Abstract
Among the various types of dementia, Alzheimer’s disease (AD) is the most prevalent and is clinically defined as the appearance of progressive deficits in cognition and memory. Considering that AD is a central nervous system disease, getting tissue from the patient to study the disease before death is challenging. The discovery of the technique called induced pluripotent stem cells (iPSCs) allows to reprogram the patient’s somatic cells to a pluripotent state by the forced expression of a defined set of transcription factors. Many studies have shown promising results and made important conclusions beyond AD using iPSCs approach. Due to the accumulating knowledge related to this topic and the important advances obtained until now, we review, using PubMed, and present an update of all publications related to AD from the use of iPSCs. The first iPSCs generated for AD were carried out in 2011 by Yahata et al. (PLoS One 6:e25788, 2011) and Yaqi et al. (Hum Mol Genet 20:4530–9, 2011). Like other authors, both authors used iPSCs as a pre-clinical tool for screening therapeutic compounds. This approach is also essential to model AD, testing early toxicity and efficacy, and developing a platform for drug development. Considering that the iPSCs technique is relatively recent, we can consider that the AD field received valuable contributions from iPSCs models, contributing to our understanding and the treatment of this devastating disorder.
Similar content being viewed by others
Introduction
Along with the aging population and as the main consequence of this, there is an increase in neurodegenerative diseases, including Alzheimer’s disease (AD) [137]. Dementia associated with several fatal clinical disorders is a considerable social, economic, and medical challenge [30]. By reaching approximately 50 million people, it has become a public health problem, with the global cost of US $818 billion for the treatment [3, 30]. Among the various types of dementia, AD is the most prevalent one and has been clinically defined as the appearance of progressive deficits in cognition and memory [10, 34].
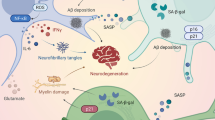
There are two types of AD: Familial AD (FAD) and Sporadic AD (SAD). Both share clinical and pathological similarities, exhibiting progressive cognitive dementia, senile plaques consisting of amyloid β (Aβ) peptide and neurofibrillary tangles (NFTs) consisting of phosphorylated tau protein [62, 137]. Axonal transport defects, synapse loss and selective neuronal death are others cellular phenotypes shared by FAD and SAD [38, 43, 137].
FAD: early-onset, accounts for 5% of cases and is caused by highly penetrant and rare autosomal mutations of the PS1, PS2 and, less frequently, amyloid precursor protein (APP) genes. APP protein is fundamental for central nervous system (CNS) function acting in the formation of synapses, neurogenesis, axonal transport, signaling and plasticity [17, 41, 43, 58, 137].
SAD: late-onset, has established risk factors beyond age including cardiovascular disease, low education, depression, and the apolipoprotein-E4 (ApoE4) gene [30]. There are no clear dominant or recessive SAD mutations; however, many genetic variants have been identified and there is clearly a strong heritable component to the disorder [6, 137]. Thus, SAD has multifactorial origins, driven in part by a complex genetic profile and in part by environmental factors and the interaction of the two [30].
AD reaches the central nervous system (CNS); it is difficult to obtain samples of the patient’s nervous tissue before his death to study the disease [137]. It is possible, using the relatively recent technique called induced pluripotent stem cells (iPSCs), to study the genesis of diseases and identify new molecular targets that recapitulate the genetic background of the individual from disease models in the laboratory.
Induced pluripotent stem cells
The models of diseases, truly representing real human diseases and their physiological peculiarities, that can be recreated in the laboratory, are needed to increase the success rate of new drug discoveries and developments [141]. In addition, the studies conducted in animal models do not efficiently show the translation of the therapeutic discovery for human use, although they are valuable in elucidating diseases and directing markers and genes associated with certain pathologies [27]. Specifically, regarding AD, vertebrate and nonvertebrate models can cause abnormal phenotypes mainly because of considerable overexpression of proteins. Notably, the mutations introduced into the endogenous mouse genes, unfortunately, do not recapitulate all the pathologies of the human AD [29, 137]. In addition, the studies already using postmortem tissue show major structural changes in the brain, both at the cellular and molecular levels.
After the discovery of the iPSCs in 2006 by Yamanaka and colleagues, it became possible to reprogram the patient’s somatic cells back to a pluripotent state, forcing the expression of a defined set of transcription factors. For this reprogramming, four transcription factors need to be introduced into fibroblasts through retroviruses. Consequently, the cells acquire a pluripotent stage with characteristics extremely similar to the embryonic stem cells [83]. The first transfection was performed on mouse fibroblasts [121], followed by transfection into human fibroblasts [120].
Considering the difficulty of obtaining CNS tissue from the patients with AD, the discovery of iPSCs shows a great potential and advantage to enable the modeling of in vitro diseases. For example, disease-specific cells from patients with AD can be produced with disorders without a clear pattern of inheritance and sporadic cases can be used in drug discovery programs [83].
Parkinson’s disease [78, 87, 101, 115], amyotrophic lateral sclerosis [25, 74], smooth muscle atrophy [31], and family dysautonomia [61] were the diseases initially studied using the iPSCs approach to model neurological diseases. These are monogenic disorders or versions of complex diseases caused by known mutations [72, 137].
Important advances in Alzheimer’s disease using iPSCs
Many studies have shown promising results and important conclusions, beyond AD, using iPSCs allowing a better understanding of cellular and molecular targets. Here we review and present an update of all publications related to AD from the use of iPSCs (Table 1). Electronic databases, including PubMed, were searched for articles related to the use of iPSCs in AD research. Only full-text English-language articles were included. If the abstract met the inclusion criteria, the full-text article was obtained and reviewed. The flow diagram below shows which terms were searched and how many articles were excluded at each step and the reasons (Fig. 1).
One of the first studies involving iPSCs generated for AD were carried out in 2011 by Yahata et al. [133] and Yaqi et al. [131]. Yahata et al., [133] successfully generated forebrain neurons from human iPSCs cells, and showed that Aβ production in neuronal cells was detectable and inhibited by some typical secretase inhibitors and modulators. According to the authors, hiPSCs cell-derived neuronal cells express functional β- and γ-secretases involved in Aβ production. However, anti-Aβ drug screening using these hiPS cell-derived neuronal cells requires sufficient neuronal differentiation. Also, Yaqi et al. [131] generated iPSCs from fibroblasts of FAD patients with mutations in PS1 (A246E) and PS2 (N141I) and characterized the differentiation of these cells into neurons. The authors demonstrated that patient-derived differentiated neurons have increased Ab42 secretion, recapitulating the pathological mechanism of FAD with PS1 and PS2 mutations.
Taken together, along with others, these two studies represent, thus, critical first steps in assessing the potential of AD iPSCs to model AD. iPSCs are a pre-clinical tool for screening therapeutic compounds.
In addition, this approach is central to toxicity and efficacy testing, in the new drug development landscape and the precise engineering of the genome and transcriptional and proteomic analyses. Thus, human cells can demonstrate pathogenic mutations in vitro, which can then be functionally validated and downstream targets can be confirmed. In the future, these models can give rise to a new preclinical model for drug discovery and even personalized therapeutics based on individual’s genetics [94].
