Abstract
Background
Owing to the heterogeneity of microbiota among individuals and populations, only Fusobacterium nucleatum and Bacteroides fragilis have been reported to be enriched in colorectal cancer (CRC) in multiple studies. Thus, the discovery of additional bacteria contributing to CRC development in various populations can be expected. We aimed to identify bacteria associated with the progression of colorectal adenoma to carcinoma and determine the contribution of these bacteria to malignant transformation in patients of Han Chinese origin.
Methods
Microbiota composition was determined through 16S rRNA V3–V4 amplicon sequencing of autologous adenocarcinomas, adenomatous polyps, and non-neoplastic colon tissue samples (referred to as “tri-part samples”) in patients with CRC. Enriched taxa in adenocarcinoma tissues were identified through pairwise comparison. The abundance of candidate bacteria was quantified through genomic quantitative polymerase chain reaction (qPCR) in tissue samples from 116 patients. Associations of candidate bacteria with clinicopathological features and genomic and genetic alterations were evaluated through odds ratio tests. Additionally, the effects of candidate bacteria on CRC cell proliferation, migration, and invasion were evaluated through the co-culture of CRC cells with bacterial cells or with conditioned media from bacteria.
Results
Prevotella intermedia was overrepresented in adenocarcinomas compared with paired adenomatous polyps. Furthermore, co-abundance of P. intermedia and F. nucleatum was observed in tumor tissues. More notably, the coexistence of these two bacteria in adenocarcinomas was associated with lymph node involvement and distant metastasis. These two bacteria also exerted additive effects on the enhancement of the migration and invasion abilities of CRC cells. Finally, conditioned media from P. intermedia promoted the migration and invasion of CRC cells.
Conclusion
This report is the first to demonstrate that P. intermedia is enriched in colorectal adenocarcinoma tissues and enhances the migration and invasion abilities of CRC cells. Moreover, P. intermedia and F. nucleatum exert additive effects on the malignant transformation of colorectal adenomas into carcinomas. These findings can be used to identify patients at a high risk of malignant transformation of colorectal adenomas or metastasis of CRC, and they can accordingly be provided optimal clinical management.
Similar content being viewed by others
Background
Colorectal cancer (CRC) is one of the most prevalent malignancies worldwide [1], and colorectal adenomas (CRAs) are considered the most crucial precursor to sporadic CRC, with the adenoma–carcinoma sequence accounting for 80–85% of all CRC cases [2]. The prevalence of CRAs is estimated to be < 20% in individuals aged ≤ 50 years and 30–40% in those aged ≥ 70 years [3]. Although CRAs are considered precancerous lesions, not all adenomas progress to cancers; only 5–6% of all individuals with noncolitis CRA develop CRC [4,5,6,7]. This statistic implies a barrier to malignant transformation, and that crucial alterations of tumor cells and the microenvironment must occur in a CRA for overcoming this barrier.
Emerging evidence strongly suggests that the alteration of the gut microbiota composition contributes to CRC tumorigenesis [8,9,10,11]. Numerous studies have reported the enrichment of B. fragilis and F. nucleatum in colorectal tumors compared with that in matched normal tissues [12,13,14,15,16,17,18]. In a multicohort meta-analysis of the human gut microbiome from 526 fecal samples, seven CRC-enriched bacteria were identified across the analyzed populations [19]. Among these bacteria, enrichment of B. fragilis and F. nucleatum was observed across four and three cohorts, respectively, whereas enrichment of the other five bacteria, including P. intermedia, was noted in two of the four cohorts. Interestingly, another meta-analysis of the overlapping samples identified P. intermedia as the CRC-enriched microbe in American, Chinese, and French cohorts [20]. Another meta-analysis of CRC data sets involving 764 fecal genomes across multiple cohorts and populations also identified P. intermedia as a microbe enriched in CRC patients compared with healthy controls [21]. However, an integrative analysis of the human gut microbiome from 1267 samples collected from populations in three continents revealed country-specific gut microbial signatures [22]. Moreover, substantial regional variation of gut microbiota was observed in a study that recruited participants of Han Chinese origin living in multiple locations in Guangdong province [23]. Notably, a previous study observed considerable heterogeneity with no single operational taxonomic unit (OTU) tested being increased in all individuals with CRC [24]. Moreover, fecal microbiota only partially reflects mucosal microbiota in patients with CRC [24, 25]. These findings indicate that although some CRC-associated pathogens are common across populations, others may be population- or region-specific. Because a single bacterium cannot explain the development of all CRC cases, additional CRC-associated bacteria specific to populations or regions must be identified, which will ultimately help in the development of more effective strategies for CRC prevention or management.
To elucidate the alterations of microbes that contribute to the malignant transformation of CRA into CRC in Han Chinese residing in Taiwan, we collected autologous pairs of adenomatous polyps and adenocarcinomas and accompanying non-neoplastic colon tissue samples (referred to as “tri-part samples”) and compared the enrichment of microbes in the adenocarcinomas with that in the adenomatous polyps through 16S rRNA sequencing. The experimental results revealed that among the candidate microbes, P. intermedia and F. nucleatum were overrepresented in the adenocarcinomas. The coexistence of these two bacteria in the adenocarcinomas was associated with lymph node involvement and distant metastasis. Both bacteria enhanced cell migration and invasion and exerted an additive effect on the aforementioned biological functions, which are characteristic functions required for malignant transformation. Intriguingly, the effects of P. intermedia on CRC cell migration and invasion were mediated by both bacteria–cell interaction and the molecules secreted by the bacteria. This study not only reveals a new candidate pathogenic bacterium that contributes to the malignant transformation of CRA into CRC but also provide insights into the contribution of oral pathogens in the pathogenesis of CRC. Finally, our findings provide evidence that P. intermedia may be a population-specific bacterium contributing to CRC progression.
Methods
Patient recruitment
Between 2010 and 2019, 116 patients with CRC were recruited for this study. These patients underwent surgical resection for primary CRC in three collaborating hospitals: Kaohsiung Medical University Hospital, Tri-Service General Hospital, and Taipei Tzu Chi General Hospital. Of these patients, 90 patients were the same as those included in our previous study [26]. All patients were of Han Chinese origin and residing in Taiwan and had both carcinomas at any pathological TNM stage and adenomas of colons or rectums discovered during colonoscopy examination. None of the patients were diagnosed as having Crohn’s disease, ulcerative colitis, familial adenomatous polyposis, hereditary non-polyposis CRC, or hamartomatous polyposis syndrome, and none received radiation or chemotherapy prior to surgery. Patients with recurrent CRC were not included. Three autologous fresh-frozen tissues, including adenocarcinomas, paired adenomatous polyps, and paired normal colon tissues located at least 10 cm away from the malignant lesions, were collected from all patients. We also collected available clinicopathological information on cancerous tissues and polyp tissues. The histological sections of tumor samples were evaluated by experienced pathologists at each hospital. The study protocol was approved by the Institutional Review Boards of Academia Sinica and the collaborating hospitals. Informed consent was obtained from all participants before the collection of their information and samples.
