Abstract
Objectives
The risk of intracranial aneurysms (IAs) development and rupture is significantly higher in patients with periodontitis (PD), suggesting an association between the two. However, the specific mechanisms of association between these two diseases have not been fully investigated.
Materials and methods
In this study, we downloaded IAs and PD data from the Gene Expression Omnibus. Differentially expressed genes (DEGs) were identified, and functional enrichment analysis was performed. The protein-protein interaction (PPI) network and weighted gene co-expression network analysis (WGCNA) was performed to identified key modules and key crosstalk genes. In addition, the immune cell landscape was assessed and the correlation of key crosstalk genes with each immune cell was calculated. Finally, transcription factors (TFs) regulating key crosstalk genes were explored.
Results
127 overlapping DEGs were identified and functional enrichment analysis highlighted the important role of immune reflection in the pathogenesis of IAs and PD. We identified ITGAX and COL4A2 as key crosstalk genes. In addition, the expression of multiple immune cells was significantly elevated in PDs and IAs compared to controls, and both key crosstalk genes were significantly negatively associated with Macrophages M2. Finally, GATA2 was identified as a potential key transcription factor (TF), which regulates two key crosstalk gene.
Conclusions
The present study identifies key crosstalk genes and TF in PD and IAs, providing new insights for further study of the co-pathogenesis of PD and IAs from an immune and inflammatory perspective. Also, this is the first study to report the above findings.
Similar content being viewed by others
Introduction
Intracranial aneurysms (IAs) are a common neurosurgical condition and a major cause of nontraumatic subarachnoid hemorrhage [1]. The prevalence in adults is 2–6%, and the pathology is characterized by local structural deterioration of the arterial wall with loss of the internal elastic layer and media rupture[2, 3]. IAs is an inflammatory disease with both genetic and environmental risk factors [4]. The former is mainly familial IAs, which are less common [5]; the vast majority of IAs are sporadic, and susceptibility factors include hypertension and smoking [6]. The consequences of a ruptured intracranial aneurysms can be catastrophic. A study showed that the risk of death at 5, 10 and 15 years after subarachnoid hemorrhage was 12.9%, 23.6% and 35.4%, respectively [7]. Therefore, research into the pathogenesis of intracranial aneurysms, especially the prevention of unruptured intracranial aneurysmsand improved detection of patients with unruptured intracranialaneurysms is essential to avoid this type of hemorrhagic stroke.
Periodontitis (PD) is a chronic inflammation of the periodontal tissue caused mainly by eriodontal pathogens, can lead to irreversible destruction of tooth-supporting tissues (gums, periodontal ligaments and alveolar bone) [8]with an overall prevalence of 45-50% [9]. Periodontal pathogens include Porphyromonas gingivalis (P. gingivalis), tannerella lengae and dense spirochetes [10]. A large number of periodontal pathogens are present in the deep periodontal pockets of patients with chronic periodontitis, some of which enter the bloodstream, leading to persistent bacteremia and the release of large amounts of inflammatory mediators that cause low-level systemic inflammation, factors that can affect and lead to organ disease away from the mouth [11]. There is now substantial evidence to support an independent association between severe periodontitis and several non-communicable diseases, including diabetes, cerebrovascular disease, and cancers of the digestive tract[12, 13, 14]. To date, more and more evidence suggests that an association between IAs and PD. First, IAs share a high degree of risk factors with PD, including smoking and obesity [15]. Second, multiple case-control studies of patients with IAs have shown that periodontitis is independently and positively associated with the risk of IAs and aneurysmal subarachnoid hemorrhage (aSAH)[16, 17]. For example, periodontitis (≥ 4 mm gingival pockets) and severe periodontitis (≥ 6 mm gingival pockets) were seen in 92% and 49% of patients with IAs, respectively, and were associated with IAs (OR 5.3, 95% CI 1.1–25.9, p < 0.000; OR 6.3, 95% CI 1.3–31.4, p < 0.001) [16]. Another longitudinal study of the relationship between unruptured intracranialaneurysms formation and periodontal disease showed that the presence of periodontal disease was significantly associated with an increased risk of unruptured intracranial aneurysms [18].
The current view is that IAs are the end result of high-flow blood flow exerting high shear forces and flow-driven inflammatory cell-mediated remodeling of the intracranial arterial wall [19]. Up to 50% of IAs have DNA of oral pathogens in the aneurysmal wall [20], such as P. gingivalis, the most important causative agent of periodontal infections [10].P. gingivalis is a gram-negative bacterium, which lurks in periodontal pockets (worsening the periodontal condition), but is also capable of increased risk of myocardial infarction. In a cross-sectional study of heart attacks, intracoronary thrombosis was found to be associated with P. gingivalis [21]. Animal study also have shown that P. gingivalis can break the blood-brain barrier in several ways, activating microglia to produce a chronic pro-inflammatory environment that is important for the development of Alzheimer’s disease [22].P. gingivalis can remain viable in human macrophages and dendritic cells and may propagate to the aneurysmal wall of IAs through macrophage infiltration, which is strongly associated with inflammatory remodeling of IAs [23]. In addition, exposure to periodontal pathogens and immune response secondary to dysfunction may cause an increased risk of IAs formation and rupture in patients with periodontal disease [24]. These findings suggest a strong association between IAs and PD, but the specific molecular mechanisms and pathological interactions are not fully understood.
