Abstract
Background
Colibacillosis in broiler chickens is associated with economic loss and localized or systemic infection. Usually, the last resort is antibacterial therapy. Insight into the disease pathogenesis, host responses and plausible immunomodulatory effects of the antibacterials is important in choosing antibacterial agent and optimization of the treatment. Selected responses of broiler chickens experimentally infected with Escherichia coli (E. coli) and also those treated with florfenicol are evaluated in this study. Chickens (n = 70, 5 weeks old) were randomly assigned to four groups. The control groups included normal control (NC) and intratracheal infection control (ITC) (received sterile bacterial medium). The experimental groups consisted of intratracheal infection (IT) that received bacterial suspension and intratracheal infection with florfenicol administration (ITF) group.
Results
Florfenicol reversed the decreased albumin/globulin ratio to the level of control groups (p > 0.05). Serum interleukin 10 (IL-10) and interferon‐gamma (IFN-γ) concentrations decreased in IT birds as compared to NC group. Florfenicol decreased the serum interleukin 6 (IL-6) concentration as compared to IT group. Milder signs of inflammation, septicemia, and left shift were observed in the leukogram of the ITF group. Florfenicol decreased the severity of histopathological lesions in lungs and liver. Depletion of lymphoid tissue was detected in spleen, thymus and bursa of IT group but was absent in ITF birds. The number of colony forming units of E. coli in liver samples of ITF group was only slightly lower than IT birds.
Conclusions
Experimental E. coli infection of chickens by intratracheal route is associated with remarkable inflammatory responses as shown by changes in biochemical and hematological parameters. Histopathological lesions in lymphoid organs (especially in the spleen) were also prominent. Florfenicol has positive immunomodulatory effects and improves many of the lesions before the full manifestation of its antibacterial effects. These effects of florfenicol should be considered in pharmacotherapy decision-making process.
Similar content being viewed by others
Background
Colibacillosis which is caused by avian pathogenic Escherichia coli(APEC) is commonly diagnosed in broiler chicken flocks already afflicted with viral diseases or other stressors. The bacteria can also be considered as a primary pathogen. The disease is associated with appreciable economic loss and adversely affects the birds’ welfare. Unfortunately, efficient vaccines are not currently available and usually the last resort is antibacterial therapy of infected flocks [18, 11].
Gaining insight into the host responses against colibacillosis and antibacterial therapy is important in tailoring control and treatment strategies. Controlled experimental studies with APEC as the primary pathogen are mainly used for evaluation of immune responses in chickens with colibacillosis. According to these experiments, the host defense against colibacillosis comprises a strong early innate immune response followed by humoral and cell-mediated adaptive responses. Many different factors related to bacteria, host and even sampling are shown to be associated with changes in host responses to APEC bacteria. Moreover; discrepancies are observed between results from ex vivo and in vivo studies [1].
Florfenicol is a broad spectrum antibacterial agent and a derivative of chloramphenicol with the advantage of applicability in food producing animals. The drug has an indication for use in broiler flocks with colibacillosis according to label instructions and shows good therapeutic effects. Florfenicol also shows immunomodulatory and anti-inflammatory properties in veterinary species [22]. In normal chickens, florfenicol has shown inconsistent immunomodulatory effects. Khalifeh et al., [12], reported that florfenicol administration to layers suppresses Newcastle disease (ND) antibody production measured by both HI and ELISA. In a study by Han et al., [7] administration of florfenicol to 1-day-old broilers for 5 consecutive days resulted in increased antibody titer against ND vaccine. The latter researchers found no change in total white blood cell (WBC) count and WBC subsets as well as serum interferon‐gamma (IFN-γ) and interleukin 4 (IL-4) in the treated broilers at the age of 21 and 42 days. Serum interleukin 2 (IL-2) content was decreased while peripheral lymphocyte transformation rate was increased in 42-day-old treated birds as compared to control group in this study. More recently, [16], reported immunotoxic effects of florfenicol administered for 6 consecutive days to 3-day-old chickens with regards to assessment of serum ND antibody titer, cytokine levels and histological features of bursa of Fabricius [16].