Human genomic DNA extraction and mutation detection
Human genomic DNA was isolated from the fresh-frozen tissues by using a Gentra Puregene Tissue kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Mutations were detected for 26 targeted genes in the genomic DNA of the carcinoma and paired adenomatous polyp samples from 72 patients with CRC by using the TruSight Tumor Sequencing Panel (Illumina, San Diego, CA, USA). A total of 30–300 ng of genomic DNA was used for strand-specific multiplex PCR in accordance with the manufacturer’s instructions. The quality and quantity of the amplicon-based library were measured using the Qubit and Agilent Technologies 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA). Equal quantities of 16 indexed DNA libraries were pooled and sequenced on the Miseq sequencing platform (Illumina) by using a paired-end (2 × 150) sequencing run. The tri-part samples of 14 patients, including 4 in whom the targeted gene mutations were detected, were subjected to whole exome sequencing. Briefly, 3 ng of genomic DNA was utilized for whole-exome enrichment and library construction by using a TruSeq Exome Library Prep Kit (Illumina) according to the manufacturer’s instructions. Then, paired-end sequencing of 150 bases was performed using the Illumina HiSeq 2000 sequencing system. Both target sequencing and exome sequencing were conducted by the National Center for Genome Medicine at Academia Sinica, Taipei, Taiwan (http://ncgm.sinica.edu.tw). Sequencing yields of at least 10 Gb for each non-neoplastic colonic tissue sample and 20 Gb for each polyp or carcinoma sample were obtained. The data analysis is described in detail in Additional file 1.
16S rRNA sequencing and analysis
Genomic DNA of the tri-part samples from 93 patients with CRC was subjected to 16S rRNA sequencing, wherein 500 ng of genomic DNA was amplified with a primer pair specific to 16S V3–V4, 341F: 5′-CCTAYGGGRBGCASCAG-3′, and 806R: 5′-GGACTACNNGGGTATCTAAT-3′. Subjects with PCR products successfully amplified from more than one tissue were included in the subsequent experiments. A total of 238 samples from 86 patients were successfully amplified, and PCR products of size 450–550 bp were purified through gel extraction. The PCR amplicons were barcoded, quantified, mixed in equal amounts, and purified through gel extraction. The pooled PCR products were then used for constructing libraries by using the TruSeq DNA PCR-Free Sample Preparation Kit (Illumina). The libraries were then subjected to sequencing on a HiSeq 2500 system (Illumina) following the manufacturer’s recommendations, and 250-bp paired-end reads were generated.
All the paired-end reads were assembled using FLASH (v.1.2.11), and low-quality reads (Q score < 20) were discarded using QIIME 1.9.1. If three consecutive bases had Q scores of < 20, the read was truncated, and the resulting read was subjected to the split_libraries_fastq.py script in QIIME and retained in the data set only if it was at least 75% of the original length. Chimeric sequences were filtered using UCHIME to obtain effective tags. Samples with fewer than 15,000 effective tags were excluded from further statistical analysis. Next, OTU picking was performed by clustering effective tags with 97% sequence identity by using the USEARCH v.7 pipeline (UPARSE function). For each representative sequence, SILVA Database version 132 was used on the basis of the RDP classifier (v.2.2) algorithm to annotate taxonomy classification. Raw OTU abundance data (QIIME script single_rarefaction.py) were then rarefied to the minimum sequences of 15,000 effective tags across qualified samples through random sampling (without replacement) to avoid sampling depth bias. 16S rRNA sequencing and analysis were conducted at BIOTOOLS Co., Ltd (New Taipei City, Taiwan).
Co-abundance group analysis
We reanalyzed the paired-end reads using mothur (v.1.39.5) for quality control and sequence alignment, and the reads were classified using the SILVA 16S rRNA database version 132 to obtain the OTU abundance table. The OTU abundance table was then used to identify microbial co-abundance groups (CAGs) by using Flemer’s method [24]. CAGs are defined as clusters of highly correlated microbes. The Euclidean distances between microbes were calculated using the Pearson correlation coefficients. The microbes were clustered using Ward’s minimum variance method with squared dissimilarities [27]. The CAGs were identified from a hierarchical cluster dendrogram with a fixed number of clusters. Vegan (Community Ecology Package, 2008) in the R environment was used for CAG identification. Pairwise comparison of the CAGs was performed using Wilcoxon tests. Significance was set as P value < 0.05.
Bacterial culture and DNA isolation
All bacterial strains used in this study were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and were cultured at 37 °C in an anaerobic chamber containing 10% CO2, 5% H2, and 85% N2. F. nucleatum subsp. nucleatum ATCC 25586 was cultured on Columbia Agar plates supplemented with 5% defibrinated sheep blood, hemin (5 µg/mL; Sigma-Aldrich, St. Louis, MO, USA), and menadione (1 µg/mL; Sigma-Aldrich). P. intermedia ATCC 25611 was cultured on ATCC medium 2722 supplemented with Tryptic Soy Broth/Agar, hemin (5 µg/mL), and menadione (1 µg/mL). Enterotoxigenic B. fragilis (Veillon and Zuber) Castellani and Chalmers (ATCC 43858) and nontoxigenic B. fragilis ATCC 25285 were cultured on the Anaerobic Blood Agar Plate (BD Diagnostics, Sparks, MD) or in the Brain Heart Infusion broth (Becton Dickinson, Franklin Lakes, NJ, USA) supplemented with hemin (15 µg/mL). The Escherichia coli strain DH5α (Invitrogen, USA) was aerobically propagated on a Luria–Bertani (LB) plate at 37 °C. Bacterial DNA was isolated from bacterial cultures by using the Gentra Puregene kit (QIAGEN, MD, USA) in accordance with the manufacturer’s instructions.
Quantification of bacteria in tissues
Primer pairs and probes specific to human genomic sequences of the PGT gene and microbial genomic sequences of P. intermedia and F. nucleatum were obtained from the literature; these primer sequences are described in Additional file 2. A primer pair and probe specific to pan-bacterial 16S rRNA were purchased from Thermo Fisher Scientific (Waltham, MA, USA; Assay ID: Ba04930791_s1). qPCR was performed using 1× TaqMan Fast Advanced Master Mix (Applied Biosystems; Thermo Fisher Scientific), 180 nM of each primer, 50 nM probe, and 40 ng of human genomic DNA in a total volume of 10 μL. Assays were performed using the ViiA 7 Real-Time PCR System (Thermo Fisher Scientific) under the following thermal cycling conditions: 2 min at 50 °C, 10 min at 95 °C, and 45 cycles of 15 s at 95 °C and 1 min at 60 °C. For absolute quantification, 10× serial dilutions of the microbial genomic DNA of P. intermedia (ATCC 25611) and F. nucleatum (ATCC 25586), starting from 1 ng/μL to 10 fg/μL, were used as templates to establish a standard curve. The absolute abundance of bacteria was calculated using the interpolation or heterodyne method and the established standard curve in QuantStudio Real-Time PCR Software v.1.3 (Thermo Fisher Scientific).
Cell culture
Human CRC cell lines were cultured as previously described [26]. HCT116 cells, HT29 cells, and HCT15 cells were cultured in McCoy’s 5A medium, Dulbecco’s Modified Eagle Medium, and RPMI 1640 medium, respectively. All cells were maintained in media supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37 °C in a 5% CO2 humidified incubator.
Cell growth curves
Cell growth curves were determined using a hemocytometer. CRC cells were seeded in 24-well plates at a density of 2 × 104 cells per well with 1 mL of antibiotic-free 10% FBS-containing complete medium. After 24 h, the cells were treated with phosphate-buffered saline (PBS)-washed bacteria at the indicated multiplicity of infection (MOI). The cells treated with PBS served as negative controls. Cell numbers were counted at 24-h intervals. In addition, the cell proliferation was measured using the Real-Time Cell Analysis (RTCA) iCELLigence System (ACEA Biosciences, San Diego, CA, USA). Briefly, CRC cells were seeded on E-plate L8 (3 × 104 cells per well) containing antibiotic-free complete medium. After 24 h, the cells were treated with PBS-washed bacterial cultures at the indicated MOI. Cellular impedance was measured every 2 h for 96 h and recorded as the cell index. All experiments were performed at 37 °C in a 5% CO2 humidified incubator. Each experiment was performed in triplicate and repeated independently at least twice.
