Abstract
Background
Chronic cough is prevalent in the clinic. The existing therapies are mostly limited to medical treatment, with poor curative effects and serious side effects. Studies have suggested that the right dorsolateral prefrontal cortex (rDLPFC) may play an active role in the inhibitory pathway of cough elicitation. Thus, this study explored the effect of transcranial direct current stimulation (tDCS) on the rDLPFC activation in relation to cough reflex and urge-to-cough sensitivity.
Methods
Twenty-three healthy young adults completed the experiment. Participants randomly received tDCS anodal stimulation, cathodal stimulation, and sham stimulation, and the interval between every two stimuli was at least one week. The tDCS (2 mA, 30 min) stimulated brain rDLPFC region. After tDCS intervention, cough reflex threshold and urge-to-cough were evaluated immediately by inhalation of citric acid-saline solution.
Results
Compared with sham stimulation, the cough reflex thresholds logC2 and logC5 have increased under tDCS anodal stimulation (1.78 ± 0.55 g/L vs. 1.57 ± 0.57 g/L, p < 0.05; 1.92 ± 0.53 g/L vs. 1.67 ± 0.56 g/L, p < 0.05), accompanied by the increase of the urge-to-cough threshold LogCu (0.76 ± 0.53 g/L vs. 0.47 ± 0.44 g/L, p < 0.05). In contrast, the urge-to-cough sensitivity expressed as UTC slope was not significantly changed (1.21 ± 0.86 point·L/g vs. 1.00 ± 0.37 point·L/g, p > 0.05), and there were no apparent changes in cough reflex thresholds Log C2 and logC5, urge-to-cough threshold LogCu, and urge-to-cough sensitivity UTC slope under tDCS cathodal stimulation, compared with sham stimulation.
Conclusions
This study found that anodal tDCS stimulation of rDLPFC could significantly decrease cough reflex sensitivity, accompanied by the increase of urge-to-cough threshold. Further investigations targeting different brain regions using multiple central intervention techniques to explore the underlying mechanisms are warranted.
Trial registration The study protocol was registered for the clinical trial in China (registration number: ChiCTR2100045618)
Similar content being viewed by others
Background
Chronic cough, lasting for more than 8 weeks, is a frequently occurring symptom in about 10% of the general adult population worldwide [1]. Persistent long-term coughing can considerably impact patients' physical and psychological health and overall quality of life as well [2]. There have been a large number of outpatients visiting the clinic every year due to seasonal coughing, which sometimes remains difficult to be completely cured. Although cold-medicine based therapy is the primary treatment option, however, the curative effect is limited and often accompanied by adverse side-effects [3]. Therefore, non-pharmaceutical treatment options are gradually coming into the limelight as an alternative approach.
The cough reflex is usually thought to be mediated at the brainstem level. Studies have demonstrated that people often sense a stimuli-induced motivation of cough-like symptoms to protect their respiratory airway, which is termed the urge-to-cough. Importantly, urge-to-cough is a critical component of the sustained motivation that mediates the cognitive responses of airway stimulation prior to coughing [4]. The intensity of the urge-to-cough has been positively correlated with that of actual cough. Furthermore, the decrease in the intensity of urge-to-cough is often accompanied by the increased cough reflex threshold [5]. Mazzone et al. [6] have found the cerebral cortex and subcortical areas, such as the insula cortex, anterior midcingulate cortex, primary sensory cortex, orbitofrontal cortex, supplementary motor area, and cerebellum, are involved in the regulations of the urge-to-cough as well as cough reflex. Comparison of the central neural responses to airway stimulation between healthy and cough hypersensitive subjects using functional Magnetic Resonance Imaging (fMRI) has revealed that patients with cough hypersensitivity are characterized by central amplification of cough sensory inputs and reduced capacity to suppress cough-associated motor behaviors [7]. Cough reflex and urge-to-cough sensitivity may be directly influenced by the regulatory cortical activity.