Available reports on the effects of florfenicol on immune responses of broilers with colibacillosis are scarce. In a study by [8], relatively high dosages (30 mg/kg or 60 mg/kg) of florfenicol were administered to broilers experimentally infected withE. coli O78 by intratracheal route. These authors found that 60 mg/kg florfenicol increases hemagglutination inhibition (HI) and enzyme-linked immunosorbent assay (ELISA) antibody titers against ND vaccination. Moreover, gene expression of interferon-inducible genes in the spleen tissue of birds increased by both dosages. In histopathological examination of bone marrow, moderate atrophy of hematopoietic lineage and increased fat cells in both florfenicol-treated groups were observed while no specific change in spleen was reported. Unfortunately, in the above mentioned study, most of the assays were performed very lately and after confirmation of E. coli clearance of the body. Therefore, the authors could not precisely relate the observed effects to immunomodulatory properties of florfenicol and deduced that the antibacterial effects of florfenicol may be involved in at least some of the observed effects.
In the present study, we evaluated selected immune responses of broilers experimentally infected with E.coli and also those treated with routine clinical dosages of florfenicol at early days after infection by considering cytokine levels, hematological changes and histopathological examination of the immune organs (spleen, thymus and bursa of Fabricius).
Materials and methods
Bacteria and experimental infection
The bacterium which was used in the study was an APEC from O2 serotype originally isolated from broiler chickens. The bacteria were provided by Razi Vaccine and Serum Research Institute, Iran. According to the result of disk diffusion testing, the bacteria were not resistant to florfenicol. A bacterial suspension was made in tryptic soy broth (TSB) medium and bacterial dose for administration to broilers was chosen based on our previous study [19]. Birds were infected with 1 mL of the bacterial suspension at the concentration of 7.1 × 108CFU/mL by intratracheal route as described by Kromann et al., [13].
Experimental design
Seventy, 1-day-old Cobb chicks from both sexes were included in the study. Chickens were purchased from a commercial local hatchery for this specific study. Birds were reared in similar conditions according to Cobb Broiler Management Guide. No vaccination or drug administration was performed during the rearing period. Biosecurity practices were followed to prevent infectious diseases. At the age of 5 weeks, birds were randomly assigned to four groups including two control groups (n = 15 each) and two experimental groups (n = 20 each). Birds in each group were allocated into 5 replicates. The control groups included normal control (NC) group which was comprised of normal birds with no specific treatment, and intratracheal infection control (ITC) group where birds received 1 mL of sterile TSB medium by intratracheal route. The experimental groups included intratracheal infection (IT) group that received 1 mL of the bacterial suspension inoculated by intratracheal route, and intratracheal infection with florfenicol administration (ITF) group that in addition to being infected was treated with florfenicol (Fluorfen®, 10% solution; Rooyan Darou Pharmaceutical Co., Tehran, Iran) at a dosage of 1 mL/L of drinking water (20 mg/kg per day) according to label instructions. Administration of florfenicol was started after overt manifestation of clinical signs (roughly 12 h. post inoculation) and lasted for 3 consecutive days.
At the end of antibacterial treatment period, 10 birds from each group were randomly selected for blood sampling from wing vein. About 4 mL blood was collected from each bird in plain and citrate sodium coated vacutainer tubes (2 mL each). Five birds from each group were euthanatized by cervical dislocation after concussion. Under aseptic conditions, right lobe of the liver was removed and transferred to sterile containers for total bacterial count. Moreover, samples of liver, lung, spleen, thymus and bursa of Fabricius of these birds were collected in 10% neutral buffered formalin for histopathological examination.
All procedures used in this study were conducted in accordance with European Union commission legislation on the protection of animals used for scientific purposes [6] and were approved by an institutional ethical review committee (code number: 1GCB3M163773).
Serum total protein, albumin and total globulin assays
For serum collection, blood samples in plain tubes were centrifuged for 5 min at 2500 rpm. Harvested sera were kept at -20 °C until use.
Serum total protein was determined by photometric test according to biuret method. Determination of albumin level was performed by using bromocresol green spectrophotometric method. Both kits were provided by Pars Azmun Co., Tehran, Iran. All methods were performed according to kit protocols. The total globulin fraction was determined by subtracting the albumin from the total protein.
