Abstract
Purpose
We evaluated the value of copy number variation sequencing (CNV-seq) and quantitative fluorescence (QF)-PCR for analyzing chromosomal abnormalities (CA) in spontaneous abortion specimens.
Methods
A total of 650 products of conception (POCs) were collected from spontaneous abortion between April 2018 and May 2020. CNV-seq and QF-PCR were performed to determine the characteristics and frequencies of copy number variants (CNVs) with clinical significance. The clinical features of the patients were recorded.
Results
Clinically significant chromosomal abnormalities were identified in 355 (54.6%) POCs, of which 217 (33.4%) were autosomal trisomies, 42(6.5%) were chromosomal monosomies and 40 (6.2%) were pathogenic CNVs (pCNVs). Chromosomal trisomy occurs mainly on chromosomes 15, 16, 18, 21and 22. Monosomy X was not associated with the maternal or gestational age. The frequency of chromosomal abnormalities in miscarriages from women with a normal live birth history was 55.3%; it was 54.4% from women without a normal live birth history (P > 0.05). There were no significant differences among women without, with 1, and with ≥ 2 previous miscarriages regarding the rate of chromosomal abnormalities (P > 0.05); CNVs were less frequently detected in women with advanced maternal age than in women aged ≤ 29 and 30–34 years (P < 0.05).
Conclusion
Chromosomal abnormalities are the most common cause of pregnancy loss, and maternal and gestational ages are strongly associated with fetal autosomal trisomy aberrations. Embryo chromosomal examination is recommended regardless of the gestational age, modes of conception or previous abortion status.
Similar content being viewed by others
Background
Conventional G-banding karyotype analysis is widely used for the genetic analysis of miscarriage samples. However, this method is limited by low resolution, culture failure, poor chromosome morphology, long turnaround time, maternal cell contamination (MCC), and submicroscopic chromosomal variations that are not visible, all of which may lead to false negative results. Other methods such as fluorescence in situ hybridization and multiplex ligation-dependent probe amplification have also been used to identify the genetic causes of miscarriage [1,2,3]. However, none of these methods detect chromosomal abnormalities at the whole-genome level. Chromosomal microarray analysis (CMA) is a powerful technology for genetic diagnosis that can detect aneuploidy, submicroscopic chromosomal variations and so on at the genome-wide level. Nonetheless, a major shortcoming of CMA is its high cost, which restricts its use as a routine detection method for spontaneous abortions [4, 5].
Low-coverage (or low-pass) whole-genome next-generation sequencing (NGS) is a low-cost technique with a short turnaround time, unprecedented resolution, reliable high-throughput, and minimal DNA requirements. It has been widely used in clinics [6]. Compared to CMA, NGS has significant advantages in terms of quality, speed, and affordability [7,8,9]. Copy number variation sequencing (CNV-seq), an NGS-based method, has been used in most pediatric and prenatal diagnostic applications as a viable alternative to CMA because of its ability to simultaneously detect aneuploidies and submicroscopic chromosomal imbalances [9,10,11]. However, CNV-seq fails to detect MCC and polyploidy, limiting its application in abortion detection. Quantitative fluorescence polymerase chain reaction (QF-PCR) is a rapid chromosomal detection method commonly used in clinical settings. It can identify MCC, some euploidies such as triploid or tetraploid, and some common aneuploidies by amplification of selected short tandem repeat (STRs) sites and quantitatively analyzing allelic dosage ratios to evaluate the number of copies of specific chromosomes [12]. Therefore, we speculated that the combination of CNV-seq and QF-PCR would be a reliable approach for chromosome detection in POCs, as confirmed prenatally [11, 13].
