-
Confidence interval
- CTD:
-
Connective-tissue disease
- CTD-ILD:
-
Connective-tissue disease-related interstitial lung disease
- CYC:
-
Cyclophosphamide
- DLCO%:
-
Percent predicted diffusion capacity for carbon monoxide
- FVC%:
-
Percent predicted forced vital capacity
- IIM:
-
Idiopathic interstitial pneumonia
- ILD:
-
Interstitial lung disease
- JBI:
-
Joanna Briggs Institute
- MMF:
-
Mycophenolate mofetil
- PFT:
-
Pulmonary function test
- PRISMA:
-
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- pSS:
-
Primary Sjögren’s syndrome
- RA:
-
Rheumatoid arthritis
- RTX:
-
Rituximab
- SSc:
-
Systemic sclerosis
- SLE:
-
Systemic lupus erythematosus
- WMD:
-
Weighted mean difference
References
Fischer A, du Bois R. Interstitial lung disease in connective tissue disorders. Lancet. 2012;380:689–98.
Castelino FV, Varga J. Interstitial lung disease in connective tissue diseases: evolving concepts of pathogenesis and management. Arthritis Res Ther. 2010;12:213.
Mathai SC, Danoff SK. Management of interstitial lung disease associated with connective tissue disease. BMJ. 2016;352: h6819.
Solomon JJ, Fischer A. Connective tissue disease-associated interstitial lung disease: a focused review. J Intensive Care Med. 2015;30:392–400.
Aduen JF, Zisman DA, Mobin SI, Venegas C, Alvarez F, Biewend M, Jolles HI, Keller CA. Retrospective study of pulmonary function tests in patients presenting with isolated reduction in single-breath diffusion capacity: implications for the diagnosis of combined obstructive and restrictive lung disease. Mayo Clin Proc. 2007;82:48–54.
Chizzolini C. T cells, B cells, and polarized immune response in the pathogenesis of fibrosis and systemic sclerosis. Curr Opin Rheumatol. 2008;20:707–12.
McGonagle D, Tan AL, Madden J, Rawstron AC, Rehman A, Emery P, Thomas S. Successful treatment of resistant scleroderma-associated interstitial lung disease with rituximab. Rheumatology (Oxford). 2008;47:552–3.
Keir GJ, Maher TM, Ming D, Abdullah R, de Lauretis A, Wickremasinghe M, Nicholson AG, Hansell DM, Wells AU, Renzoni EA. Rituximab in severe, treatment-refractory interstitial lung disease. Respirology. 2014;19:353–9.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6: e1000100.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019; 10:Ed000142.
Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7:7.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Daoussis D, Liossis SC, Tsamandas AC, Kalogeropoulou C, Paliogianni F, Sirinian C, Yiannopoulos G, Andonopoulos AP. Effect of long-term treatment with rituximab on pulmonary function and skin fibrosis in patients with diffuse systemic sclerosis. Clin Exp Rheumatol. 2012;30(2 Suppl 71): S17–22.
Md Yusof MY, Kabia A, Darby M, Lettieri G, Beirne P, Vital EM, Dass S, Emery P. Effect of rituximab on the progression of rheumatoid arthritis-related interstitial lung disease: 10 years’ experience at a single centre. Rheumatology (Oxford). 2017;56:1348–57.
Lepri G, Avouac J, Airo P, Anguita Santos F, Bellando-Randone S, Blagojevic J, Garcia Hernandez F, Gonzalez Nieto JA, Guiducci S, Jordan S, et al. Effects of rituximab in connective tissue disorders related interstitial lung disease. Clin Exp Rheumatol. 2016;34(Suppl 100):181–5.
Fitzgerald DB, Moloney F, Twomey M, O’Connell JO, Cronin O, Harty L, Harney S, Henry MT. Efficacy and safety of rituximab in connective tissue disease related interstitial lung disease. Sarcoidosis Vasc Diffuse Lung Dis. 2015;32:215–21.
Allenbach Y, Guiguet M, Rigolet A, Marie I, Hachulla E, Drouot L, Jouen F, Jacquot S, Mariampillai K, Musset L, et al. Efficacy of rituximab in refractory inflammatory myopathies associated with anti-synthetase auto-antibodies: an open-label, Phase II Trial. PLoS ONE. 2015;10: e0133702.