Future directions
With the advancement of research using the iPSCs, the customization of the treatment for patients with AD is possible, reaching new insights associated with the pathogenesis and discovery of new drugs for the treatment and/or prevention, which is economically impractical at present [83]. The treatment is individualized based on the behavior of the cellular model for possibly defining the AD subgroups [83]. At present, we can model in vitro diseases to allow patient-specific therapies from newly derived AD-iPSCs to be used by considering the appropriate characterization of AD patient groups through genetic profiles and biomarkers [83].
In addition, iPSCs have some limitations. One can consider the immature and fetal population of neurons that are obtained from the iPSCs that model the AD in case of an illness of the aging [69, 73, 118, 127]. Therefore, there is a possibility to express a mutant form of LMNA, which is known to cause premature aging [46].
The challenges in the enhancement of iPSCs are directly related to modeling protocols. In iPSCs, we can highlight the level of maturity of the neurons, lack of efficient protocols to generate microglia, and few protocols of 3D differentiation that appropriately mimic the in vivo environment of the brain [107]. Now, to generate and differente iPSCs are still a time-consuming and expensive processes; however, with the improvement and development of new protocols, iPSCs can be used from an individual to direct their appropriate treatment through personalized medicine, improving the patient’s life [46].
During the formation of iPSCs, there are a concern about introducing harmful mutations. Therefore, new genome editing techniques, such as the clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9 (Cas9) nuclease system, reduce the risk of introduction and spread of undesired mutations [134, 110]. Paquet et al. [86] accurately and efficiently generated both homozygous and heterozygous dominant AD-causing mutations using CRISPR/Cas9 [110].
Essentially, neurodifferentiated cells from iPSCs exhibit the pathological characteristics of an individual with AD in less than two months, demonstrating that cultured cells are more susceptible to display the disease characteristics than those that occur in a patient’s brain. Moreover, it is not known whether one or two differentiated cell types from iPSCs may represent the complicated disease phenotype [134].
Another limitation of iPSCs is the fact that they represent models in two dimensions, thus lacking cellular diversity, having structural complexity, and presenting physical architecture in vivo. Therefore, a fundamental approach for the development of a physiologically relevant model is to make a three-dimensional (3D) model of the neurons and glia [4, 32], revealing heterogeneous and naturally organized cellular models [4]. Normal cortical folding [66], microcephaly [56], and lissencephaly [11] are some successful organoids used to model neurodevelopmental processes and diseases [4]. Neurodegenerative disease models are still scarce; they can give new insight to model AD [4]. According to our literature survey, some authors are already using organoids in AD research, which originate from iPSCs [1, 102, 116, 117]. Therefore, defined radial glial cells can be obtained using these organoids; these cells are crucial in brain development and function, as well as associated with the organization and morphology similar to the developing human cortex [4, 93]. This model has made considerable contributions over time. Thus, supporting cells that develop along the first neurons can be crucial in modeling the onset and progression of the disease [4, 95].
Along with the progressive neurodegeneration of patients with AD, memory and the ability to learn and perform daily activities are also impaired over time. In an aging society is necessary and urgent to develop an efficient drug to treat AD, thus clinical AD may need to be reclassified into different subtypes, and the prediction of drug responsiveness may be possible based on the different subtypes [55, 47].
Considering that therapies for AD are mostly palliative, a great deal of effort is made by the scientific community to discover a drug; however, several promising candidate drugs have failed in recent clinical trials [20]. Semagacestat, for example, is a potential nontransitional state analog of γ-secretase inhibitor (GSI). All GSI studies for AD, including Semagacestat, were unsuccessful [112, 109]. Arguments against the efficacy of reducing Aβ levels in the brain are based on the results obtained while aiming a therapy for AD [12, 33, 53, 109]; the expected effect was exactly the opposite, considering that Semagacestat and another potential GSI Avagacestat worsened the cognitive decline [22, 26, 109].
Recently, quantifying small residual peptides, Tagami et al. [109], addressed the effects of Semagacestat on PS/g-secretase activity, generated during sequential cleavages upon Aβ production. The authors demonstrated seemingly contradictory actions of Semagacestat, by decreasing levels of extracellular Aβ and intracellular amyloid protein precursor intracellular cytoplasmic domain along with increased bAPP-C-terminal fragment stubs. These Semagacestat effects are clearly different from those caused by a loss of functional PSs. According to the authors, Semagacestat is a pseudo-GSI and may inhibit the liberation of product peptides by g-secretase (g-byproducts) from the membrane to the soluble space. This allows g-byproducts to accumulate in living cells. A comprehensive assessment related to g-secretase activity will allow the discovery of clinical application of g-secretase-modulating compounds [109].
From AD diagnosis, an individual has four to five years of life span. Neuroreplaced therapies will not compensate for the neuronal loss but may be used to improve existing circuits temporarily, contributing to cognitive function and quality of life [30]. Cell replacement therapy has been the most challenging because of the multifactorial nature of AD. Earlier studies in animal models with AD have shown that the transplantation of neural stem cells can improve cognition, reduce neuronal loss, and increase synaptic plasticity. This is probably because of the mechanisms that are involved in neuroprotection and trophic support rather than those involved in neuronal substitution [20].
Future studies with iPSCs need to define the cell type and which cell type is impacted in a disease phenotype [24, 35, 40]. If the genetic identity of a natural cell is defined, it is possible to correlate with the modified cell in vitro. Similar studies are already proposed regarding the retina [105, 75].
With the increased genetic information, it becomes a growing priority to translate these genotypes into their functional biological results. The formation of subtypes of neurons can contribute to the evaluation of variants within discrete cell populations, defining specific genetic contributions to disease within each cell class [107].
The involvement of the cell type in the disease, with the possibility of modulating a specific gene expression profile, will help monitor the effect on downstream pathway members and consequently allow the modification of the pathways associated with the disease by modifying the proposed disease-relevant pathways. The evaluation of the phenotypic results of these alterations will show the biological effects of the gene expression, classifying whether the gene is relevant to the disease or not [108].
The prevalence of AD is higher in women, but in men the congenital decline is more severe and early [57, 110]. Hormonal and metabolic differences in the brain may explain these distinctions between the sexes [138, 110]. The study on the sexual dimorphism of microglia phenotype, for example, in the cortex and cerebellum, has strengthened [106, 111, 103, 7]. According to Streit et al. [119], microglia may be involved through the “microglia dysfunction hypothesis”. Therefore, to elucidate the influence of sex and its contribution to neuroinflammation in the AD, future studies may include endogenous microglia and inflammation as a phenotype in chimeric models [110].
Goldstein et al. [39] pointed some suggestions for future research, such as working in isogenic systems, which are described by Woodruff et al. [129], considering the known genome variability and human physiology. In addition, working on cell -nes that have been completely sequenced and determining a true diploid sequence to the level of the genome described to date have been suggested [63, 39]. Because of the complex nature of the pathophysiology of AD, a multimodal approach may be necessary, incorporating the pharmacological segmentation of the pathology, stimulation of endogenous neurogenesis and synaptogenesis, and exogenous neuroreplacement [30].
Regarding sporadic AD, a greater challenge exists to elucidate the factors that result in the disease. It is known that there is a hereditary component and that each individual with its unique genetic background has variants that may predispose or protect for the disease. Therefore, the research seeks to discover the genetic contribution to the AD if there are phenotypic consequences in an individual with a genetic background that contains genetic variants of risk [137]. In this manner, possible paths can be disclosed, and in the future may be used to determine the factors that cause AD and to test new possible therapeutics strategies [137]. Yang et al. [134] highlighted that an appropriate control group needs to be selected; iPSCs derived from healthy individuals or family members may have totally different genetic background compared with those from individuals with AD [134].