Miscarriage is the spontaneous loss of a pregnancy at less than 28 weeks, or the spontaneous loss of the fetus with a weight less than 1000 g. When miscarriage occurs before 13 gestational weeks, it is called first- trimester miscarriage or early abortion; when it occurs from 13 to 28 gestational weeks it is called second-trimester miscarriage or late abortion [14]. Stillbirth is the death of a fetus in the uterus after 20 weeks of gestation [15]. The incidence of miscarriage is approximately 15–20%, with 25% of females experiencing at least one spontaneous abortion [16, 17]. Studies have shown that genetic factors play an important role in miscarriage, with 50% of cases caused by chromosomal abnormalities [18]. On the other hand, the risk factors for stillbirth (≥ 28 gestational weeks) are mainly immune and environmental factors [19]. Researchers have found that fetal chromosomal aneuploidy is the primary cause of miscarriage [20], with aneuploidy of chromosomes 13, 16, 18, 21, 22 and sex chromosomes being ubiquitous [21, 22]. Previous studies have focused on populations with specific clinical factors such as early or recurrent spontaneous abortion, and there have been few cross-sectional comparative studies of populations with these different factors. In this study, we aimed to evaluate the combined application of CNV-seq and QF-PCR as a tool for the identification of chromosomal abnormalities. We investigate the frequency and type of chromosomal aberrations in the POCs of participants under different clinical conditions to provide evidence for clinical advice and genetic counseling.
Materials and methods
A total of 650 fetal specimens, including 597 chorionic villi and 53 fetal muscle tissues, were obtained from female participants who had undergone spontaneous abortion between April 2018 and December 2020. The mean age of the patients was 31.29 years old (19–46 years), and the mean gestational age was 9.1 weeks (5–25 weeks). Clinical information including early miscarriage history, normal live birth history, and mode of conception was recorded. Maternal age was classified into the following four groups: ≤ 29, 30–34, 35–39, and ≥ 40 years. The number of previous early miscarriages was classified into four groups: 0, 1, 2 and ≥ 3. The normal live birth history was categorized as “0” and “≥ 1” groups. The modes of conception were categorized as assisted and natural concepts.
This study was approved by the Protection of Human Ethics Committee of the Fujian Provincial Maternity and Children’s Hospital, which is affiliated with the Hospital of Fujian Medical University. Written informed consent was obtained from the individual or guardian participants.
Copy number variation sequencing
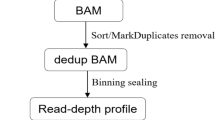
CNV-seq was carried out in accordance with the manufacturer’s instructions. In brief, total genomic DNA was extracted from tissue samples using the Amp Genomic DNA Kit (TIANGEN Biotech, Beijing, China). After shearing the genomic DNA to an average size of 200 bp, 2.5 ng of the fragmented DNA was used to create the sequencing library. 8-bp bar-coded sequencing adaptors were legated to the DNA fragments, and PCR was performed to amplify the ligation products. The generated libraries were then pooled and sequenced on a NextSeq CN 500 high-throughput platform at approximately 1× depth after purification of the PCR product using magnetic beads. For each sample, 8–10 million of 35-bp single-end raw reads were produced. Short reads were aligned to the human reference genome (hg19) using the BWA aligner after sequencing quality control and trimming. Each reference chromosome was divided equally by a 100-kb window and the number of uniquely mapped reads in each window of each chromosome was counted. The LOWESS model was used to adjust the GC-bias of per window read counts. The corrected read counts were contrasted with an internal reference database created from a collection of 100 samples with a normal karyotype that was verified using G-banded karyotype analysis. A full description of the algorithms employed for the bioinformatics analysis was detailed in the previous literature [23]. Mosaicism was reported when the detection threshold of 10% was exceeded, CNVs detected by the platform had an effective minimum resolution of 100 kb.
QF-PCR
Maternal peripheral blood samples were obtained via QF-PCR. DNA was extracted from maternal blood and POCs using a QIAGEN kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. Multiple QF-PCRs were performed using a Chromosome (13/18/21/X/Y) multiplex STR Genotyping Kit (Guangzhou Darui Biotechnology Co., Ltd.) containing 20 STR markers (14 STR markers on autosomes 13, 18, and 21, four on chromosome X-linked markers, one on amelogenin, and SRY on chromosome Y). PCR products were separated on an ABI 3500 (Applied Biosystems, Foster City, CA, USA) capillary genetic analyzer and the results were analyzed using ABI Genemapper 6.0. The informative markers present in the POC DNA samples were compared with those in the maternal DNA samples to estimate the presence of maternal cell contamination.