Vadillo C, Nieto MA, Romero-Bueno F, Leon L, Sanchez-Pernaute O, Rodriguez-Nieto MJ, Freites D, Jover JA, Alvarez-Sala JL, Abasolo L. Efficacy of rituximab in slowing down progression of rheumatoid arthritis-related interstitial lung disease: data from the NEREA Registry. Rheumatology (Oxford). 2020;59(8):2099–108.
Sircar G, Goswami RP, Sircar D, Ghosh A, Ghosh P. Intravenous cyclophosphamide vs rituximab for the treatment of early diffuse scleroderma lung disease: open label, randomized, controlled trial. Rheumatology (Oxford). 2018;57:2106–13.
Andersson H, Sem M, Lund MB, Aalokken TM, Gunther A, Walle-Hansen R, Garen T, Molberg O. Long-term experience with rituximab in anti-synthetase syndrome-related interstitial lung disease. Rheumatology (Oxford). 2015;54:1420–8.
Daoussis D, Melissaropoulos K, Sakellaropoulos G, Antonopoulos I, Markatseli TE, Simopoulou T, Georgiou P, Andonopoulos AP, Drosos AA, Sakkas L, Liossis SN. A multicenter, open-label, comparative study of B-cell depletion therapy with Rituximab for systemic sclerosis-associated interstitial lung disease. Semin Arthritis Rheum. 2017;46:625–31.
Sari A, Guven D, Armagan B, Erden A, Kalyoncu U, Karadag O, Apras Bilgen S, Ertenli I, Kiraz S, Akdogan A. Rituximab experience in patients with long-standing systemic sclerosis-associated interstitial lung disease: a series of 14 patients. J Clin Rheumatol. 2017;23:411–5.
Chartrand S, Swigris JJ, Peykova L, Fischer A. Rituximab for the treatment of connective tissue disease-associated interstitial lung disease. Sarcoidosis Vasc Diffuse Lung Dis. 2016;32:296–304.
Sharp C, McCabe M, Dodds N, Edey A, Mayers L, Adamali H, Millar AB, Gunawardena H. Rituximab in autoimmune connective tissue disease-associated interstitial lung disease. Rheumatology (Oxford). 2016;55:1318–24.
Duarte AC, Cordeiro A, Fernandes BM, Bernardes M, Martins P, Cordeiro I, Santiago T, Seixas MI, Ribeiro AR, Santos MJ. Rituximab in connective tissue disease-associated interstitial lung disease. Clin Rheumatol. 2019;38:2001–9.
Doyle TJ, Dhillon N, Madan R, Cabral F, Fletcher EA, Koontz DC, Aggarwal R, Osorio JC, Rosas IO, Oddis CV, Dellaripa PF. Rituximab in the treatment of interstitial lung disease associated with antisynthetase syndrome: a multicenter retrospective case review. J Rheumatol. 2018;45:841–50.
Marie I, Dominique S, Janvresse A, Levesque H, Menard JF. Rituximab therapy for refractory interstitial lung disease related to antisynthetase syndrome. Respir Med. 2012;106:581–7.
Fui A, Bergantini L, Selvi E, Mazzei MA, Bennett D, Pieroni MG, Rottoli P, Bargagli E. Rituximab Therapy in Interstitial Lung Disease associated with Rheumatoid Arthritis. Intern Med J. 2020;50(3):330–6.
Chen MH, Chen CK, Chou HP, Chen MH, Tsai CY, Chang DM. Rituximab therapy in primary Sjogren’s syndrome with interstitial lung disease: a retrospective cohort study. Clin Exp Rheumatol. 2016;34:1077–84.
Ebata S, Yoshizaki A, Fukasawa T, Miura S, Takahashi T, Sumida H, Asano Y, Sato S. Rituximab therapy is more effective than cyclophosphamide therapy for Japanese patients with anti-topoisomerase I-positive systemic sclerosis-associated interstitial lung disease. J Dermatol. 2019;46:1006–13.
Sem M, Molberg O, Lund MB, Gran JT. Rituximab treatment of the anti-synthetase syndrome: a retrospective case series. Rheumatology (Oxford). 2009;48:968–71.