Transwell migration and invasion assays
Migration and invasion assays were performed in a 24-well plate with Corning 8-µm transwell inserts, as previously described [26]. The cells were harvested and resuspended at a concentration of 1 × 106/mL in antibiotic-free, serum-free medium. Next, 200 µL of the cell suspension was added to the top chamber of each 8-µm transwell insert. Bacteria pellets were then collected after centrifugation at 6000 rpm for 20 min, then washed with PBS twice, and added to the top chamber at an MOI of 100:1. To test the effect of the bacterial secretome on cell migration and invasion, 10 mL of medium from overnight-cultured bacteria was collected and centrifuged at 6000 rpm for 20 min, and the supernatant was filtered through a 0.22-µm cellulose filter (Millipore, Burlington, MA, USA). The filtered supernatant was added to the top chamber with the cells. The lower chamber contained complete medium with 10% FBS. After 18-h incubation at 37 °C, the membrane surface was fixed with paraformaldehyde and methanol, and the cells were stained with Giemsa’s staining solution and counted under a microscope. For the invasion assay, each transwell insert was precoated with 100 µL of Matrigel (Corning, NY, USA) at 0.4 µg/mL. Each experiment was performed in triplicate, and the average of the three independent experiments was calculated. To inactivate proteins in the conditioned medium, the filtered supernatant was treated with proteinase K at a final concentration of 0.1 mg/mL at 56 °C for 24 h, followed by inactivation with proteinase K at 90 °C for 10 min. The effect of the treated supernatant on cell migration was then examined. To examine the efficiency of proteinase K treatment, 30 µl of the treated and untreated conditioned media was separated using SDS-PAGE and stained using the ProteoSilver™ Silver Stain Kit (Sigma-Aldrich).
Statistical analysis
Pairwise comparison of the relative abundance of the annotated taxa at the species level based on 16S rRNA sequencing was performed using the Wilcoxon signed-rank test. Correlations of the presence of P. intermedia and F. nucleatum with clinicopathological features and genomic or genetic alterations were determined on the basis of odds ratios (ORs). Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA). Differences in the absolute amounts of individual bacteria between the paired carcinoma samples and the polyp samples were determined using the Wilcoxon signed-rank test. Fisher’s exact test was performed to determine associations of the presence of P. intermedia and F. nucleatum in tumor tissues. The effects of the bacteria on biological functions of CRC cells were assessed using a paired t test. Statistical analyses were conducted using GraphPad Prism 9 (GraphPad Inc., San Diego, CA, USA). A two-tailed P value of < 0.05 was considered statistically significant.
Results
Relative abundance of Prevotella intermedia in adenocarcinomas
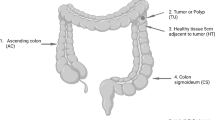
The clinicopathological characteristics of the 116 patients with CRC are presented in Table 1. Genomic DNA of the tri-part samples from 93 patients was subjected to 16S-rRNA V3–V4 sequencing because this subset was available when the assay was performed (Fig. 1). Samples without successful PCR product amplification or sufficient sequencing reads were excluded from downstream analysis. Finally, a total of 196 tissue samples from 76 patients with CRC with more than 15,000 effective sequencing tags were included in a pairwise comparison to identify enriched microbes in adenocarcinoma tissues compared with those in paired adenomatous polyps and normal mucosa tissues. Only taxa annotated at the species level were analyzed (Additional file 3). The relative abundance of each bacterium in individual samples was obtained on the basis of the abundance data rarefied to 15,000 reads. Because the composition of microbiota varied considerably among individuals, we did not set a P value for the selection of enriched bacteria. Instead, we identified the top 10 overrepresented bacteria in the carcinoma samples by using the Wilcoxon signed-rank test (Table 2 and Additional file 4). Among these bacteria, B. fragilis was relatively enriched in carcinoma tissues compared with paired non-neoplastic colon tissues, as reported in previous studies [12, 15, 28].
We were particularly interested in bacteria that were relatively enriched in adenocarcinomas compared with paired adenomatous polyps because these bacteria may be involved in the malignant transformation of adenomas into adenocarcinomas. Among the top 10 bacteria, the present study focused on P. intermedia. P. intermedia was reported to be enriched in the fecal samples of patients with CRC compared with healthy controls in the Austrian, Chinese, American, and French populations, but not in German population [19, 20]. However, no study has reported the abundance of P. intermedia in colorectal carcinoma tissues.
Co-abundance of Prevotella intermedia and Fusobacterium nucleatum in carcinoma tissues compared with paired polyp tissues validated through qPCR
To verify the 16S rRNA sequencing result, we performed genomic qPCR to measure the absolute amount of P. intermedia in adenocarcinoma tissues and paired polyp tissues from the 76 patients with CRC (Fig. 1). Forty more patients with CRC were included in the qPCR assays to increase the statistical power. Although F. nucleatum was not identified in the sequencing analysis because of the low taxonomical resolution of the V3-V4 amplicon, CAG analysis of the OTUs revealed that Prevotella and Fusobacterium were co-abundant in cancer tissues compared with polyp tissues (Fig. 2 and Additional file 5). CAG Cluster 46 comprised f_Prevotellaceae; g_Prevotella, f_Peptococcaceae; g_SCADC1-2-3, f_Fusobacteriaceae; g_Fusobacterium, Fusobacteriales, and f_Methylomonaceae; g_Methylobacter and exhibited higher abundance in adenocarcinomas than in adenomatous polyps (P value = 0.0046, Fig. 2) and normal mucosa tissues (P value = 0.0146, Additional file 5). Therefore, we also measured the quantity of F. nucleatum through qPCR.
With the threshold set at cycle 45, P. intermedia and F. nucleatum were detected in at least one of the tri-part tissue samples of 25 patients (21.6%) and 60 patients (51.7%), respectively. More specifically, P. intermedia and F. nucleatum were detected in the carcinoma tissues of 22 (19.0%) and 54 (46.6%) patients, respectively. According to the available information on the histological types of polyps, five patients had adenocarcinoma lesions within their adenoma tissues and were excluded from the paired tissue comparison. The absolute abundance of P. intermedia in carcinoma tissues was significantly higher than that in paired polyp tissues (P value = 0.0003, Fig. 3A). A similar result was obtained for F. nucleatum (P value < 0.0001, Fig. 3B). The qPCR result was consistent with that of the 16S rRNA sequencing data enrichment analysis. Notably, this result indicated that P. intermedia and F. nucleatum coexisted in both the carcinoma tissues (Fig. 3C, P value = 0.0003, Pearson’s correlation coefficient = 0.85) and polyp tissues (Fig. 3D, P value = 0.0433, Pearson’s correlation coefficient = 0.99) of the patients with CRC. Moreover, the coexistence of these two bacteria was significantly associated with polyps with adenocarcinoma lesions (P value = 0.046, Fig. 3E). In addition, of the five polyps with higher abundance of P. intermedia compared with the paired adenocarcinomas, two had carcinoma lesions within adenoma tissues. These findings strongly suggest that P. intermedia is associated with the malignant transformation of CRA into CRC.