In this study, we utilized publicly available datasets from the GEO database, including the test datasets GSE54083 [IAs] and GSE10334 [PD] and the validation datasets GSE75436 [IAs] and GSE16134 [PD]. We used a combination of differential analysis, weighted correlation network analysis (WGCNA) and immune infiltration analysis to confirm that ITGAX and COL4A2 genes play an important role in the common pathogenesis of IAs and PD. Thus, our study may provide new clues to the co-pathogenesis of IAs and PD.
Materials and methods
Data source
First we show the flow chart of this study (Fig. 1). The expression data of IAs and PDs were obtained from the GEO data base (https://www.ncbi.nlm.nih.gov/geo). The search strategy for this study included: (1) subject searches for “intracranial aneurysms” and “periodontitis”, respectively; (2) Study type option selection “Expression profiling by array”; (3) samples were obtained from Homo sapiens; (4) the dataset contained normal control group samples. The mRNA sequencing of the data set GSE54083 was based on GPL4133 Agilent-014850 Whole Human Genome Microarray 4 × 44 K G4112F (Feature Number version). The mRNA sequencing of GSE10334 samples was based on GPL570 [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0. The former includes 8 ruptured intracranial aneurysms samples, 5 unruptured aneurysm samples and 10 Superficial temporal artery samples, but the ruptured intracranial aneurysms samples will be excluded in this study. The latter contains 183 PD-affected gingival tissue samples and 64 unaffected gingival tissue samples.
Differentially expression analysis
Our preliminary analyses of the dataset were based on R software (version 4.2.1; https://cran.r-project.org). Before conducting data analysis, we performed data cleaning, which included normalization using the “normalizeBetweenArrays” function and log2 transformation. DEGs were identified by the"Limma” package [25]. The genes with |log2 fold change| > 0.5 and adjusted P-value < 0.05 were identified as DEGs. Genes that were both up-regulated or down-regulated in both sets of DEGs were defined as overlapping DEGs. Veen plots of overlapping DEGs were plotted with the help of the “ggvenn” package.
Enrichment analysis of overlapping DEGs
We used the “clusterProfiler” package to perform enrichment analysis of overlapping DEGs, including Gene Ontology Biological Process (GO_BP) enrichment analysis [26]. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis [27].Terms with FDR < 0.05 were considered to be significantly enriched.
Protein-protein interaction (PPI) network construction
We use the STRING database V11.5 (http://string-db.org) to build a PPI network with overlapping DEGs and set the “minimum required interaction score” parameter to 0.4 to hide the unconnected nodes. We obtained the interaction score of the overlapping DEGs, which was then imported the Cytoscape software V3.9.1 [28]for visualization. The MCODE plug in was used to filter clusters with high connectivity, thus dividing the PPI network into multiple clusters with default parameters. Finally, the genes in the clusters were analyzed for functional enrichment.
Weighted gene co-expression network analysis (WGCNA)
The WGCNA analysis can identify genes with high correlation, i.e., genes that are grouped into modules with similar functions, and can perform correlation analysis between the modules and phenotypic data to discover potential key genes. The top 5000 genes with the highest absolute median difference in expression in the test data set were screened for WGCNA analysis using the “WGCNA” package [29].First, a soft threshold was obtained using the pickSoftThreshold function. Then, a weighted adjacency matrix was constructed. Finally, the correlation between each module and the disease was calculated. The module with the highest correlation with the grouped traits was defined as the key module. The genes within the key module were correlated with the grouped traits.
Identification and validation of key crosstalk genes
First, we use the CytoHubba plugin of Cytoscape software to filter the hub genes in the PPI network [30]. The genes that were ranked in the top 20 using different algorithms [Maximal Clique Centrality (MCC); Maximum Neighborhood Component (MNC); Edge Percolated Component (EPC) ] were identified as PPI key genes [30]. Next, the PPI key genes were intersected with the key module genes of WGCNA, and these intersected genes were defined as key crosstalk genes. Subsequently, we compared the mRNA expression levels of key crosstalk genes between the case and control groups using the Mann-Whitney U test, and P < 0.05 was considered statistically significant, and visualized by “ggplot2”. Finally, we tested the diagnostic efficacy of key crosstalk genes in the test dataset using the receiver operating characteristic curves (ROCs) of the “pROC” package.
Validation of key crosstalk genes in an independent external dataset
When selecting an external validation dataset, we followed the same screening principles to minimize bias. We validated the mRNA expression levels of key crosstalk genes in independent external datasets GSE75436 and GSE16134 samples. GSE75436 included 15 normal superficial temporal artery samples, 15 intracranial aneurysms samples, and mRNA sequencing of the samples was based on GPL570 [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array. GSE16134 contains 241 PD-affected gingival tissue samples and 69 unaffected gingival tissue samples, mRNA sequencing of the samples is based on GPL570 [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array.
Immuno-infiltration analysis
First, immune cell expression levels in the test dataset were analyzed in the case and control groups using the “cibersort” algorithm [31]. We then calculated the Pearson correlation coefficient and P-value between the expression of key genes that cause cross-talk and the expression of immune cells., P < 0.05 was considered statistically significant and was visualized by “ggplot2”.
Identification of transcription factors (TFs) of key crosstalk genes
TFs of key crosstalk genes were predicted by NetworkAnalyst 3.0 (https://www.networkanalyst.ca) [32]. Subsequently, we compared the average expression levels of TFs in the test set and validation set samples using the Mann-Whitney U test. Finally, TFs that were typically upregulated in the case set were identified as potential key TFs in IAs and PDs.
