Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory airway disease caused by inhalation of cigarette smoke (CS) and other harmful gases and particles.
Methods
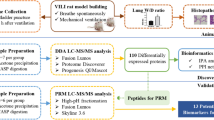
This study aimed to explore potential urinary biomarkers for CS-induced COPD based on LC–MS/MS analysis.
Results
A total of 340 urinary proteins were identified, of which 79 were significantly changed (30, 31, and 37 at week 2, 4 and 8, respectively). GO annotation of the differential urinary proteins revealed that acute-phase response, response to organic cyclic compounds, complement activation classical pathway, and response to lead ion were significantly enriched at week 2 and 4. Another four processes were only enriched at week 8, namely response to oxidative stress, positive regulation of cell proliferation, thyroid hormone generation, and positive regulation of apoptotic process. The PPI network indicated that these differential proteins were biologically connected in CS-exposed rats. Of the 79 differential proteins in CS-exposed rats, 56 had human orthologs. Seven proteins that had changed at week 2 and 4 when there were no changes of pulmonary function and pathological morphology were verified as potential biomarkers for early screening of CS-induced COPD by proteomic analysis. Another six proteins that changed at week 8 when obvious airflow obstruction was detected were verified as potential biomarkers for prognostic assessment of CS-induced COPD.
Conclusions
These results reveal that the urinary proteome could sensitively reflect pathological changes in CS-exposed rats, and provide valuable clues for exploring COPD biomarkers.
Similar content being viewed by others
Background
Chronic obstructive pulmonary disease (COPD) is a common, preventable, and treatable disease characterized by persistent respiratory symptoms and airflow limitation. It is an important public health challenge, and is now the third leading cause of death worldwide [1]. COPD is projected to continue to contribute to an increase in the overall worldwide burden of disease in the coming decades [2]. The airway and/or alveolar abnormalities were usually caused by exposure to cigarette smoking (CS) and other noxious gases or particles. The main mechanisms underlying COPD include amplified inflammation, oxidative stress, protease-antiprotease imbalance, and peribronchiolar and interstitial fibrosis [3, 4].
Despite increasing knowledge regarding COPD pathophysiology, substantial gaps remain regarding diagnosis and, in particular, early detection. Spirometry is by far the primary diagnostic approach, according to the criteria provided by the Global Initiative for Chronic Obstructive Lung Disease (GOLD), the American Thoracic Society (ATS), the European Respiratory Society (ERS), and the Japanese Respiratory Society (JRS). However, it cannot be reliably used as the only diagnostic test because of its weak specificity, and it is not recommended for the evaluation of airflow limitations when testing respiratory function in patients without respiratory symptoms [5]. Mounting evidence suggests that COPD is either underdiagnosed or misdiagnosed in approximately two-thirds of patients at risk of COPD [6]. Early COPD diagnosis has remained challenging due to the small impact of early lung function loss. In this study, we intend to explore new non-invasive biomarkers for the diagnosis of early COPD to enable timely and accurate interventions.
There is growing awareness of the need to identify new non-invasive biomarkers for the early screening and detection of COPD. In recent decades, mass spectrometry (MS)-based proteomics has dramatically improved and emerged as an important tool for identifying biomarkers. Several potential biomarkers of COPD have been described and categorized as primarily blood and sputum biomarkers [7, 8]. Some of these candidate blood protein biomarkers include C-reactive protein (CRP), fibrinogen, surfactant protein D, club cell protein 16, brain natriuretic peptide, soluble receptor for advanced glycation end-products and immunoglobulins [9], and lipocalins, matrix metalloproteinases, several inflammatory cytokines, and polymeric immunoglobulin receptor are some of the promising sputum biomarkers [8]. However, none of these candidate biomarkers could be successfully translated clinically. Shotgun proteomics of blood and sputum have been largely disappointing as the discovered protein biomarkers have lacked resolution or specificity to the condition.
Urine can be both sampled noninvasively and continuously. Moreover, compared with blood, urine proteome may reflect changes in disease progression at the early stage, for lack of mechanisms for maintaining homeostasis. Urinary proteomic studies have discovered several candidate biomarkers for pulmonary diseases, such as lung cancer [10], pulmonary fibrosis [11], and ventilation-induced lung injury [12]. The urinary proteome showed obvious changes even in the absence of clinical manifestations or histopathological damage to lung tissue, as in the bleomycin-induced pulmonary fibrosis rat model [11] and the ovalbumin (OVA)-induced asthma mouse model [13]. Therefore, the urinary proteome might sensitively reflect pathophysiological changes in the lung at an early stage and is a promising resource for studying the biomarkers of pulmonary diseases.