Co-abundance of Prevotella intermedia and Fusobacterium nucleatum increases from polyps to paired carcinoma tissues. The absolute abundance of A Prevotella intermedia and B Fusobacterium nucleatum increases from polyps (TA) to paired carcinomas (TC). The quantification of bacteria is presented as the amount of microbial genomic DNA per ng of genomic DNA isolated from corresponding tissues. TA adenomatous polyp, TC carcinoma tissue. Tissue samples from the same patients with CRC are linked by lines. ***P value < 0.001; ****P value < 0.0001. The numbers of tissues with the presence or absence of Prevotella intermedia (PI) and Fusobacterium nucleatum (FN) in C cancer tissues and D polyp tissues are presented. E The coexistence of Prevotella intermedia (PI) and Fusobacterium nucleatum (FN) is predominant in polyps with adenocarcinoma lesions
Associations of bacteria with clinicopathological features and genomic alterations
Next, we determined the associations of the presence of the two bacteria in carcinoma samples with clinicopathological features (Table 3). The abundance of a bacterium was categorized as a dichotomous variable (present vs. absent), and ORs were calculated. The coexistence of P. intermedia and F. nucleatum was significantly associated with distant metastasis (OR = 5.31, 95% confidence interval [CI] = 1.27–22.2). The association of the presence of P. intermedia or F. nucleatum alone with distant metastasis was marginally significant (OR = 3.96, 95% CI = 0.97–16.19 and OR = 4.47, 95% CI = 0.89–22.5, respectively). Although not statistically significant, the coexistence of these two bacteria were associated with the TNM stage (OR = 2.89, 95% CI = 0.99–8.48) and lymph node involvement (OR = 2.89, 95% CI = 0.99–8.48). The association of the presence of F. nucleatum with the TNM stage and lymph node involvement was statistically significant (OR = 2.27, 95% CI = 1.08–4.8). Gender, tumor location, or primary tumor was not associated with the presence of either bacterium.
We further examined the associations of the presence of the two bacteria in the carcinoma samples with genomic and genetic alterations wherever the data were available. Our previous study demonstrated that Chr20q amplification and Chr13q amplification were the characteristic genomic alterations involved in the malignant transformation of CRA into CRC [26]. APC, KRAS, and TP53 genes are the most frequently mutated genes in CRC. Therefore, we examined the association of the two bacteria with these mutational events, and the results revealed that the coexistence of P. intermedia and F. nucleatum was associated with the TP53 mutation; however, this association did not reach statistical significance (OR = 3.87, 95% CI = 0.81–18.58). No other associations were observed between other mutational events and either of the two bacteria.
Effects of Prevotella intermedia on CRC cell growth in various cell lines and at different MOIs
To investigate whether P. intermedia is involved in CRC development, we first examined whether the growth of CRC cells was influenced by the direct interaction between bacteria and cancer cells. Compared with PBS-treated cells, the growth of HCT116 cells was not affected by P. intermedia at an MOI of 100 (Fig. 4A). The growth of HT29 cells increased after 48–72-h incubation with P. intermedia at an MOI of 100 (Fig. 4B). We co-cultured F. nucleatum with CRC cells as a control. Unexpectedly, the growth of HCT116 cells was suppressed, whereas that of HT29 cells was promoted after 24-h incubation at an MOI of 100 (Fig. 4A, B). Escherichia coli DH5α treatment resulted in cell damage in both cell lines after 24-h incubation at an MOI of 100 (Fig. 4A, B). We then employed cell index measurement to examine the growth of CRC cells incubated with P. intermedia at various MOIs. The cell index of HCT116 cells was similar between the PBS control group and the groups treated with bacteria at an MOI of 100, except after 40-h incubation. However, the cell index of HCT116 cells was lower after incubation with P. intermedia at an MOI of 250 or 500 compared with that of the cells treated with PBS only (Fig. 4C). In HT29 cells, the cell index increased at an MOI of 100 but decreased at an MOI of 250 or 500 (Fig. 4D).
Effects of Prevotella intermedia on cell growth varied across cell lines and MOIs. A HCT116 cells and B HT29 cells were treated with Prevotella intermedia (PI), Fusobacterium nucleatum (FN), or E. coli DH5a at an MOI of 100 on day 0. Cell growth curves were measured using a hemocytometer. Cells treated with PBS served as controls. C HCT116 cells and D HT29 cells were co-cultured with Prevotella intermedia at various MOIs, and cell growth was measured using the RTCA iCELLigence System. The arrowhead indicates the time when the bacteria were added to the cell culture. *P value < 0.05; **P value < 0.01; ****P value < 0.0001
Prevotella intermedia and Fusobacterium nucleatum additively enhanced the migration and invasion of CRC cells
Migration and invasion are hallmarks of malignant transformation. We investigated whether P. intermedia influenced the migration and invasion abilities of CRC cells. Compared with PBS treatment and nontoxigenic B. fragilis, P. intermedia significantly promoted the migration of HCT116, HT29, and HCT15 cells at an MOI of 100 (Fig. 5A–C). Moreover, P. intermedia substantially enhanced cell invasion in the three CRC cell types (Fig. 5D–F). To examine whether the ability of P. intermedia to promote cell migration and invasion was reinforced by an increased number of bacteria, we incubated CRC cells with bacteria at a higher MOI; the results revealed that the percentages of migrated and invaded cells were similar at MOIs of 100 and 250, suggesting no dose-dependent effect (Additional file 6).
Prevotella intermedia and Fusobacterium nucleatum additively enhances CRC cell migration and invasion. A–C Migration and D–F invasion assays were performed on CRC cells cultured with Prevotella intermedia (PI), Fusobacterium nucleatum (FN), PI plus FN (PI + FN), or nontoxigenic B. fragilis (NTBF) at an MOI of 100. Cells treated with PBS served as untreated controls, and NTBF served as a negative control. The relative percentage of migrated and invaded cells is presented as mean ± standard deviation. Data were obtained from three independent experiments. *P value < 0.05; ****P value < 0.0001; ns not significant
Consistent with the findings of other studies [29, 30], F. nucleatum promoted the migration and invasion of the three CRC cell lines at an MOI of 100. Because P. intermedia and F. nucleatum were observed to coexist in human carcinoma tissues, we further investigated whether P. intermedia in combination with F. nucleatum exerted a synergistic effect on cell migration or invasion. We observed that both migration and invasion were significantly promoted in CRC cells co-cultured with the combination of the two pathogens compared with those co-cultured with either pathogen alone. These findings revealed an additive effect of P. intermedia and F. nucleatum on the promotion of migration and invasion of CRC cells.
Secretome of Prevotella intermedia enhanced migration and invasion of CRC cells
Few studies have explored the cancer pathogenicity of P. intermedia. Therefore, the present study investigated whether P. intermedia stimulate the migration or invasion of CRC cells through its secretory factors or through direct contact with cells. Conditioned medium from P. intermedia significantly enhanced the migration and invasion of HCT116, HT29, and HCT15 cells compared with growth medium alone (Fig. 6A–F). However, when the conditioned medium was treated with proteinase K, the effects were eliminated (Fig. 6G–I). Moreover, the secreted proteins were destroyed, which was verified through gel electrophoresis (Additional file 7). Collectively, these results indicate that P. intermedia can exert its effects on cell migration and invasion through secreted proteins as well as through direct cell contact.