Transcranial direct current stimulation (tDCS) is a noninvasive brain stimulation technique to regulate the activity of cerebral cortical neurons. It can modulate neural plasticity and regulate cerebral cortex function by applying mild direct current to the scalp through one or two electrodes placed thereon. Anodal stimulation can induce neuronal depolarization, thus improving the excitability of the cortical neurons, while cathodal stimulation exerts exactly the reverse effects [8]. tDCS has been broadly used in various clinical fields. For example, Bornheim et al. [9] applied tDCS to stroke patients by inducing anodal stimulation at stroke lesions in the primary motor cortex. Therapeutically, the patient receives tDCS intervention (2 mA, 20 min) 5 times per week for 4 weeks, combined with traditional rehabilitation treatment. It has been found that the motor and somatosensory functions of stroke patients significantly improve after tDCS therapy. Moreover, Suntrup-Krueger et al. [10] applied tDCS, with a current intensity of 1 mA for 20 min each time, to the contralesional motor cortical areas in stroke patients with acute dysphagia. The results showed significant improvement in swallowing function after tDCS intervention for four consecutive days.
Whether the cough reflex and urge-to-cough sensitivity of subjects can be modulated by tDCS intervention remains unclear to date. Studies have shown that tDCS can significantly improve swallowing dysfunction and pain [11, 12], since the cough reflex, swallowing reflex, and pain mechanism operate under the same central regulatory hub in the brain [13, 14]. Thus, we speculated that tDCS might influence individual’s cough reflex. Furthermore, studies have shown that right dorsolateral prefrontal cortex (rDLPFC) brain activity is simultaneously induced when the individual’s urge-to-cough decreases, suggesting that rDLPFC may play a vital role in the inhibitory pathway of cough symptoms [15]. Similarly, the placebo analgesia studies reported elevated brain activity in the dorsolateral prefrontal cortex, suggesting an essential role the region may play in regulating sensorimotor responses in the brain [16]. To date, there is no study exploring the effects of tDCS on rDLPFC stimulation related to cough reflex sensitivity. In this study, two stimulation protocols, anodal and cathodal stimulation, were followed to conduct the investigation on the rDLPFC with sham stimulation used as a control to reveal the effects of the two different tDCS modes on the cough reflex and urge-to-cough sensitivities.
Methods
Subjects
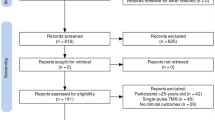
Initially, 25 healthy young adults, including 14 males and 11 females, were enrolled in this study. After one male and one female participant dropped out, 23 adults were finally recruited to this trial. All participants were initially recruited via WeChat on the campus of Capital Medical University. The mean age was 22.5 ± 3.6 (S.D.) years. Subjects without a history of respiratory diseases, respiratory infection, and/or seasonal allergy within past 4 weeks were selected. Participants having menstruation, pregnancy, or lactation, had metal implantation, had a history of neuropsychiatric diseases, alcohol or drug abuse were excluded from this study. All participants signed written informed consent for their participation in this study. The study protocol was approved by the review board of the medical ethics committee of Beijing Friendship Hospital affiliated with Capital Medical University (approval number: 2021-P2-014-02) and registered for the clinical trial in China (registration number: ChiCTR2100045618).
Cough reflex threshold and urge-to-cough
Citric acid was selected as a tussive agent for the cough reflex test. Firstly, citric acid was dissolved in 0.9% saline solution. The initial concentration was 0.7 g/L, providing a two-fold incremental concentration to 360 g/L. An ultrasonic nebulizer (NE-C900, Omron medical devices Co Ltd, Beijing, China) delivered a tidal nebulized citric acid solution. The mean mass median diameter of the particles generated by the nebulizer was 3.0 ± 1.0 µm, and the output speed was 0.25 mL/min. Each subject inhaled physiologic saline as the control and then inhaled the citric acid solution in a progressively increasing concentration until five or more coughs were elicited. Each inhalation time was set to 1 min, and the interval between two consecutive inhalations was 2 min. The number of coughs was counted by technicians blinded to the experimental grouping and the study purpose. The lowest citric acid concentrations that elicited two or more coughs and five or more coughs were defined as the lower and upper cough reflex thresholds, recorded as C2 and C5, respectively. The maximum citric acid concentration that caused zero time of cough was recorded as C0max.