Results
Identify overlapping DEGs of IAs and PDs
The “PCA” function was used to downscale the high-latitude data, and significant sample differences were found between the test and control groups in the two datasets (Fig. 2A-D). In the dataset GSE54083, the “Limma” R package identified 4333 DEGs, of which 2364 were up-regulated and 1969 were down-regulated (Fig. 2E). In the dataset GSE10334, the “Limma” R package identified 1353 DEGs, of which 813 were up-regulated and 540 were down-regulated (Fig. 2F). Subsequently, we identified 258 intersecting DEGs (Fig. 2G). Finally, the above genes were examined for trend checked and 127 overlapping DEGs were obtained (S1).
DEGs of test datasets GSE54083 and GSE10334. (A), (C), and (E) are PCA plots, heat maps, and volcano plots of GSE54083 DEG analysis, respectively. (B), (D), and (F) are PCA plots, heat maps, and volcano plots of GSE10334 DEG analysis, respectively. (G). DEGs of GSE54083 and GSE10334 are crossed to obtain 258 intersecting DEGs
Functional enrichment analysis of overlapping DEGs
The GO_BP analysis showed that the most significantly enriched terms were immune response-regulating signaling pathway, extracellular matrix organization, extracellular structure organization, external encapsulating structure organization, leukocyte migration, and activation of immune response (Fig. 3A). kEGG analysis showed that overlapping DEGs may be associated with Regulation of actin cytoskeleton, Axon guidance, Complement and coagulation cascades, and Leukocyte transendothelial migration (Fig. 3B). Thus, overlapping DEG functions are clearly associated with immune and inflammatory processes.
Functional enrichment analysis and PPI network construction for overlapping DEGs of the test dataset. (A) GP-BP enrichment analysis of overlapping DEGs. (B) KEGG enrichment analysis of overlapping DEGs. (C) PPI network constructed for overlapping DEGs. (D-E). PPI network obtained by the Mcode algorithm for cluster 1 and cluster 2. (F-G). GP-BP enrichment analysis and KEGG enrichment analysis of genes contained in cluster 1 and cluster 2. enrichment analysis and KEGG enrichment analysis
Building PPI networks with overlapping DEGs
Overlapping DEGs were entered into the STRING database and a PPI network with 81 nodes and 123 edges (PPI enrichment p-value < 4.33e-09) was constructed at a medium confidence level (0.4). The PPI network was visualized using Cytoscape software (Fig. 3C). Two clusters with high connectivity were identified by the Mcode plug-in (Fig. 3D, E). Cluster 1 contains 8 nodes and 17 edges; Cluster 2 contains 4 nodes and 6 edges. Subsequently, we performed GP-BP enrichment analysis and KEGG enrichment analysis on the genes contained in Cluster 1 and Cluster 2 (Fig. 3F, G). The functional enrichment of GO-BP for cluster 1 intronic genes mainly included lymphocyte differentiation, mononuclear cell differentiation, lymphocyte activation involved in immune response, leukocyte activation involved in immune response and T cell differentiation involved in immune response, etc. KEGG enrichment analysis for cluster 1 intronic gene mainly includes Fc epsilon RI signaling pathway, Viral protein interaction with cytokine and cytokine receptor and Sphingolipid signaling pathway, etc. The functional enrichment of GO-BP for cluster 2 intronic genes mainly included extracellular matrix organization, extracellular structure organization, external encapsulating structure organization, collagen fibril organization and collagen-activated signaling pathwa, etc. KEGG enrichment analysis for cluster 2 intronic genemainly included ECM-receptor interaction, AGE-RAGE signaling pathway in diabetic complications, Amoebiasis, Relaxin signaling pathway and Focal adhesion, etc.
Weighted gene co-expression network construction and key module screening
The top 5000 genes with the highest absolute median difference in expression in the test dataset were screened for WGCNA analysis. β = 16 and β = 18 (scale-free R2 = 0.85) were chosen as soft thresholds for IAs and PDs, respectively, to ensure scale-free networks (Fig. 4A and B). 13 modules were identified in GSE54083 and 8 modules in GSE10334 by WGCNA analysis (Fig. 4C and D). Finally, a heat map of module-trait relationships was generated based on Pearson correlation coefficients. The results showed that the green module was the most correlated with IAs (0.854, p = 5.2E-05) and contained 661 genes; the turquoise module was the most correlated with PD (0.783, p = 5E-11) and included 705 genes (Fig. 4E and F).
Weighted gene co-expression network analysis (WGCNA) of the test dataset. (A-B). Soft threshold selection for GSE54083 and GSE10334. (C-D). Clustering dendrogram of the top 5000 genes with the highest absolute median difference in expression between GSE54083 and GSE10334 based on differential measures. (E-F). Module-trait relationship between GSE54083 and GSE10334 in relation to the modules and traits. Different colors represent different modules and contain the corresponding correlations and p-values
Identification and validation of key crosstalk genes
We used the CytoHubba plugin to sort the top 20 genes under four algorithms (Degree, EPC, MCC and MNC). The Upset plot showed that ITGAX and COL4A2 were present in both the top 20 genes of the four algorithms and in the two key modules (Fig. 5A). Therefore, these two genes were identified as key crosstalk genes. In addition, the ITGAX and COL4A2 mRNA expression levels were significantly higher in the case group than in the control group in GSE54083 and GSE10334 (Fig. 5B, C). In addition, ITGAX and COL4A2 had good diagnostic ability for IAs and PD according to ROC curves. The AUC values of ITGAX in GSE54083 and GSE10334 were 1.000 and 0.857, respectively. And the AUC values of COL4A2in GSE54083 and GSE10334 were 1.000 and 0.848, respectively (Fig. 5D, E).