Evaluation of serum cytokines levels
Chicken specific ELISA kits were used for the assay of IFN-γ, interleukin 6 (IL-6) and IL-10 in sera. All kits were provided by ZellBio GmbH, Ulm, Germany. All kits were based on one-step biotin double antibody sandwich ELISA method with intra assay and inter assay coefficients of variation (CVs) of < 10% and < 12%, respectively. All assays were performed according to manufacturer’s instructions.
Determination of total WBC, WBC subsets and thrombocytes in blood
Total WBC count and thrombocyte count were determined by manual technique. To determine the differential leukocyte counts (heterophil, lymphocyte, monocyte, and immature white blood cells), a drop of blood was thinly spread over a glass slide, air-dried, and stained with the Giemsa staining technique. One hundred cells are then counted and classified. Then absolute number of WBC subsets was calculated by using their percentage and total WBC count [21, 24].
Histopathological examination of lymphoid organs, lung and liver
After fixation, samples were routinely processed and embedded in paraffin. Five μm-thick sections from paraffin blocks were made and stained with hematoxylin and eosin for examination under light microscope [3]. Different histopathological lesions were determined in each tissue (Nakamura et al., [17] and Usman et al. [23] with modifications). Lesions in all tissues were semi quantitatively scored from 0–3. The scoring system was as follows: 0: no lesion, 1: mild, 2: moderate and 3: severe lesion.
Total bacterial count of liver
Liver samples were immediately used for bacterial count. Samples were weighed and then were placed in boiling normal saline for 4 s for surface sterilization. Then samples were transferred to sterile bags and sterile normal saline was added to each bag at 9:1 w/w ratio. After mechanical homogenization, serial dilutions (10–2 to 10–6) were made in sterile micro tubes. Ten µL of each dilution was transferred to MacConkey agar plates and incubated for 18–24 h at 37 °C. Plates containing less than 250 colonies were used for colony count and total bacterial CFU count/g tissue was calculated (Adzitey and Yildiz [2] with modifications).
Statistical analysis
Shapiro-Wilks’s normality test was performed on all data sets. Based on the results, data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test or Kruskal–Wallis test followed by Dunn's multiple comparisons test where appropriate. P < 0.05 was considered as the level of significance for statistical analysis.
Results
It should be noted that data related to clinical signs, mortality, gross pathology, pathogenesis, etc. are reported in our previously published paper [19].
Serum total protein, albumin and total globulin levels
Birds in IT group showed significantly increased serum levels of total protein and total globulin as compared to control birds in NC group (p < 0.01 and p < 0.0001, respectively). Although a slight decrease was observed in serum albumin levels of IT group in comparison with control groups, the change was not significant (p > 0.05). Florfenicol administration to birds in ITF group was associated with a significant decrease in serum total protein and globulin concentrations as compared to untreated birds in IT group (p < 0.01 and p < 0.0001, respectively). No significant difference was observed in these parameters among ITF and control groups (p > 0.05). The albumin/globulin (A/G) ratio was significantly decreased in IT group as compared to control groups (p < 0.05 for both comparisons). Birds in ITF group showed statistically the same A/G ratio as compared to control groups and IT birds (p > 0.05) (Fig. 1).
Serum levels (mean and SD) of total protein, albumin, globulin and A/G ratio in different groups. NC: normal control, normal birds with no specific treatment, ITC: intratracheal infection control, birds received sterile medium by intratracheal route; IT: Intratracheal infection group, birds received bacterial suspension inoculated by intratracheal route and ITF: intratracheal infection with florfenicol administration group, in addition to being infected, birds were treated with florfenicol. Values in columns without a common letter are significantly different at p < 0.05
Serum levels of cytokines
Induction of colibacillosis in birds of IT group was associated with significantly lower serum concentrations of IL-10 and IFN-γ as compared to normal birds of NC group (p < 0.01 for both comparisons). IL-6 levels remained statistically similar between these two groups (p > 0.05). Administration of florfenicol to birds with colibacillosis (ITF group) resulted in appreciable decrease in serum concentration of IL-6 as compared to birds in IT group (p < 0.05). Antibiotic therapy of birds in ITF group had no significant effect on serum IL-10 or IFN-γ as compared to IT birds (p > 0.05) (Fig. 2).