Each subject was assessed for the urge-to-cough sensitivity using a modified Borg scale immediately after completion of each inhalation. The Borg scale ranges from no need-to-cough (0) to maximum urge-to-cough (10). The urge-to-cough scale was placed in front of the subjects, and subjects were instructed to point to the corresponding scale score based on their actual feelings, which the experimenter recorded. In order to evaluate the intensity of urge-to-cough, subjects were required to ignore other feelings, such as dyspnea, burning in the throat, choking. During the inhalation of citric acid, subjects were made aware of the fact that their sensitivity to urge-to-cough could increase, decrease, or remain unchanged, and their Borg score should reflect the degree of sensitivity. According to previous studies [5], there is a linear correlation between the Borg score of urge-to-cough and the concentration of citric acid using log–log transformation. Therefore, we used linear regression analysis to calculate the slope on a log–log scale. The citric acid concentration corresponding to the first Borg scale score of not 0 was defined as the urge-to-cough threshold, recorded as Cu.
Transcranial direct current stimulation (tDCS)
Direct current was applied using a saline-soaked pair of surface sponge electrodes (5 × 7 cm2) and delivered using a battery-driven, constant current stimulator (IS200, Zhineng Co. Ltd, Chengdu, China) [17]. A constant current of 2 mA (0.057 mA/cm2) was applied for 30 min. There were three different stimulation modes. In mode 1 (the anodal mode), the anode was placed on the rDLPFC, and the cathode on the left shoulder (Fig. 1). In mode 2 (the cathodal mode), the cathode was placed on the rDLPFC, and the anode on the left shoulder (Fig. 1). In mode 3 (the sham mode), anode and cathode were randomly placed on the rDLPFC and the left shoulder. The stimulator produced electricity only for the first 30 s. The current intensity was gradually increased in the initial 15 s and then gradually decreased in the following 15 s. There was no current in the rest time. According to the 10–20 system 32-lead electrode distribution position, the rDLPFC corresponded to F4, so that the top center was 8 cm forward along the sagittal line, and then a vertical line on the sagittal section was drawn at this point, with a side opening of 6 cm.
Experimental protocol
The researcher introduced the whole experimental procedure to all subjects before it started. All subjects first underwent a pulmonary function examination and then randomly received tDCS interventions in three different stimulation modes, namely anodal stimulation (anodal mode), cathodal stimulation (cathodal mode), or sham stimulation (sham mode). Immediately after the tDCS intervention, the subjects underwent citric acid challenge to evaluate their cough reflex thresholds and urge-to-cough sensitivities. In order to avoid the sustained effect of tDCS, the interval between two consecutive stimulation modes was at least one week. Previous studies have reported that [18] people may experience adverse effects after tDCS intervention, such as dizziness, headache, neck pain, and scalp burning. Therefore, subjects were asked whether they had any uncomfortable sensations after each intervention.
Data analysis
Data are expressed as mean ± SD except otherwise stated. The group variances were tested using the Shapiro–Wilk normality test and found no significant difference, other than urge-to-cough log–log slope. The paired sample t-test and Wilcoxon test were used to compare anodal stimulation or cathodal stimulation with sham stimulation. A p < 0.05 was considered significant.