Identification and validation of key crosstalk genes. (A). ITGAX and COL4A2 were both present in the four algorithms and two WGCNA key modules. (B-C). ITGAX and COL4A2 expression levels were higher in the case group than in the control group in GSE54083 and GSE10334. (D-E). ITGAX and COL4A2 had good diagnostic capabilities in GSE54083 and GSE10334. (F-G). The expression levels of ITGAX and COL4A2 were higher in the GSE75436 and GSE16134 case groups than in the control group. (H-I). ITGAX and COL4A2 had good diagnostic capabilities in GSE75436 and GSE16134. (***, p < 0.0001)
Validation of key crosstalk genes in an independent external datasets
To increase the confidence level, we validated the expression of key crosstalk genes in two additional independent external datasets (GSE75436 and GSE16134). Consistent with the results of the test set, ITGAX and COL4A2 mRNA expression levels were elevated in the case set (Fig. 5F, G), while showing good diagnostic efficiency. The AUC values of ITGAX in GSE75436 and GSE16134 were 0.831 and 0.834, respectively. And the AUC values of COL4A2in GSE75436 and GSE16134 were 0.827 and 0.871, respectively (Fig. 5H, I).
Immuno-infiltration analysis
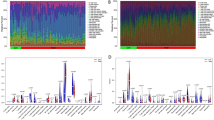
By performing enrichment analysis of overlapping DEGs, we found that immune and inflammatory processes are involved in crosstalk between IAs and PD. Therefore, we used the cibersort algorithm to analyze the proportion of immune cells in the case group versus control samples in both test datasets. We found that the immune landscape of IAs and PD was significantly altered in the case group (Fig. 6A and B). Furthermore, correlation analysis showed that ITGAX and COL4A2 expression levels were significantly negatively correlated with Macrophages M2 (Fig. 6C-F).
Immune infiltration analysis of the test dataset. (A-B). The immune landscape was significantly altered in the GSE54083 and GSE10334 case groups. (C-D). ITGAX and COL4A2 expression levels in GSE54083 were significantly negatively correlated with Macrophages M2. (D-F). ITGAX and COL4A2 expression levels in GSE10334 were significantly negatively correlated with Macrophages M2
Exploring key transcription factors (TFs) that regulate key crosstalk genes
Further, we explored potential TFs that may regulate ITGAX and COL4A2 genes using NetworkAnalyst 3.0 and compared the expression levels of case and control groups in all datasets by Mann-Whitney U test. We found that GATA2 interacted with both ITGAX and COL4A2 (Fig. 7A), and the expression level of GATA2 was significantly elevated in all case groups (Fig. 7B-E). Thus, GATA2 may be a potential key TF regulating two key crosstalk genes in the pathological process of IAs and PD.
GATA2 was identified as a potential key TF shared in IAs and PD. (A). NetworkAnalyst 3.0 suggests that GATA2 regulates both ITGAX and COL4A2. (B-C). GATA2 was significantly elevated in the test dataset (GSE54083 and GSE10334) case set. (D-E). GATA2 was significantly elevated in the external validation dataset (GSE75436 and GSE16134) case group (*, P < 0.01; **, P < 0.001; ***, P < 0.0001)
Discussion
Current studies suggest that periodontitis is not only associated with abdominal aortic aneurysm [33], coronary heart disease [34] and atherosclerosis cardiovascular disease risk [35], but also with the occurrence and rupture of intracranial aneurysms[16, 24].However, the specific mechanisms of the effect of periodontitis on intracranial aneurysms have not been well studied. In the present study, we focused on exploring the key crosstalk genes (ITGAX and COL4A2) shared by intracranial aneurysms and periodontitis as well as the transcription factor (GATA2) regulating key crosstalk genes by bioinformatics approach, which was fully validated in an external independent datasets. To our knowledge, this is the first study to report the above findings.
Through a comprehensive analysis of the datasets, ITGAX and COL4A2 were identified as key crosstalk genes in the comorbidity of IAs and PD. ITGAX, also known as CD11c, complement receptor 4 (CR4), is responsible for encoding integrin αX chain proteins, a marker shared by macrophages and dendritic cells [36].ITGAX can promotes monocyte adhesion and chemotaxis, regulates immune response, and plays an important role in the pathogenesis of infection and atherosclerosis [37]. Macrophages and the inflammatory signals they mediate play an important role in the pathogenesis of IAs[38, 39]. Meanwhile, macrophage levels were significantly higher in periodontitis samples than in healthy samples [40], they are central players in the destruction and repair phases of periodontal disease [41].COL4A2, which encodes the α2 chain of type IV collagen, is a major structural component of basement membranes (BMs) and plays a fundamental and crucial role in vessel wall integrity; Abnormal COL4A2 expression can lead to a broad phenotypic spectrum involving the nervous system, kidneys, and other organs, but the main site of vascular damage is the brain[42, 43]. Compared to familial intracranial aneurysms, abnormal COL4A2 expression in sporadic aneurysms may be associated with aneurysm development[44, 45]. Periodontitis is characterized by irreversible and progressive degeneration of periodontal tissue. Periodontal ligament stem cells (PDLSC) were involved in periodontal tissue regeneration, and COL4A2 in the tissue-specific extracellular matrix plays an important role in this process [46]. Therefore, two upregulated key crosstalk genes may be involved in IAs and PD comorbidity mechanisms.
