Abstract
Background
Acute exacerbation of idiopathic pulmonary fibrosis (AE-IPF) is associated with high mortality. However, few studies have so far reviewed analyses of autopsy findings in patients with AE-IPF.
Methods
We retrospectively reviewed 52 consecutive patients with AE-IPF who underwent autopsies at five university hospitals and one municipal hospital between 1999 and 2013. The following variables were abstracted from the medical records: demographic and clinical data, autopsy findings and complications during the clinical course until death.
Results
The median age at autopsy was 71 years (range 47–86 years), and the subjects included 38 (73.1%) males. High-dose corticosteroid therapy was initiated in 45 (86.5%) patients after AE-IPF. The underling fibrotic lesion was classified as having the usual interstitial pneumonia (UIP) pattern in all cases. Furthermore, 41 (78.8%) patients had diffuse alveolar damage (DAD), 15 (28.8%) exhibited pulmonary hemorrhage, nine (17.3%) developed pulmonary thromboembolism and six (11.5%) were diagnosed with lung carcinoma. In addition, six (11.5%) patients developed pneumothorax prior to death and 26 (53.1%) developed diabetes that required insulin treatment after the administration of high-dose corticosteroid therapy. In addition, 15 (28.8%) patients presented with bronchopneumonia during their clinical course and/or until death, including fungal (seven, 13.5%), cytomegalovirus (six, 11.5%) and bacterial (five, 9.6%) infections.
Conclusions
The pathological findings in patients with AE-IPF represent not only DAD, but also a variety of pathological conditions. Therefore, making a diagnosis of AE-IPF is often difficult, and the use of cautious diagnostic approaches is required for appropriate treatment.
Similar content being viewed by others
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, fibrosing form of interstitial pneumonia with a median survival after diagnosis of three to five years [1]-[4]. Acute exacerbation of IPF (AE-IPF) has an extremely poor prognosis and is believed to occur in 5-10% of patients with IPF annually [5],[6]. In 1993, AE-IPF was first described in a case report by Kondoh et al. as acute clinical deterioration in three IPF patients in the absence of an identified infection [7]. The American Thoracic Society and European Respiratory Society subsequently introduced the notion of AE-IPF in 2002 [8], and Collard and colleagues focused on establishing a worldwide consensus for AE-IPF in 2007 [9]. This consensus statement is currently the most widely used definition of AE-IPF, having been used in several clinical studies and being referred to in the 2011 IPF guidelines [10].
Lung tissue derived from patients with IPF shows a characteristic histopathological pattern known as usual interstitial pneumonia (UIP), which includes the presence of fibroblastic foci. The pathological findings of AE-IPF comprise diffuse alveolar damage (DAD) superimposed on underlying UIP; this is the most commonly described finding on surgical lung biopsies [9],[11],[12]. On the other hand, several studies have reported the detection of organizing pneumonia (OP) without evidence of organizing DAD or extensive fibroblastic foci [13],[14]. In addition, although previous reports have focused on the postmortem pathological findings of patients with IPF [15],[16], no studies have focused on the characteristics of “acute exacerbation”, and the pathological findings and clinical manifestations of AE-IPF remain only partially understood.
The current study is a retrospective review of an autopsy series designed to describe and evaluate the pathological findings, including concomitant and infectious diseases, observed during the clinical course until death in patients with AE-IPF.