Valuable findings have already been obtained regarding the development of AD; however, several studies still need to elucidate its effects. For example, new information related to genetic variants in individual genomes and their influence on the neuronal phenotype will facilitate to identify the chance to developing AD through the identification of molecular and biochemical phenotypes caused by these genetic variants [137]. This 3D approach can reveal the connection between neurons and glia, and these genetic factors can be advantageous to drugs discovery of when the supporting cells are crucial, or the route of interest is unknown [4, 32].
Conclusion
The AD field of research has received valuable contributions from iPSC models. Considering that the iPSC technique is relatively recent, discovered in 2006, it is important to recognize associated advances obtained so far in AD research. The possibility to study neurons from a patient with AD in a culture dish allows observation of relevant cellular phenotypes and behaviors, following the earliest events in dementia. These research models have allowed the observation of direct molecular effects of FAD mutations and genetic risk variants, which may lead to more efficacious treatments targeting this devastating disorder.
Abbreviations
- AD:
-
Alzheimer’s disease
- ApoE4:
-
Apolipoprotein-E4
- APP:
-
Amyloid precursor protein
- ASM:
-
Acid sphingomyelinase
- Aβ:
-
Amyloid-beta
- BFCNs:
-
Basal forebrain cholinergic neurons
- Cdk5:
-
Cyclin-dependent kinase 5
- CNS:
-
Central Nervous System
- CTF:
-
C-terminal fragment
- DIAN:
-
Dominantly Inherited Alzheimer Network
- DM:
-
Diabetes mellitus
- DMD:
-
Duchenne type muscular dystrophy
- ESCs:
-
Embryonic stem cells
- FAD:
-
Familial AD
- GCSF:
-
Granulocyte colony stimulating factor
- GSI:
-
γ-secretase inhibitors
- GSM:
-
γ-secretase modulator
- HCE:
-
Human chemical exposome
- HLC:
-
Human lymphoblast cells
- hTFAM:
-
Human mitochondrial transcriptional factor A
- IMGLs:
-
Human microglial-like cells
- iPSCs:
-
Induced pluripotent stem cells
- MSC:
-
Mesenchimal stem cells
- mtDNA:
-
Mitochondrial DNA
- NFTs:
-
Neurofibrillary tangles.
- non-TSA:
-
Non-transition state analog
- NPCs:
-
Neural progenitor cells
- PBMCs:
-
Peripheral blood mononuclear cells
- PD:
-
Parkinson’s disease
- PS1:
-
Presenilin-1
- PS2:
-
Presenilin-2
- SAD:
-
Sporadic AD
- sAPPα:
-
Soluble amyloid precursor protein-alpha
References
Abud EM, Ramirez RN, Martinez ES, Healy LM, Nguyen CHH, Newman SA, Yeromin AV, et al. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron. 2017;94(2):278–293.e9. https://doi.org/10.1016/j.neuron.2017.03.042.
Akhtar MW, Sanz-Blasco S, Dolatabadi N, Parker J, Chon K, Lee MS, Soussou W, et al. Elevated glucose and oligomeric β-amyloid disrupt synapses via a common pathway of aberrant protein S-nitrosylation. Nat Commun. 2016;7:10242. https://doi.org/10.1038/ncomms10242.
Alzheimers Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11:332.
Arber C, Lovejoy C, Wray S. Stem cell models of Alzheimer's disease: progress and challenges. Alzheimers Res Ther. 2017;9(1):42. Published 2017 Jun 13. https://doi.org/10.1186/s13195-017-0268-4.
Armijo E, Gonzalez C, Shahnawaz M, Flores A, Davis B, Soto C. Increased susceptibility to Aβ toxicity in neuronal cultures derived from familial Alzheimer's disease (PSEN1-A246E) induced pluripotent stem cells. Neurosci Lett. 2017;639:74–81. https://doi.org/10.1016/j.neulet.2016.12.060.
Avramopoulos D. Genetics of Alzheimer’s disease: recente advances. Genome Med. 2009;1:34.
Bakkour A, Morris JC, Wolk DA, Dickerson BC. The effects of aging and Alzheimer's disease on cerebral cortical anatomy: specificity and differential relationships with cognition. Neuroimage. 2013;76:332–44. https://doi.org/10.1016/j.neuroimage.2013.02.059 Epub 2013 Mar 16.
Balez R, Steiner N, Engel M, Muñoz SS, Lum JS, Wu Y, Wang D, Vallotton P, Sachdev P, et al. Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer's disease. Sci Rep. 2016;6:31450. https://doi.org/10.1038/srep31450.
Ben Halima S, Mishra S, Raja KMP, Willem M, Baici A, Simons K, Brüstle O, Koch P, Haass C, Caflisch A, Rajendran L. Specific Inhibition of β-Secretase Processing of the Alzheimer DiseaseAmyloid Precursor Protein. Cell Rep. 2016;14(9):2127–41. https://doi.org/10.1016/j.celrep.2016.01.076.
Berchtold NC, Cotman CW. Evolution in the conceptualization of dementia and Alzheimer’s disease: Greco-Roman period to the 1960s. Neurobiol Aging. 1998;19(3):173–89.
Bershteyn M, Nowakowski TJ, Pollen AA, Di Lullo E, Nene A, Wynshaw-Boris A, et al. Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Cell Stem Cell. 2017;20(4):435–49.e4.
Blennow K, Zetterberg H, Haass C, Finucane T. Semagacestat’s fall: where next for AD therapies? Nat. Med., 19 (2013), pp. 1214–1215.
Birnbaum JH, Wanner D, Gietl AF, Saake A, Kündig TM, Hock C, Nitsch RM, Tackenberg C. Oxidative stress and altered mitochondrial protein expression in the absence of amyloid-β and tau pathology in iPSC-derived neurons from sporadic Alzheimer's disease patients. Stem Cell Res. 2018;27:121–30. https://doi.org/10.1016/j.scr.2018.01.019.
Brookhouser N, Zhang P, Caselli R, Kim JJ, Brafman DA. Generation and characterization of human induced pluripotent stem cell (hiPSC) lines from an Alzheimer's disease (ASUi003-A) and non-demented control (ASUi004-A) patient homozygous for the Apolipoprotein e4 (APOE4) risk variant. Stem Cell Res. 2017;25:266–9. https://doi.org/10.1016/j.scr.2017.07.003.
Brookhouser N, Zhang P, Caselli R, Kim JJ, Brafman DA. Generation and characterization of human induced pluripotent stem cell (hiPSC) lines from an Alzheimer's disease (ASUi001-A) and non-demented control (ASUi002-A) patient homozygous for the Apolipoprotein e4 (APOE4) risk variant. Stem Cell Res. 2017;24:160–3. https://doi.org/10.1016/j.scr.2017.06.003.
Brownjohn PW, Smith J, Portelius E, Serneels L, Kvartsberg H, De Strooper B, Blennow K, et al. Phenotypic Screening Identifies Modulators of Amyloid Precursor Protein Processing in Human Stem Cell Models of Alzheimer's Disease. Stem Cell Reports. 2017;8(4):870–82. https://doi.org/10.1016/j.stemcr.2017.02.006.