Evaluation of CNVs
Databases (ISCA, DGV, Decipher, Ensemble, OMIM, ClinGen, UCSC and PubMed) were used to analyze the suspected pathogenic regions. Pathogenicity of CNVs was evaluated according to the American College of Medical Genetics (ACMG) guidelines [24, 25]. CNVs were classified into three major categories: pathogenic, variants of uncertain significance (VOUS), and benign. Only pathogenic CNVs and VOUS were reported in this study.
Statistical analysis
SPSS software (version 22.0) was used for the data analysis. Quantitative data were expressed as mean ± standard deviation (X ± S), and comparisons between groups were performed using the t-test. Qualitative data were represented as the number of cases (percentage), and comparisons between groups were performed using the paired chi-square test. Logistic regression analysis was used to analyze the factors related to chromosomal abnormalities. Differences were considered statistically significant at P < 0.05.
Results
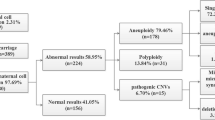
CNV-seq and QF-PCR were used to analyze 650 samples of aborted tissues collected during early and middle pregnancy. The success rate of all the tests was 100%. The rate of chromosomal abnormalities was 54.6% (355/650), of which 37.1% (241/650) were single aneuploidies, 2.8% (18/650) were multiple aneuploidies, 5.2% (34/650) were polyploidy, 3.5% (23/650) were mosaic aneuploidies, and 6.2% (40/650) were pathogenic copy number variations (pCNVs). VOUS were identified in 60 cases (9.2%), and normal results were identified in 235 cases (36.2%). Most aneuploidies were autosomal trisomies (217/650, 33.4%), whereas the others were monosomies found on chromosomes X (39/241, 16.2%) and 21 (3/241, 1.2%) (Table 1).
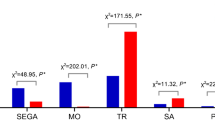
Different distributions of chromosomal abnormalities were detected between first- and second-trimester abortions. In the first trimester of pregnancy loss, all chromosomes were involved in trisomies (except for chromosome 1), with T16 being the most common finding, followed by T22, T21, T18, and T15. Monosomy X was the most frequently encountered sex chromosome abnormality, with an incidence of 6.0% (Fig. 1). Seventeen abnormalities occurred in second-trimester miscarriages, and these abnormalities mainly involved T18, T21, 45, X and pCNVs. The most frequent karyotype was trisomy 18 (29.4%, 5/17), followed by monosomy X (23.5%, 4/17), trisomy 21 (23.5%, 4/17), and pCNVs (17.6%, 3/17) (Fig. 2).
Associations between chromosomal abnormalities and gestational age, maternal age, previous miscarriages, live birth history, and mode of conception are shown in Table 2. The rate of chromosomal abnormalities in the first-trimester pregnancy loss (56.6%) was significantly higher than that in the second-trimester pregnancy loss (32.1%) (P < 0.05). Autosomal trisomy was less common in second-trimester pregnancy loss than in first-trimester pregnancy loss (P < 0.05), but no statistical difference was found in the frequency of 45, X (Table 3). Similar incidences of chromosomal abnormalities were found among women aged ≤ 29, 30–34, and 35–39 years (P > 0.05), and were all significantly lower than those in women ≥ 40 years (P < 0.05). The incidence of autosomal trisomy also increased with maternal age (P < 0.05). The frequency of 45, X decreased with maternal age; however, the difference was not statistically significant (P > 0.05). The frequency of chromosomal abnormalities in miscarriages from women with a normal live birth history was 55.3%, it was 54.4% from women without a normal live birth history (P > 0.05), indicating that there was no significant difference in the frequency of CA between women with and without a normal live birth history. There were no significant differences in the rate of chromosomal abnormalities among miscarriages from women without, with 1, and with ≥ 2 previous miscarriages (P > 0.05). No significant differences were observed between miscarriages from women with different modes of conception (P > 0.05).