Serum levels (mean and SD) of cytokines in different groups. NC: normal control, normal birds with no specific treatment, ITC: intratracheal infection control, birds received sterile medium by intratracheal route; IT: Intratracheal infection group, birds received bacterial suspension inoculated by intratracheal route and ITF: intratracheal infection with florfenicol administration group, in addition to being infected, birds were treated with florfenicol. MS: missed samples. Values in columns without a common letter are significantly different at p < 0.05
White Blood cells and thrombocytes
Birds in IT group showed a significant increase in number of WBCs as compared to control groups (p < 0.0001 for both comparisons). Birds in ITF group had significantly lower number of WBCs as compared to IT group (p < 0.05), however this parameter value was statistically higher in ITF birds than NC or ITC groups (p < 0.01 for both comparisons).
Although the percentage of heterophils was statistically the same among groups, birds in IT and ITF groups showed significantly higher number of heterophils as compared to birds in NC group (p < 0.0001 and p < 0.001, respectively). Florfenicol administration resulted in an appreciable decrease in heterophils count as compared to IT group (p < 0.05).
Lymphocytes counts in birds of IT and ITF groups were significantly higher than NC birds (p < 0.001 and p < 0.05, respectively). Birds in IT and ITF groups showed statistically the same counts of lymphocytes (p > 0.05). The percentage of lymphocytes in IT and ITF groups was lower than NC birds (p < 0.001 and p < 0.05, respectively). The values of this parameter were not significantly different between IT and ITF groups (p > 0.05).
Regarding monocytes, birds in IT and ITF groups showed significantly higher numbers of these cells as compared to NC birds (p < 0.0001 and p < 0.01, respectively). Birds in ITF group had lower number of monocytes in comparison with birds in IT group (p < 0.001). Percentage of monocytes in IT and ITF groups was also higher than NC birds (p < 0.0001 and p < 0.01). Birds in ITF group showed lower percentage of monocytes as compared to IT birds (p < 0.05).
Birds in IT group had significantly higher counts and percentage of immature white blood cells as compared to NC group (p < 0.0001 for both comparisons). Number and percentage of these cells in ITF group were statistically the same as NC birds (p > 0.05) and significantly lower than IT group (p < 0.0001 for both comparisons).
Number of thrombocytes was statistically the same among all groups (p > 0.05).
Data related to blood cells are summarized in Table 1.
Descriptive parameters of blood cells
Blood cells in NC and ITC groups showed completely normal appearances. In IT group, signs of severe acute inflammation and septicemia were present. Toxic heterophils with vacuolated cytoplasm were abundantly observed. Severe left shift with presence of toxic myelocytes, metamyelocytes and band heterophils was detected in blood smears. Polychromatophilic erythrocytes were detected relatively more in IT group than ITF birds. The severity of changes was generally lower in ITF group as shown by the presence of some band heterophils and heterophils that only showed mildly vacuolated cytoplasms. Red blood cells were normal. Figure 3 represents some of these changes in IT and ITF groups.
Representative photomicrographs of birds in intratracheal infection group (IT) (A and B) and intratracheal infection with florfenicol administration group (ITF) (C and D). Short thin arrow: Toxic heterophil with vacuolated cytoplasm; Long thin arrow: Toxic metamyelocyte, vacuoles and dark toxic granules are present in cytoplasm; Star: Polychromatophilic erythrocyte; Thick arrow: Toxic myelocyte, vacuoles and dark toxic granules are present in cytoplasm; Curved arrow: heterophil with mildly vacuolated cytoplasm; #: Normal monocytes. Giemsa staining, Magnification: 1000X
Histopathological findings of liver and lung
Lungs and livers of birds in NC and ITC groups did not show any lesions and looked normal in histopathological examination. Infiltration of inflammatory cells, presence of necrotic foci, accumulation of eosinophilic substances in parabronchi and intravascular fibrin thrombi were the most profound lesions that were observed in lungs of birds in IT group. Hemorrhage was not detected in lungs of these birds. Except congestion, the severity of lesions was lower in birds of ITF group as compared to IT group although the only parameter that showed significantly lower scores was the accumulation of eosinophilic substances in parabronchi (p < 0.05).
Fatty changes, intravascular fibrin thrombi, perihepatitis, accumulation of heterophils around portal areas and congestion were detected in livers of birds in IT group. The only parameter that showed significantly lower scores in ITF group compared to IT birds was accumulation of heterophils around portal areas (p < 0.05) (Table 2).