Immune infiltration plays an important role in the co-pathogenesis of IAs and PD. Crosstalk gene enrichment analysis suggests that immune and inflammatory processes are involved in IAs and PD comorbidity mechanisms. Immune cell infiltration analysis revealed a significantly different immune landscape in IAs and PD disease groups compared to controls, and two key crosstalk genes expression was significantly negatively correlated with Macrophages M2. Several studies in both human and animal models have confirmed the presence of macrophage-dominated immune cell infiltration in IAs and PD tissues. Histopathological analysis of human IAs tissues revealed that the number of macrophage infiltration in ruptured IAs tissues was higher than that in unruptured IAs, suggesting that increased macrophage infiltration in tissues had already occurred prior to the rupture of IAs [47]. Another study in a rat model of IAs suggested that macrophage infiltration in the vessel wall occurs at the time of IAs formation, and that the degree of infiltration increases with the pathological progression of IAs [48]. Thus, macrophage-mediated cellular and molecular inflammation is a key factor in aneurysm formation and rupture [49].At the level of gene transcription, mRNA expression of IL-1β and TNF-α was increased in monocytes isolated from peripheral blood of patients with IAs compared to the healthy population, while IL-10 mRNA did not change significantly, indicating that monocytes showed preferential transcription to M1-type macrophages in patients with IAs [50]. It was also shown that balanced transfer of macrophages to M2 could prevent aneurysm formation and rupture [51]. Similarly, Macrophages play different roles through polarization in different stages of PD [52]. In the early or active phase of periodontitis, macrophage polarization phenotype as M1 type is mainly induced by Th1 production of IFN-γ and LPS-dominated microbial-associated factors [53], while the proportion of M1 is positively correlated with the progression of periodontal inflammatory activity; in the repair phase of PD, M2-associated factor expression, Th2 and Treg cell immunosuppression and repair effects increased [54, 55]. Thus, M1 polarization is a major feature of macrophages in periodontitis and is responsible for the development and progression of periodontal tissue destruction in periodontitis [56].
In summary, we hypothesize the following mechanisms of co-morbidity between IAs and PD. First, periodontal pathogens such as P. gingivalis proliferate in periodontal pockets and release large amounts of inflammatory mediators that recruit large numbers of monocytes and macrophages. P. gingivalis uses complex strategies to evade the major antimicrobial mechanisms of macrophages and inhibit macrophage M2 polarization [57, 58], leading to the onset and progression of periodontitis. In our study, ITGAX was highly expressed and inversely correlated with M2 macrophages. This implies that ITGAX is associated with inhibition of macrophage M2 polarization. Second, when these pathogen-carrying immune cells entered the cerebral arteries with the circulation, underwent transendothelial migration and clustered in the cerebral artery wall [59], matrix metalloproteinases were upregulated, mediated accelerated degradation of type IV collagen, and moreover induced de novo synthesis of new proteins and maintained tissue homeostasis of the basement membrane [60]. COL4A2 encodes the α2 chain of type IV collagen, a major structural component of almost all basement membranes (BMs) and plays a fundamental and critical role in vascular wall integrity. Abnormal COL4A2 expression can lead to a broad spectrum of phenotypes involving the nervous system, kidney, and other organs, but the major site of vascular injury is the brain [42, 43]. Genetic studies have shown that COL4A2 mutations can lead to congenital aneurysms [61]. In addition, COL4A2 is essential for aneurysm development according to a label-free quantitative proteomics study of human intracranial aneurysms [44]. Upregulation of COL4A2 expression may reflect the process of basement membrane remodeling after accelerated degradation of the basement membrane, which is also necessary for the growth of IAs. Therefore, we hypothesize that the immune process mediated by ITGAX and COL4A2 is central to the co-morbid mechanism of intracranial aneurysm and periodontitis.
For further investigate the candidate regulatory mechanisms of key crosstalk genes (ITGAX and COL4A2) shared by intracranial aneurysms and periodontitis, we constructed a target gene-TF network. Our study showed that the potential regulatory network of ITGAX and COL4A2 consists of 9 TFs, in which GATA2 interacts closely with both of these genes. Recent studies have shown that GATA2 may play an important role in regulating the immune response in the mechanism of IAs formation [62]. Meanwhile, phagocytic GATA2 overexpression drives atherosclerosis formation [63, 64]. These findings are consistent with the results of the present study. Currently, no studies have focused on the potential role of GATA2 in the pathogenesis of PD. According to the available data, GATA2 is a zinc-finger transcription factor that is mainly expressed in the hematopoietic system.GATA2 regulates various biological processes to directly or directly influence the progression of atherosclerosis, including aortic neovascularization, hematopoiesis, adipogenesis, and inflammation [65, 66]. For example, it improves monocyte adhesion and promotes leakage through the arterial wall by upregulating VCAM-1 [66].
This study identifies key crosstalk genes and TFs in IAs and PD from an immune and inflammatory perspective, thus providing new insights into the mechanisms of comorbidity in IAs and PD. It is important to note that the conclusion needs more clinical validation in the future. In addition, the specific functions of the crosstalk genes remain to be validated in vivo and in vitro.
