Abstract
Background
Chest computed tomography (CT) is a widely used method to assess morphological and dynamic abnormalities in chronic obstructive pulmonary disease (COPD). The small pulmonary vascular cross-section (CSA), quantitatively extracted from volumetric CT, is a reliable indicator for predicting pulmonary vascular changes. CSA is associated with the severity of symptoms, pulmonary function tests (PFT) and emphysema and in COPD patients the severity increases over time. We analyzed the correlation longitudinal changes in pulmonary vascular parameters with clinical parameters in COPD patients.
Materials and methods
A total of 288 subjects with COPD were investigated during follow up period up to 6 years. CT images were classified into five subtypes from normal to severe emphysema according to percentage of low-attenuation areas less than -950 and -856 Hounsfield units (HU) on inspiratory and expiratory CT (LAA-950, LAA-856exp). Total number of vessels (Ntotal) and total number of vessels with area less than 5 mm2 (N<5 mm) per 1 cm2 of lung surface area (LSA) were measured at 6 mm from the pleural surface.
Results
Ntotal/LSA and N<5 mm/LSA changed from 1.16 ± 0.27 to 0.87 ± 0.2 and from 1.02 ± 0.22 to 0.78 ± 0.22, respectively, during Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage progression. Both parameters changed from normal to severe emphysema according to CT subtype from 1.39 ± 0.21 to 0.74 ± 0.17 and from 1.18 ± 0.19 to 0.67 ± 0.15, respectively. LAA-950 and LAA-856exp were negatively correlated with Ntotal/LSA (r = − 0.738, − 0.529) and N<5 mm /LSA (r = − 0.729, -− .497). On the other hand, pulmonary function test (PFT) results showed a weak correlation with Ntotal/LSA and N<5 mm/LSA (r = 0.205, 0.210). The depth in CT subtypes for longitudinal change both Ntotal/LSA and N<5 mm/LSA was (− 0.032, − 0.023) and (− 0.027) in normal and SAD, respectively.
Conclusions
Quantitative computed tomography features faithfully reflected pulmonary vessel alterations, showing in particular that pulmonary vascular alteration started.
Similar content being viewed by others
Background
Chronic obstructive pulmonary disease (COPD) is a multifaceted disease characterized by airflow obstruction, and is associated with chronic inflammatory response of the airways, often involving destruction of adjacent alveoli and vasculature [1, 2]. COPD have been known as a heterogeneous and complex condition with a variety of pathological and clinical compartments [3, 4]. Pulmonary vascular alteration is a major pathophysiological characteristic of COPD [5]. It is estimated that 30–70% of COPD patients have some degree of pulmonary vascular abnormalities due to pulmonary hypertension [6, 7]. Passive vascular compression by emphysema and hypoxic pulmonary vasoconstriction are thought to be critical for the pathogenesis of vascular changes, and recent studies have suggested that endothelial dysfunction is associated with vascular alterations in patients with COPD [2, 8, 9].
The gold standard for evaluating pulmonary vascular abnormality and hemodynamics is right heart catheterization, which is too invasive in clinical practice [2]. Angiographic studies of smokers showed narrowing and reduction of small pulmonary arteries in regions severely affected by emphysema [10, 11]. Chest computed tomography (CT) is widely used to evaluate the morphologic and dynamic abnormalities of COPD. The cross-sectional areas (CSAs) of the small pulmonary vessels, quantitatively extracted from volumetric CT, are reliable indicators of pulmonary vascular alteration [9]. CSAs are associated with symptoms, pulmonary function test (PFT), and severity of emphysema [5, 8, 9]. The extent of emphysema increases over time in patients with COPD [12]. However, there are few studies on the changes in vascular alterations during longitudinal follow-up in patients with COPD.
In this study, we conducted a quantitative analysis based on volumetric CT scans to identify vessel alterations in patients with COPD. The purpose of our study was to determine the differences in pulmonary vascular parameters measured by volumetric CT according to disease severity and CT phenotype, and to assess their correlations with clinical parameters. In addition, we observed longitudinal vascular changes in the subjects, classified by Global Initiative for Chronic Obstructive Lung Disease (GOLD) grade and CT subtype, during a follow-up period of up to 6 years.