To determine the sample size for the study, a pilot trial of six subjects were enrolled to evaluate both cough reflex sensitivity and UTC. Based on the pilot trial results, the sample size was calculated by the difference in LogC5 between the anodal and sham groups by PASS 11.0. With a power of 0.9 and a significance level of 0.05, the sample size was calculated to be sufficient with 18 participants. With uncertainty about variability and an expected drop-out rate of 10%, we aimed to include 20 healthy young adults. We finally recruited 23 healthy young adults.
Results
All 23 subjects completed the experiment without any difficulty or side effects. The characteristics of subjects are summarized in Table 1. All subjects had normal pulmonary functions.
Figure 2 shows the comparison of cough reflex threshold under the anodal mode and the sham mode, as expressed by LogC2 and LogC5. The LogC2 in the anodal mode shown in Fig. 2A was significantly higher than that in the sham mode (1.78 ± 0.55 g/L vs. 1.57 ± 0.57 g/L, p < 0.05). The LogC5 between the anodal and sham modes was shown in Fig. 2B. Compared with the sham mode, the cough reflex threshold LogC5 in the anodal mode was also significantly higher (1.92 ± 0.53 g/L vs. 1.67 ± 0.56 g/L, p < 0.05). Figure 3 shows the urge-to-cough sensitivity between the anodal and sham modes. As shown in Fig. 3A, LogCu in the anodal mode was significantly increased than that of the sham mode (0.76 ± 0.53 g/L vs. 0.47 ± 0.44 g/L, p < 0.05). Figure 3B shows LogC0max of 1.19 ± 0.62 g/L, 1.42 ± 0.60 g/L for the sham and anodal modes, respectively. LogC0max was significantly higher in the anodal mode than that for the sham mode (p < 0.05). As shown in Fig. 3C, the urge-to-cough log–log slopes were 1.00 ± 0.37 point·L/g, 1.21 ± 0.86 point·L/g in the sham mode and anodal mode, respectively. There was no significant difference between the two groups.
Comparison of cough reflex threshold between the sham and anodal modes. A LogC2 in each group. B LogC5 in each group. Open circles indicate the value of each subject. Closed circles and error bars indicate the mean and S.D. in each group, respectively. LogC2 = the log transformation of the lowest concentration of citric acid that elicited two or more coughs. LogC5 = the log transformation of the lowest concentration of citric acid that elicited five or more coughs
Comparison of urge-to-cough (UTC) between the sham and anodal modes. A LogCu in each group. B LogC0max in each group. C The UTC log–log slope in each group. Open circles indicate the value of each subject. Closed circles and error bars indicate the mean and S.D. in each group, respectively. LogCu = the log transformation of concentration of citric acid at a threshold of UTC. LogC0max = the maximum concentration of citric acid causing zero time of cough. UTC log–log slope = the slope between UTC scores and citric acid concentration on a log–log scale
Figure 4 shows the comparison of cough reflex threshold under the cathodal mode and the sham mode, as expressed by LogC2 and LogC5. There was no significant difference in LogC2 between the cathodal and the sham modes (1.65 ± 0.60 g/L vs. 1.57 ± 0.57 g/L, p > 0.05), as shown in Fig. 4A. Similarly, no significant difference was observed in LogC5 between the cathodal mode and the sham mode (1.72 ± 0.55 g/L vs. 1.67 ± 0.56 g/L, p > 0.05), as shown in Fig. 4B. Figure 5 shows the urge-to-cough sensitivity between the cathodal mode and the sham mode. As shown in Fig. 5A, the difference in LogCu was not significant between the cathodal and sham modes (0.62 ± 0.45 g/L vs. 0.47 ± 0.44 g/L, p > 0.05). Figure 5B shows LogC0max of 1.19 ± 0.62 g/L and 1.33 ± 0.63 g/L for the sham and cathodal modes, respectively, and there was no significant difference in LogC0max between the cathodal and sham modes. In Fig. 5C, the urge-to-cough log–log slopes were 1.00 ± 0.37 point·L/g and 1.11 ± 0.40 point·L/g in the sham and cathodal modes, respectively, and no significant differences was seen between the two groups.