Conditioned medium from Prevotella intermedia enhances CRC cell migration and invasion. CRC cells were incubated in the conditioned medium from Prevotella intermedia in the top chamber for migration (A–C) and invasion (D–F) assays. G–I Effect of proteinase K-treated conditioned medium from Prevotella intermedia on cell migration. The relative percentages of the migrated and invaded cells are presented as mean ± standard deviation. Data were obtained from three independent experiments. ****P value < 0.0001
Discussion
Because of the low resolution of the taxonomy assignment based on 16S rRNA V3-V4 sequencing, most studies have reported microbiota dysbiosis of CRC at the genus level. For example, Liu et al. reported that the genus Prevotella was more abundant in biopsy samples from patients with CRC than in those from patients with CRA in one of the two cohorts from two geographically separated cities in China [31]. However, no information at the species level was available. In addition, because of the heterogeneity of microbiota among individuals and populations, only F. nucleatum and B. fragilis have been clearly identified as species enriched in fecal samples and tumor samples taken from patients with CRC across studies. Although P. intermedia has been identified as a CRC-enriched microbe, this finding was based on a metagenomic analysis of fecal samples; no study has demonstrated the presence of this bacterium in tumor tissues. The present study demonstrated that P. intermedia and F. nucleatum were enriched in carcinoma tissues compared with autologous adenomatous polyp tissues. Notably, this finding was confirmed by the absolute quantification of these two bacteria in 116 patients with CRC. To the best of our knowledge, the present study included the largest collection of samples of paired colorectal carcinoma and polyp tissues to date. The effects of P. intermedia on the malignant properties of CRC cells strongly suggest that P. intermedia is a pathogenic microbe that contributes to the malignant transformation of CRA into CRC. Intriguingly, the results revealed that the coexistence of P. intermedia and F. nucleatum was associated with polyp tissues with carcinoma lesions. These findings support the pathogenic role of the combination of P. intermedia and F. nucleatum in the progression of CRA to CRC.
Our finding of the coexistence of P. intermedia and F. nucleatum in cancerous colon tissue is consistent with the previous findings that Fusobacterium cooccurs with gram-negative anaerobic oral bacteria [13, 32, 33]. In particular, the co-abundance of the genera Fusobacterium and Prevotella was observed in a subset of CRC samples in another study [24]. Both F. nucleatum and P. intermedia are oral bacteria and are implicated in the pathogenesis of periodontitis. One study involving the in vitro co-culture of F. nucleatum and P. intermedia demonstrated that these two bacteria were coaggregated [34]. Moreover, in other studies, a co-culture of F. nucleatum and P. intermedia significantly enhanced biofilm formation compared with the single culture of F. nucleatum [34, 35]. These two bacteria may be a part of a strong symbiotic network that originates in oral cavities and colonizes the gut to promote malignant transformation. Avuthu and Guda performed a meta-analysis of existing metagenomic data sets from diverse geographic regions to identify a set of global biomarkers for CRC [20]. The co-occurrence network analysis of fecal microbes revealed that a cluster of oral microbes including F. nucleatum and P. intermedia was associated with CRC. This result is in line with our findings for tumor tissues. In the future, we will employ murine models to elucidate the potential oncogenic role of P. intermedia and how it cooperates with F. nucleatum to promote CRC metastasis. Moreover, whether P. intermedia, in addition to F. nucleatum, in stool samples can serve as a diagnostic biomarker for CRC in Chinese population will be evaluated. Our result of the co-abundance of these two bacteria in human colorectal carcinoma tissues supports that the alteration of the ecosystem consisting of the oral microbiome is involved in CRC development [24, 33]. Because the coexistence of P. intermedia and F. nucleatum was found in only a subset of patients with CRC in this study, we suspect that other oral pathogens may also coexist with F. nucleatum. Therefore, shot-gun metagenomic sequencing of tissue samples should be used in the future to demonstrate the other bacterial species coexisting with F. nucleatum. Moreover, using the metadata collected from CRC patients, we will implement a model in explainable artificial intelligence (AI) to predict the stages of CRC based on the gut microbiota and selected features from metadata. This will help identify the most important microbiota community and features, thereby enabling the development of more sensitive models to detect CRC at the early stage.
The TP53 mutation is a critical genetic event contributing to the malignant transformation of CRA into CRC with chromosomal instability. In this study, the trend of association of the coexistence of P. intermedia and F. nucleatum with the TP53 mutation, suggesting that these two bacteria may contribute to the malignant transformation of CRA into CRC through genotoxicity. The most widely known example of genotoxin-induced TP53 mutation is the synergistic effect of aflatoxin produced by fungi and chronic hepatitis B virus infection in hepatocellular carcinoma [36]. Similarly, we propose that along with the invasion of CRA tissues with F. nucleatum, the to-be-identified secreted proteins of P. intermedia may induce host gene mutations. The clonal expansion of cells with TP53 mutations and their relatively high ability of both migration and invasion render benign tumors malignant. Studies have reported the associations of the number of F. nucleatum with high microsatellite instability (MSI) cancer tissue [37,38,39]; however, no such association was observed in the present study. This inconsistency is likely due to the small number of MSI-high samples included in this study. Additionally, the associations of F. nucleatum with right-sided tumors, advanced stage tumors, and BRAF-mutated tumors were reported in a Brazilian cohort [40]. The present study observed an association of F. nucleatum with the high TNM stage, but not with right-colon cancers or BRAF mutations in our cohort. Similarly, no association between F. nucleatum abundance and KRAS-mutation-positive CRC was observed in a Chinese cohort [39], which is consistent with our finding. Therefore, we speculate that the associations of the abundance of F. nucleatum with clinicopathological and molecular features may be population-specific. Although studies with more diverse populations and larger sample sizes are required to determine whether population-specific bacteria are responsible for CRC progression and are associated with clinicopathological characteristics, our results strongly suggest that P. intermedia and F. nucleatum are critical pathogens for CRC development in individuals of Han Chinese origin. Moreover, our recent study demonstrated that F. nucleatum is closely associated with treatment outcomes in patients with metastatic CRC receiving chemotherapy and targeted therapy [41].
To investigate the potential pathogenic role of P. intermedia in CRC progression, we conducted a series of experiments involving the incubation of CRC cells with bacterial cells or conditioned medium from bacteria. The results revealed that P. intermedia significantly enhanced cell migration and invasion, and these effects were consistently observed in the three CRC cell lines. Moreover, the combination of P. intermedia and F. nucleatum exerted additive effects on the promotion of CRC cell migration and invasion. The findings regarding the co-abundance of these two bacteria in carcinoma tissues compared with paired polyp tissues and the association of the coexistence of these two bacteria with lymph node involvement and distant metastasis strongly suggest that P. intermedia is involved in the malignant transformation of CRA and metastasis in combination with F. nucleatum. In a recent study, Ternes and colleagues reported that gut microbial metabolite formate is a key player in CRC progression through the activation of AhR signaling [42]. The microbiome-derived formate induces cancer stemness, promotes colon cancer cell invasion, and ultimately induces metastatic dissemination. The authors suggest that F. nucleatum is one of the microbes enriched in CRC tissues that produce formate at the tumor site, and that bacterial species other than F. nucleatum may also contribute to formate production. Intriguingly, the bacterium P. intermedia contains a series of enzymes essential for formate formation, and it can metabolize glucose into formate [43]. The additive effect of P. intermedia and F. nucleatum on the migration and invasion of CRC cells may be explained by the increasing concentration of formate. However, confirming whether tumor-associated P. intermedia contributes to formate formation in patients with CRC is difficult because it coexists with Fusobacteria nucleatum in our study cohort. Nevertheless, based on the association of the coexistence of these two bacteria with malignant transformation and metastasis of CRC and considering that formate may function as a major oncometabolite through the activation of AhR signaling, AhR inhibitors are of potential therapeutic interest in patients with CRC with both the bacteria detected [44]. We propose that the follow-up visit intervals for patients with both bacteria present in adenoma tissues should be shortened. Moreover, we propose that the formate levels of CRC patients with the presence of these two bacteria in the cancerous tissues should be detected to monitor disease progression. Additionally, AhR inhibitors may be administered to this subgroup of patients to prevent metastasis when the drugs are available.