Conclusion
In summary, we identified ITGAX and COL4A2 as key crosstalk genes in IAs and PD by multiple bioinformatics analysis methods, and they may be involved in crosstalk between IAs and PD through immune pathways. In addition, GATA2 was identified as a potential key TF in IAs and PD. To our knowledge, this is the first study to report the above findings. The present study provides new insights into the co-pathogenesis of IAs and PD. This will provide important guidance for the treatment of intracranial aneurysms, particularly the pharmacological treatment and prevention of unruptured aneurysms.
Data availability
The datasets generated and analysed during the current study are available in the NCBI Gene Expression Omnibus (GEO) repository, Accession Number GSE54083, GSE10334, GSE75463 and GSE16134.
Abbreviations
- IAs:
-
Intracranial aneurysms
- PD:
-
Periodontitis
- GEO:
-
Gene Expression Omnibus
- DEG:
-
Differentially expressed gene
- PPI:
-
Protein-protein Interaction
- WGCNA:
-
Weighted Gene Co-expression Network Analysis
- GO_BP:
-
Gene Ontology Biological Process
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- ROCs:
-
Receiver operating characteristic curves
References
Alshekhlee A, Mehta S, Edgell RC, Vora N, Feen E, Mohammadi A, Kale SP. Cruz-Flores, Hospital mortality and complications of electively clipped or coiled unruptured intracranial aneurysm. Stroke. 2010;41:1471–6.
van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–18.
Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10:626–36.
Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke. 2013;44:3613–22.
Bor AS, Rinkel GJ, Adami J, Koffijberg H, Ekbom A, Buskens E, Blomqvist P, Granath F. Risk of subarachnoid haemorrhage according to number of affected relatives: a population based case-control study. Brain. 2008;131:2662–5.
Karhunen V, Bakker MK, Ruigrok YM, Gill D, Larsson SC. Modifiable risk factors for intracranial aneurysm and Aneurysmal Subarachnoid Hemorrhage: a mendelian randomization study. J Am Heart Assoc. 2021;10:e022277.
Sharma M, Brown B, Madhugiri V, Cuellar-Saenz H, Sonig A, Ambekar S, Nanda A. Unruptured intracranial aneurysms: comparison of perioperative complications, discharge disposition, outcome, and effect of calcification, between clipping and coiling: a single institution experience. Neurol India. 2013;61:270–6.
Papapanou P.N., Sanz M, Buduneli N, Dietrich T, Feres M, Fine D H., Flemmig T.F., Garcia R, Giannobile W.V., Graziani F., Greenwell H., Herrera D., Kao R.T., Kebschull M., Kinane D.F., Kirkwood K.L., Kocher T., Kornman K.S., Kumar P.S., Loos B.G., Machtei E., Meng H., Mombelli A., Needleman I., Offenbacher S., Seymour G.J., Teles R., Tonetti M.S. Periodontitis: Consensus report of workgroup 2 of the 2017 World workshop on the classification of Periodontal and Peri-implant diseases and conditions. J Periodontol. 2018;89 Suppl 1:S173–82.
Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res. 2014;93:1045–53.
Mysak J, Podzimek S, Sommerova P, Lyuya-Mi Y, Bartova J, Janatova T, Prochazkova J, Duskova J. Porphyromonas gingivalis: major periodontopathic pathogen overview. J Immunol Res. 2014;2014:476068.
Ding F, Lyu Y, Han X, Zhang H, Liu D, Hei W, Liu Y. Detection of periodontal pathogens in the patients with aortic aneurysm. Chin Med J (Engl). 2014;127:4114–8.
Nwizu N, Wactawski-Wende J, Genco RJ. Periodontal disease and cancer: epidemiologic studies and possible mechanisms. Periodontol 2000. 2020;83:213–33.
Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, Herrera D, Jepsen S, Lione L, Madianos P, Mathur M, Montanya E, Shapira L, Tonetti M, Vegh D. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. 2018;45:138–49.
Chen YL, Bai L, Dilimulati D, Shao S, Qiu C, Liu T, Xu S, Bai XB, Du LJ, Zhou LJ, Lin WZ, Meng XQ, Jin YC, Liu Y, Zhang XH, Duan SZ, Jia F. Periodontitis Salivary Microbiota aggravates ischemic stroke through IL-17A. Front Neurosci. 2022;16:876582.
Hallikainen J, Keranen S, Savolainen J, Narhi M, Suominen AL, Ylostalo P, Kellokoski J, Pyysalo M, Pussinen P, Rauramaa T, Frosen J. Role of oral pathogens in the pathogenesis of intracranial aneurysm: review of existing evidence and potential mechanisms. Neurosurg Rev. 2021;44:239–47.
Hallikainen J, Lindgren A, Savolainen J, Selander T, Jula A, Narhi M, Koivisto T, Kellokoski J, Ylostalo P, Suominen AL, Frosen J. Periodontitis and gingival bleeding associate with intracranial aneurysms and risk of aneurysmal subarachnoid hemorrhage. Neurosurg Rev. 2020;43:669–79.
Hallikainen J, Pessi T, Vehkalahti M, Suominen AL, Pyysalo M, Frosen J. Unlike severe periodontitis, caries does not associate with intracranial aneurysms or aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien). 2023;165:169–75.