Methods
Patients
A total of 52 patients with AE-IPF who underwent autopsies at five university hospitals and one municipal hospital between January 1, 1999 and December 31, 2013 were identified based on medical records. The Ethics Committee of University of Occupational and Environmental Health in Kitakyushu, Japan approved this study (approval number H26-12, April 3, 2014), with a waiver for informed consent due to the retrospective study design. AE-IPF was defined according to a previous report [9] and included the following items: [1] a previous or concurrent diagnosis of idiopathic pulmonary fibrosis; [2] unexplained worsening or new development of dyspnea within 30 days; [3] high-resolution computed tomography (HRCT) findings showing new sites of bilateral ground-glass opacity; [4] abnormalities and/or consolidation superimposed on a background of a reticular or honeycomb pattern consistent with the UIP pattern; [5] no evidence of pulmonary infection on an endotracheal aspirate or bronchoalveolar lavage (BAL) sample and [6] the exclusion of left heart failure, pulmonary embolism and other identifiable causes of acute lung injury. Regarding infectious causes in the current study, we carefully excluded possible infections based on the findings of a sputum examination, laboratory tests and physical examinations, although we did not perform endotracheal aspiration or BAL in all cases due to the presence of severe hypoxemia. In addition, we attempted to rule out pulmonary embolism as far as possible using enhanced computed tomography. The following variables were abstracted from the medical records: demographic data (age, sex, smoking history, symptoms, comorbidities and prior treatment for IPF), clinical data (hospitalization before death, laboratory results and HRCT findings) and the autopsy findings, including infectious causes.
Autopsy findings in the patients with AE-IPF
All autopsied lung materials were fixed in 10% formalin for more than seven days. At least one tissue block was prepared from the gross AE-IPF lesions in each lobe, and the tissue blocks were embedded in paraffin. Sections (4 μm thick) were cut and stained with hematoxylin and eosin (H&E) using standard procedures. The pathological diagnosis of UIP was made according to the current guidelines [10]. The criteria for DAD were the presence of hyaline membranes in addition to at least one of the following findings: alveolar type I cell or endothelial cell necrosis, edema, organizing interstitial fibrosis or prominent alveolar type II cell proliferation [15]. We also performed phosphotungstic acid hematoxylin staining for fibrin in patients without a hyaline membrane on H&E staining. OP was pathologically defined as the presence of buds of granulation tissue in the distal air spaces progressing from fibrin exudates to loose collagen-containing fibroblasts [16]. Alveolar hemorrhage was diagnosed in cases involving acute hemorrhage in the alveoli and airways as well as the presence of macrophages (i.e., siderophages) that stained positively for hemosiderin with Berlin-blue stain. Right ventricular hypertrophy was defined as a right ventricular free wall thickness of ≥ 5 mm [17].
Screening for infectious diseases in the autopsied lungs
Four-micrometer-thick sections were cut from the lung tissue blocks and stained with H&E for the histopathological examinations in addition to Periodic acid-Schiff (PAS) stain, Grocott's methenamine silver stain and Gram stain to identify any infectious pathogens in the presence of bronchopneumonia. Bronchopneumonia (i.e., concomitant infection) was conclusively diagnosed based on histopathological evidence, such as neutrophil infiltration, fibrinopurulent exudate accumulation or abscess formation in the broncho-bronchioloalveolar space. For immunohistochemical detection of cytomegalovirus (CMV) antigens, the sections were incubated with a mouse monoclonal anti-CMV antibody (DAKO, Glostrup, Denmark; diluted 1:20) for 30 minutes. Secondary antibody peroxidase-linked polymers were then applied, and the sections were incubated with a solution consisting of 20 mg of 3.3’-diaminobenzidine tetrahydrochloride, 65 mg of sodium azide and 20 ml of 30% H2O2 in 100 ml of Tris–HCl (50 mM, pH 7.6). After counterstaining with Meyer’s hematoxylin, the sections were observed under a light microscope.
Results
Patient characteristics
A total of 52 patients with AE-IPF underwent autopsies during the study period. The demographics of the patients are reported in Table 1. All patients had a known diagnosis of IPF in addition to either unexplained worsening or the development of dyspnea within 30 days and were hospitalized at the time of death. A large majority of patients experienced acute episodes of AE-IPF, including 41 (78.8%) patients with coughing and 22 (42.3%) patients with a fever. Only one patient was diagnosed with pulmonary hypertension prior to AE-IPF, whereas 12 patients were additionally diagnosed with pulmonary hypertension at the onset of AE-IPF based on the findings of right heart catheterization and/or transthoracic echocardiography [18]. The median survival period for the patients with AE-IPF was approximately 29 days (range: 1 to 134 days) from admission.