In this study, we establish a rat model of short-term CS exposure to simulate the pathogenesis of human early COPD [14]. We intend to explore potential urinary protein biomarkers to screen for early COPD based on proteomics technology.
Materials and methods
Animals
Male Wistar’s rats (weight range: 180–200 g; 8 weeks of age) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. The rats were acclimatized for 1 week before the experiment. The animal experiments were reviewed and approved by Qingdao Municipal Hospital Medical Ethics Committee. All methods were carried out in accordance with relevant guidelines and regulations of the National Health Commission and the Ministry of Science and Technology and performed in accordance with the guidelines for animal research.
Model establishment
The rats with COPD-like lung disease were established by the CS method. The rats were randomly divided into a control group (room air-exposed, n = 18) and a CS group (CS-exposed, n = 18). Commercial non-filtered cigarettes (trade name: DA QIAN MEN) containing 11 mg tar and 0.8 mg nicotine per cigarette were used in this study. In detail, three rats were kept in a chamber with size of 36 cm (length) × 20 cm (width) × 28 cm (height) and exposed to successive periods of CS at a rate of approximately 10 min per cigarette. At intervals of 1 min, the smoke of a new cigarette was delivered into the chamber and 6 cigarettes for 1 h in the morning and 6 cigarettes 1 h in the afternoon for 6 days per week. After exposure, the rats were returned to their cages. Control animals (sham group) inhaled clean (filtered) air only. All of the rats were maintained throughout the study in specific-pathogen-free conditions ventilated with clean air at 20–25 °C. The lights were on a 12-h cycle. Water and diets were provided ad libitum, excluding the CS exposure period.
Pulmonary function test and lung histopathology
Pulmonary function was evaluated by the AniRes2005 animal lung function analysis system (Beijing Beilanbo Technology). The forced vital capacity (FVC) and forced expiratory volume in 0.3 s (FEV0.3), expiratory resistance (RE), and dynamic lung compliance (Cdy) were measured, and the ration of FEV0.3/FVC was calculated.
The lung was harvested at week 2, 4 and 8 and fixed in 4% paraformaldehyde for 24 h. The fixed tissues were embedded in paraffin, sectioned at 4 μm and stained with hematoxylin and eosin (HE) and alcian blue-periodic acid-Schiff (AB-PAS) to reveal histopathological lesions.
Urine collection and sample preparation
Urine samples from the COPD rat model induced by smoking were taken at week 2, 4 and 8. After collection, the urine was centrifuged at 4 °C for 30 min at 3000×g and then at 12,000×g to remove pellets. Three volumes of ethanol (− 20 °C precooling) were added to the supernatant, which was shaken well and then precipitated in a – 20 °C refrigerator overnight. The next day, the urine was centrifuged at 4 °C at 12,000×g for 30 min, and the supernatant was discarded. The pellet was then resuspended in lysis buffer (8 M urea, 2 M thiourea, 50 mM Tris, and 25 mM DTT). The protein concentrations were measured using the Bradford method. Proteins were digested with trypsin (Trypsin Gold, 122 Mass Spec Grade, Promega, Fitchburg, Wisconsin, USA) using filter-aided sample 123 preparation methods [15]. The peptide mixtures were desalted using Oasis HLB cartridges (Waters, Milford, MA) and dried by vacuum evaporation.
Liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS) analysis
The digested peptides were acidified with 0.1% formic acid and then loaded onto a reversed-phase micro-capillary column using the Thermo EASY-nLC 1200 HPLC system. The MS data were acquired using the Thermo Orbitrap Fusion Lumos (Thermo Fisher Scientific, Bremen, Germany). The elution gradient for the analytical column was 95% mobile phase A (0.1% formic acid; 99.9% water) to 40% mobile phase B (0.1% formic acid; 89.9% acetonitrile) over 60 min at a flow rate of 300 nL/min.
Label-free proteome quantification
The LC–MS/MS results were analyzed using Mascot software and Progenesis software. The database used was the SwissProt_Rat database (8091 sequences). The search conditions were trypsin digestion, fixed modification: carbamidomethylation of cysteines, variable modification: oxidation of ethionine, and the tolerances of the parent ion and fragment ion were both 0.02 Da. After normalization, the mass spectrometry peak intensity was used to analyze differential proteins between the control and CS groups.