Keir GJ, Maher TM, Hansell DM, Denton CP, Ong VH, Singh S, Wells AU, Renzoni EA. Severe interstitial lung disease in connective tissue disease: rituximab as rescue therapy. Eur Respir J. 2012;40:641–8.
Daoussis D, Liossis SC, Tsamandas AC, Kalogeropoulou C, Paliogianni F, Sirinian C, Yiannopoulos G, Andonopoulos AP. Effect of long-term treatment with rituximab on pulmonary function and skin fibrosis in patients with diffuse systemic sclerosis. Clin Exp Rheumatol. 2012;30:S17–22.
Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390:1685–99.
Morales-Cárdenas A, Pérez-Madrid C, Arias L, Ojeda P, Mahecha MP, Rojas-Villarraga A, Carrillo-Bayona JA, Anaya JM. Pulmonary involvement in systemic sclerosis. Autoimmun Rev. 2016;15:1094–108.
Cavagna L, Nuño L, Scirè CA, Govoni M, Longo FJ, Franceschini F, Neri R, Castañeda S, Sifuentes Giraldo WA, Caporali R, et al. Clinical spectrum time course in anti Jo-1 positive antisynthetase syndrome: results from an international retrospective multicenter study. Medicine (Baltimore). 2015;94: e1144.
Mirrakhimov AE. Antisynthetase syndrome: a review of etiopathogenesis, diagnosis and management. Curr Med Chem. 2015;22:1963–75.
Hamaguchi Y, Fujimoto M, Matsushita T, Kaji K, Komura K, Hasegawa M, Kodera M, Muroi E, Fujikawa K, Seishima M, et al. Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PLoS ONE. 2013;8: e60442.
Hervier B, Benveniste O. Clinical heterogeneity and outcomes of antisynthetase syndrome. Curr Rheumatol Rep. 2013;15:349.
Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, Vassallo R, Gabriel SE, Matteson EL. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62:1583–91.
Carrasco Cubero C, Chamizo Carmona E, Vela Casasempere P. Systematic review of the impact of drugs on diffuse interstitial lung disease associated with rheumatoid arthritis. Reumatol Clin. 2020. https://doi.org/10.1016/j.reumae.2020.04.010
Roca F, Dominique S, Schmidt J, Smail A, Duhaut P, Levesque H, Marie I. Interstitial lung disease in primary Sjogren’s syndrome. Autoimmun Rev. 2017;16:48–54.
Natalini JG, Johr C, Kreider M. Pulmonary Involvement in Sjögren Syndrome. Clin Chest Med. 2019;40:531–44.
Verstappen GM, van Nimwegen JF, Vissink A, Kroese FGM, Bootsma H. The value of rituximab treatment in primary Sjogren’s syndrome. Clin Immunol. 2017;182:62–71.
Connors GR, Christopher-Stine L, Oddis CV, Danoff SK. Interstitial lung disease associated with the idiopathic inflammatory myopathies: what progress has been made in the past 35 years? Chest. 2010;138:1464–74.
Barba T, Fort R, Cottin V, Provencher S, Durieu I, Jardel S, Hot A, Reynaud Q, Lega JC. Treatment of idiopathic inflammatory myositis associated interstitial lung disease: a systematic review and meta-analysis. Autoimmun Rev. 2019;18:113–22.
So H, Wong VTL, Lao VWN, Pang HT, Yip RML. Rituximab for refractory rapidly progressive interstitial lung disease related to anti-MDA5 antibody-positive amyopathic dermatomyositis. Clin Rheumatol. 2018;37:1983–9.
Cassia MA, Alberici F, Jones RB, Smith RM, Casazza G, Urban ML, Emmi G, Moroni G, Sinico RA, Messa P, et al. Rituximab as maintenance treatment for systemic lupus erythematosus: a multicenter observational study of 147 patients. Arthritis Rheumatol. 2019;71:1670–80.
Kishi J, Nanki T, Watanabe K, Takamura A, Miyasaka N. A case of rituximab-induced interstitial pneumonitis observed in systemic lupus erythematosus. Rheumatology (Oxford). 2009;48:447–8.
Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, Arriola E, Silver R, Strange C, Bolster M, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655–66.
Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, Goldin J, Arriola E, Volkmann ER, Kafaja S, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4:708–19.