Nearly half of the patients had received corticosteroid therapy before AE-IPF. The therapeutic regimens for AE-IPF are shown in Table 2. Forty-five (86.5%) patients had received high-dose corticosteroid therapy, 43 (82.7%) had received antibacterial drugs and 27 (51.9%) were on mechanical ventilation due to hypoxemia. Sulfamethoxazole/trimethoprim was used to prevent pneumocystis pneumonia in 21 (40.4%) patients. Six (11.5%) patients developed pneumothorax prior to death and 26 (57.8%) developed insulin-dependent diabetes after receiving high-dose corticosteroid therapy.
Pathological findings
The mean duration between death and autopsy was 286 minutes (range: 60 to 990 minutes). Overall, the median weights of the right and left lungs were 658 g (range: 320 to 1,330 g) and 552 g (range: 260 to 1,000 g), respectively. These median weights were remarkably heavier than the standard lung weights reported for Japanese males (right and left lung weights: 474 and 404 g, respectively) [19]. The underlying pulmonary fibrotic lesion was classified as exhibiting the UIP pattern in all cases. Forty-one (78.8%) patients had DAD (Figure 1A-D), 15 (28.8%) patients had pulmonary hemorrhage (Figure 2A), nine (17.3%) patients had pulmonary thromboembolism (Figure 2B) and six (11.5%) patients had lung carcinoma (Table 3). Only two of the 15 AE-IPF patients with pulmonary hemorrhage received anticoagulant therapy.
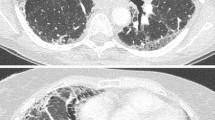
A 78-year-old male with acute exacerbation of idiopathic pulmonary fibrosis (AE-IPF). High resolution computed tomography (HRCT) images at the initial diagnosis of IPF showed subpleural-predominant interstitial fibrosis, traction bronchiectasis (arrow) and honeycombing (arrowhead) (A). HRCT images at the onset of acute exacerbation (12 months after the initial diagnosis) showed diffuse areas of ground glass attenuations superimposed on underlying fibrotic opacities (B). The underling fibrotic lesions were classified as the UIP pattern, including dense interstitial fibrosis with focal squamous or bronchial metaplasia (inset), alternating with only bland-looking alveolar walls (H&E, ×100) (C). DAD with hyaline membranes (inset) superimposed on a background fibrotic and edematous lung with several fibroblastic foci (H&E, ×100) (D).
The histopathological findings of the lung specimens obtained at autopsy. The microscopic findings of the lung showed diffuse alveolar hemorrhage (arrows) without any specific evidence of vasculitis (A) or pulmonary thromboembolism (B) (H&E, ×10). The macroscopic findings of the heart showed overt right ventricular hypertrophy, confirmed by an increased (≥5 mm) right ventricular wall thickness (double-headed arrows) (C). Immunohistochemical staining of an inflamed lung tissue specimen revealed a number of specific cytomegalovirus-positive cells (proliferating type II pneumocytes) (D).
The pathological findings in the 11 patients with AE-IPF who met the 2007 criteria but did not have DAD were indicative of UIP alone (n = 5), alveolar hemorrhage (n = 3), pulmonary thromboembolism (n = 1), OP (n = 1) and lung adenocarcinoma (n = 1) (Figure 3). There were no significant differences in any of the patient characteristics between the UIP with DAD and UIP without DAD groups on admission. In addition, there were no correlations or significant differences between the degree of pulmonary hypertension and histological variation. Among the 52 patients with AE-IPF, 13 (25.0%) exhibited gastrointestinal hemorrhage and 18 (34.6%) displayed right ventricular hypertrophy (Figure 2C) as extrapulmonary findings.