Comparison of cough reflex threshold between the sham and cathodal modes. A LogC2 in each group. B LogC5 in each group. Open circles indicate the value of each subject. Closed circles and error bars indicate the mean and S.D. in each group, respectively. LogC2 = the log transformation of the lowest concentration of citric acid that elicited two or more coughs. LogC5 = the log transformation of the lowest concentration of citric acid that elicited five or more coughs
Comparison of urge-to-cough (UTC) between the sham and cathodal modes. A LogCu in each group. B LogC0max in each group. C The UTC log–log slope in each group. Open circles indicate the value of each subject. Closed circles and error bars indicate the mean and S.D. in each group, respectively. LogCu = the log transformation of concentration of citric acid at a threshold of UTC. LogC0max = the maximum concentration of citric acid causing zero time of cough. UTC log–log slope = the slope between UTC scores and citric acid concentration on a log–log scale
Discussion
Our study explored the effect of tDCS on the cough reflex. The results showed that single anodal tDCS applied to the rDLPFC could significantly increase the cough reflex threshold, accompanied by the increase in urge-to-cough threshold but not the sensitivity.
This study, for the first time, reported the effects of the direct intervention of the central cerebral cortex on cough reflex sensitivity and urge-to-cough. The cough reflex is mediated by the higher-order neuronal circuitry [19], including the activation and inhibition circuits [20]. The primary sensory cortex, orbitofrontal cortex, insula cortex, anterior midcingulate cortex, and other brain regions are involved in the activation circuit [6]. The rDLPFC and parietal cortex are likely to play a role in the inhibition circuit [15]. The activity of the rDLPFC decreases significantly in patients with cough hypersensitivity [7]. Studies on the mechanism of placebo antitussive therapy have found that the magnitude of the rDLPFC activation was significantly related to the magnitude of the placebo antitussive effect. Since the brain regions involved in cough and breathing suppression, including the right ventral inferior frontal gyrus, anterior insula, and the middle cingulate gyrus, exhibit no significant difference between the placebo and control groups [15]. Thus, it is suggested that the rDLPFC might play a vital role in the inhibition of cough. Moreover, pain and cough reflex activities share overlapping central mechanisms [21]. In addition, a study exploring the central mechanism of analgesia has found that the analgesic effect caused by the placebo is related to the increased activation of the bilateral DLPFC during harmful stimulation [22]. Given that noninvasive brain stimulation technology can produce an analgesic effect by stimulating the DLPFC [23]. Therefore, we speculated that the rDLPFC intervention through noninvasive brain stimulation technology might affect cough reflex and urge-to-cough sensitivity.
tDCS is a noninvasive brain stimulation technology, which applies mild direct current to the scalp to modulate cortical excitability. Previous studies have reported that tDCS (2 mA, 20 min, electrode size: 3 × 5 cm) can produce an analgesic effect by stimulating the left DLPFC [24]. A randomized controlled study also showed that after tDCS intervention in the swallowing motor cortex, the swallowing function of stroke patients was significantly improved [10]. Notably, pain, swallowing reflex, and cough reflex mechanisms overlap in the central regulatory pathway. When painful stimuli were applied to the volunteers, fMRI showed the activation of several brain regions, such as the prefrontal cortex, insular cortex, primary somatosensory cortex, similar to the brain regions activated when individual coughs [25]. Thus, tDCS stimulation of the cerebral cortex might also influence the cough reflex sensitivity. Our results showed that individuals’ cough reflex and urge-to-cough threshold were significantly increased after the anodal tDCS stimulation of the rDLPFC activity, indicating the possibility of modulating cough reflex through the regulation of the central nervous system (CNS).