Methods
Subjects
A total 504 of subjects were collected from the COPD in Dusty Areas (CODA) cohort, which consisted of Korean subjects residing near cement plants. As a prospective study, all subjects underwent medical interviews, PFTs, laboratory tests, and chest CT. COPD was diagnosed in subjects with post-bronchodilator forced expiratory volume in 1 s (FEV1) / forced vital capacity (FVC) ratio < 0.7 at baseline [13]. We excluded 206 subjects due to FEV1/FVC ratio ≥ 0.7 (n = 162), lung surgery (n = 4), CT quantification error (n = 10), and severe lung parenchymal distortion by tuberculosis sequelae and pneumoconiosis with progressive massive fibrosis (n = 30). Thus 288 subjects with COPD were finally investigated in the current study, of which 147 were investigated at least two chest CT scans within 3 years from baseline and 88 were followed up with CT scans at least two for up to 6 years (Fig. 1). Institutional Review Board approval for all processes of this study was obtained from Kangwon National University Hospital (KNUH 2012-06-007), and written informed consent was obtained from all subjects.
Clinical and pulmonary function parameters
All subject data were obtained from interviews and assessments of physical condition using questionnaires, including demographic data, medical history, exposure environment, and respiratory symptoms. Dyspnea assessment was conducted using the modified Medical Research Council (mMRC) scale, and quality of life related to health was assessed by calculating the sum of scores on the subject-reported COPD Assessment Test (CAT).
PFTs were performed using the Easy One Kit (NDD, Zurich, Switzerland), before and after inhalation of 400 μg salbutamol. Specifically, the airflow limitation on spirometry for the severity of COPD is defined using the FEV1 and the FEV1/FVC ratio, and divided into four GOLD grades: grade 1 (≥ 80%), grade 2 (50–79%), grade 3 (30–49%), or grade 4 (< 30%)[13]. The number of subjects in grades 3 and 4 was insufficient compared to that of early stage patients, thus grades 3 and 4 were combined into one group.
Chest CT acquisition
All volumetric CT scan images were obtained at full inspiration and expiration in the supine position. Intravenous contrast medium administration was not required. The CT scanners used in this study are first-generation dual-source CT scanners manufactured by Siemens Healthcare (Somatom Definition; Forchheim, Germany) with the following parameters:140 kVp, 100 mA, beam pitch 0.9–1, slice thickness 0.6 and 3 mm. All acquired CT images were reconstructed using the soft convolution kernel B30f.
Quantitative analysis of CT images
Lung segmentation and quantification of emphysema, air trapping, and pulmonary vessels were performed using an Aview® system (Coreline Soft Inc., Seoul, South Korea). The extent of emphysematous lung was measured by quantifying the fraction of low-attenuation areas less than -950 Hounsfield units (HU) on inspiratory CT scan (LAA-950) (Fig. 2). Air trapping was used to assess the percentage of low attenuation less than or equal to -856 HU measured on expiratory CT scan (LAA-856exp) [14]. CT images were classified into five subtypes according to LAA-950 and LAA-856exp: normal (LAA-950 < 5% and LAA-856exp < 20%), small airway disease (SAD, LAA-950 < 5% and LAA-856exp ≥ 20%), mild emphysema (LAA-950 ≥ 5% and < 10%), moderate emphysema (LAA-950 ≥ 10% and < 15%), and severe emphysema (LAA-950 ≥ 15%) [15].