Bioinformatic analysis
GO analysis was performed on the 79 differential urinary proteins identified in CS-induced COPD rat model (http://www.geneontology.org/) [16, 17]. In this study, significant GO enrichment was defined at p < 0.05. STRING database (http://www.string-db.org) was used to construct protein–protein interaction (PPI) networks. The database of known and predicted protein interactions, including direct (physical) and indirect (functional) associations.
The ‘Wu Kong’ platform (https://www.omicsolution.org/wkomics/main/) was used for statistical analysis. The differential proteins were selected using one-way ANOVA, and p-values were adjusted using the Benjamini–Hochberg method. Significance was set at a fold change of 1.5 and a p-value of < 0.05.
Results
Characterization of CS-induced COPD in rats
There was no difference in the baseline body-weight between the two groups. However, the body weight of the CS group was reduced compared to the control group after CS exposure for 3 weeks, (p < 0.01, Fig. 1a). FEV0.3/FVC were significantly lower in the CS group than that in the control group on week 8, indicating CS exposure caused obvious airflow obstruction compared with room-air exposure controls (p < 0.01, Fig. 1b).
Clinical characterization of the cigarette smoke-induced COPD rat model. a Body weight changes in the CS-induced COPD rat model (n = 12, *p < 0.01); b Pulmonary function in rats (n = 10, *p < 0.01); d HE staining of alveolar tissue at an original magnification 200 × ; d AB-PAS staining for mucus expression in the epithelium of the bronchus at an original magnification 200 ×
The H&E staining showed bronchial epithelial detachment and expansion and rupture of the alveolar space after CS exposure for 4 weeks. The rat lung bronchial epithelial cells were denatured, adhered, and partially detached and alveolar wall thinning occurred, while the alveolar space expanded, ruptured or had bullae formed in it after 8 weeks CS exposure (Fig. 1c). AB-PAS staining showed bronchial epithelial goblet cells in the rats became larger after 4 weeks CS exposure. And the number of goblet cells (in blue) in the bronchial epithelium was dramatically elevated and the size of goblet cells was enlarged with hypertrophy and hypersecretion after 8 weeks CS exposure (Fig. 1D).
Dynamic urinary proteome changes in CS-induced COPD rats
After LC–MS/MS analysis, 340 urinary proteins were identified with at least 2 unique peptides (FDR 1%). All identification and quantitation details are listed in Additional file 1: Table S1. Among these, 79 proteins were significantly changed (fold change-1.5, p < 0.05), and 56 proteins had human orthologs. There were 30, 31, and 37 differential proteins after CS exposure for 2, 4 and 8 weeks, respectively, (Table 1). The overlap of the differential proteins identified at different COPD stages is shown as a Venn diagram (Fig. 2).
Gene ontology (GO) analysis of the differential proteins
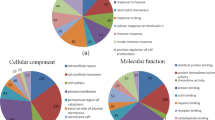
GO enrichment analysis was performed on the 79 differential urinary proteins in CS-induced COPD rats. GO revealed that these differential proteins were involved in the regulation of a host of biological processes (Fig. 3). Five biological processes were enriched at week 2 and 4 only, namely acute-phase response, response to organic cyclic compound, complement activation classical pathway, and response to lead ion. Another five biological processes were only enriched at week 8, namely positive regulation of acute inflammatory response, response to oxidative stress, positive regulation of cell proliferation, thyroid hormone generation, and positive regulation of the apoptotic process.
Most of the differential urinary proteins were associated with extracellular exosomes, extracellular space, blood microparticle, and extracellular region among the cellular components (Fig. 3). In the molecular function category, endopeptidase inhibitor activity, serine-type endopeptidase inhibitor activity, cysteine-type endopeptidase inhibitor activity, protease binding, and peptidase activity were overrepresented at week 2 and 4. The steroid binding, antigen binding, endopeptidase activity, and metallodipeptidase activity were overrepresented at week 8 (Fig. 3). These results indicate that the urine proteome can reflect biological responses in the body during the progression of CS exposure.