Infectious causes
Of the 52 autopsies performed, death was attributed to bronchopneumonia in 15 (28.8%) patients (Table 4). In these cases, the pulmonary infectious lesions were not diagnosed until autopsy. The causes of infection included fungal infection in seven cases (13.5%), CMV infection (Figure 2D) in six cases (11.5%) and bacterial infection in five cases (9.6%). One patient with pulmonary aspergillosis died on the first day after AE-IPF; however, the postmortem pathological findings of the lungs in this case primarily showed a DAD pattern, with only slight bronchopneumonia induced by aspergillosis. Gram staining demonstrated the infectious bacteria to be Gram-negative rods and Gram-positive cocci. Pneumocystis pneumonia was not detected in any patient in the present study. All patients with AE-IPF, except one, had received high-dose corticosteroid therapy and/or immunosuppressive agents. There were also no significant differences in the time interval between the diagnosis of AE-IPF and death based on whether the patient was diagnosed with infectious disease (29.8 vs 31.1 days, p = 0.87).
Discussion
In this study, we retrospectively analyzed the autopsy findings of AE-IPF and clarified that AE-IPF exhibits a variety of pathological findings in addition to DAD. Moreover, the AE-IPF patients were diagnosed with various infectious diseases and complications during their clinical course after AE-IPF.
DAD has been reported to be the focal pathological finding of AE-IPF [9]. DAD also accounted for many cases of AE-IPF in the present investigation, involving a mixture of pulmonary hemorrhage, pulmonary thromboembolism and OP. However, DAD was not observed in all cases. In particular, pulmonary hemorrhage was a representative pathological finding in the AE-IPF patients without DAD. Pulmonary hemorrhage is rarely encountered during the course of IPF [20]-[22], and it is difficult to make a premortem diagnosis in actual clinical practice without performing BAL. Nevertheless, careful attention is required in such cases, as the use of BAL in IPF patients may increase the risk of acute exacerbation [23]. Moreover, pulmonary thromboembolism frequently occurs as a complication during the course of IPF [24] and can be an important differential diagnosis of AE-IPF.
Regarding acute respiratory distress syndrome (ARDS), in which the diagnostic criteria are based on clinical findings, in the same manner as AE-IPF, Patel S.R. et al. reported that they investigated the pathological findings of 57 cases satisfying the diagnostic criteria for ARDS via open-lung biopsy, with DAD observed in 23 patients (40.3%) [25]. On the other hand, another study reported specific infections in eight patients (14.0%), diffuse alveolar hemorrhage in five patients (8.8%) and bronchiolitis obliterans organizing pneumonia in five patients (8.8%). Moreover, Esteban A. et al. found that, although DAD was observed in 112 of 127 autopsy cases satisfying the diagnostic criteria for ARDS, the following diseases were observed in patients without DAD, in order of descending prevalence: pneumonia, pulmonary hemorrhage, pulmonary edema and pulmonary embolism [26]. The findings of such diverse pathological features are very similar to the results of the present study. To date, very few investigations have been carried out regarding the pathological findings of AE-IPF, and the present study is the first report to summarize the features of autopsy cases involving the “acute exacerbation” of IPF. In this study, although the diagnosis was made while checking the findings of AE-IPF against the diagnostic criteria, in the same manner as diagnosing ARDS, not all of the patients with AE-IPF exhibited DAD, namely only 78.8% of the AE-IPF patients demonstrated DAD. Moreover, a significant number of patients who satisfied the diagnostic criteria for AE-IPF did not have remarkable DAD (11/52, 21.2%). In addition, we found no findings associated with acute fibrinous or organizing pneumonia in the AE-IPF patients without DAD [27]. In these cases, genuine respiratory failure accompanied the progression of pulmonary fibrosis, which may constitute the natural course of IPF. Notably, the results of the present study are supported by the findings of a previous report in which only extensive fibroblastic foci were observed as a pathological finding of an acute pattern [13]. The incidence of gastrointestinal hemorrhage and right ventricular hypertrophy, both of which are extrapulmonary findings, was 25.0% and 34.6%, respectively. Furthermore, the effects of hypoxic, physical and psychosomatic stress [28] and high-dose corticosteroid therapy [29] must be considered with respect to gastrointestinal hemorrhage. Right ventricular hypertrophy may be caused by pulmonary hypertension [30] at the end stage of AE-IPF. Moreover, the relationship between IPF and pulmonary hypertension is important, and Judge and colleagues recently reported that pulmonary hypertension is associated with acute disease exacerbation as well as poor survival [31]. Therefore, it is necessary to monitor the potential for pulmonary hypertension in patients with IPF.