Selected lesions in lungs and livers of birds are shown in Fig. 4.
Representative photomicrographs of liver (A) and lung (B) of birds experimentally infected with E. coli by intratracheal route (IT group). Long arrow: perihepatitis; #: Lymphatic cells accumulation; Star: accumulation of eosinophilic substances in parabronchi; Short arrow: Intravascular thrombus, hematoxylin and eosin staining
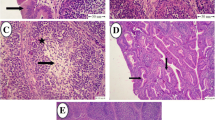
Histopathological findings of immune organs
Spleen, bursa of Fabricius and thymus of birds in NC and ITC groups were normal without any considerable lesions. Among the three immune organs that were histopathologically examined, spleen was the most affected organ of birds in IT group where almost all of the assayed parameters showed a median score of 3 (the highest severity score). Congestion, hemorrhage, intravascular fibrin thrombi, heterophil accumulation foci, depletion of lymphoid cells in white pulp and focal areas of necrosis were profoundly detected in the spleens of IT birds. The scores of all these parameters were statistically lower in ITF birds as compared to IT group (p < 0.05) and were reversed to normal values of NC group (p > 0.05). Moreover, birds in ITF group showed hyperplasia in white pulp which was not observed in any other groups.
Congestion, intravascular fibrin thrombi, heterophil accumulation, depletion of lymphoid tissues and edema were detected in thymi of birds in IT group. Thymi of birds in ITF group showed normal structural features without detectable lesions in histopathological examination.
Depletion of lymphoid cells, cyst formation, interfollicular edema and distended lymphatic vessels were observed in bursa of Fabricius of birds in IT group. Except for interfollicular edema, the bursas of birds in ITF group looked almost normal in histopathological evaluation.
Figure 5 shows selected lesions in lymphoid organs of birds in IT group.
Table 3 summarizes the scores of histopathological findings in different groups.
E. coli count in liver
As shown in Fig. 6, no E. coli growth was observed in liver samples collected from control groups (NC and ITC). Incubation of liver samples from both infected groups (IT and ITF) resulted in E. coli bacterial growth. The number of colony forming units (CFUs) of E. coli in liver samples of ITF group was only slightly lower than IT birds (p > 0.05).
E. coli count (mean and SD) in liver samples of birds in different groups. NC: normal control, normal birds with no specific treatment, ITC: intratracheal infection control, birds received sterile medium by intratracheal route; IT: Intratracheal infection group, birds received bacterial suspension inoculated by intratracheal route and ITF: intratracheal infection with florfenicol administration group, in addition to being infected, birds were treated with florfenicol. Values in columns without a common letter are significantly different at p < 0.05
Discussion
This study is focused on certain aspects of responses of chickens experimentally infected with APEC via intra tracheal route with or without florfenicol treatment.
In acute or chronic inflammatory conditions, total protein may increase due to elevated globulin fraction. In these situations, albumin concentrations often decrease. The combined effect of these changes is a decrease in the A/G ratio [15]. Consistently, in the present study, hyperproteinemia and decreased A/G ratio was observed in birds of IT group which was due to increased serum globulins. Hyperglobulinemia in chickens with colibacillosis has been reported by other investigators [20, 5]. As previously stated, in the present study birds in ITF group showed statistically similar levels of serum globulin and A/G ratio compared to control groups which can be related to a suppressed inflammatory condition following florfenicol administration to these birds.
In 2024, Usman et al., observed that serum concentration of IL-6 significantly increases in chickens that were inoculated via intra nasal route by O78:K80 E. coli three days post inoculation. In a study by Elnagar et al., [4]; broiler chickens which were orally infected with E. coli O78, O26, O55, or O44 showed increased mRNA expression of IL-6 in ileal tissue two days post infection. Conversely, in our study serum level of IL-6 was not significantly changed in IT birds. It is worth to mention that in the study performed by Elnagar et al., the level of increase in mRNA expression of IL-6 cytokine was different between the E. coli strains. Therefore, the reason for the observed discrepancy, might be the difference in E. coli strain used as well as time of sampling, inoculation route and type of the sample.