In this study, conditioned medium from P. intermedia exerted a more prominent effect on cell migration and invasion compared with the bacterial cells. The virulence factors mediating the contribution of P. intermedia to malignant transformation may be secreted proteins given that the proteinase K treatment of the conditioned medium eliminated these biological activities. In a recent report, an analysis of the functional protein association network of P. intermedia secretomes revealed that carbohydrate metabolism constitutes one of the three major groups in the network [45]. Among the components of the sugar metabolism network, glucose-6-phosphate isomerase (GPI), also known as autocrine motility factor (AMF), was identified. AMF secreted from tumor cells exhibits non-canonical function and signaling through its receptor gp78 and drives epithelial–mesenchymal transition (EMT) in several cancers [46,47,48]. AMF has also been demonstrated to stimulate invasion and metastasis in various cancers [47, 49, 50]. We speculate that AMF is one of the molecules secreted by P. intermedia, and that it is responsible for promoting CRC cell motility and ultimately metastasis. Future studies should attempt to detect AMF in colorectal cancer tissues with the presence of P. intermedia. Because AMF-induced metastasis occurs through the non-canonical pathway, targeting AMF to inhibit its contribution to metastasis without affecting its physiological functions is feasible. Efforts toward developing monoclonal antibodies against AMF have demonstrated encouraging results [51, 52]. Moreover, studies including more clinical samples and applying animal models [38] for the identification of more secreted molecules and the mechanisms by which they promote CRC progression may assist in the prevention of malignant transformation and metastasis of CRC. Together, these results suggest that P. intermedia—both the bacterial cells and their secreted components—may play a pathogenic role in the malignant transformation of CRA into carcinoma.
Cell proliferation is another key feature of tumorigenesis. In this study, we demonstrated various effects of P. intermedia on CRC cell growth. Specifically, P. intermedia selectively promoted the growth of HT29 cells, but not that of HCT116 cells, at an MOI of 100. This effect may partially be explained by the varying gene mutations in different cell lines, which are associated with different signaling responses and metabolic pathways. To the best of our knowledge, the effect of P. intermedia on cancer cell growth has not been reported previously. However, one study demonstrated that some bacterial cells as well as their secretomes were associated with either the enhancement or inhibition of cancer cell growth [53]. Studies have demonstrated that F. nucleatum can increase the proliferation of HCT116, HT29, and SW480 CRC cells [16, 30] and can promote cell growth through Toll-like Receptor 4 signaling and microRNA-21 upregulation through its binding to FadA. However, we observed that F. nucleatum inhibited the growth of HCT116 cells but enhanced the growth of HT29 cells at an MOI of 100. Moreover, after incubation with bacteria at higher MOIs of up to 1000, the growth of CRC cells was arrested, and the cells were damaged (Additional file 8). Therefore, differences in MOI or the experimental materials cannot explain the contrasting results, because we used the same bacterial strain (ATCC 25586), culture medium, and assay techniques. However, differences in co-culture conditions may exist among the study groups. To sustain cell growth, we established the co-culture in an aerobic chamber unsuitable for anaerobic bacterial growth. Notably, one recent report revealed that Fusobacteriaceae significantly inhibited CRC cell growth [53], suggesting that the bacterial characteristics must be elucidated to further explain the inconsistent results. Notably, we observed no association of the presence of P. intermedia or F. nucleatum with the size or extent of the primary tumor (T0/T1/T2 vs. T3/T4). This finding is consistent with the result of the cell proliferation assay in this study.
This study has several limitations. First, the study patients were of Han Chinese origin and were residing in Taiwan. Therefore, it requires further investigation to determine the generalization of the results to CRC patients of Han Chinese origin residing in other locations and to other populations. Second, no independent cohort was available in this study for replication. Autologous adenomas and adenocarcinomas from additional patients with CRC should be collected. Finally, the low resolution of 16S rRNA sequencing is an obstacle to the detection of bacteria at species level. Some important bacterial species responsible for malignant transformation may not be identified by 16S rRNA sequencing. Shotgun metagenomic sequencing coupled with complete depletion of host DNA from tissue samples will provide a comprehensive list of microbes that contribute to malignant transformation of CRA at the species and even strain levels.
Conclusion
This study demonstrated that P. intermedia plays a crucial role in the promotion of both migration and invasion of CRC cells. Furthermore, the coexistence of P. intermedia and F. nucleatum exerted an additive effect on the migration and invasion of CRC cells and was associated with both lymph node metastasis and distant metastasis. Therefore, patients with these two bacteria present in their precancerous tissues may be at a relatively high risk of developing colorectal carcinomas, and patients with these two bacteria present in cancerous tissues may be at a relatively high risk of metastasis. Both these conditions necessitate the increased monitoring of patients. In addition, the identification of the pathogenic molecules from P. intermedia and the response molecules from host cells as well as the relevant mechanical pathways warrants further investigation. The discovery of these mechanisms can help establish optimal strategies for preventing the malignant transformation of CRA and metastasis of CRC.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional files.
Abbreviations
- CRC:
-
Colorectal carcinoma
- qPCR:
-
Quantitative polymerase chain reaction
- P. intermedia :
-
Prevotella intermedia
- F. nucleatum :
-
Fusobacterium nucleatum
- CRA:
-
Colorectal adenoma
- B. fragilis :
-
Bacteroides fragilis
- OUT:
-
Operational taxonomic unit
- CAGs:
-
Co-abundance groups
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- CIN:
-
Chromosomal instability
- MSI:
-
Microsatellite instability
- MOI:
-
Multiplicity of infection
- GPI:
-
Glucose-6-phosphate isomerase
- AMF:
-
Autocrine motility factor
- EMT:
-
Epithelial–mesenchymal transition
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-386.
Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–67.
Corley DA, Jensen CD, Marks AR, Zhao WK, de Boer J, Levin TR, Doubeni C, Fireman BH, Quesenberry CP. Variation of adenoma prevalence by age, sex, race, and colon location in a large population: implications for screening and quality programs. Clin Gastroenterol Hepatol. 2013;11(2):172–80.
Risio M. The natural history of adenomas. Best Pract Res Clin Gastroenterol. 2010;24(3):271–80.
Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology. 1987;93(5):1009–13.
Shinya H, Wolff WI. Morphology, anatomic distribution and cancer potential of colonic polyps. Ann Surg. 1979;190(6):679–83.
Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36(6):2251–70.
Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. 2019;16(11):690–704.
Keku TO, Dulal S, Deveaux A, Jovov B, Han X. The gastrointestinal microbiota and colorectal cancer. Am J Physiol Gastrointest Liver Physiol. 2015;308(5):G351-363.
Gao Z, Guo B, Gao R, Zhu Q, Qin H. Microbiota disbiosis is associated with colorectal cancer. Front Microbiol. 2015;6:20.
Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(10):661–72.
Zhou Y, He H, Xu H, Li Y, Li Z, Du Y, He J, Zhou Y, Wang H, Nie Y. Association of oncogenic bacteria with colorectal cancer in South China. Oncotarget. 2016;7(49):80794–802.