Protein–protein interaction (PPI) network of the differential proteins
To better understand the pathogenic mechanisms in COPD, the PPI network of the 79 differential proteins was constructed by STRING (Fig. 4). The number of included nodes was 68, the details were listed in Additional file 1: Table S2. The average node degree is 4.29, and the average local clustering coefficient is 0.491 (p < 1.0e–16). The results revealed that the differential urinary proteins had more intra- and intermolecular interactions than expected for a random set of proteins of similar size, drawn from the genome. Such an enrichment indicates that the differential proteins are closely biologically connected as a group.
Urinary candidate biomarker for CS-induced COPD
To find more reliable urinary differential proteins associated with CS-induced COPD, the twenty remaining urine samples were validated by LC–MS/MS. Thirteen urinary proteins with human orthologs were verified as potential biomarkers of CS-induced COPD (Table 2). Of these 13 candidate biomarkers, eight proteins have been reported as biomarkers of certain diseases. In addition, four proteins are known to be associated with COPD, namely urokinase-type plasminogen activator, plasminogen, fibronectin, and trefoil factor 2. At week 2 and 4, seven differential proteins were verified as early screening biomarkers of CS-induced COPD, when no obvious changes in pulmonary function or pathological morphology were observed. At week 8, six differential proteins were verified as diagnostic biomarkers of CS-induced COPD when obvious airflow obstruction compared with room-air exposure controls was detected.
Discussion
COPD is a prevalent respiratory disease showing an annual increase in morbidity and mortality rates. Given the prevalence and negative impact of comorbidities in individuals with COPD, early screening and detection that leads to meaningful interventions may improve patients’ outcomes and quality of life. However, early COPD diagnosis has remained challenging due to small impact of early lung function loss. In this study, we aimed to explore potential urinary biomarkers for CS-induced COPD. Overall, we systematically investigated dynamic changes in urinary proteome in a CS-induced COPD rat model for the first time based on proteomics analysis. A total of 340 urinary proteins were identified, of which 79 were significantly changed (30, 31, and 37 at week 2, 4 and 8, respectively). And 13 urinary proteins with human orthologs were verified as potential biomarkers for CS-induced COPD (Table 2). Of these 13 candidate biomarkers, eight proteins have been reported as biomarkers of certain diseases, and four proteins are known to be associated with COPD.
At week 2 and 4, seven differential proteins were verified as early screening biomarkers of CS-induced COPD, when no obvious changes in pulmonary function and pathological morphology were observed. Of these seven early screening biomarkers, Fn has been reported as a serum biomarker of COPD [7, 18]. Fn is a high molecular weight glycoprotein that is present in the body as two major isomers: a soluble circulating form and an insoluble extracellular matrix isomer [19]. Although serum Fn has many functions, its primary role is to promote wound repair following injury or infection by mediating cellular adhesion, motility, differentiation, apoptosis and hemostasis [20]. Using immunohistochemical analysis, the expression of Fn in bronchial vessels has been negatively correlated with FEV1 values in patients with COPD [21]. Moreover, in a study of 4787 subjects with mild-to-moderate COPD, Man et al. observed that the circulating Fn to CRP ratio was independently associated with all-cause mortality of the COPD patients at more than 7 years follow-up [18]. In the current study, the urinary Fn content increased nearly twofold in 2 weeks in CS exposed rats, indicating that urinary Fn may be a promising biomarker for early screening of COPD.
In addition, Plg and uPA have also been implicated in the progression of COPD [22]. The plasminogen activator system, including Plg, uPA, tPA and PAI-1, have diverse functions related to the inflammatory response in mammals [23]. Following injury, Plg extravasates into lung tissue, and cleavage of Plg to plasmin by uPA, stimulates inflammatory and epithelial cell cytokine production and mesenchymal cell proliferation. Immunohistochemical staining analysis has revealed marked elevation of uPA expression in the small airway epithelia of COPD patients by [24]. According to an in vitro study, upregulation of uPA expression might modulate the small airway remodeling in COPD by promoting epithelial-mesenchymal transition [25]. Our previous study revealed that urinary Plg increased 1.6-fold in OVA-induced asthma mice compared to controls [13]. In the current study, the expressions of uPA and Plg in the urinary proteome were both up-regulated nearly twofold in the CS group at week 2 and 4.