We observed that ITF birds show significantly lower levels of IL-6 as compared to IT group. The suppressive effects of florfenicol on IL-6 serum levels has also been previously reported in mice challenged with LPS [27]. These researchers also showed that florfenicol inhibits the translocation of LPS-induced nuclear factor-κB (NF-κB) from cytoplasm into the nucleus in RAW 264.7 macrophages. Therefore, they suggested that the effects of florfenicol on early cytokine responses can be due to blocking of NF-κB pathway.
Interleukin 6 is a multifunctional cytokine in chickens with major roles in immune responses including activating B and T lymphocytes and encouraging macrophage production [25]. As an important cytokine in innate immune responses, IL-6 alerts the immune system about the presence of the pathogen. However, improper over production of this molecule may also be damaging [4]. On the other hand, suppressed production of this cytokine may help to the spread of infection as shown for Salmonella gallinarum [10]. Therefore, the suppressive effect of florfenicol on this cytokine level in chickens with colibacillosis should be considered conservatively.
It is well stablished that IL-10 is an inducible feedback regulator of immune response in chickens and acts as an anti-inflammatory cytokine [26]. It is reported that IL-10 mRNA expression decreases in ileal tissue of chickens with colibacillosis [4]. Consistently, in the present study, we observed decreased serum levels of IL-10 in birds of IT group. These birds also showed decreased serum levels of IFN-γ. It has been shown that administration of IFN-γ to chickens with colibacillosis enhances immune responses against the disease although it does not mitigate the development of air sac lesions [9]. Therefore the decreased serum level of IFN-γ may negatively affect the immune responses of chickens with colibacillosis.
In a study by Zhang et al., [27], florfenicol prolonged IL-10 expression in serum of mice challenged with LPS while had no effect on IL-10 production by LPS-induced RAW 264.7 cells in vitro. Administration of florfenicol to SPF chicks at the age of 3 days for six consecutive days has been associated with decreased serum levels of IFN-γ compared to control group in the early stages of drug withdrawal [16]. In contrast, in the present study, florfenicol administration had no effect on the levels of IL-10 or IFN-γ in chickens with colibacillosis. The differences in the nature and conditions of the mentioned studies might have a role in the discrepancy observed in the results.
In the present study, florfenicol improved the hematological profile of birds with colibacillosis as shown by milder signs of inflammation, toxemia and left shift presented by WBCs. Leukocytosis and monocytosis were also ameliorated by florfenicol administration. Moreover, florfenicol decreased the severity of some of the lesions observed in lung (accumulation of eosinophilic substances in parabronchi) and liver (congestion and heterophil accumulation in portal area). Although it was not addressed in the present study, these effects of florfenicol at cellular level may improve organ function and subsequently expedite the recovery and escalate health status of the bird. Regarding the lymphoid organs, administration of florfenicol resulted in remarkable decrease of lesion severity especially with regard to the spleen which was the most affected lymphoid organ in IT birds. Depletion of lymphoid tissue was observed in spleens, thymus and bursa of Fabricius of birds in IT group. Lymphocytic depletion of bursa and thymus of chickens infected with E. colihas been previously reported by Nakamura et al., [17]. Interestingly, florfenicol administration protected these organs against lymphoid tissue depletion and even resulted in mild hyperplasia of white pulp in the spleen of chickens in ITF group. Consistently, in a study by Lis et al. [14], florfenicol increased percentage and absolute number of T lymphocytes in mesenteric lymph nodes of mice.
An important question is that whether the observed effects of florfenicol in this study are related to its antibacterial effect (reversal of lesions and changes after bacterial clearance) and/or its plausible immunomodulatory effects? As it was confirmed by the results related to bacterial count performed on liver samples, birds in both infected groups were still afflicted with systemic colibacillosis. Interestingly, although the bacteria were sensitive to florfenicol (based on sensitivity test), administration of this drug was not associated with a drastic decrease in bacterial load at the time of sampling. This can be related to the fact that florfenicol administration in this study was continued for relatively short period (3 days) before sampling and a very high load of bacteria was used for inoculation as it is a routine procedure in studies that use experimental models of colibacillosis. Therefore, there is a high chance that the observed beneficial effects are related to the immunomodulatory effects of the drug, although we cannot completely rule out the possibility that antibacterial effects of the drug could be still involved since we could not count bacteria in all afflicted organs.