Although there is no established standard therapy for AE-IPF [10], high-dose corticosteroid therapy [11],[12],[14],[32]-[34] and immunosuppressive agents [35],[36] are commonly used in clinical practice. However, a previous study [37] suggested that these drugs increase the rates of infectious complications. In addition to the immunodeficient state caused by corticosteroids and/or immunosuppressants, the application of intensive antimicrobial treatment following the acute exacerbation of pulmonary infection may have affected the results of the present study. Regardless, similar to recently published data [38] from a study of gene expression profiling of patients with AE-IPF, our data demonstrated that an infectious etiology may not be the main cause of AE-IPF. In fact, all patients underwent treatment with high-dose corticosteroids and/or immunosuppressive agents, excluding only one patient, with mechanical ventilation carried out in more than half of the AE-IPF patients. Moreover, the pulmonary infections detected in the present study may have affected, at least in part, the occurrence of respiratory failure leading to death, although there were no significant differences in the time interval between the diagnosis of AE-IPF and death based on the presence of infectious disease. Therefore, in addition to the detection of pathological findings based on DAD, AE-IPF eventually causes respiratory failure due to the effects of accompanying infectious diseases, with patients thus exhibiting a variety of histopathological features. The present investigation was carried out among autopsy cases only; therefore, the efficacy of high-dose corticosteroid therapy against AE-IPF cannot be debated based on our results. However, taking into consideration the fact that many patients with diabetes and pneumothorax following acute disease exacerbation underwent high-dose corticosteroid therapy, such therapy should be carefully administered based on the tolerability and efficacy of the treatment in conjunction with multidisciplinary therapies. Physicians should therefore be aware of the appropriate therapeutic strategies when treating patients with complications induced by treatment for AE-IPF. Furthermore, the results of the present study indicate that it is difficult to distinguish acute exacerbation from other disorders, even if the patient meets the diagnostic criteria for AE-IPF. Given the results of this study, it is very important for clinicians to be alert to the possibility of other treatable disorders, such as infectious diseases, as no effective treatment regimen for AE-IPF has been established to date.
There are several limitations associated with this study. First, only patients who died and underwent autopsy were included; therefore, the results may differ based on the status of onset of AE-IPF. In other words, no patients who survived after AE-IPF were included, and the present findings thus do not reflect all aspects of the entity of AE-IPF. Second, we did not perform endotracheal aspiration or bronchoalveolar lavage due to the presence of severe hypoxemia in all cases. However, we carefully excluded patients based on the findings of sputum, laboratory and physical examinations. In addition, a previous study reported that the features of clinically suspected acute exacerbation diagnosed based on the radiological and clinical course are similar to those of acute exacerbation diagnosed based on the results of several intensive examinations, including BAL [39]. Third, this study was carried out jointly across multiple facilities, primarily university hospitals, including only Japanese IPF patients. Hence, potential biases, including racial and institutional selection, must be considered when interpreting the results. Finally, 52% of the patients with AE-IPF in the present study required mechanical ventilation on admission, which suggests that these patients had a relatively more severe condition. Therefore, the results of this study may not be extrapolated to all cases of AE-IPF.
Conclusions
The pathological findings of AE-IPF include DAD as well as a variety of pathological conditions, and making a definitive diagnosis of AE-IPF is difficult. Patients with AE-IPF therefore are at risk of death due to the occurrence of several complications during their clinical course, including the effects of treatment with high-dose corticosteroid therapy. In patients with AE-IPF, it is very important to treat the disease by monitoring the patient’s condition and timely intervening with appropriate treatment.