At week 8, six differential proteins were verified as diagnostic biomarkers of CS-induced COPD, when obvious airflow obstruction compared with room-air exposure controls was detected. Of these six diagnostic biomarkers, TFF2 has been reported as a serum and a bronchioalveolar lavage fluid (BAL) biomarker of COPD [26, 27]. TFFs 1, 2 and 3 are co-secreted with mucin throughout the body and proposed to be involved in tissue regeneration, proliferation and protection [28]. Mounting evidence shows that TFFs have therapeutic potential in lung disease, and TFF2 promoted repair and had anti-apoptotic effects on epithelial cells in the lung [29]. Viby et al. reported a fourfold increase of TFF2 in serum samples from COPD patients; however, a similar increase was not detected in the sputum [26]. Subsequently, they reported an increased level of TFF2 in BAL fluids from COPD patients and a positive correlation between the level of TFF2 and lung function [27]. Interestingly, we found that the urinary concentration of TFF2 was extremely down-regulated in CS-induced COPD rats, which suggests that the excretion of TFF2 is reduced upon the CS exposure. Taken together, we speculated that the exogenous delivery of TFF2 may prevent the progression of COPD, but this hypothesis needs further investigation.
Furthermore, we compared the urinary differential protein profile of CS-induced COPD with those of OVA-induced asthma [13]. We only identified five differential proteins shared by these two disease states, and these proteins shared a common change trend. The five differential proteins are alpha-1-antiproteinase, beta-2-microglobulin, plasminogen, protein AMBP and haptoglobin. This may be because OVA-induced asthma is a more TH2 based eosinophilic inflammation, while in the CS-induced COPD model is more TH1 based inflammation. Of the 13 candidate biomarkers for CS-induced COPD, eight proteins have been reported as biomarkers of certain diseases (Table 2). And most of these diseases were mainly Th2 response, such as lung adenocarcinoma, hepatocellular carcinoma, ovarian cancer, breast cancer, colon cancer, pancreatic cancer. Taken together, the urinary proteome could be a promising resource for studies of COPD biomarkers.
As a preliminary study, we found candidate urinary biomarkers associated with the development of COPD based on a CS-exposed rat model. Variables that impact clinical samples, such as medication, surgery, and patients’ living habits, were excluded. In the future, proteomics studies in large derivation, along with validation cohorts of patients with well-phenotype COPD and other obstructive lung diseases, may be required for the translation of urinary biomarkers into clinical settings. Furthermore, a combined panel of biomarkers capturing different pathways related to COPD pathophysiology may be required for use in clinical practice.
Conclusions
In conclusion, we investigated dynamic changes of urinary proteome in CS-induced COPD rat model. Our results reveal that urinary proteome could sensitively reflect pathophysiological changes in the development of COPD, and may advance the knowledge of pathogenesis of COPD in CS exposure. More important, we identified candidate urinary biomarkers which may be utilized for early screening, diagnosis and/or prognosis of COPD.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BAL:
-
Bronchioalveolar lavage fluid
- COPD:
-
Chronic obstructive pulmonary disease
- CS:
-
Cigarette smoking
- Fn:
-
Fibronectin
- LC–MS/MS:
-
Liquid chromatography coupled with tandem mass spectrometry
- PPI:
-
Protein–protein interaction
- Plg:
-
Plasminogen
- uPA:
-
Urokinase-type plasminogen activator
- TFF2:
-
Trefoil factor 2
References
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380:2095–128.
Halpin DMG, Celli BR, Criner GJ, Frith P, Lopez Varela MV, Salvi S, Vogelmeier CF, Chen R, Mortimer K, Montes de Oca M, et al. The GOLD Summit on chronic obstructive pulmonary disease in low- and middle-income countries. Int J Tuberc Lung Dis. 2019;23:1131–41.
Lange P, Ahmed E, Lahmar ZM, Martinez FJ, Bourdin A. Natural history and mechanisms of COPD. Respirology. 2021;26:298–321.
Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298:L715-731.
Hashimoto N, Wakahara K, Sakamoto K. The importance of appropriate diagnosis in the practical management of chronic obstructive pulmonary disease. Diagnostics (Basel). 2021;11:618.
Laucho-Contreras ME, Cohen-Todd M. Early diagnosis of COPD: myth or a true perspective. Eur Respir Rev. 2020;29:200131.
Serban KA, Pratte KA, Bowler RP. Protein biomarkers for COPD outcomes. Chest. 2021;159:2244–53.
Moon JY, Leitao Filho FS, Shahangian K, Takiguchi H, Sin DD. Blood and sputum protein biomarkers for chronic obstructive pulmonary disease (COPD). Expert Rev Proteomics. 2018;15:923–35.