Woo HG, Chang YK, Lee JS, Song TJ. Association of Periodontal Disease with the occurrence of Unruptured cerebral aneurysm among adults in Korea: a Nationwide Population-based Cohort Study TRANSLATE with x EnglishArabicHebrewPolishBulgarianHindiPortugueseCatalanHmong DawRomanianChinese SimplifiedHungarianRussianChinese TraditionalIndonesianSlovakCzechItalianSlovenianDanishJapaneseSpanishDutchKlingonSwedishEnglishKoreanThaiEstonianLatvianTurkishFinnishLithuanianUkrainianFrenchMalayUrduGermanMalteseVietnameseGreekNorwegianWelshHaitian CreolePersian// TRANSLATE with COPY THE URL BELOW back EMBED THE SNIPPET BELOW IN YOUR SITE enable collaborative features and customize widget: Bing Webmaster PortalBack. Med (Kaunas) 57 (2021).
Leite FRM, Nascimento GG, Scheutz F, Lopez R. Effect of smoking on Periodontitis: a systematic review and Meta-regression. Am J Prev Med. 2018;54:831–41.
Beukers NG, van der Heijden GJ, van Wijk AJ, Loos BG. Periodontitis is an independent risk indicator for atherosclerotic cardiovascular diseases among 60 174 participants in a large dental school in the Netherlands. J Epidemiol Community Health. 2017;71:37–42.
Joshi C, Bapat R, Anderson W, Dawson D, Hijazi K, Cherukara G. Detection of periodontal microorganisms in coronary atheromatous plaque specimens of myocardial infarction patients: a systematic review and meta-analysis. Trends Cardiovasc Med. 2021;31:69–82.
Muller Herde A, Schibli R, Weber M, Ametamey SM. Metabotropic glutamate receptor subtype 5 is altered in LPS-induced murine neuroinflammation model and in the brains of AD and ALS patients. Eur J Nucl Med Mol Imaging. 2019;46:407–20.
Hu S, Yu N, Li Y, Hao Z, Liu Z, Li MH. A Meta-analysis of risk factors for the formation of de novo intracranial aneurysms. Neurosurgery. 2019;85:454–65.
Liu K, Sun J, Shao L, He H, Liu Q, Li Y, Ge H. Correlation of periodontal diseases with intracranial aneurysm formation: novel predictive indicators. Chin Neurosurg J. 2021;7:31.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W. Smyth, limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–9.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504.
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559.
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8(4):S11.
Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–7.
Zhou G, Soufan O, Ewald J, Hancock REW, Basu N, Xia J. NetworkAnalyst 3.0: a visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019;47:W234–41.
Salhi L, Sakalihasan N, Okroglic AG, Labropoulos N, Seidel L, Albert A, Teughels W, Defraigne JO, Lambert F. Further evidence on the relationship between abdominal aortic aneurysm and periodontitis: a cross-sectional study. J Periodontol. 2020;91:1453–64.
Nocini R, Favaloro EJ, Sanchis-Gomar F, Lippi G. Periodontitis, coronary heart disease and myocardial infarction: treat one, benefit all. Blood Coagul Fibrinolysis. 2020;31:339–45.
Kebschull M, Demmer RT, Papapanou PN. Gum bug, leave my heart alone!--epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dent Res. 2010;89:879–902.
Liu P, Yu YR, Spencer JA, Johnson AE, Vallanat CT, Fong AM, Patterson C, Patel DD. CX3CR1 deficiency impairs dendritic cell accumulation in arterial intima and reduces atherosclerotic burden. Arterioscler Thromb Vasc Biol. 2008;28:243–50.
Cole JE, Park I, Ahern DJ, Kassiteridi C, Danso Abeam D, Goddard ME, Green P, Maffia P, Monaco C. Immune cell census in murine atherosclerosis: cytometry by time of flight illuminates vascular myeloid cell diversity. Cardiovasc Res. 2018;114:1360–71.
Shimizu K, Kushamae M, Mizutani T, Aoki T. Intracranial aneurysm as a macrophage-mediated inflammatory disease. Neurol Med Chir (Tokyo). 2019;59:126–32.
Stratilova MH, Koblizek M, Steklacova A, Benes V, Sames M, Hejcl A, Zamecnik J. Increased macrophage M2/M1 ratio is associated with intracranial aneurysm rupture. Acta Neurochir (Wien). 2023;165:177–86.
Garaicoa-Pazmino C, Fretwurst T, Squarize CH, Berglundh T, Giannobile WV, Larsson L, Castilho RM. Characterization of macrophage polarization in periodontal disease. J Clin Periodontol. 2019;46:830–9.
Zhou LN, Bi CS, Gao LN, An Y, Chen F, Chen FM. Macrophage polarization in human gingival tissue in response to periodontal disease. Oral Dis. 2019;25:265–73.
Volonghi I, Pezzini A, Del Zotto E, Giossi A, Costa P, Ferrari D, Padovani A. Role of COL4A1 in basement-membrane integrity and cerebral small-vessel disease. The COL4A1 stroke syndrome. Curr Med Chem. 2010;17:1317–24.
Meuwissen ME, Halley DJ, Smit LS, Lequin MH, Cobben JM, de Coo R, van Harssel J, Sallevelt S, Woldringh G, van der Knaap MS, de Vries LS, Mancini GM. The expanding phenotype of COL4A1 and COL4A2 mutations: clinical data on 13 newly identified families and a review of the literature. Genet Med. 2015;17:843–53.
Liu Y, Song Y, Liu P, Li S, Shi Y, Yu G, Quan K, Fan Z, Li P, An Q, Zhu W. Comparative bioinformatics analysis between proteomes of rabbit aneurysm model and human intracranial aneurysm with label-free quantitative proteomics. CNS Neurosci Ther. 2021;27:101–12.