Abbreviations
- IPF:
-
Idiopathic pulmonary fibrosis
- AE-IPF:
-
Acute exacerbation of idiopathic pulmonary fibrosis
- UIP:
-
Usual interstitial pneumonia
- DAD:
-
Diffuse alveolar damage
- OP:
-
Organizing pneumonia
- HRCT:
-
High-resolution computed tomography
- BAL:
-
Bronchoalveolar lavage
- H&E:
-
Hematoxylin and eosin
- PAS:
-
Periodic acid-Schiff
- CMV:
-
Cytomegalovirus
- RV:
-
Right ventricular
- LV:
-
Left ventricular
- ARDS:
-
Acute respiratory distress syndrome
References
Bjoraker JA, Ryu JH, Edwin MK, Myers JL, Tazelaar HD, Schroeder DR, Offord KP: Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998, 157: 199-203. 10.1164/ajrccm.157.1.9704130.
Flaherty KR, Toews GB, Travis WD, Colby TV, Kazerooni EA, Gross BH, Jain A, Strawderman RL, Paine R, Flint A, Lynch JP, Martinez FJ: Clinical significance of histological classification of idiopathic interstitial pneumonia. Eur Respir J. 2002, 19: 275-283. 10.1183/09031936.02.00182002.
Nicholson AG, Colby TV, du Bois RM, Hansell DM, Wells AU: The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med. 2000, 162: 2213-2217. 10.1164/ajrccm.162.6.2003049.
Rudd RM, Prescott RJ, Chalmers JC, Johnston ID: British Thoracic Society Study on cryptogenic fibrosing alveolitis: Response to treatment and survival. Thorax. 2007, 62: 62-66. 10.1136/thx.2005.045591.
Kondoh Y, Taniguchi H, Katsuta T, Kataoka K, Kimura T, Nishiyama O, Sakamoto K, Johkoh T, Nishimura M, Ono K, Kitaichi M: Risk factors of acute exacerbation of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010, 27: 103-110.
Ley B, Collard HR, King TE: Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011, 183: 431-440. 10.1164/rccm.201006-0894CI.
Kondoh Y, Taniguchi H, Kawabata Y, Yokoi T, Suzuki K, Takagi K: Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest. 1993, 103: 1808-1812. 10.1378/chest.103.6.1808.
Society AT, Society ER: American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002, 165: 277-304. 10.1164/ajrccm.165.2.ats01.
Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE, Lasky JA, Loyd JE, Noth I, Olman MA, Raghu G, Roman J, Ryu JH, Zisman DA, Hunninghake GW, Colby TV, Egan JJ, Hansell DM, Johkoh T, Kaminski N, Kim DS, Kondoh Y, Lynch DA, Müller-Quernheim J, Myers JL, Nicholson AG, Selman M, Toews GB, Wells AU, Martinez FJ: Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007, 176: 636-643. 10.1164/rccm.200703-463PP.
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, et al: An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011, 183: 788-824. 10.1164/rccm.2009-040GL.
Kim DS, Park JH, Park BK, Lee JS, Nicholson AG, Colby T: Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J. 2006, 27: 143-150. 10.1183/09031936.06.00114004.
Ambrosini V, Cancellieri A, Chilosi M, Zompatori M, Trisolini R, Saragoni L, Poletti V: Acute exacerbation of idiopathic pulmonary fibrosis: report of a series. Eur Respir J. 2003, 22: 821-826. 10.1183/09031936.03.00022703.
Churg A, Müller NL, Silva CI, Wright JL: Acute exacerbation (acute lung injury of unknown cause) in UIP and other forms of fibrotic interstitial pneumonias. Am J Surg Pathol. 2007, 31: 277-284. 10.1097/01.pas.0000213341.70852.9d.