Li F, Xu D, Wang J, Jing J, Li Z, Jin X. Comparative proteomics analysis of patients with quick development and slow development Chronic Obstructive Pulmonary Disease (COPD). Life Sci. 2020;256: 117829.
Zhang C, Leng W, Sun C, Lu T, Chen Z, Men X, Wang Y, Wang G, Zhen B, Qin J. Urine proteome profiling predicts lung cancer from control cases and other tumors. EBioMedicine. 2018;30:120–8.
Wu J, Li X, Zhao M, Huang H, Sun W, Gao Y. Early detection of urinary proteome biomarkers for effective early treatment of pulmonary fibrosis in a rat model. Proteomics Clin Appl. 2017;11:1700103.
Qin W, Zhang X, Chen L, Li Q, Zhang B, Sun L, Han W. Differential urine proteome analysis of a ventilator-induced lung injury rat model by label-free quantitative and parallel reaction monitoring proteomics. Sci Rep. 2021;11:21446.
Qin W, Wang T, Liu G, Sun L, Han W, Gao Y. Dynamic urinary proteome changes in ovalbumin-induced asthma mouse model using data-independent acquisition proteomics. J Asthma Allergy. 2021;14:1355–66.
Nie YC, Wu H, Li PB, Luo YL, Zhang CC, Shen JG, Su WW. Characteristic comparison of three rat models induced by cigarette smoke or combined with LPS: to establish a suitable model for study of airway mucus hypersecretion in chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2012;25:349–56.
Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359–62.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. Nat Genet. 2000;25:25–9.
Consortium TGO. The gene ontology resource: 20 years and still GOing strong. Nucleic Acids Res. 2019;47:D330–8.
Man SF, Xing L, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Zhang X, Vessey R, Walker TG, Celli BR, Sin DD. Circulating fibronectin to C-reactive protein ratio and mortality: a biomarker in COPD? Eur Respir J. 2008;32:1451–7.
Limper AH, Roman J. Fibronectin. A versatile matrix protein with roles in thoracic development, repair and infection. Chest. 1992;101:1663–73.
Mosesson MW, Amrani DL. The structure and biologic activities of plasma fibronectin. Blood. 1980;56:145–58.
Kranenburg AR, Willems-Widyastuti A, Moori WJ, Sterk PJ, Alagappan VK, de Boer WI, Sharma HS. Enhanced bronchial expression of extracellular matrix proteins in chronic obstructive pulmonary disease. Am J Clin Pathol. 2006;126:725–35.
Schuliga M, Westall G, Xia Y, Stewart AG. The plasminogen activation system: new targets in lung inflammation and remodeling. Curr Opin Pharmacol. 2013;13:386–93.
Baker SK, Strickland S. A critical role for plasminogen in inflammation. J Exp Med. 2020; 217.
Zhang Y, Xiao W, Jiang Y, Wang H, Xu X, Ma D, Chen H, Wang X. Levels of components of the urokinase-type plasminogen activator system are related to chronic obstructive pulmonary disease parenchymal destruction and airway remodelling. J Int Med Res. 2012;40:976–85.
Wang Q, Wang Y, Zhang Y, Zhang Y, Xiao W. Involvement of urokinase in cigarette smoke extract-induced epithelial-mesenchymal transition in human small airway epithelial cells. Lab Invest. 2015;95:469–79.
Viby NE, Pedersen L, Lund TK, Kissow H, Backer V, Nexo E, Thim L, Poulsen SS. Trefoil factor peptides in serum and sputum from subjects with asthma and COPD. Clin Respir J. 2015;9:322–9.
Viby NE, Nexo E, Kissow H, Andreassen H, Clementsen P, Thim L, Poulsen SS. Trefoil factors (TFFs) are increased in bronchioalveolar lavage fluid from patients with chronic obstructive lung disease (COPD). Peptides. 2015;63:90–5.
White SR. Trefoil peptides in airway epithelium: an important addition to the plethora of peptides. Am J Respir Cell Mol Biol. 2001;25:401–4.
Royce SG, Moodley Y, Samuel CS. Novel therapeutic strategies for lung disorders associated with airway remodelling and fibrosis. Pharmacol Ther. 2014;141:250–60.