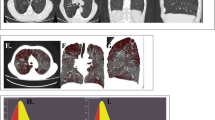
CT quantification of emphysema and pulmonary vessels. A CT coronal reconstructed image of a 54-year-old male with COPD GOLD grade 1 reveals centrilobular and paraseptal emphysema. B The emphysema was measured using a threshold of -950HU (shown in green; LAA-950, 12.3%). C Pulmonary vessels are automatically extracted and segmented (shown in red; Ntotal/LSA, 1.05; N5mm/LSA, 0.97), and the green contours show the lung surface area at 6 mm from the pleural surface. CT computed tomography, LAA low attenuation area
The methodology for pulmonary vessel quantification is described in detail elsewhere [16]. Pulmonary vessels were extracted using a threshold of -750 HU. The extracted initial vessels were refined in detail as twigs of lung vascular structures using region growing and weighted minimum spanning tree (MST) algorithms with an orientation vector field. After pulmonary vascular structure reconstruction, the lung surface area (LSA) at a depth of 6 mm from the pleural surface was computed [17]. For each surface area, the total number of vessels (Ntotal) and total number of vessels with vessel area less than 5 mm2 (N< 5 mm) were counted as robust values, and reported as values per 1 cm2 of LSA (Ntotal/LSA; N<5 mm/LSA).
Statistical analysis
Continuous variables were expressed as mean ± standard deviation. Differences between two groups were evaluated using independent sample t-tests and the chi-square statistics. One-way ANOVA was used to analyze the differences between the measured quantitative and qualitative pulmonary vessel changes and parameters. A mixed model was used to longitudinally analyze the changes in time of qualitatively and quantitatively measured pulmonary vessel parameters during the follow-up period of up to 6 years. The missing values that occurred in each patient during the entire follow-up period using the results within the remaining period were imputed to analyze the mixed model by multiple imputation (MI) method [18]. Pearson’s correlation analysis was used to measure the associations between pulmonary vessel parameters and clinical parameters such as FEV1, FEV1/FVC, CAT, LAA-950, and LAA-856exp. For all statistical analyses, p-values < 0.05 were considered statistically significant. All statistical analyses were performed using SAS (Version 9.4, SAS Institute, Cary, NC) and R (Version 3.6.3, The R Foundation for 211 Statistical Computing, Vienna, Austria; 64-bit platform).
Results
Patient characteristics
We present the demographic characteristics of the study cohort (Table 1 and Additional file 1: Table S1). In particular, by presenting the background of all subjects and subjects with smoking history, respectively, we considered the changes of vascular due to smoking. The 288 subjects included 236 (81.9%) men and 52 (18.1%) women. The mean age was 72.88 ± 7.27 (range 44.0 to 96.0) years. Regarding smoking history, the subjects included 71 non-smokers, 138 former smokers, and 76 current smokers, with mean pack-years 21.03 ± 25.49. The mean body mass index (BMI) of the subjects was 23.17 ± 3.13 kg/m2, the mMRC score 1.48 ± 1.16, and the CAT score 17.06 ± 9.68. The mean pulmonary function evaluation result (FEV1/FVC) was 58.80 ± 8.64.
Vessel quantification according to subtypes
Pulmonary vascular parameters were assessed according to the GOLD grade of the subjects (Table 2). The measured Ntotal/LSA and N<5 mm/LSA decreased as the GOLD grade increased. Ntotal/LSA was 1.16 ± 0.27 in GOLD 1, and decreased to 1.12 ± 0.29 and 0.87 ± 0.27 for GOLD 2 and 3/4, respectively. N<5 mm/LSA was 1.02 ± 0.22, 0.99 ± 0.23 and 0.78 ± 0.22 for GOLD 1, 2 and 3/4, respectively, thus more decreased than Ntotal/LSA. The decrease of Ntotal/LSA and that of N<5 mm/LSA were statistically significant (both p < 0.001).
In addition, we measured pulmonary vascular parameters according to CT subtype (Table 3). The measured Ntotal/LSA and N<5 mm/LSA were 1.39 ± 0.21 and 1.18 ± 0.19, respectively, in the normal CT subtype. Both Ntotal/LSA and N<5 mm /LSA decreased to 1.28 ± 0.17 and 1.12 ± 0.14, respectively, in the SAD subtype, and to 1.05 ± 0.19 and 0.95 ± 0.16 in the mild emphysema subtype. In the moderate and severe emphysema subtypes Ntotal/LSA was 0.90 ± 0.18 and 0.74 ± 0.17, respectively, while N<5 mm/LSA was 0.82 ± 0.15 and 0.67 ± 0.15, showing more decreased numbers than Ntotal/LSA for increasing emphysema severity. The decrease of both pulmonary vascular parameters was statistically significant (both p < 0.001).