Parambil JG, Myers JL, Ryu JH: Histopathologic features and outcome of patients with acute exacerbation of idiopathic pulmonary fibrosis undergoing surgical lung biopsy. Chest. 2005, 128: 3310-3315. 10.1378/chest.128.5.3310.
Katzenstein AL, Bloor CM, Leibow AA: Diffuse alveolar damage–the role of oxygen, shock, and related factors. A review. Am J Pathol. 1976, 85: 209-228.
Sulavik SB: The concept of “organizing pneumonia”. Chest. 1989, 96: 967-969. 10.1378/chest.96.5.967.
Forman MB, Wilson BH, Sheller JR, Kopelman HA, Vaughn WK, Virmani R, Friesinger GC: Right ventricular hypertrophy is an important determinant of right ventricular infarction complicating acute inferior left ventricular infarction. J Am Coll Cardiol. 1987, 10: 1180-1187. 10.1016/S0735-1097(87)80116-X.
Nadrous HF, Pellikka PA, Krowka MJ, Swanson KL, Chaowalit N, Decker PA, Ryu JH: Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest. 2005, 128: 2393-2399. 10.1378/chest.128.4.2393.
Sawabe M, Saito M, Naka M, Kasahara I, Saito Y, Arai T, Hamamatsu A, Shirasawa T: Standard organ weights among elderly Japanese who died in hospital, including 50 centenarians. Pathol Int. 2006, 56: 315-323. 10.1111/j.1440-1827.2006.01966.x.
Husari A, Beydoun A, Sheik Ammar A, Maakaron JE, Taher A: The untold story of Dabigatran etexilate: alveolar hemorrhage in an elderly patient with interstitial pulmonary fibrosis. J Thromb Thrombolysis. 2013, 35: 81-82. 10.1007/s11239-012-0784-9.
Perri D, Cole DE, Friedman O, Piliotis E, Mintz S, Adhikari NK: Azathioprine and diffuse alveolar haemorrhage: the pharmacogenetics of thiopurine methyltransferase. Eur Respir J. 2007, 30: 1014-1017. 10.1183/09031936.00026107.
Birnbaum J, Danoff S, Askin FB, Stone JH: Microscopic polyangiitis presenting as a “pulmonary-muscle” syndrome: is subclinical alveolar hemorrhage the mechanism of pulmonary fibrosis?. Arthritis Rheum. 2007, 56: 2065-2071. 10.1002/art.22633.
Sakamoto K, Taniguchi H, Kondoh Y, Wakai K, Kimura T, Kataoka K, Hashimoto N, Nishiyama O, Hasegawa Y: Acute exacerbation of IPF following diagnostic bronchoalveolar lavage procedures. Respir Med. 2012, 106: 436-442. 10.1016/j.rmed.2011.11.006.
Sprunger DB, Olson AL, Huie TJ, Fernandez-Perez ER, Fischer A, Solomon JJ, Brown KK, Swigris JJ: Pulmonary fibrosis is associated with an elevated risk of thromboembolic disease. Eur Respir J. 2012, 39: 125-132. 10.1183/09031936.00041411.
Patel SR, Karmpaliotis D, Ayas NT, Mark EJ, Wain J, Thompson BT, Malhotra A: The role of open-lung biopsy in ARDS. Chest. 2004, 125: 197-202. 10.1378/chest.125.1.197.
Esteban A, Fernández-Segoviano P, Frutos-Vivar F, Aramburu JA, Nájera L, Ferguson ND, Alía I, Gordo F, Ríos F: Comparison of clinical criteria for the acute respiratory distress syndrome with autopsy findings. Ann Intern Med. 2004, 141: 440-445. 10.7326/0003-4819-141-6-200409210-00009.
Beasley MB, Franks TJ, Galvin JR, Gochuico B, Travis WD: Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med. 2002, 126: 1064-1070.
Cook DJ, Fuller HD, Guyatt GH, Marshall JC, Leasa D, Hall R, Winton TL, Rutledge F, Todd TJ, Roy P: Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med. 1994, 330: 377-381. 10.1056/NEJM199402103300601.