To identify significant CNVs related to miscarriage, cases with numerical chromosomal abnormalities were excluded from CNV analysis. As a result, a total of 60 pCNVs in 40 cases were subjected to further analysis, including 29 with duplications in 28cases and 31 with deletions in 29cases. The pCNVs of deletions and duplications ranged in size from 450 Kb–35.6 Mb and 0.38 Mb–217.86 Mb, respectively. The distribution of all detected pCNVs on all chromosomes was shown in Table 4. Deletions occurred mostly on chromosome 4, followed by chromosomes 8 and X. Duplications occurred mostly on chromosome 16. CNVs were less frequently detected in women with advanced maternal age than in women aged ≤ 29 years and 30–34 years (P < 0.05). However, no statistically significant differences were found in the frequency of CNVs at different gestational ages (P > 0.05). The results of the logistic regression analysis identified a trend suggesting that the percentage of fetal chromosomal abnormalities was significantly higher in advanced maternal age (OR = 1.810, 95% CI 1.217-2.693), and lower gestational age (OR = 0.361, 95% CI: 0.196–0.665) (Table 5).
Discussion
The overall detection rate of clinically significant chromosomal abnormalities was 54.6%. Additionally, the rate of VOUS was 9.2%, which is in accordance with previous studies [13, 26]. We found that the largest proportion of chromosomal abnormalities was autosomal trisomy (33.4%), followed by CNVs (15.4%), and monosomy (6.0%). The frequencies of aneuploidy and polyploidy (39.9% and 5.2%, respectively) in the present study were similar to those obtained in a large-scale study (42.5% and 7.5%, respectively) conducted by Sahoo et al. [13]. Trisomies T16 and T22 were the most common, followed by T21, T15, T18, and T13. Trisomy was detected on all chromosomes except T1.
The rate of chromosomal abnormalities in second-trimester miscarriages was as high as 32.1% in this study but was lower than that in early miscarriages (56.6%). The lower frequency of other chromosomal trisomies may be because most trisomic embryos end in embryo implantation failure, and not all embryos have the opportunity to manifest abortion after implantation. In contrast, fetus withT16, T22, and T15 routinely have no opportunity to survive; therefore, these fetuses are almost always miscarried in early pregnancy, implying that T16, T22, and T15 may affect embryo development more than implantation. The risk of chromosomal abnormalities was significantly lower in the mid-trimester group than in the early pregnancy group (26.4% vs. 50.4%, P < 0.05); however, it still had a high risk of occurrence during this period, and was the leading cause of embryonic abortion in the mid-trimester. Therefore, chromosomal testing is necessary to identify the cause of miscarriages, even in the second trimester. According to previous studies, 45,X, T21, and T18 are miscarried in early pregnancy, whereas some continue to develop and survive in mid and late pregnancy. Further scientific studies are needed to reveal the underlying mechanisms [27] that also support self-repair during further embryo development – including apoptosis and selective differentiation [28], resulting in a substantial decrease in the proportion of abnormal chromosomal mosaicism during mid-pregnancy. In our study, the incidence of polyploidy in early pregnancy was as high as 10.0% (34/338); no polyploidy was detected in the second-trimester, and 97.1% (33/34) were triploid.
Advanced maternal age (≥ 35 years ) is a well-known independent factor associated with the frequency of chromosomal abnormalities during miscarriages [29, 30]. In this study, the frequencies of chromosomal abnormalities in women aged up to 30 years and 30–34 years were similar, but lower than those in women aged 35–39 years; all of them were significantly lower than those in women aged ≥ 40 years. This tendency was consistent with that of autosomal trisomy, confirming a close association between maternal age and viable autosomal trisomy. In recent years, some studies have proposed that the incidence of post-meiotic abnormalities such as structural abnormalities is not directly related to maternal age [31, 32]. In our study, a higher frequency of aneuploidy and lower frequency of CNVs were identified in the advanced maternal age group. Our results further support the theory that the incidence of embryonic aneuploidy increases with maternal age. The frequent detection of CNVs in miscarriages from young women is interesting; however, the overall sample size was small, and CNV-seq was not applied for parents in this study. Parental analysis is very promising for future research, because it allowed to detect de novo and inherited CNV. The latter may have a considerable interest for couples with recurrent pregnancy loss. Monosomy X is the most common viable sex chromosome abnormality. Unlike viable autosomal trisomy, the frequency of monosomy X did not increase with maternal age, which agrees with previous reports [7, 29, 33]. Hassold et al. [33, 34] found that paternal sex chromosome loss was the most common error leading to 45, X. They speculated that monosomy X was more likely derived from a meiotic error of the father than the mother. There are two possible reasons for this: an increase in the frequency of monosomy X conceptions related to events in meiosis, fertilization, or early zygotic division; or an increase in the rate of survival of monosomy X conceptions to the stage of recognizable pregnancies.