Brunholz S, Sisodia S, Lorenzo A, Deyts C, Kins S, Morfini G. Axonal transport of APP and the spatial regulation of APP cleavage and function in neuronal cells. Exp Brain Res. 2012;217:353–64.
Cha MY, Kwon YW, Ahn HS, Jeong H, Lee YY, Moon M, Baik SH, Kim DK, Song H, et al. Protein-Induced pluripotent Stem Cells Ameliorate Cognitive Dysfunction and Reduce Aβ Deposition in a Mouse Model of Alzheimer's Disease. Stem Cells Transl Med. 2017;6(1):293–305. https://doi.org/10.5966/sctm.2016-0081.
Chang CY, Chen SM, Lu HE, Lai SM, Lai PS, Shen PW, Chen PY, Shen CI, et al. N-butylidenephthalide attenuates Alzheimer's disease-like cytopathy in Down syndrome induced pluripotent stem cell-derived neurons. Sci Rep. 2015;5:8744. https://doi.org/10.1038/srep08744.
Chen WW, Blurton-Jones M. Concise review: can stem cells be used to treat or model Alzheimer's disease? Stem Cells. 2012;30(12):2612–8.
Chen M, Lee HK, Moo L, Hanlon E, Stein T, Xia W. Common proteomic profiles of induced pluripotent stem cell-derived three-dimensional neurons and brain tissue from Alzheimer patients. J Proteome. 2018;182:21–33. https://doi.org/10.1016/j.jprot.2018.04.032.
Coric V, van Dyck CH, Salloway S, Andreasen N, Brody M, Richter RW, Soininen H, Thein S, Shiovitz T, Pilcher G, Colby S, Rollin L, Dockens R, Pachai C, Portelius E, Andreasson U, Blennow K, Soares H, Albright C, Feldman HH, Berman RM.Safety and tolerability of the γ-secretase inhibitor avagacestat in a phase 2 study of mild to moderate Alzheimer disease. Arch Neurol. 2012;69(11):1430–40.
Dashinimaev EB, Artyuhov AS, Bolshakov AP, Vorotelyak EA, Vasiliev AV. Neurons Derived from Induced pluripotent Stem Cells of Patients with Down Syndrome Reproduce Early Stages of Alzheimer's Disease Type Pathology in vitro. J Alzheimers Dis. 2017;56(2):835–47. https://doi.org/10.3233/JAD-160945.
DeFelipe J, Lopez-Cruz PL, Benavides-Piccione R, Bielza C, Larranaga P, Anderson S, Burkhalter A, Cauli B, Fairen A, Feldmeyer D, Fishell G, Fitzpatrick D, Freund TF, Gonzalez-Burgos G, Hestrin S, Hill S, Hof PR, Huang J, Jones EG, Kawaguchi Y, Kisvarday Z, Kubota Y, Lewis DA, Marin O, Markram H, McBain CJ, Meyer HS, Monyer H, Nelson SB, Rockland K, Rossier J, Rubenstein JLR, Rudy B, Scanziani M, Shepherd GM, Sherwood CC, Staiger JF, Tamas G, Thomson A, Wang Y, Yuste R, Ascoli GA. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nature Reviews Neuroscience. 2013; 14:202–216. PMID: 23385869.
Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–21.
Doody RS, Raman R, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, He F, Sun X, Thomas RG, Aisen PS; Alzheimer's Disease Cooperative Study Steering Committee, Siemers E, Sethuraman G, Mohs R; Semagacestat Study Group. A phase 3 trial of semagacestat for treatment of Alzheimer's disease. N Engl J Med. 2013;369(4):341–50. https://doi.org/10.1056/NEJMoa1210951
Dragunow M. The adult human brain in preclinical drug development. Nat Rev Drug Discov. 2008;7(8):659–66.
Duan L, Bhattacharyya BJ, Belmadani A, Pan L, Miller RJ, Kessler JA. Stem cell derived basal forebrain cholinergic neurons from Alzheimer's disease patients are more susceptible to cell death. Mol Neurodegener. 2014;9:3. https://doi.org/10.1186/1750-1326-9-3.
Duff K, Suleman F. Transgenic mouse models of Alzheimer’s disease: how useful have they been for therapeutic development? Brief Funct. Genomic Proteomic. 2004;3:47–59.
Duncan T, Valenzuela M. Alzheimer's disease, dementia, and stem cell therapy. Stem Cell Res Ther. 2017;8(1):111. https://doi.org/10.1186/s13287-017-0567-5.
Ebert AD, Yu J, Rose FF Jr, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–80.
Edmondson R, Adcock AF, Yang L, Liu G, Diot A, Xirodimas D. Influence of matrices on 3D-cultured prostate cancer cells’ drug response and expression of drug-action associated proteins. PLoS One. 2016;11:e0158116.
Extance A. Alzheimer's failure raises questions about disease-modifying strategies. Nat Rev Drug Discov. 2010;9(10):749–51. https://doi.org/10.1038/nrd3288.
Fan X, Sun D, Tang X Cai Y, Yin ZQ, and Xu H. Stem cell challenges in the treatment of Alzheimer’s disease: a long way from bench to bedside. Med. Res. Rev. 2014; 34, 957–978.
Fishell G, Heintz N. The Neuron Identity Problem: Form Meets Function. Neuron. 2013; 80:602–612. PMID: 24183013. https://doi.org/10.1016/j.neuron.2013.10.035
Fong LK, Yang MM, Dos Santos CR, Reyna SM, Langness F, Woodruff G, Roberts EA, Young JE, Goldstein LSB. Full-length amyloid precursor protein regulates lipoprotein metabolism and amyloid-β clearance in human astrocytes. J Biol Chem. 2018;293(29):11341–57. https://doi.org/10.1074/jbc.RA117.000441.
García-León JA, Cabrera-Socorro A, Eggermont K, Swijsen A, Terryn J, Fazal R, Nami F, Ordovás L, et al. Generation of a human induced pluripotent stem cell-based model for tauopathies combining three microtubule-associated protein tau mutations which displays several phenotypes linked to neurodegeneration. Alzheimers Dement. 2018;18:30161–4. https://doi.org/10.1016/j.jalz.2018.05.007 pii: S1552–5260.
Goldstein LS. Axonal transport and neurodegenerative disease: can we see the elephant? Prog Neurobiol. 2012. https://doi.org/10.1016/j.pneurobio.2012.03.006.
Goldstein LS, Reyna S, Woodruff G. Probing the secrets of Alzheimer's disease using human-induced pluripotent stem cell technology. Neurotherapeutics. 2015;12(1):121–5. https://doi.org/10.1007/s13311-014-0326-6.
Grange P, Bohland JW, Okaty BW, Sugino K, Bokil H, Nelson SB, Ng L, Hawrylycz M, Mitra PP. Cell-type-based model explaining coexpression patterns of genes in the brain. Proceedings of the National Academy of Sciences of the United States of America. 2014; 111:5397–5402.
Gunawardena S, Goldstein LS. Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in drosophila. Neuron. 2001;32:389–401.