Correlation between vessel parameters and clinical/quantitative CT parameters
We also investigated the correlation between the pulmonary vascular parameters and clinical/CT quantitative parameters (Table 4). FEV1 showed weak but significant positive correlation with Ntotal/LSA and N<5 mm/LSA (correlation coefficient 0.205 and 0.210, respectively, both p < 0.001), and FEV1/FVC had a positive correlation with the same parameters (0.332 and 0.337 with Ntotal/LSA and N<5 mm/LSA, respectively, both p < 0.001).
LAA-950 and LAA-856exp showed strong negative correlation with Ntotal/LSA and N<5 mm/LSA (LAA-950: correlation coefficients -0.738 and -0.729 with Ntotal/LSA and N<5 mm/LSA, respectively; LAA-856exp: − 0.529 and − 0.497, p < 0.001). However, pulmonary vascular parameters had no statistically significant correlations with FVC and CAT scores.
Longitudinal changes over a follow up period
We analyzed the pattern of pulmonary vascular parameter changes for the all subjects and subjects with smoking during the entire follow-up period of up to 6 years from baseline (Table 5 and Additional file 1: Table S2). Calibration was performed using covariates such as age, gender, and smoking status in individual subjects for the effective results. The results were presented as coefficients with 95% confidence interval (CI). Changes over time were observed according to CT subtypes and GOLD grades (Fig. 3). The longitudinal analysis of pulmonary vascular parameters showed a tendency for N<5 mm/LSA to decrease during the follow-up period as the severity increased from GOLD 1 to GOLD 3/4. However, the same pattern of change was not observed for Ntotal/LSA, and neither vascular parameter showed a statistically significant change pattern.
Longitudinal changes of vessel quantification during up to 6 years by GOLD grade and CT subtype. No significant differences were observed according to GOLD grade. However, the number of vessels during the initial COPD symptom progression (CT subtype normal to SAD) was markedly reduced, although without visually structural changes in the CT image. In other words, the decrease of vessels counts over 6 years was more pronounced in the airway disease phenotype than in the emphysema phenotype. GOLD Global Initiative for Chronic Obstructive Lung Disease, CT computed tomography, SAD small airway disease
Unlike GOLD grades, CT subtypes based on volumetric quantitative analysis results of emphysema and air trapping severity would clearly indicate a decline. The CT image-based quantitative volumetric scan results reflect the longitudinal changes over a follow-up period of up to 6 years of the Ntotal/LSA and N<5 mm/LSA from the normal to the severe stage (Additional file 1: Tables S3 and S4). Both pulmonary vascular parameters exhibited a more pronounced decrease from normal to mild, than from moderate to severe. The results of depth of longitudinal change in Ntotal /LSA progressed − 0.032 and -0.023 in normal and SAD, respectively. In addition, the change in N<5mm/LSA was − 0.027 and − 0.027 in the same subtypes. Moreover, only Ntotal/LSA showed a statistically significant result (p value of 0.031) over the entire follow-up period.
Discussion
In this study, we performed a quantitative analysis of pulmonary vessel changes according to GOLD grade and CT subtype. As the GOLD grade based on PFT and the emphysema severity based on volumetric chest CT increased, the number of pulmonary vessels consistently decreased. In addition, quantitative longitudinal analysis up to 6 years demonstrated that the number of vessels decreased more significantly in the normal and SAD subtypes than in the emphysema subtypes, while no significant differences were observed according to GOLD grades.