Sub-microscopic genomic imbalances or CNVs have been shown to play an important role in prenatal ultrasound anomalies and neuron-developmental disorders such as intellectual disability, autism, and epilepsy [35, 36]. Several attempts have been made to identify lethal CNVs in humans. Analysis of the functions of the genes contained in the CNVs showed that the percentage of pathogenic CNV in miscarriage tissues ranged from 6 to 15% [32, 37, 38]. The detection rate of CNVs in our study was 15.4%, including 6.2% of pathogenic CNVs. Among these cases, 4p16.3 microdeletion, 8p23.3microdeletion, 16p13.3microdeletion, 16p13.3 duplications and 16q24.3 duplications were found, some of which have also been reported in other studies concerning miscarriage [39, 40]. These microdeletions and microduplications might be related to pregnancy loss by comparing the prevalence of CNVs in miscarriage products and the general population; however, there is still no definite conclusion owing to the lack of more powerful evidence. Therefore, large-scale studies are required to confirm whether these CNVs cause miscarriages.
The present study had some limitations. First, the overall sample size was small, particularly for the mid-trimester. More cases, especially those of mid-trimester miscarriages, should be collected in future studies, and further functional studies should be performed on CNVs and genes associated with miscarriage. Second, parental karyotyping was not offered to couples whose POC revealed pCNVs abnormalities. Third, the distribution of patients was unequal between the age groups.
Conclusions
Our results confirmed that chromosomal abnormalities were the most common cause of pregnancy loss. Maternal and gestational ages are strongly associated with fetal chromosomal aberrations. Embryo chromosomal examination is recommended regardless of the gestational age, mode of conception or previous abortion status. Some useful and accurate genetic etiology information was obtained, which provides useful genetic guidance for high-risk pregnancies.
Availability of data and materials
All data relevant to the study are included in the article. The datasets generated during the current study are available in the SRA repository, https://www.ncbi.nlm.nih.gov/sra/PRJNA961959.
Abbreviations
- CNV-seq:
-
Copy number variation sequencing
- CA:
-
Chromosomal abnormalities
- POCs:
-
Products of conception
- pCNVs:
-
Pathogenic copy number variants
- MCC:
-
Maternal cell contamination
- CMA:
-
Chromosomal microarray analysis
- NGS:
-
Next-generation sequencing
- QF-PCR:
-
Quantitative fluorescence polymerase chain reaction
- STRs:
-
Short tandem repeat
- VOUS:
-
Variants of uncertain significance
References
Zimowski JG, Massalska D, Pawelec M, et al. First-trimester spontaneous pregnancy loss - molecular analysis using multiplex ligation-dependent probe amplification. Clin Genet. 2016;89:620–4.
Wei P, Li Y, Chen C, Xi N, et al. Detection of chromosomal aneuploidies in spontaneous abortion samples by fluorescence in situ hybridization. Zhong Hua Yi Xue Yi Chuan Xue Za Zhi. 2015;32:229–32.
Jobanputra V, Esteves C, Sobrino A, Warburton D, et al. Using FISH to increase the yield and accuracy of karyotypes from spontaneous abortion specimens. Prenat Diagn. 2011;31:755–9.
Sahoo T, Dzidic N, Strecker MN, et al. Comprehensive genetic analysis of pregnancy loss by chromosomal microarrays: outcomes, benefits, and challenges. Genet Med. 2017;19:83–9.
Petracchi F, Paez C, Igarzabal L. Cost-effectiveness of cytogenetic evaluation of products of conception by chorionic villus sampling in recurrent miscarriage. Prenat Diagn. 2017;37:282–8.