Zamora AC, Wolters PJ, Collard HR, Connolly MK, Elicker BM, Webb WR, King TE Jr, Golden JA. Use of mycophenolate mofetil to treat scleroderma-associated interstitial lung disease. Respir Med. 2008;102:150–5.
Volkmann ER, Tashkin DP, Li N, Roth MD, Khanna D, Hoffmann-Vold AM, Kim G, Goldin J, Clements PJ, Furst DE, Elashoff RM. Mycophenolate mofetil versus placebo for systemic sclerosis-related interstitial lung disease: an analysis of scleroderma lung studies I and II. Arthritis Rheumatol. 2017;69:1451–60.
Oldham JM, Lee C, Valenzi E, Witt LJ, Adegunsoye A, Hsu S, Chen L, Montner S, Chung JH, Noth I, et al. Azathioprine response in patients with fibrotic connective tissue disease-associated interstitial lung disease. Respir Med. 2016;121:117–22.
Matteson EL, Kelly C, Distler JHW, Hoffmann-Vold AM, Seibold JR, Mittoo S, Dellaripa PF, Aringer M, Pope J, Distler O, et al. Nintedanib in patients with autoimmune disease-related progressive fibrosing interstitial lung diseases: subgroup analysis of the INBUILD trial. Arthritis Rheumatol. 2022;74:1039–47.
Wells AU, Flaherty KR, Brown KK, Inoue Y, Devaraj A, Richeldi L, Moua T, Crestani B, Wuyts WA, Stowasser S, et al. Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir Med. 2020;8:453–60.
Duarte AC, Vinagre F, Soares J, Cordeiro A. Antifibrotics in interstitial lung disease related to connective tissue diseases—a paradigm shift in treatment and outcome. Acta Reumatol Port. 2019;44:161–2.
Volkmann ER, Tashkin DP, LeClair H, Roth MD, Kim G, Goldin J, Clements PJ, Furst DE, Khanna D. Treatment with mycophenolate and cyclophosphamide leads to clinically meaningful improvements in patient-reported outcomes in scleroderma lung disease: results of scleroderma lung study II. ACR Open Rheumatol. 2020;2:362–70.
Kimby E. Tolerability and safety of rituximab (MabThera). Cancer Treat Rev. 2005;31:456–73.
Vikse J, Jonsdottir K, Kvaloy JT, Wildhagen K, Omdal R. Tolerability and safety of long-term rituximab treatment in systemic inflammatory and autoimmune diseases. Rheumatol Int. 2019;39:1083–90.
Hadjinicolaou AV, Nisar MK, Parfrey H, Chilvers ER, Ostör AJ. Non-infectious pulmonary toxicity of rituximab: a systematic review. Rheumatology (Oxford). 2012;51:653–62.
Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I(2) is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8:5–18.
Tashkin DP, Elashoff R, Clements PJ, Roth MD, Furst DE, Silver RM, Goldin J, Arriola E, Strange C, Bolster MB, et al. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med. 2007;176:1026–34.
Hoyles RK, Ellis RW, Wellsbury J, Lees B, Newlands P, Goh NS, Roberts C, Desai S, Herrick AL, McHugh NJ, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006;54:3962–70.
Mathai SC. Pulmonary hypertension associated with connective tissue disease. Cardiol Clin. 2022;40:29–43.
Lin CY, Ko CH, Hsu CY, Chen HA. Epidemiology and mortality of connective tissue disease-associated pulmonary arterial hypertension: a national cohort study in taiwan. Semin Arthritis Rheum. 2020;50:957–62.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
YZ and YG contributed equally to this work. YZ and YG performed the systematic review of databases, study data abstraction, and YZ, YG, TP, WC, CT, and XZ completed the meta-analysis. All authors contributed to manuscript writing. YZ and TM are guarantors of this manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Study was approved by Mayo Clinic Institutional Review Board prior to study initiation.
Consent for publication
None.
Competing interests
All authors report no related disclosures or conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Results of search terms and strategies. Table S2. Risk of biasassessment.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, Y., Gao, Y., Petnak, T. et al. Effect size of rituximab on pulmonary function in the treatment of connective-tissue disease-related interstitial lung disease: a systematic review and meta-analysis. Respir Res 23, 164 (2022). https://doi.org/10.1186/s12931-022-02082-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-022-02082-x