Pulmonary vascular alteration is an important complication in the natural history of COPD, but its pathophysiologic mechanisms are still poorly understood [19]. Endothelial dysfunction is a major cause of vascular remodeling and emphysema [2, 8, 20]. Chest CT could quantitatively assess macroscopic pulmonary vascular alterations in subjects with COPD. The ratio of the main pulmonary artery to the ascending aorta diameter has been suggested as an important marker for pulmonary vascular disease [19]. Approximately 66% of subjects with COPD have some degree of pulmonary hypertension, and a pulmonary artery-to-ascending-aorta ratio > 1 was associated with acute exacerbation (AE) and mortality in patients with COPD [21,22,23]. Our previous study showed that the pulmonary artery-to-ascending aorta ratio was correlated with FEV1 in patients with mild to moderate COPD [24]. In addition, a study using CT and cardiac magnetic resonance imaging reported that pulmonary artery enlargement is associated with the loss of blood volume in the distal pulmonary vessels in patients with COPD [25].
The CSAs of the small pulmonary vessels can be evaluated quantitatively on CT to identify pulmonary vascular alterations in patients with COPD [19, 26]. Several studies found the CSAs of small pulmonary vessels to be associated with symptoms, pulmonary artery pressure, pulmonary function, exercise capacity, AE of COPD, and mortality [5, 27, 28]. In our study, similar to other studies, Ntotal/LSA and N<5 mm/LSA showed a distinct decrease as the GOLD grades progressed. Histological studies have shown that a greater degree of emphysema and SAD are associated with pulmonary vascular alteration [29, 30]. Downregulation of lung vascular endothelial growth factor (VEGF) and upregulation of inducible nitric oxide synthase (iNOS), which can lead to endothelial dysfunction, play crucial roles in the development of vascular alteration and emphysema [6, 20, 31]. Previous studies have found a relationship between quantitative CT vascular parameters and emphysema [8, 17]. Likewise, the current study showed that Ntotal/LSA and N<5 mm/LSA were negatively correlated with LAA-950. In addition, the quantitative assessment of pulmonary vascular alterations may be more strongly associated with the extent of emphysema than the PFT results.
COPD is a heterogeneous disease with various clinical and pathologic characteristics, and can traditionally be distinguished into two phenotypes: emphysema and airway disease [32, 33]. VEGF is a potential mediator of pulmonary vascular remodeling, and its expression increases in the airway of bronchitis-type patients, leading to abnormal proliferation of endothelial and vascular smooth muscle cells in pulmonary vessels [34]. A study reported that pulmonary vascular alteration was more strongly associated to the emphysema phenotype than to the bronchitis phenotype in patients with COPD [8]. In our study, the analysis was conducted by dividing the patients into five subtypes based on quantitative CT analysis. Compared with the emphysema phenotype, Ntotal/LSA and N<5 mm/LSA were significantly higher in the SAD phenotype.
In the past, pulmonary vascular disease was considered an end-stage feature of COPD, and pulmonary hypertension was observed in 90% of patients with GOLD grade 4 COPD [17, 35]. However, recent studies have shown that pulmonary vascular alteration occurs in the setting of subclinical and early stage COPD by an impairment of endothelial function in pulmonary vessels [4, 19, 26, 36, 37]. Emphysema and air trapping progressed over time in smokers [12]. Some studies reported that emphysema increased over 2–3 years, whereas the CSAs of small pulmonary vessels did not decrease [9, 38]. In our study, there were no changes in each GOLD grade, but the number of vessels decreased in the normal and SAD CT subtype over the follow-up period of up to 6 years. This suggests that vessel changes over time were more prominent in the normal and SAD phenotype than in the emphysema phenotype. However, various factors could have affected this result, because the pulmonary hemodynamics affecting quantitative CSA assessment can be changed by breath-holding, circulating blood volume, and treatment [9, 38, 39].
This study has several limitations. First, we quantitatively measured the pulmonary vessel count change based on volumetric chest CT but could not distinguish between the pulmonary artery and vein. Second, the gold standard for assessing pulmonary vascular abnormality and pulmonary hypertension is right heart catheterization, but this was not done in our study because of the invasiveness of the method. Third, a longitudinal analysis was performed over the 6-year follow-up period, but the number of subjects gradually decreased. Thus, we performed to analyze after missing value correction using the MI method [18]. However, the statistical power to detect statistical significance in longitudinal observations of pulmonary vascular changes was lack. In addition, we considered quantitatively and qualitatively the emphysema index for all subjects and subjects with smoking to observe longitudinal change but the results were not shown the significance of statistical results. Therefore, validation of our results in a large cohort study is necessary. The last one is that the cohort used in this study was collected for subjects in a dust area. In order words, it includes non-smoking COPD caused by dust other than cigarette smoking, generalization might be limited. In addition, 52 subjects with asthma were included but the diagnosis of asthma is unclear due to collecting based on questionnaire survey. Therefore, it was difficult to exclude asthma patients.