Hardwick SA, Deveson IW, Mercer TR. Reference standards for next generation sequencing. Nat Rev Genet. 2017;18:473–84.
Hawan D, Padh H. Pharmacogenetics: technologies to detect copy number variations. Curr Opin Mol Ther. 2009;11:670–80.
Zhu X, Li J, Ru T, et al. Identification of copy number variations associated with congenital Heart Disease by chromosomal microarray analysis and next-generation sequencing. Prenat Diagn. 2016;36:321–7.
Wang H, Dong Z, Zhang R, et al. Low-pass genome sequencing versus chromosomal microarray analysis: implementation in prenatal diagnosis. Genet Med. 2020;22:500–10.
Dong Z, Zhang J, Hu P, et al. Low-pass whole-genome sequencing in clinical cytogenetics: a validated approach. Genet Med. 2016;18:940–8.
Wang J, Chen L, Zhou C, et al. Prospective chromosome analysis of 3429 amniocentesis samples in China using copy number variation sequencing. Am J Obstet Gynecol. 2018;219:287.e1–e18.
Nicolini U, Lalatta F, Natacci F, et al. The introduction of QF-PCR in prenatal diagnosis of fetal aneuploidies: time for reconsideration. Hum Reprod Update. 2004;10:541–8.
Wang J, Chen L, Zhou C, et al. Identification of copy number variations among fetuses with ultrasound soft markers using nextgeneration sequencing. Sci Rep. 2018;8:8134.
Van den Berg MM, van Maarle MC, van Wely M, et al. Genetics of early miscarriage. Biochim Biophys Acta. 2012;12:1951–9.
Smith LK, Dickens J, Bender Atik R, et al. Parents’ experiences of care following the loss of a baby at the margins between miscarriage, stillbirth and neonatal death: a UK qualitative study. BJOG. 2020;127(7):868–74.
Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368:601–11.
Dai R, Li L, Zhu H, et al. Effect of maternal age on spontaneous abortion during the first trimester in Northeast China. J Matern Fetal Neonatal Med. 2018;31:1824–9.
Ozawa N, Ogawa K, Sasaki A, et al. Maternal age, history of miscarriage, and embryonic/fetal size are associated with cytogenetic results of spontaneous early miscarriages. J Assist Reprod Genet. 2019;36:749–57.
Meng L, Wang Z, Reilly M, et al. Amniotic immune biomarkers as risk factors in women with different symptoms of threatened late miscarriage. Am J Reprod Immunol. 2020;83(5):e13232.
Petracchi F, Colaci DS, Igarzabal L, et al. Cytogenetic analysis of first trimester pregnancy loss. Int J Gynaecol Obstet. 2009;104:243–4.
Russo R, Sessa AM, Fumo R, et al. Chromosomal anomalies in early spontaneous abortions: interphase FISH analysis on 855 FFPE first trimester abortions. Prenat Diagn. 2016;36:186–91.
An N, Li LL, Zhang XY, et al. Result and pedigree analysis of spontaneously abortion villus chromosome detecting by FISH. Genet Mol Res. 2015;14:16662–6.
Qi H, Xuan ZL, Du Y, Cai LR, Zhang H, Wen XH, Kong XD, Yang K, Mi Y, Fu XX, Cao SB, Wang J, Chen CJ, Liang JB. High resolution global chromosomal aberrations from spontaneous miscarriages revealed by low coverage whole genome sequencing. Eur J Obstet Gynecol Reprod Biol. 2018;224:21–8.
Brandt T, Sack LM, Arjona D, et al. Adapting ACMG/AMP sequence variant classification guidelines for single-Gene Copy Number variants. Genet Med. 2020;22:336–44.
Riggs ER, Andersen EF, Cherry AM, et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). Genet Med. 2020;22:245–57.
Shen JD, Wu W, Gao C, et al. Humphrey Ochin Chromosomal copy number analysis on chorionic villus samples from early spontaneous miscarriages by high throughput genetic technology. Mol Cytogenet. 2016;9:7.