Su SC, Lin CW, Yang WE, Fan WL, Yang SF. The urokinase-type plasminogen activator (uPA) system as a biomarker and therapeutic target in human malignancies. Expert Opin Ther Targets. 2016;20:551–66.
Zhu C, Jiang L, Xu J, Ren A, Ju F, Shu Y. The urokinase-type plasminogen activator and inhibitors in resectable lung adenocarcinoma. Pathol Res Pract. 2020;216: 152885.
Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y, Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015;21:10573–83.
Wu D, Zhang S, Xie Z, Chen E, Rao Q, Liu X, Huang K, Yang J, Xiao L, Ji F, et al. Plasminogen as a prognostic biomarker for HBV-related acute-on-chronic liver failure. J Clin Invest. 2020;130:2069–80.
Cynthia Martin F, Hiller M, Spitali P, Oonk S, Dalebout H, Palmblad M, Chaouch A, Guglieri M, Straub V, Lochmuller H, et al. Fibronectin is a serum biomarker for Duchenne muscular dystrophy. Proteomics Clin Appl. 2014;8:269–78.
Kuk C, Gunawardana CG, Soosaipillai A, Kobayashi H, Li L, Zheng Y, Diamandis EP. Nidogen-2: a new serum biomarker for ovarian cancer. Clin Biochem. 2010;43:355–61.
Zeng L, Zhong J, He G, Li F, Li J, Zhou W, Liu W, Zhang Y, Huang S, Liu Z, Deng X. Identification of nucleobindin-2 as a potential biomarker for breast cancer metastasis using iTRAQ-based quantitative proteomic analysis. J Cancer. 2017;8:3062–9.
Lee CH, Im EJ, Moon PG, Baek MC. Discovery of a diagnostic biomarker for colon cancer through proteomic profiling of small extracellular vesicles. BMC Cancer. 2018;18:1058.
Lim JH, Lee CH, Kim KY, Jung HY, Choi JY, Cho JH, Park SH, Kim YL, Baek MC, Park JB, et al. Novel urinary exosomal biomarkers of acute T cell-mediated rejection in kidney transplant recipients: a cross-sectional study. PLoS ONE. 2018;13: e0204204.
Jahan R, Ganguly K, Smith LM, Atri P, Carmicheal J, Sheinin Y, Rachagani S, Natarajan G, Brand RE, Macha MA, et al. Trefoil factor(s) and CA19.9: a promising panel for early detection of pancreatic cancer. EBioMedicine. 2019;42:375–85.
Jahan R, Shah A, Kisling SG, Macha MA, Thayer S, Batra SK, Kaur S. Odyssey of trefoil factors in cancer: diagnostic and therapeutic implications. Biochim Biophys Acta Rev Cancer. 2020;1873: 188362.
Solimando AG, Brandl A, Mattenheimer K, Graf C, Ritz M, Ruckdeschel A, Stuhmer T, Mokhtari Z, Rudelius M, Dotterweich J, et al. JAM-A as a prognostic factor and new therapeutic target in multiple myeloma. Leukemia. 2018;32:736–43.
Rosager AM, Sorensen MD, Dahlrot RH, Boldt HB, Hansen S, Lathia JD, Kristensen BW. Expression and prognostic value of JAM-A in gliomas. J Neurooncol. 2017;135:107–17.
Acknowledgements
The authors would like to thank Yanyan Sui and Lixin Sun for pulmonary function test support and Xuefei Qi for lung histopathology analysis.
Statement on ARRIVE guidelines
We declared that this study was carried out in compliance with the ARRIVE guidelines.
Funding
National Natural Science Foundation of China (81973012, 82000881), National Key Research and Development Program of China (2018YFC0910202), Beijing cooperative construction project (110651103), Beijing Normal University (11100704).
Author information
Authors and Affiliations
Contributions
YHG and WH conceived and designed the experiments; WWQ, YTD and HH performed the experiments and analyzed the data, WWQ, YHG and WH wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Male Wistar rats (180–200 g) were purchased from Charles River China (Beijing, China). The animal experiments were reviewed and approved by Qingdao Municipal Hospital Medical Ethics Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
All identification and quantitation details in COPD rats. Table S2. The details of the nodes in PPI.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Qin, W., Huang, H., Dai, Y. et al. Proteome analysis of urinary biomarkers in a cigarette smoke-induced COPD rat model. Respir Res 23, 156 (2022). https://doi.org/10.1186/s12931-022-02070-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-022-02070-1