Conclusion
Quantitative pulmonary vascular parameters measured using volumetric chest CT were significantly associated with clinical measures of COPD severity. Quantitative CT features faithfully reflected pulmonary vessel alterations in patients with COPD. In addition, we performed a longitudinal analysis of pulmonary vessel changes for up to 6 years according to GOLD grade and CT subtype. The long-term follow-up revealed that pulmonary vessel change was more severe in the normal and SAD subtype than in the emphysema subtype.
Availability of data and materials
The datasets used for the current study are available from the corresponding author on reasonable request.
Abbreviations
- AE:
-
Acute exacerbation
- CAT:
-
COPD assessment test
- CODA:
-
COPD in dusty area
- COPD:
-
Chronic obstructive lung disease
- CSAs:
-
Cross-sectional areas
- CT:
-
Computed tomography
- EI:
-
CT-emphysema index
- FEV1 :
-
Forced expiratory volume in 1 s
- FVC:
-
Forced vital capacity
- GOLD:
-
Global initiative for obstructive lung disease
- HU:
-
Hounsfield unit
- iNOS:
-
Inducible nitric oxide synthase (iNOS)
- LAA-950:
-
Low-Attenuation Areas less than-950
- LSA:
-
Lung surface area
- mMRC:
-
Modified medical research council
- Ntotal :
-
Total number of vessels as values per 1 cm2
- N< 5 mm :
-
Total number of vessels with vessel area less than 5 mm2
- PFT:
-
Pulmonary function test
- VEGF:
-
Lung vascular endothelial growth factor
References
Stringer WW, Porszasz J, Bhatt SP, McCormack MC, Make BJ, Casaburi R. Physiologic insights from the COPD genetic epidemiology study. J COPD F. 2019;6:256–66.
Kovacs G, Agusti A, Barberà JA, Celli B, Criner G, Humbert M, et al. Pulmonary vascular involvement in chronic obstructive pulmonary disease. Is there a pulmonary vascular phenotype? Am J Respir Crit Care Med. 2018;198:1000–11.
Celli BR, MacNee W. ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46.
Barberà JA, Riverola A, Roca J, Ramirez J, Wagner PD, Ros D, et al. Pulmonary vascular abnormalities and ventilation-perfusion relationships in mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;149:423–9.
Yang T, Chen C, Chen Z. The CT pulmonary vascular parameters and disease severity in COPD patients on acute exacerbation: a correlation analysis. BMC Pulm Med. 2021;21:34.
Estépar RSJ, Kinney GL, Black-Shinn JL, Bowler RP, Kindlmann GL, Ross JC, et al. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med. 2013;188:231–9.
Labaki WW, Martinez CH, Martinez FJ, Galbán CJ, Ross BD, Washko GR, et al. The role of chest computed tomography in the evaluation and management of the patient with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196:1372–9.
Matsuoka S, Washko GR, Dransfield MT, Yamashiro T, San Jose Estepar R, Diaz A, et al. Quantitative CT measurement of cross-sectional area of small pulmonary vessel in COPD: correlations with emphysema and airflow limitation. Acad Radiol. 2010;17:93–9.
Saruya S, Matsuoka S, Yamashiro T, Matsushita S, Fujikawa A, Yagihashi K, et al. Quantitative CT measurements of small pulmonary vessels in chronic obstructive pulmonary disease: do they change on follow-up scans? Clin Physiol Funct Imaging. 2016;36:211–7.
Scarrow GD. The pulmonary angiogram in chronic bronchitis and emphysema. Proc R Soc Med. 1965;58:684–7.