In addition, our results further supported the active role of the rDLPFC upon anodal tDCS stimulation in the cough inhibition pathway. On the other hand, cathodal tDCS reduced rDLPFC neural activity but did not affect individuals’ cough reflex and urge-to-cough sensitivity. A systematic meta-analysis [26] has revealed that following the cathodal tDCS intervention, the behavioral results related to cognitive function may not change significantly. Several studies on the motor and a few studies on cognition have found that the inhibition can rarely be caused by the cathode tDCS [27,28,29]. One possible interpretation for the findings is that the bilateral interactions support the contralateral compensation [26]. When cathodal tDCS inhibits the neural activity in the stimulated cerebral hemisphere, corresponding brain regions in the contralateral cerebral hemisphere may be activated to maintain the stability of physiological functions [27]. Furthermore, brain regions in the cough activation pathway should be targeted for patients who need to improve urge-to-cough sensitivity, such as elderly patients with aspiration pneumonia, and the rDLPFC may be a potential target of central antitussive therapy in the future.
At present, antitussive therapy includes both pharmacological and nonpharmacological interventions. The traditional antitussive drugs mainly include codeine, morphine, gabapentin, pregabalin, amitriptyline, but the efficacy is poor and exhibits adverse effects. Although several studies have focused on identifying new targeted antitussive drugs for the peripheral pathway, however, these new antitussive drugs are mostly in the pre-clinical stage, and their clinical efficacies need to be further confirmed [3]. The existing nonpharmacological therapies mainly include health education, cough suppression, breathing training, vocal hygiene and hydration, psychological counseling, and laryngeal massage. Unfortunately, patients need long-term persistence to have effective therapeutic benefits, and only about half of those patients finally adhered to the treatment [30]. Hence, this study demonstrated a short-term therapeutic intervention strategy by applying anodal tDCS to the rDLPFC to inhibit cough reflex. Moreover, tDCS therapy is cheaper, portable, well-tolerated, and safer [31, 32]. Therefore, this technique may be clinically used as a new therapy to treat chronic cough in the future.
Despite these therapeutic advantages, there are also certain limitations in the study. (1) The subjects were all healthy young adults. Belvisi et al. showed that the reduction in experimentally evoked cough might not reliable to predict the antitussive effects [33]. Hence, whether tDCS can be applied to patients with chronic cough and cough hypersensitivity need further study. (2) Only one cough reflex inhibitory targeted brain region was investigated. Whether other brain regions are involved in cough reflex should be investigated further. (3) The long-term effects of treatment should be investigated.
Conclusions
This study confirmed that anodal tDCS stimulation of rDLPFC could increase cough reflex threshold and urge-to-cough threshold significantly, but the urge-to-cough sensitivity exhibited no significant change. Further research is necessary to reveal the underlying mechanisms and develop novel intervention targets for the central nervous system.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- LogC 2 :
-
The log transformation of the lowest concentration of citric acid that elicited two or more coughs
- LogC5 :
-
The log transformation of the lowest concentration of citric acid that produced five or more coughs
- LogCu :
-
The log transformation of concentration of citric acid at a threshold of urge-to-cough
- LogC0max :
-
The maximum concentration of citric acid causing zero time of cough
- UTC Slope:
-
Urge-to-cough log–log slope, the slope between urge-to-cough scores and citric acid concentration on a log–log scale
- fMRI:
-
Functional magnetic resonance imaging
- tDCS:
-
Transcranial direct current stimulation
- rDLPFC:
-
Right dorsolateral prefrontal cortex
References
Song WJ, Chang YS, Faruqi S, Kim JY, Kang MG, Kim S, Jo EJ, Kim MH, Plevkova J, Park HW, Cho SH, Morice AH. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J. 2015;45(5):1479–81.
McGarvey LP, Carton C, Gamble L, Heaney L, Shepherd R, Ennis M, MacMahon J. Prevalence of psychomorbidity among patients with chronic cough. Cough. 2006;2:4.
Song WJ, Chung KF. Pharmacotherapeutic options for chronic refractory cough. Expert Opin Pharmacother. 2020;21(11):1345–58.