Haenseler W, Sansom SN, Buchrieser J, Newey SE, Moore CS, Nicholls FJ, Chintawar S, et al. A Highly Efficient Human Pluripotent Stem Cell Microglia Model Displays a Neuronal-Co-culture-Specific Expression Profile and Inflammatory Response. Stem Cell Reports. 2017;8(6):1727–42. https://doi.org/10.1016/j.stemcr.2017.05.017.
Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci. Transl. Med. 2011;3:77sr71.
Hossini AM, Megges M, Prigione A, Lichtner B, Toliat MR, Wruck W, Schröter F, et al. Induced pluripotent stem cell-derived neuronal cells from a sporadic Alzheimer's disease donor as a model for investigating AD-associated gene regulatory networks. BMC Genomics. 2015;16:84. https://doi.org/10.1186/s12864-015-1262-5.
Hossini AM, Quast AS, Plötz M, Grauel K, Exner T, Küchler J, Stachelscheid H, et al. PI3K/AKT Signaling Pathway Is Essential for Survival of Induced pluripotent Stem Cells. PLoS One. 2016;11(5):e0154770. https://doi.org/10.1371/journal.pone.0154770.
Imm J, Kerrigan TL, Jeffries A, Lunnon K. Using induced pluripotent stem cells to explore genetic and epigenetic variation associated with Alzheimer's disease. Epigenomics. 2017;9(11):1455–68. https://doi.org/10.2217/epi-2017-0076 Epub 2017 Oct 3.
Inoue H, Nagata N, Kurokawa H, Yamanaka S. iPS cells: a game changer for future medicine. EMBO J. 2014;33(5):409–17.
Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, et al. Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells. Nature. 2012;482(7384):216–20. https://doi.org/10.1038/nature10821.
Izuo N, Murakami K, Sato M, Iwasaki M, Izumi Y, Shimizu T, Akaike A, Irie K, Kume T. Non-toxic conformer of amyloid β may suppress amyloid β-inducedtoxicity in rat primary neurons: implications for a novel therapeutic strategy for Alzheimer's disease. Biochem Biophys Res Commun. 2013;438(1):1–5. https://doi.org/10.1016/j.bbrc.2013.05.106.
Jang J, Yoo JE, Lee JA, Lee DR, Kim JY, Huh YJ, Kim DS, Park CY, Hwang DY, Kim HS, Kang HC, Kim DW. Disease-specific induced pluripotent stem cells: a platform for human disease modeling and drug discovery. Exp Mol Med. 2012;44(3):202–13. https://doi.org/10.3858/emm.2012.44.3.015.
Jones VC, Atkinson-Dell R, Verkhratsky A, Mohamet L. Aberrant iPSC-derived human astrocytes in Alzheimer's disease. Cell Death Dis. 2017;8(3):e2696. https://doi.org/10.1038/cddis.2017.89.
Karch CM, Hernández D, Wang JC, Marsh J, Hewitt AW, Hsu S, et al. Dominantly Inherited Alzheimer Network (DIAN), Pébay A2,3, Goate AM12. Human fibroblast and stem cell resource from the Dominantly Inherited Alzheimer Network. Alzheimers Res Ther. 2018;10(1):69.
Karran E, Hardy J. A critique of the drug discovery and phase 3 clinical programs targeting the amyloid hypothesis for Alzheimer disease. Ann. Neurol., 76 (2014), pp. 185–205.
Koch P, Tamboli IY, Mertens J, Wunderlich P, Ladewig J, Stüber K, Esselmann H, Wiltfang J, Brüstle O, Walter J. Presenilin-1 L166P mutant human pluripotent stem cell-derived neurons exhibit partial loss of γ-secretase activity in endogenous amyloid-β generation. Am J Pathol. 2012;180(6):2404–16. https://doi.org/10.1016/j.ajpath.2012.02.012.
Kondo T, Asai M, Tsukita K, Kutoku Y, Ohsawa Y, Sunada Y, Imamura K, Egawa N, Yahata N, et al. Modeling Alzheimer's disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness. Cell Stem Cell. 2013;12(4):487–96. https://doi.org/10.1016/j.stem.2013.01.009.
Lancaster MA, Renner M, Martin C-A, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–9.
Laws KR., Irvine K, Gale TM. Sex differences in cognitive impairment in Alzheimer’s disease. World J. Psychiatry. 2016;6(1):54–65. https://doi.org/10.5498/wjp.v6.i1.54
Lazarov O, Demars MP. All in the family: how the APPs regulate neurogenesis. Front Neurosci. 2012;6:81.
Lee HK, Morin P, Xia W. Peripheral blood mononuclear cell-converted induced pluripotent stem cells (iPSCs) from an early onset Alzheimer's patient. Stem Cell Res. 2016;16(2):213–5. https://doi.org/10.1016/j.scr.2015.12.050.
Lee JK, Jin HK, Park MH, Kim BR, Lee PH, Nakauchi H, Carter JE, He X, Schuchman EH, Bae JS. Acid sphingomyelinase modulates the autophagic process by controlling lysosomal biogenesis in Alzheimer's disease. J Exp Med. 2014;211(8):1551–70. https://doi.org/10.1084/jem.20132451.
Lee G, Papapetrou EP, Kim H, Chambers SM, Tomishima MJ, Fasano CA, Ganat YM, Menon J, Shimizu F, Viale A, et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 2009;461:402–6.
Lee VM, Brunden KR, Hutton M, Trojanowski JQ. Developing therapeutic approaches to tau, selected kinases, and related neuronal protein targets. Cold Spring Harb Perspect Med. 2011;1:a006437.
Levy S, Sutton G, Ng PC, et al. The diploid genome sequence of an individual human. PLoS Biol. 2007;5:e254.
Li T, Pires C, Nielsen TT, Waldemar G, Hjermind LE, Nielsen JE, Dinnyes A, Holst B, Hyttel P, Freude KK. Generation of induced pluripotent stem cells (iPSCs) from an Alzheimer's disease patient carrying a M146I mutation in PSEN1. Stem Cell Res. 2016;16(2):334–7. https://doi.org/10.1016/j.scr.2016.01.001.
Li T, Pires C, Nielsen TT, Waldemar G, Hjermind LE, Nielsen JE, Dinnyes A, Hyttel P, Freude KK. Generation of induced pluripotent stem cells (iPSCs) from an Alzheimer's disease patient carrying an A79V mutation in PSEN1. Stem Cell Res. 2016;16(2):229–32. https://doi.org/10.1016/j.scr.2016.01.002.
Li Y, Muffat J, Omer A, Bosch I, Lancaster MA, Sur M, et al. Induction of expansion and folding in human cerebral organoids. Cell Stem Cell. 2017;20(3):385–96.e3.
Liao MC, Muratore CR, Gierahn TM, Sullivan SE, Srikanth P, De Jager PL, Love JC, Young-Pearse TL. Single-Cell Detection of Secreted Aβ and sAPPα from Human IPSC-Derived Neurons and Astrocytes. J Neurosci. 2016;36(5):1730–46. https://doi.org/10.1523/JNEUROSCI.2735-15.2016.
Liu Q, Waltz S, Woodruff G, Ouyang J, Israel MA, Herrera C, Sarsoza F, Tanzi RE, Koo EH, et al. Effect of potent γ-secretase modulator in human neurons derived from multiple presenilin 1-induced pluripotent stem cell mutant carriers. JAMA Neurol. 2014;71(12):1481–9. https://doi.org/10.1001/jamaneurol.2014.2482.