Nakatsu G, Li X, Zhou H, Sheng J, Wong SH, Wu WK, Ng SC, Tsoi H, Dong Y, Zhang N, He Y, Kang Q, Cao L, Wang K, Zhang J, Liang Q, Yu J, Sung JJ. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun. 2015;6:8727.
Mira-Pascual L, Cabrera-Rubio R, Ocon S, Costales P, Parra A, Suarez A, Moris F, Rodrigo L, Mira A, Collado MC. Microbial mucosal colonic shifts associated with the development of colorectal cancer reveal the presence of different bacterial and archaeal biomarkers. J Gastroenterol. 2015;50(2):167–79.
Boleij A, Hechenbleikner EM, Goodwin AC, Badani R, Stein EM, Lazarev MG, Ellis B, Carroll KC, Albesiano E, Wick EC, Platz EA, Pardoll DM, Sears CL. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin Infect Dis. 2015;60(2):208–15.
Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, Meyerson M, Garrett WS. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14(2):207–15.
Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J, Baselga J, Liu C, Shivdasani RA, Ogino S, Birren BW, Huttenhower C, Garrett WS, Meyerson M. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22(2):292–8.
Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, Barnes R, Watson P, Allen-Vercoe E, Moore RA, Holt RA. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22(2):299–306.
Dai Z, Coker OO, Nakatsu G, Wu WKK, Zhao L, Chen Z, Chan FKL, Kristiansen K, Sung JJY, Wong SH, Yu J. Multi-cohort analysis of colorectal cancer metagenome identified altered bacteria across populations and universal bacterial markers. Microbiome. 2018;6(1):70.
Avuthu N, Guda C. Meta-analysis of altered gut microbiota reveals microbial and metabolic biomarkers for colorectal cancer. Microbiol Spectr. 2022;10(4): e0001322.
Thomas AM, Manghi P, Asnicar F, Pasolli E, Armanini F, Zolfo M, Beghini F, Manara S, Karcher N, Pozzi C, Gandini S, Serrano D, Tarallo S, Francavilla A, Gallo G, Trompetto M, Ferrero G, Mizutani S, Shiroma H, Shiba S, Shibata T, Yachida S, Yamada T, Wirbel J, Schrotz-King P, Ulrich CM, Brenner H, Arumugam M, Bork P, Zeller G, Cordero F, Dias-Neto E, Setubal JC, Tett A, Pardini B, Rescigno M, Waldron L, Naccarati A, Segata N. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat Med. 2019;25(4):667–78.
Li J, Jia H, Cai X, Zhong H, Feng Q, Sunagawa S, Arumugam M, Kultima JR, Prifti E, Nielsen T, Juncker AS, Manichanh C, Chen B, Zhang W, Levenez F, Wang J, Xu X, Xiao L, Liang S, Zhang D, Zhang Z, Chen W, Zhao H, Al-Aama JY, Edris S, Yang H, Wang J, Hansen T, Nielsen HB, Brunak S, Kristiansen K, Guarner F, Pedersen O, Dore J, Ehrlich SD, Meta HITC, Bork P, Wang J, Meta HITC. An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol. 2014;32(8):834–41.
He Y, Wu W, Zheng HM, Li P, McDonald D, Sheng HF, Chen MX, Chen ZH, Ji GY, Zheng ZD, Mujagond P, Chen XJ, Rong ZH, Chen P, Lyu LY, Wang X, Wu CB, Yu N, Xu YJ, Yin J, Raes J, Knight R, Ma WJ, Zhou HW. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat Med. 2018;24(10):1532–5.
Flemer B, Lynch DB, Brown JM, Jeffery IB, Ryan FJ, Claesson MJ, O’Riordain M, Shanahan F, O’Toole PW. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut. 2017;66(4):633–43.
Flemer B, Warren RD, Barrett MP, Cisek K, Das A, Jeffery IB, Hurley E, O’Riordain M, Shanahan F, O’Toole PW. The oral microbiota in colorectal cancer is distinctive and predictive. Gut. 2018;67(8):1454–63.
Chuang TP, Wang JY, Jao SW, Wu CC, Chen JH, Hsiao KH, Lin CY, Chen SH, Su SY, Chen YJ, Chen YT, Wu DC, Li LH. Over-expression of AURKA, SKA3 and DSN1 contributes to colorectal adenoma to carcinoma progression. Oncotarget. 2016;7(29):45803–18.
Legendre FMP. Ward’s hierarchical agglomerative clustering method: which algorithms implement ward’s criterion? J Classif. 2014;31:274–95.
Shariati A, Razavi S, Ghaznavi-Rad E, Jahanbin B, Akbari A, Norzaee S, Darban-Sarokhalil D. Association between colorectal cancer and Fusobacterium nucleatum and Bacteroides fragilis bacteria in Iranian patients: a preliminary study. Infect Agent Cancer. 2021;16(1):41.
Casasanta MA, Yoo CC, Udayasuryan B, Sanders BE, Umana A, Zhang Y, Peng H, Duncan AJ, Wang Y, Li L, Verbridge SS, Slade DJ. Fusobacterium nucleatum host-cell binding and invasion induces IL-8 and CXCL1 secretion that drives colorectal cancer cell migration. Sci Signal. 2020;13(641): eaba9157.
Yang Y, Weng W, Peng J, Hong L, Yang L, Toiyama Y, Gao R, Liu M, Yin M, Pan C, Li H, Guo B, Zhu Q, Wei Q, Moyer MP, Wang P, Cai S, Goel A, Qin H, Ma Y. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor-kappaB, and up-regulating expression of microRNA-21. Gastroenterology. 2017;152(4):851-866.e824.
Liu W, Zhang X, Xu H, Li S, Lau HC, Chen Q, Zhang B, Zhao L, Chen H, Sung JJ, Yu J. Microbial community heterogeneity within colorectal neoplasia and its correlation with colorectal carcinogenesis. Gastroenterology. 2021;160(7):2395–408.
Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. 2014;15(3):317–28.
Warren RL, Freeman DJ, Pleasance S, Watson P, Moore RA, Cochrane K, Allen-Vercoe E, Holt RA. Co-occurrence of anaerobic bacteria in colorectal carcinomas. Microbiome. 2013;1(1):16.
Okuda T, Kokubu E, Kawana T, Saito A, Okuda K, Ishihara K. Synergy in biofilm formation between Fusobacterium nucleatum and Prevotella species. Anaerobe. 2012;18(1):110–6.
Keskin M. A comparative analysis of biofilm characteristics of dual-species periodontopathogenic biofilm based on Fusobacterium nucleatum and the DualSpecies biofilm response in the presence of antimicrobial peptide. Aurum J Health Sci. 2020;2:1–10.
Kew MC. Synergistic interaction between aflatoxin B1 and hepatitis B virus in hepatocarcinogenesis. Liver Int. 2003;23(6):405–9.
Jin M, Shang F, Wu J, Fan Q, Chen C, Fan J, Liu L, Nie X, Zhang T, Cai K, Ogino S, Liu H. Tumor-associated microbiota in proximal and distal colorectal cancer and their relationships with clinical outcomes. Front Microbiol. 2021;12: 727937.
Chen YC, Miao ZF, Yip KL, Cheng YA, Liu CJ, Li LH, Lin CY, Wang JW, Wu DC, Cheng TL, Wang JY. Gut fecal microbiota transplant in a mouse model of orthotopic rectal cancer. Front Oncol. 2020;10: 568012.