Hernández-Díaz S, Rodríguez LA: Steroids and risk of upper gastrointestinal complications. Am J Epidemiol. 2001, 153: 1089-1093. 10.1093/aje/153.11.1089.
Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF: Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006, 129: 746-752. 10.1378/chest.129.3.746.
Judge EP, Fabre A, Adamali HI, Egan JJ: Acute exacerbations and pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Eur Respir J. 2012, 40: 93-100. 10.1183/09031936.00115511.
Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, Taguchi Y, Nagai S, Itoh H, Ohi M, Sato A, Kudoh S: Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005, 171: 1040-1047. 10.1164/rccm.200404-571OC.
Kubo H, Nakayama K, Yanai M, Suzuki T, Yamaya M, Watanabe M, Sasaki H: Anticoagulant therapy for idiopathic pulmonary fibrosis. Chest. 2005, 128: 1475-1482. 10.1378/chest.128.3.1475.
Kim DS, Collard HR, King TE: Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006, 3: 285-292. 10.1513/pats.200601-005TK.
Inase N, Sawada M, Ohtani Y, Miyake S, Isogai S, Sakashita H, Miyazaki Y, Yoshizawa Y: Cyclosporin A followed by the treatment of acute exacerbation of idiopathic pulmonary fibrosis with corticosteroid. Intern Med. 2003, 42: 565-570. 10.2169/internalmedicine.42.565.
Sakamoto S, Homma S, Miyamoto A, Kurosaki A, Fujii T, Yoshimura K: Cyclosporin A in the treatment of acute exacerbation of idiopathic pulmonary fibrosis. Intern Med. 2010, 49: 109-115. 10.2169/internalmedicine.49.2359.
Stuck AE, Minder CE, Frey FJ: Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989, 11: 954-963. 10.1093/clinids/11.6.954.
Konishi K, Gibson KF, Lindell KO, Richards TJ, Zhang Y, Dhir R, Bisceglia M, Gilbert S, Yousem SA, Song JW, Kim DS, Kaminski N: Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009, 180: 167-175. 10.1164/rccm.200810-1596OC.
Collard HR, Yow E, Richeldi L, Anstrom KJ, Glazer C: Suspected acute exacerbation of idiopathic pulmonary fibrosis as an outcome measure in clinical trials. Respir Res. 2013, 14: 73-10.1186/1465-9921-14-73.
Acknowledgements
This research was partly supported by a grant to the Diffuse Lung Diseases Research Group from the Ministry of Health, Labour and Welfare, Japan and was a Ministry of Education, Science, Sports and Culture Grant-in-Aid for Scientific Research (B), 2013–2014 (25860665, Keishi Oda).
The authors thank all test personnel for their work during the data collection at the six institutions involved in the study: University of Occupational and Environmental Health, Japan, Oita University Faculty of Medicine, Fukuoka University School of Medicine, Steel Memorial Yawata Hospital, Nagasaki University School of Medicine and Miyazaki University School of Medicine. The authors also thank Drs. Chiharu Yoshii, Yukiko Kawanami, Toshinori Kawanami, Chinatsu Nishida, Kei Yamasaki, Shingo Noguchi, Takaaki Ogoshi, Kentarou Akata, Kaori Kato, Tetsuya Hanaka, and Masahiro Tahara for their cooperation in this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KO, HI and SY made substantial contributions to the conception and design of the study. KO, HK, HI, TI, TH, YI, NM and KN acquired the data. HI and KY analyzed and interpreted the data. KO, HI, MN, JK, KW, SK and HM participated in drafting the article and critically revising it for important intellectual content. All authors have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Oda, K., Ishimoto, H., Yamada, S. et al. Autopsy analyses in acute exacerbation of idiopathic pulmonary fibrosis. Respir Res 15, 109 (2014). https://doi.org/10.1186/s12931-014-0109-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-014-0109-y