Livesey MR, Magnani D, Hardingham GE, Chandran S, Wyllie DJA. Functional properties of in vitro excitatory cortical neurons derived from human pluripotent stem cells. J Physiol. 2016;594(22):6573–82.
Mahairaki V, Ryu J, Peters A, Chang Q, Li T, Park TS, Burridge PW, Talbot CC Jr, Asnaghi L, Martin LJ, Zambidis ET, Koliatsos VE. Induced pluripotent stem cells from familial Alzheimer's diseasepatients differentiate into mature neurons with amyloidogenic properties. Stem Cells Dev. 2014;23(24):2996–3010. https://doi.org/10.1089/scd.2013.0511.
Maloney JA, Bainbridge T, Gustafson A, Zhang S, Kyauk R, Steiner P, van der Brug M, Liu Y, Ernst JA, Watts RJ, Atwal JK. Molecular mechanisms of Alzheimer disease protection by the A673T allele of amyloid precursor protein. J Biol Chem. 2014;289(45):30990–1000. https://doi.org/10.1074/jbc.M114.589069.
Marchetto MC, Brennand KJ, Boyer LF, Gage FH. Induced pluripotent stem cells (iPSCs) and neurological disease modeling: progress and promises. Hum Mol Genet. 2011;20:R109–15.
Mariani J, Simonini MV, Palejev D, et al. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109(31):12770–5.
Mitne-Neto M, Machado-Costa M, Marchetto MC, Bengtson MH, Joazeiro CA, Tsuda H, Bellen HJ, Silva HC, Oliveira AS, Lazar M, et al. Downregulation of VAPB expression in motor neurons derived from induced pluripotent stem cells of ALS8 patients. Hum Mol Genet. 2011;20:3642–52.
Mungenast AE, Siegert S, Tsai LH. Modeling Alzheimer's disease with human induced pluripotent stem (iPS) cells. Mol Cell Neurosci. 2016 Jun;73:13–31. https://doi.org/10.1016/j.mcn.2015.11.010 Epub 2015 Dec 4.
Muratore CR, Rice HC, Srikanth P, Callahan DG, Shin T, Benjamin LN, Walsh DM, Selkoe DJ, Young-Pearse TL. The familial Alzheimer's disease APPV717I mutation alters APP processing and Tau expression in iPSC-derived neurons. Hum Mol Genet. 2014;23(13):3523–36. https://doi.org/10.1093/hmg/ddu064.
Muratore CR, Zhou C, Liao M, Fernandez MA, Taylor WM, Lagomarsino VN, Pearse RV 2nd, et al. Cell-type Dependent Alzheimer's Disease Phenotypes: Probing the Biology of Selective Neuronal Vulnerability. Stem Cell Reports. 2017;9(6):1868–84. https://doi.org/10.1016/j.stemcr.2017.10.015.
Nguyen HN, Byers B, Cord B, Shcheglovitov A, Byrne J, Gujar P, Kee K, Schule B, Dolmetsch RE, Langston W, et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011;8:267–80.
Nieweg K, Andreyeva A, van Stegen B, Tanriöver G, Gottmann K. Alzheimer's disease-related amyloid-β induces synaptotoxicity in human iPS cell-derived neurons. Cell Death Dis. 2015;6:e1709. https://doi.org/10.1038/cddis.2015.72.
Ochalek A, Mihalik B, Avci HX, Chandrasekaran A, Téglási A, Bock I, Giudice ML, et al. Neurons derived from sporadic Alzheimer's disease iPSCs reveal elevated TAU hyperphosphorylation, increased amyloid levels, and GSK3B activation. Alzheimers Res Ther. 2017;9(1):90. https://doi.org/10.1186/s13195-017-0317-z.
Oka S, Leon J, Sakumi K, Ide T, Kang D, LaFerla FM, Nakabeppu Y. Human mitochondrial transcriptional factor A breaks the mitochondria-mediated vicious cycle in Alzheimer's disease. Sci Rep. 2016;6:37889. https://doi.org/10.1038/srep37889.
Oksanen M, Petersen AJ, Naumenko N, Puttonen K, Lehtonen Š, Gubert Olivé M, Shakirzyanova A, et al. PSEN1 Mutant iPSC-Derived Model Reveals Severe Astrocyte Pathology in Alzheimer's Disease. Stem Cell Reports. 2017;9(6):1885–97. https://doi.org/10.1016/j.stemcr.2017.10.016.
Ooi L, Sidhu K, Poljak A, Sutherland G, O'Connor MD, Sachdev P, Münch G. Induced pluripotent stem cells as tools for disease modelling and drug discovery in Alzheimer's disease. J Neural Transm (Vienna). 2013;120(1):103–11. https://doi.org/10.1007/s00702-012-0839-2 Epub 2012 Jun 13.
Ortiz-Virumbrales M, Moreno CL, Kruglikov I, Marazuela P, Sproul A, Jacob S, Zimmer M, et al. CRISPR/Cas9-Correctable mutation-related molecular and physiological phenotypes in iPSC-derived Alzheimer's PSEN2 N141I neurons. Acta Neuropathol Commun. 2017;5(1):77. https://doi.org/10.1186/s40478-017-0475-z.
Ovchinnikov DA, Korn O, Virshup I, Wells CA, Wolvetang EJ. The Impact of APP on Alzheimer-like Pathogenesis and Gene Expression in Down Syndrome iPSC-Derived Neurons. Stem Cell Reports. 2018;11(1):32–42. https://doi.org/10.1016/j.stemcr.2018.05.004.
Paquet D, Kwart D, Chen A, Sproul A, Jacob S, Teo S, Olsen KM, Gregg A, et al. Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature. 2016;533(7601):125–9. https://doi.org/10.1038/nature17664.
Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–86.
Pires C, Schmid B, Petræus C, Poon A, Nimsanor N, Nielsen TT, Waldemar G, Hjermind LE, et al. Generation of a gene-corrected isogenic control cell line from an Alzheimer's disease patient iPSC line carrying a A79V mutation in PSEN1. Stem Cell Res. 2016;17(2):285–8. https://doi.org/10.1016/j.scr.2016.08.002.
Poon A, Li T, Pires C, Nielsen TT, Nielsen JE, Holst B, Dinnyes A, Hyttel P, Freude KK. Derivation of induced pluripotent stem cells from a familial Alzheimer's disease patient carrying the L282F mutation in presenilin 1. Stem Cell Res. 2016;17(3):470–3. https://doi.org/10.1016/j.scr.2016.09.016.
Poon A, Schmid B, Pires C, Nielsen TT, Hjermind LE, Nielsen JE, Holst B, Hyttel P, Freude KK. Generation of a gene-corrected isogenic control hiPSC line derived from a familial Alzheimer's disease patient carrying a L150P mutation in presenilin 1. Stem Cell Res. 2016;17(3):466–9. https://doi.org/10.1016/j.scr.2016.09.018.
Portelius E, Durieu E, Bodin M, Cam M, Pannee J, Leuxe C, Mabondzo A, Oumata N, et al. Specific Triazine Herbicides Induce Amyloid-β42 Production. J Alzheimers Dis. 2016;54(4):1593–605.