Vanneste E, Voet T, Le Caignec C, et al. Chromosome instability is common in human cleavage stage embryos. Nat Med. 2009;15:577–83.
Cram DS, Leigh D, Handyside A, et al. PGDIS position Statement on the transfer of mosaic embryos 2019. Reprod Biomed Online. 2019;39(Suppl 1):e1–1e4.
Menasha J, Levy B, Hirschhorn K, Kardon N. Incidence and spectrum of chromosome abnormalities in spontaneous abortions: new insights from a 12-year study. Genet Med. 2005;7:251–63.
Tamura Y, Santo M, Araki Y, et al. Chromosomal copy number analysis of products of conception by conventional karyotyping and next-generation sequencing. Reprod Med Biol. 2021;20:71–5.
Simpson JL, Rechitsky S, Kuliev A. Before the beginning: the genetic risk of a couple aiming to conceive. Fertil Steril. 2019;112:622–30.
Chen L, Wang L, Tang F, et al. Copy number variation sequencing combined with quantitative fluorescence polymerase chain reaction in clinical application of pregnancy loss. J Assist Reprod Gene. 2021;38:2397–404.
Hassold T, Arnovitz K, Jacobs PA, May K, Robinson D. The parental origin of the missing or additional chromosome in 45, X and 47, XXX females. Birth Defects Orig Artic Ser. 1990;26:297–304.
Hassold T, Benham F, Leppert M. Cytogenetic and molecular analysis of sex-chromosome monosomy. Am J Hum Genet. 1988;42:534–41.
Levy B, Wapner R. Prenatal diagnosis by chromosomal microarray analysis. Fertil Steril. 2018;109:201–12.
Deshpande A, Weiss LA. Recurrent reciprocal copy number variants: roles and rules in neurodevelopmental disorders. DevNeurobiol. 2018;78:519–30.
Viaggi CD, Cavani S, Malacarne M, et al. First-trimester euploid miscarriages analyzed by array-CGH. J Appl Genet. 2013;54:353–9.
Rajcan-Separovic E, Qiao Y, Tyson C, et al. Genomic changes detected by array CGH in human embryos with developmental defects. Mol Hum Reprod. 2010;16:125–34.
Liu S, Song L, Cram DS, et al. Traditional karyotyping vs copy number variation sequencing for detection of chromosomal abnormalities associated with spontaneous miscarriage. Ultrasound Obstet Gynecol. 2015;46:472–7.
Zhu X, Li J, Zhu Y, et al. Application of chromosomal microarray analysis in products of miscarriage. Mol Cytogenet. 2018;11:44.
Acknowledgements
The authors acknowledge the patients who participating in this study.
Patient consent for publication
Not required.
Funding
The Natural Science Foundation of Fujian Province, China (No. 2020J01339).
Author information
Authors and Affiliations
Contributions
Author Contributions: Dr Xu LP,Lin N had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Drs Dai YF, Wu XQ , Xu LP and Lin N contributed equally to this work. Study concept and design: Dai YF, Wu XQ, and Huang HL. Acquisition, analysis, or interpretation of data: Dai YF, Wu XQ, Huang HL, He SQ,Guo DH ,Li Y, Xu LP,Lin N. Drafting of the manuscript: Dai YF, Wu XQ, Xu LP,Lin N. Critical revision of the manuscript for important intellectual content: Huang HL,Xu LP,Lin N.Statistical analysis: Dai YF, Wu XQ, He SQ,Guo DH ,Li Y.Administrative, technical, or material support: Dai YF, Wu XQ, Huang HL, He SQ,Guo DH ,Li Y, Xu LP,Lin N.Study supervision: Huang HL Li Y, Xu LP,Lin N.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Protection of Human Ethics Committee of Fujian Provincial Maternity and Children’s Hospital, which is affiliated with the Hospital of Fujian Medical University. Informed consent was obtained from all patients. All methods were carried out in accordance with relevant guidelines and regulations.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dai, YF., Wu, XQ., Huang, HL. et al. Experience of copy number variation sequencing applied in spontaneous abortion. BMC Med Genomics 17, 15 (2024). https://doi.org/10.1186/s12920-023-01699-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-023-01699-1