Cordasco EM, Beerel FR, Vance JW, Wende RW, Toffolo RR. Newer aspects of the pulmonary vasculature in chronic lung disease. A comparative study. Angiology. 1968;19:399–407.
Pompe E, Strand M, van Rikxoort EM, Hoffman EA, Barr RG, Charbonnier JP, et al. Five-year progression of emphysema and air trapping at CT in smokers with and those without chronic obstructive pulmonary disease: results from the COPDGene study. Radiology. 2020;295:218–26.
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49:557–82.
Bhatt SP, Washko GR, Hoffman EA, Newell JD, Bodduluri S, Diaz AA, et al. Imaging advances in chronic obstructive pulmonary disease. Insights from the genetic epidemiology of chronic obstructive pulmonary disease (COPDGene) study. Am J Respir Crit Care Med. 2019;199:286–301.
Park J, Hobbs BD, Crapo JD, Make BJ, Regan EA, Humphries S, et al. Subtyping COPD by using visual and quantitative CT imaging features. Chest. 2020;157:47–60.
Park S, Lee SM, Kim N, Seo JB, Shin H. Automatic reconstruction of the arterial and venous trees on volumetric chest CT. Med Phys. 2013;40:071906.
Cho YH, Lee SM, Seo JB, Kim N, Bae JP, Lee JS, et al. Quantitative assessment of pulmonary vascular alterations in chronic obstructive lung disease: Associations with pulmonary function test and survival in the KOLD cohort. Eur J Radiol. 2018;108:276–82.
Pompe E, Galbán CJ, Ross BD, Koenderman L, Nick HT, Postma DS, van den Berge M, de Jong PA, Lammers JW, Hoesein FA, et al. Parametric response mapping on chest computed tomography associates with clinical and functional parameters in chronic obstructive pulmonary disease. Respir Med. 2017;123:48–55.
Blanco I, Tura-Ceide O, Peinado VI, Barberà JA. Updated Perspectives on Pulmonary Hypertension in COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:1315–24.
Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med. 2001;163:737–44.
Aluja Jaramillo F, Gutierrez FR, Díaz Telli FG, Yevenes Aravena S, Javidan-Nejad C, Bhalla S. Approach to pulmonary hypertension: from CT to clinical diagnosis. Radiographics. 2018;38:357–73.
Shin S, King CS, Brown AW, Albano MC, Atkins M, Sheridan MJ, et al. Pulmonary artery size as a predictor of pulmonary hypertension and outcomes in patients with chronic obstructive pulmonary disease. Respir Med. 2014;108:1626–32.
Wells JM, Washko GR, Han MK, Abbas N, Nath H, Mamary AJ, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367:913–21.
Hahm CR, Lim MN, Kim HY, Hong S-H, Han S-S, Lee S-J, et al. Implications of the pulmonary artery to ascending aortic ratio in patients with relatively mild chronic obstructive pulmonary disease. J Thorac Dis. 2016;8:1524–31.
Wells JM, Iyer AS, Rahaghi FN, Bhatt SP, Gupta H, Denney TS, Lloyd SG, Dell’Italia LJ, Nath H, Estepar RS, Washko GR. Pulmonary artery enlargement is associated with right ventricular dysfunction and loss of blood volume in small pulmonary vessels in chronic obstructive pulmonary disease. Circulation. 2015;8(4):e002546.
Uejima I, Matsuoka S, Yamashiro T, Yagihashi K, Kurihara Y, Nakajima Y. Quantitative computed tomographic measurement of a cross-sectional area of a small pulmonary vessel in nonsmokers without airflow limitation. Jpn J Radiol. 2011;29:251–5.
Yoshimura K, Suzuki Y, Uto T, Sato J, Imokawa S, Suda T. Morphological changes in small pulmonary vessels are associated with severe acute exacerbation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11:1435–45.
Washko GR, Nardelli P, Ash SY, Vegas Sanchez-Ferrero G, Rahaghi FN, Come CE, et al. Arterial vascular pruning, right ventricular size, and clinical outcomes in chronic obstructive pulmonary disease. A longitudinal observational study. Am J Respir Crit Care Med. 2019;200:454–61.