Prakash A, Medhi B, Chopra K. Granulocyte colony stimulating factor (GCSF) improves memory and neurobehavior in an amyloid-β induced experimental model of Alzheimer's disease. Pharmacol Biochem Behav. 2013;110:46–57. https://doi.org/10.1016/j.pbb.2013.05.015.
Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165:1238–54.
Robbins JP, Price J. Human induced pluripotent stem cells as a research tool in Alzheimer's disease. Psychol Med. 2017;47(15):2587–92.
Rodríguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A. Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience. 2016;323:170–82.
Rose SE, Frankowski H, Knupp A, Berry BJ, Martinez R, Dinh SQ, Bruner LT, et al. Leptomeninges-Derived Induced pluripotent Stem Cells and Directly Converted Neurons From Autopsy Cases With Varying Neuropathologic Backgrounds. J Neuropathol Exp Neurol. 2018;77(5):353–60. https://doi.org/10.1093/jnen/nly013.
Saurat NG, Livesey FJ, Moore S. Cortical Differentiation of Human Pluripotent Cells for In Vitro Modeling of Alzheimer's Disease. Methods Mol Biol. 2016;1303:267–78. https://doi.org/10.1007/978-1-4939-2627-5_16.
Schröter F, Sleegers K, Cuyvers E, Bohndorf M, Wruck W, Van Broeckhoven C, Adjaye J. Lymphoblast-derived integration-free iPS cell line from a female 67-year-old Alzheimer's disease patient with TREM2 (R47H) missense mutation. Stem Cell Res. 2016;17(3):553–5. https://doi.org/10.1016/j.scr.2016.10.005.
Schröter F, Sleegers K, Cuyvers E, Bohndorf M, Wruck W, Van Broeckhoven C, Adjaye J. Lymphoblast-derived integration-free iPS cell line from a 65-year-old Alzheimer's disease patient expressing the TREM2 p.R47H variant. Stem Cell Res. 2016;16(1):113–5. https://doi.org/10.1016/j.scr.2015.12.017.
Schröter F, Sleegers K, Van Cauwenberghe C, Bohndorf M, Wruck W, Van Broeckhoven C, Adjaye J. Lymphoblast-derived integration-free iPSC lines from a female and male Alzheimer's disease patient expressing different copy numbers of a coding CNV in the Alzheimer risk gene CR1. Stem Cell Res. 2016;17(3):560–3. https://doi.org/10.1016/j.scr.2016.10.003.
Seibler P, Graziotto J, Jeong H, Simunovic F, Klein C, Krainc D. Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells. J Neurosci. 2011;31:5970–6.
Seo J, Kritskiy O, Watson LA, Barker SJ, Dey D, Raja WK, Lin YT, Ko T, Cho S, Penney J, et al. Inhibition of p25/Cdk5 Attenuates Tauopathy in Mouse and iPSC Models of Frontotemporal Dementia. J Neurosci. 2017;37(41):9917–24. https://doi.org/10.1523/JNEUROSCI.0621-17.2017.
Schwarz JM., Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J. Neurochem. 2012;120(6):948–963.
Shi Y, Kirwan P, Smith J, MacLean G, Orkin SH, Livesey FJ. A human stem cell model of early Alzheimer's disease pathology in Down syndrome. Sci Transl Med. 2012;4(124):124ra29. https://doi.org/10.1126/scitranslmed.3003771.
Siegert S, Cabuy E, Scherf BG, Kohler H, Panda S, Le YZ, Fehling HJ, Gaidatzis D, Stadler MB, Roska B. Transcriptional code and disease map for adult retinal cell types. Nat Neurosci. 2012; 15:487–95. S1–2. PMID: 22267162. https://doi.org/10.1038/nn.3032.
Sjöbeck M, Englund E. Alzheimer’s disease and the cerebellum: a morphologic study on neuronal and glial changes. Dement. Geriatr. Cogn. Disord. 2001;12(3):211–218. https://doi.org/10.1159/000051260
Sullivan SE, Young-Pearse TL.Induced pluripotent stem cells as a discovery tool for Alzheimer's disease. Brain Res. 2017;1656:98–106. https://doi.org/10.1016/j.brainres.2015.10.005. Epub 2015 Oct 13.
Sullivan SE, Young-Pearse TL. Induced pluripotent stem cells as a discovery tool for Alzheimer׳s disease. Brain Res. 2015;1656:98–106.
Tagami S, Yanagida K, Kodama TS, Takami M, Mizuta N, Oyama H, Nishitomi K, Chiu Y-w, Okamoto T, Ikeuchi T, Sakaguchi G, Kudo T, Matsuura Y, Fukumori A, Takeda M, Ihara Y, Okochi M. Semagacestat Is a Pseudo-Inhibitor of γ-Secretase. Cell Rep. 2017;21(1):259–73.
Tong G, Izquierdo P, Raashid RA. Human Induced Pluripotent Stem Cells and the Modelling of Alzheimer's Disease: The Human Brain Outside the Dish. Open Neurol J. 2017;11:27-38. Published 2017 Sep 30. https://doi.org/10.2174/1874205X01711010027.
Tong M, Dominguez C, Didsbury J, de la Monte SM. Targeting Alzheimer's Disease Neuro-Metabolic Dysfunction with a Small Molecule Nuclear Receptor Agonist (T3D-959) Reverses Disease Pathologies. J Alzheimers Dis Parkinsonism. 2016;6(3):238.
Selkoe DJ.Preventing Alzheimer's disease. Science. 2012;337(6101):1488–92.
Shirotani K, Matsuo K, Ohtsuki S, Masuda T, Asai M, Kutoku Y, Ohsawa Y, et al. A simplified and sensitive method to identify Alzheimer's diseasebiomarker candidates using patient-derived induced pluripotent stem cells (iPSCs). J Biochem. 2017;162(6):391–4. https://doi.org/10.1093/jb/mvx058.
Siegel G, Gerber H, Koch P, Bruestle O, Fraering PC, Rajendran L. The Alzheimer's Disease γ-Secretase Generates Higher 42:40 Ratios for β-Amyloid Than for p3 Peptides. Cell Rep. 2017;19(10):1967–76. https://doi.org/10.1016/j.celrep.2017.05.034.
Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, Hargus G, Blak A, Cooper O, Mitalipova M, et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–77.
Sproul AA, Jacob S, Pre D, Kim SH, Nestor MW, Navarro-Sobrino M, Santa-Maria I, et al. Characterization and molecular profiling of PSEN1 familial Alzheimer's disease iPSC-derived neural progenitors. PLoS One. 2014;9(1):e84547. https://doi.org/10.1371/journal.pone.0084547.
Sproul AA, Vensand LB, Dusenberry CR, Jacob S, Vonsattel JP, Paull DJ, Shelanski ML, Crary JF, Noggle SA. Generation of iPSC lines from archived non-cryoprotected biobanked dura mater. Acta Neuropathol Commun. 2014;2:4. https://doi.org/10.1186/2051-5960-2-4.
Stein JL, De La Torre-Ubieta L, Tian Y, et al. A quantitative framework to evaluate modeling of cortical development by neural stem cells. Neuron. 2014;83(1):69–86.