Mohan D, Munteanu V, Coman T, Ciurea AV. Genetic factors involves in intracranial aneurysms–actualities. J Med Life. 2015;8:336–41.
Wen Y, Yang H, Wu J, Wang A, Chen X, Hu S, Zhang Y, Bai D, Jin Z. COL4A2 in the tissue-specific extracellular matrix plays important role on osteogenic differentiation of periodontal ligament stem cells. Theranostics. 2019;9:4265–86.
Frosen J, Piippo A, Paetau A, Kangasniemi M, Niemela M, Hernesniemi J, Jaaskelainen J. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004;35:2287–93.
Aoki T, Kataoka H, Morimoto M, Nozaki K, Hashimoto N. Macrophage-derived matrix metalloproteinase-2 and– 9 promote the progression of cerebral aneurysms in rats. Stroke. 2007;38:162–9.
Muhammad S, Chaudhry SR, Dobreva G, Lawton MT, Niemela M, Hanggi D. Vascular macrophages as therapeutic targets to treat intracranial aneurysms. Front Immunol. 2021;12:630381.
Zhang HF, Zhao MG, Liang GB, Yu CY, He W, Li ZQ, Gao X. Dysregulation of CD4(+) T cell subsets in intracranial aneurysm. DNA Cell Biol. 2016;35:96–103.
Nowicki KW, Hosaka K, Walch FJ, Scott EW, Hoh BL. M1 macrophages are required for murine cerebral aneurysm formation. J Neurointerv Surg. 2018;10:93–7.
Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15:123–47.
Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44.
Yu T, Zhao L, Huang X, Ma C, Wang Y, Zhang J, Xuan D. Enhanced activity of the macrophage M1/M2 phenotypes and phenotypic switch to M1 in Periodontal infection. J Periodontol. 2016;87:1092–102.
Hussain QA, McKay IJ, Gonzales-Marin C, Allaker RP. Detection of adrenomedullin and nitric oxide in different forms of periodontal disease. J Periodontal Res. 2016;51:16–25.
Almubarak A, Tanagala KKK, Papapanou PN, Lalla E. Momen-Heravi, disruption of Monocyte and Macrophage Homeostasis in Periodontitis. Front Immunol. 2020;11:330.
Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16:745–59.
Li C, Yin W, Yu N, Zhang D, Zhao H, Liu J, Liu J, Pan Y, Lin L. miR-155 promotes macrophage pyroptosis induced by Porphyromonas gingivalis through regulating the NLRP3 inflammasome. Oral Dis. 2019;25:2030–9.
Yamamoto R, Aoki T, Koseki H, Fukuda M, Hirose J, Tsuji K, Takizawa K, Nakamura S, Miyata H, Hamakawa N, Kasuya H, Nozaki K, Hirayama Y, Aramori I, Narumiya S. A sphingosine-1-phosphate receptor type 1 agonist, ASP4058, suppresses intracranial aneurysm through promoting endothelial integrity and blocking macrophage transmigration. Br J Pharmacol. 2017;174:2085–101.
Chistiakov DA, Sobenin IA, Orekhov AN. Vascular extracellular matrix in atherosclerosis. Cardiol Rev. 2013;21:270–88.
Gunda B, Mine M, Kovacs T, Hornyak C, Bereczki D, Varallyay G, Rudas G, Audrezet MP, Tournier-Lasserve E. COL4A2 mutation causing adult onset recurrent intracerebral hemorrhage and leukoencephalopathy. J Neurol. 2014;261:500–3.
Li S, Xiao J, Yu Z, Li J, Shang H, Zhang L. Integrated analysis of C3AR1 and CD163 associated with immune infiltration in intracranial aneurysms pathogenesis. Heliyon. 2023;9:e14470.
Yin C, Vrieze AM, Rosoga M, Akingbasote J, Pawlak EN, Jacob RA, Hu J, Sharma N, Dikeakos JD, Barra L, Nagpal AD, Heit B. Efferocytic defects in early atherosclerosis are driven by GATA2 overexpression in macrophages. Front Immunol. 2020;11:594136.
Luo S, Wang F, Chen S, Chen A, Wang Z, Gao X, Kong X, Zuo G, Zhou W, Gu Y, Ge Z, Zhang J. NRP2 promotes atherosclerosis by upregulating PARP1 expression and enhancing low shear stress-induced endothelial cell apoptosis. FASEB J. 2022;36:e22079.
Izadpanah P, Khabbzi E, Erfanian S, Jafaripour S, Shojaie M. Case-control study on the association between the GATA2 gene and premature myocardial infarction in the Iranian population. Herz. 2021;46:71–5.
Luo C, Tang B, Qin S, Yuan C, Du Y, Yang J. GATA2 regulates the CAD susceptibility gene ADTRP rs6903956 through preferential interaction with the G allele. Mol Genet Genomics. 2021;296:799–808.
Acknowledgements
Not applicable.
Funding
This article did not receive sponsorship for publication.
Author information
Authors and Affiliations
Contributions
Jian-huang Huang analyzed the data and wrote this manuscript. Yao Chen, Yuan-bao Kang and Jian-hua Song assisted in analyzing the data and revising the manuscript. Yao Chen, Zheng-jian Yao critically read and edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, Y., Huang, Jh., Kang, Yb. et al. Bioinformatics analysis revealed the potential crosstalk genes and molecular mechanisms between intracranial aneurysms and periodontitis. BMC Med Genomics 17, 114 (2024). https://doi.org/10.1186/s12920-024-01864-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-024-01864-0