Davenport PW. Urge-to-cough: what can it teach us about cough? Lung. 2008;186(Suppl 1):S107–11.
Davenport PW, Bolser DC, Vickroy T, Berry RB, Martin AD, Hey JA, Danzig M. The effect of codeine on the Urge-to-Cough response to inhaled capsaicin. Pulm Pharmacol Ther. 2007;20(4):338–46.
Mazzone SB, McLennan L, McGovern AE, Egan GF, Farrell MJ. Representation of capsaicin-evoked urge-to-cough in the human brain using functional magnetic resonance imaging. Am J Respir Crit Care Med. 2007;176(4):327–32.
Ando A, Smallwood D, McMahon M, Irving L, Mazzone SB, Farrell MJ. Neural correlates of cough hypersensitivity in humans: evidence for central sensitisation and dysfunctional inhibitory control. Thorax. 2016;71(4):323–9.
Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17(1):37–53.
Bornheim S, Croisier JL, Maquet P, Kaux JF. Transcranial direct current stimulation associated with physical-therapy in acute stroke patients—a randomized, triple blind, sham-controlled study. Brain Stimul. 2020;13(2):329–36.
Suntrup-Krueger S, Ringmaier C, Muhle P, Wollbrink A, Kemmling A, Hanning U, Claus I, Warnecke T, Teismann I, Pantev C, Dziewas R. Randomized trial of transcranial direct current stimulation for poststroke dysphagia. Ann Neurol. 2018;83(2):328–40.
Marchina S, Pisegna JM, Massaro JM, Langmore SE, McVey C, Wang J, Kumar S. Transcranial direct current stimulation for post-stroke dysphagia: a systematic review and meta-analysis of randomized controlled trials. J Neurol. 2021;268(1):293–304.
O’Connell NE, Marston L, Spencer S, Desouza LH, Wand BM. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2018;3(3):CD008208.
Amin MR, Belafsky PC. Cough and swallowing dysfunction. Otolaryngol Clin North Am. 2010;43(1):35–42.
Gracely RH, Undem BJ, Banzett RB. Cough, pain and dyspnoea: similarities and differences. Pulm Pharmacol Ther. 2007;20(4):433–7.
Leech J, Mazzone SB, Farrell MJ. Brain activity associated with placebo suppression of the urge-to-cough in humans. Am J Respir Crit Care Med. 2013;188(9):1069–75.
Amanzio M, Benedetti F, Porro CA, Palermo S, Cauda F. Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum Brain Mapp. 2013;34(3):738–52.
Zhang X, Liu B, Li N, Li Y, Hou J, Duan G, Wu D. Transcranial direct current stimulation over prefrontal areas improves psychomotor inhibition state in patients with traumatic brain injury: a pilot study. Front Neurosci. 2020;14:386.
Russo C, Souza Carneiro MI, Bolognini N, Fregni F. Safety review of transcranial direct current stimulation in stroke. Neuromodulation. 2017;20(3):215–22.
Mazzone SB, Mcgovern AE, Yang SK, Woo A, Phipps S, Ando A, Leech J, Farrell MJ. Sensorimotor circuitry involved in the higher brain control of coughing. Cough. 2013;9(1):7.
Mazzone SB, Cole LJ, Ando A, Egan GF, Farrell MJ. Investigation of the neural control of cough and cough suppression in humans using functional brain imaging. J Neurosci. 2011;31(8):2948–58.
Mazzone SB, McGovern AE, Koo K, Farrell MJ. Mapping supramedullary pathways involved in cough using functional brain imaging: comparison with pain. Pulm Pharmacol Ther. 2009;22(2):90–6.
Altas LY, Wager TD. A meta-analysis of brain mechanisms of placebo analgesia: consistent findings and unanswered questions. Handb Exp Pharmacol. 2014;225:37–69.