Streit WJ, Braak H, Xue QS, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta Neuropathol. 2009; 118:475–85. PMID: 19513731. https://doi.org/10.1007/s00401-009-0556-6
Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Takamatsu K, Ikeda T, Haruta M, Matsumura K, Ogi Y, Nakagata N, Uchino M, et al. Degradation of amyloid beta by human induced pluripotent stem cell-derived macrophages expressing Neprilysin-2. Stem Cell Res. 2014;13(3 Pt A):442–53. https://doi.org/10.1016/j.scr.2014.10.001.
Tubsuwan A, Pires C, Rasmussen MA, Schmid B, Nielsen JE, Hjermind LE, Hall V, Nielsen TT, et al. Generation of induced pluripotent stem cells (iPSCs) from an Alzheimer's disease patient carrying a L150P mutation in PSEN-1. Stem Cell Res. 2016;16(1):110–2. https://doi.org/10.1016/j.scr.2015.12.015.
Usenovic M, Niroomand S, Drolet RE, Yao L, Gaspar RC, Hatcher NG, Schachter J, et al. Internalized Tau Oligomers Cause Neurodegeneration by Inducing Accumulation of Pathogenic Tau in Human Neurons Derived from Induced pluripotent Stem Cells. J Neurosci. 2015;35(42):14234–50. https://doi.org/10.1523/JNEUROSCI.1523-15.2015.
Vazin T, Ball KA, Lu H, Park H, Ataeijannati Y, Head-Gordon T, Poo MM, Schaffer DV. Efficient derivation of cortical glutamatergic neurons from human pluripotent stem cells: a model system to study neurotoxicity in Alzheimer's disease. Neurobiol Dis. 2014;62:62–72. https://doi.org/10.1016/j.nbd.2013.09.005.
Wang Z, Zhang P, Wang Y, Shi C, Jing N, Sun H, Yang J, Liu Y, Wen X, et al. Establishment of induced pluripotent stem cell line (ZZUi010-A) from an Alzheimer's disease patient carrying an APP gene mutation. Stem Cell Res. 2017;25:213–6. https://doi.org/10.1016/j.scr.2017.10.025.
Weick JP. Functional properties of human stem cell-derived neurons in health and disease. Stem Cells Int. 2016;2016:4190438.
Woodruff G, Reyna SM, Dunlap M, Van Der Kant R, Callender JA, Young JE, Roberts EA, Goldstein LS. Defective Transcytosis of APP and Lipoproteins in Human iPSC-Derived Neurons with Familial Alzheimer's Disease Mutations. Cell Rep. 2016;17(3):759–73. https://doi.org/10.1016/j.celrep.2016.09.034.
Woodruff G, Young JE, Martinez FJ, Buen F, Gore A, Kinaga J, Li Z, Yuan SH, Zhang K, Goldstein LS. The presenilin-1 ΔE9 mutation results in reduced γ-secretase activity, but not total loss of PS1 function, in isogenic human stem cells. Cell Rep. 2013;5(4):974–85. https://doi.org/10.1016/j.celrep.2013.10.018.
Yagi T, Ito D, Okada Y, Akamatsu W, Nihei Y, Okano H, Suzuki N. Modeling familial Alzheimer's disease with induced pluripotent stem cells. Rinsho Shinkeigaku. 2012;52(11):1134–6.
Yagi T, Ito D, Okada Y, Akamatsu W, Nihei Y, Yoshizaki T, Yamanaka S, Okano H, Suzuki N. Modeling familial Alzheimer's disease with induced pluripotent stem cells. Hum Mol Genet. 2011;20(23):4530–9. https://doi.org/10.1093/hmg/ddr394.
Yagi T, Kosakai A, Ito D, Okada Y, Akamatsu W, Nihei Y, Nabetani A, Ishikawa F, Arai Y, Hirose N, Okano H, Suzuki N. Establishment of induced pluripotent stem cells from centenarians for neurodegenerative disease research. PLoS One. 2012;7(7):e41572. https://doi.org/10.1371/journal.pone.0041572.
Yahata N, Asai M, Kitaoka S, Takahashi K, Asaka I, Hioki H, Kaneko T, Maruyama K, Saido TC, et al. Anti-Aβ drug screening platform using human iPS cell-derived neurons for the treatment of Alzheimer's disease. PLoS One. 2011;6(9):e25788. https://doi.org/10.1371/journal.pone.0025788.
Yang Y, Zhang X, Yi L, et al. Naïve Induced Pluripotent Stem Cells Generated From β-Thalassemia Fibroblasts Allow Efficient Gene Correction With CRISPR/Cas9. Stem Cells Transl Med. 2016; 5(1): 8–19.
Yang J, Zhao H, Ma Y, Shi G, Song J, Tang Y, Li S, Li T, Liu N, Tang F, Gu J, et al. Early pathogenic event of Alzheimer's disease documented in iPSCs from patients with PSEN1 mutations. Oncotarget. 2017;8(5):7900–13. https://doi.org/10.18632/oncotarget.13776.
Young JE, Boulanger-Weill J, Williams DA, Woodruff G, Buen F, Revilla AC, et al. Elucidating molecular phenotypes caused by the SORL1 Alzheimer's disease genetic risk factor using human induced pluripotent stem cells. Cell Stem Cell. 2015;16(4):373–85. https://doi.org/10.1016/j.stem.2015.02.004.
Young JE, Goldstein LS. Alzheimer's disease in a dish: promises and challenges of human stem cell models. Hum Mol Genet. 2012;21(R1):R82-9. Epub 2012 Aug 2.
Zagni E, Simoni L, Colombo D. Sex and Gender Differences in Central Nervous System-Related Disorders. Neurosci J. 2016;2016:2827090. https://doi.org/10.1155/2016/2827090 Epub 2016 May 30.
Zhang D, Pekkanen-Mattila M, Shahsavani M, Falk A, Teixeira AI, Herland A. A 3D Alzheimer's disease culture model and the induction of P21-activated kinase mediated sensing in iPSC derived neurons. Biomaterials. 2014;35(5):1420–8. https://doi.org/10.1016/j.biomaterials.2013.11.028.
Zhang S, Lv Z, Zhang S, Liu L, Li Q, Gong W, Sha H, Wu H. Characterization of human induced pluripotent stem cell (iPSC) line from a 72year old male patient with later onset Alzheimer's disease. Stem Cell Res. 2017;19:34–6. https://doi.org/10.1016/j.scr.2016.12.024.
Zhang R, Zhang LH, Xie X. iPSCs and small molecules: a reciprocal effort towards better approaches for drug discovery. Acta Pharmacol Sin. 2013;34(6):765–76.
Zollo A, Allen Z, Rasmussen HF, Iannuzzi F, Shi Y, Larsen A, Maier TJ, Matrone C. Sortilin-Related Receptor Expression in Human Neural Stem CellsDerived from Alzheimer's Disease Patients Carrying the APOE Epsilon 4 Allele. Neural Plast. 2017;2017:1892612. https://doi.org/10.1155/2017/1892612.
Acknowledgements
We thank Pandurata Alimentos Ltda for financial support to the English editing of text. FM is the recipient postdoctoral grant from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (Capes).
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
FM and DRM drafted the manuscript and wrote the article. DCM and JCDC participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Majolo, F., Marinowic, D.R., Machado, D.C. et al. Important advances in Alzheimer’s disease from the use of induced pluripotent stem cells. J Biomed Sci 26, 15 (2019). https://doi.org/10.1186/s12929-019-0501-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12929-019-0501-5