Wright JL, Lawson L, Paré PD, Hooper RO, Peretz DI, Nelems JM, et al. The structure and function of the pulmonary vasculature in mild chronic obstructive pulmonary disease. The effect of oxygen and exercise. Am Rev Respir Dis. 1983;128:702–7.
Hale KA, Niewoehner DE, Cosio MG. Morphologic changes in the muscular pulmonary arteries: relationship to cigarette smoking, airway disease, and emphysema. Am Rev Respir Dis. 1980;122:273–8.
Weissmann N. Chronic obstructive pulmonary disease and pulmonary vascular disease. A comorbidity? Ann Am Thorac Soc. 2018;15:S278–81.
Filley GF, Beckwitt HJ, Reeves JT, Mitchell RS. Chronic obstructive bronchopulmonary disease. II. Oxygen transport in two clinical types. Am J Med. 1968;44:26–38.
Vanfleteren LEGW, Spruit MA, Groenen M, Gaffron S, van Empel VPM, Bruijnzeel PLB, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:728–35.
Kanazawa H, Asai K, Nomura S. Vascular endothelial growth factor as a non-invasive marker of pulmonary vascular remodeling in patients with bronchitis-type of COPD. Respir Res. 2007;8:22.
Chaouat A, Bugnet A-S, Kadaoui N, Schott R, Enache I, Ducoloné A, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:189–94.
Synn AJ, Li W, San José Estépar R, Zhang C, Washko GR, O’Connor GT, et al. Radiographic pulmonary vessel volume, lung function and airways disease in the Framingham Heart Study. Eur Respir J. 2019. https://doi.org/10.1183/13993003.00408-2019.
Santos S, Peinado VI, Ramírez J, Melgosa T, Roca J, Rodriguez-Roisin R, et al. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J. 2002;19:632–8.
Takayanagi S, Kawata N, Tada Y, Ikari J, Matsuura Y, Matsuoka S, et al. Longitudinal changes in structural abnormalities using MDCT in COPD: do the CT measurements of airway wall thickness and small pulmonary vessels change in parallel with emphysematous progression? Int J Chron Obstruct Pulmon Dis. 2017;12:551–60.
Matsuura Y, Kawata N, Yanagawa N, Sugiura T, Sakurai Y, Sato M, et al. Quantitative assessment of cross-sectional area of small pulmonary vessels in patients with COPD using inspiratory and expiratory MDCT. Eur J Radiol. 2013;82:1804–10.
Acknowledgements
Not applicable.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF 2018R1D1A1B07049670) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2021R1A4A5032806).
Author information
Authors and Affiliations
Contributions
SHB takes responsibility for the content of the manuscript, including the data and analysis. SHB and WJK were responsible for conceptualization; SHB and WJK conducted data collection; S.W.P., MNL, and SHB performed data curation; SWP, MNL, SHB conducted formal analysis; SHB and WJK were responsible for funding acquisition; SWP, MNL, and SHP performed investigations and methodology; SWP, SHB were responsible for project administration; SHB and WJK supervised the study; and SWP, and SHB wrote the original draft. All authors contributed to writing, reviewing, and editing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by Institutional Review Board of Kangwon National University Hospital (KNUH, 2012-06-007). All participants gave their written informed consent. Our study was conducted in accordance with the amended Declaration of Helsinki.
Consent for publication
Not Applicable.
Competing interests
The authors have no financial conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Characteristics of subjects with COPD in the CODA cohort (Smoking group). Table S2. Longitudinal changes over a follow up period up to 6 years for subjects with smoking. Table S3. The GOLD results reflecting the longitudinal changes of pulmonary vascular up to 6 years. Table S4. The CT-based results reflecting the longitudinal changes of pulmonary vascular up to 6 years
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Park, S.W., Lim, MN., Kim, W.J. et al. Quantitative assessment the longitudinal changes of pulmonary vascular counts in chronic obstructive pulmonary disease. Respir Res 23, 29 (2022). https://doi.org/10.1186/s12931-022-01953-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-022-01953-7