Gao R, Kong C, Huang L, Li H, Qu X, Liu Z, Lan P, Wang J, Qin H. Mucosa-associated microbiota signature in colorectal cancer. Eur J Clin Microbiol Infect Dis. 2017;36(11):2073–83.
de Carvalho AC, de Mattos Pereira L, Datorre JG, Dos Santos W, Berardinelli GN, Matsushita MM, Oliveira MA, Duraes RO, Guimaraes DP, Reis RM. Microbiota profile and impact of Fusobacterium nucleatum in colorectal cancer patients of Barretos cancer hospital. Front Oncol. 2019;9:813.
Chen YC, Chuang CH, Miao ZF, Yip KL, Liu CJ, Li LH, Wu DC, Cheng TL, Lin CY, Wang JY. Gut microbiota composition in chemotherapy and targeted therapy of patients with metastatic colorectal cancer. Front Oncol. 2022;12: 955313.
Ternes D, Tsenkova M, Pozdeev VI, Meyers M, Koncina E, Atatri S, Schmitz M, Karta J, Schmoetten M, Heinken A, Rodriguez F, Delbrouck C, Gaigneaux A, Ginolhac A, Nguyen TTD, Grandmougin L, Frachet-Bour A, Martin-Gallausiaux C, Pacheco M, Neuberger-Castillo L, Miranda P, Zuegel N, Ferrand JY, Gantenbein M, Sauter T, Slade DJ, Thiele I, Meiser J, Haan S, Wilmes P, Letellier E. The gut microbial metabolite formate exacerbates colorectal cancer progression. Nat Metab. 2022;4(4):458–75.
Takahashi N, Yamada T. Glucose metabolism by Prevotella intermedia and Prevotella nigrescens. Oral Microbiol Immunol. 2000;15(3):188–95.
Paris A, Tardif N, Galibert MD, Corre S. AhR and cancer: from gene profiling to targeted therapy. Int J Mol Sci. 2021;22(2):752.
Karched M, Bhardwaj RG, Qudeimat M, Al-Khabbaz A, Ellepola A. Proteomic analysis of the periodontal pathogen Prevotella intermedia secretomes in biofilm and planktonic lifestyles. Sci Rep. 2022;12(1):5636.
Williams D, Fingleton B. Non-canonical roles for metabolic enzymes and intermediates in malignant progression and metastasis. Clin Exp Metastasis. 2019;36(3):211–24.
Li Y, Jia Y, Bian Y, Tong H, Qu J, Wang K, Wan XP. Autocrine motility factor promotes endometrial cancer progression by targeting GPER-1. Cell Commun Signal. 2019;17(1):22.
Ahmad A, Aboukameel A, Kong D, Wang Z, Sethi S, Chen W, Sarkar FH, Raz A. Phosphoglucose isomerase/autocrine motility factor mediates epithelial–mesenchymal transition regulated by miR-200 in breast cancer cells. Cancer Res. 2011;71(9):3400–9.
Tsutsumi S, Yanagawa T, Shimura T, Kuwano H, Raz A. Autocrine motility factor signaling enhances pancreatic cancer metastasis. Clin Cancer Res. 2004;10(22):7775–84.
Torimura T, Ueno T, Kin M, Harada R, Nakamura T, Kawaguchi T, Harada M, Kumashiro R, Watanabe H, Avraham R, Sata M. Autocrine motility factor enhances hepatoma cell invasion across the basement membrane through activation of beta1 integrins. Hepatology. 2001;34(1):62–71.
Jung HS, Lee SI, Kang SH, Wang JS, Yang EH, Jeon B, Myung J, Baek JY, Park SK. Monoclonal antibodies against autocrine motility factor suppress gastric cancer. Oncol Lett. 2017;13(6):4925–32.
Gallardo-Perez JC, Rivero-Segura NA, Marin-Hernandez A, Moreno-Sanchez R, Rodriguez-Enriquez S. GPI/AMF inhibition blocks the development of the metastatic phenotype of mature multi-cellular tumor spheroids. Biochim Biophys Acta. 2014;1843(6):1043–53.
Taddese R, Garza DR, Ruiter LN, de Jonge MI, Belzer C, Aalvink S, Nagtegaal ID, Dutilh BE, Boleij A. Growth rate alterations of human colorectal cancer cells by 157 gut bacteria. Gut Microbes. 2020;12(1):1–20.
Acknowledgements
The authors would like to thank the participants. We would like to acknowledge the National Center for Genome Medicine of the National Core Facility Program for Biotechnology, Ministry of Science and Technology, for the technical/bioinformatics support of mutation detection, Dr. Yu-Lun Kuo at BIOTOOLS Co., Ltd in Taiwan for kindly supporting the analysis of 16S rRNA sequencing data, and Dr. Jeffrey J. Y. Yen for his critical comments regarding the manuscript.
Funding
VGH, TSGH, NDMC, AS Joint Research Program (to L.-H. Li), Academia Sinica GMM (to Y.-T. Chen), the Ministry of Science and Technology, Taiwan, Republic of China (MOST 107-2321-B037-003, MOST 108-2321-B-037-001, and MOST 109-2314-B-037-046-MY3) and Kaohsiung Medical University Center for Cancer Research (KMU-TC111A04-1) (to J.-Y. Wang), Kaohsiung Medical University (KMUH107-7R02) and Kaohsiung Medical University Research Center Grant (KMU-TC111A02-2) (to D.-C. Wu). The funding body does not play any role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
LHL, JYW, and DCW conceived the study and aims. LHL and JYW designed the research. LHL directed and coordinated study and oversaw the project. CHL and LHL performed the experiments, analyzed data, wrote the original draft, and wrote the revised manuscript. JYW, DCW, SWJ, CCW, JHC, KHH, and YTC were involved in resources. CHC, YBL and CYL performed bioinformatics analysis. CHC and YTC assisted in the statistical analysis. YJC collected and curated the clinicopathological information. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Internal Review Boards of Academia Sinica (AS-IRB01-12024), Kaohsiung Medical University Hospital (KMUH-IRB-980278), Tri-Service General Hospital (098-05-156), and Taipei Tzu Chi General Hospital (00-IRB-005XD). Informed consent was obtained from all participants before information and samples were collected.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Details of data analysis of tumor-gene panel sequencing and exome sequencing.
Additional file 2.
Primer and probe sequences specific to species for bacterial DNA quantification. Primer and probe sequences specific to species for bacterial DNA quantification.
Additional file 3.
Relative abundance of taxa at species level. Relative abundance of taxa annotated at species level of 196 tissue samples from 76 CRC patients.
Additional file 4.
Wilcoxon signed-rank test. Wilcoxon signed-rank test of the relative abundance of species in pairwise comparison.
Additional file 5.
Genera included in co-abundance groups with significant alteration. T-test results and genera of the co-abundance groups with significant alteration among cancer tissues, polyp tissues, and non-neoplastic colon tissues.
Additional file 6: Figure S1.
Migration and invasion of CRC cells incubated with Prevotella intermedia at various MOIs.
Additional file 7: Figure S2.
Silver staining of conditioned medium collected from Prevotella intermedia.
Additional file 8: Figure S3.
Growth of CRC cells incubated with Prevotella intermedia at various MOIs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lo, CH., Wu, DC., Jao, SW. et al. Enrichment of Prevotella intermedia in human colorectal cancer and its additive effects with Fusobacterium nucleatum on the malignant transformation of colorectal adenomas. J Biomed Sci 29, 88 (2022). https://doi.org/10.1186/s12929-022-00869-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12929-022-00869-0