Costa B, Ferreira I, Trevizol A, Thibaut A, Fregni F. Emerging targets and uses of neuromodulation for pain. Expert Rev Neurother. 2019;19(2):109–18.
Mariano TY, Van’t Wout M, Garnaat SL, Rasmussen SA, Greenberg BD. Transcranial direct current stimulation (tDCS) targeting left dorsolateral prefrontal cortex modulates task-induced acute pain in healthy volunteers. Pain Med. 2016;17(4):737–45.
O’Neill J, McMahon SB, Undem BJ. Chronic cough and pain: Janus faces in sensory neurobiology? Pulm Pharmacol Ther. 2013;26(5):476–85.
Jacobson L, Koslowsky M, Lavidor M. tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp Brain Res. 2012;216(1):1–10.
Baumert A, Buchholz N, Zinkernagel A, Clarke P, MacLeod C, Osinsky R, Schmitt M. Causal underpinnings of working memory and Stroop interference control: testing the effects of anodal and cathodal tDCS over the left DLPFC. Cogn Affect Behav Neurosci. 2020;20(1):34–48.
Tanaka S, Hanakawa T, Honda M, Watanabe K. Enhancement of pinch force in the lower leg by anodal transcranial direct current stimulation. Exp Brain Res. 2009;196(3):459–65.
Sparing R, Dafotakis M, Meister IG, Thirugnanasambandam N, Fink GR. Enhancing language performance with non-invasive brain stimulation–a transcranial direct current stimulation study in healthy humans. Neuropsychologia. 2008;46(1):261–8.
Chamberlain S, Birring SS, Garrod R. Nonpharmacological interventions for refractory chronic cough patients: systematic review. Lung. 2014;192(1):75–85.
Zaghi S, Heine N, Fregni F. Brain stimulation for the treatment of pain: a review of costs, clinical effects, and mechanisms of treatment for three different central neuromodulatory approaches. J Pain Manag. 2009;2(3):339–52.
Krishnan C, Santos L, Peterson MD, Ehinger M. Safety of noninvasive brain stimulation in children and adolescents. Brain Stimul. 2015;8(1):76–87.
Belvisi MG, Birrell MA, Wortley MA, Maher SA, Satia I, Badri H, Holt K, Round P, McGarvey L, Ford J, Smith JA. XEN-D0501, a novel transient receptor potential vanilloid 1 antagonist, does not reduce cough in patients with refractory cough. Am J Respir Crit Care Med. 2017;196(10):1255–63.
Acknowledgements
We thank the participants of the study with respect.
Funding
This study was supported by the National Natural Science Foundation of China (Grant number: 81800098), Beijing Hospital Authority Youth Program (Grant number: QML20200109), and Beijing Talents Fund (Grant number: 2018000021469G204).
Author information
Authors and Affiliations
Contributions
PG: Conceptualization, Methodology, Investigation, Formal analysis, Supervision, Writing- Original Draft, Writing- Review and Editing. LW: Conceptualization, Methodology, Investigation, Formal analysis, Writing- Original Draft. LG: Methodology, Investigation, Writing- Review and Editing. CW: Methodology, Investigation, Writing- Review and Editing. BZ: Methodology, Investigation, Writing- Review and Editing. CC: Methodology, Investigation, Writing- Review and Editing. YX: Conceptualization, Methodology, Investigation, Formal analysis, Supervision, Writing- Review and Editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the review board of the medical ethics committee of Beijing Friendship Hospital affiliated with Capital Medical University (approval number: 2021-P2-014-02). All participants signed written informed consent for their participation in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interest among the authors, and the article’s publication will not be affected by individuals' relationships.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gui, P., Wang, L., Guo, L. et al. Effects of transcranial direct current stimulation on cough reflex and urge-to-cough in healthy young adults. Respir Res 23, 99 (2022). https://doi.org/10.1186/s12931-022-02020-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-022-02020-x