In conclusion, experimental E. coli infection of chickens by intratracheal route results in remarkable inflammatory responses associated with changes in serum cytokine levels (IL-10 and IFN-γ) as well as in biochemical (decreased A/G ratio) and hematological (severe left shit with presence of toxic myelocytes, leukocytosis and monocytosis) parameters. Histopathological lesions in lymphoid organs (especially in spleen) were also prominent in these birds. Florfenicol administration ameliorated inflammatory responses and improved many of the lesions when it has not yet dominated the bacteria. These anti-inflammatory and beneficial effects of florfenicol should be considered in pharmacotherapy decision-making process and might help clinicians select a more effective antimicrobial agent among options to which bacteria may be susceptible. Of course, effects of florfenicol (suppressive or stimulant) on other response parameters that have a role in host defense mechanisms and the outcome of chickens with colibacillosis need to be clarified in future studies.
Availability of data and materials
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Alber A, Stevens MP, Vervelde L. The bird’s immune response to avian pathogenic Escherichia coli. Avian Pathol. 2021Oct;50(5):382–91. https://doi.org/10.1080/03079457.2021.1873246.
Adzitey F, Yildiz, F. Incidence and antimicrobial susceptibility of Escherichia coli isolated from beef (meat muscle, liver and kidney) samples in Wa Abattoir, Ghana. Cogent Food & Agriculture, (2020). 6(1). https://doi.org/10.1080/23311932.2020.1718269.
Bancroft JD, Layton C. The haematoxylins and eosin in Bancroft’s Theory and Practice of Histological Techniques. (eds. Suvarna SK, Layton C and Bancroft JD), 8th edition. Elsevier; 2019:126–38.
Elnagar R, Elkenany R, Younis G. Interleukin gene expression in broiler chickens infected by different Escherichia coli serotypes. Vet World. 2021;14(10):2727–34.https://doi.org/10.14202/vetworld.2021.2727-2734.
El-Tahawy AO, Said AA, Shams GA, Hassan HM, Hassan AM, Amer SA, El-Nabtity SM. Evaluation of cefquinome’s efficacy in controlling avian colibacillosis and detection of its residues using high performance liquid chromatography (HPLC). Saudi J Biol Sci. 2022;29(5):3502–10. https://doi.org/10.1016/j.sjbs.2022.02.029.
European Parliament. Council of the European Union Directive 2010/63/EU of Sep 22, 2010, on the protection of animals used for scientific purposes, Document 32010L0063. Off J Eur Union. 2010;L276:33–79.
Han C, Wang X, Zhang D, Wei Y, Cui Y, Shi W, Bao Y. Synergistic use of florfenicol and Salvia miltiorrhiza polysaccharide can enhance immune responses in broilers. Ecotoxicol Environ Saf. 2021;1(210):111825.
Hassanin O, Abdallah F, Awad A. Effects of florfenicol on the immune responses and the interferon-inducible genes in broiler chickens under the impact of E. coli infection. Vet Res Commun. 2014;38(1):51–8. https://doi.org/10.1007/s11259-013-9585-7.
Janardhana V, Ford ME, Bruce MP, Broadway MM, O'Neil TE, Karpala AJ, Asif M, Browning GF, Tivendale KA, Noormohammadi AH, Lowenthal JW, Bean AG. IFN-gamma enhances immune responses to E. coli infection in the chicken. J Interferon Cytokine Res. 2007;27(11):937–46. https://doi.org/10.1089/jir.2007.0020. PMID: 18052728.
Kaiser P, Rothwell L, Galyov EE, Barrow PA, Burnside J, Wigley P. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium. Salmonella enteritidis and Salmonella gallinarum Microbiology (Reading). 2000;146(Pt 12):3217–26. https://doi.org/10.1099/00221287-146-12-3217.
Kathayat D, Lokesh D, Ranjit S, Rajashekara G. Avian Pathogenic Escherichia coli (APEC): An Overview of Virulence and Pathogenesis Factors, Zoonotic Potential, and Control Strategies. Pathogens. 2021;10(4):467. https://doi.org/10.3390/pathogens10040467.
Khalifeh MS, Amawi MM, Abu-Basha EA, Yonis IB. Assessment of humoral and cellular-mediated immune response in chickens treated with tilmicosin, florfenicol, or enrofloxacin at the time of Newcastle disease vaccination. Poult Sci. 2009;88(10):2118–24. https://doi.org/10.3382/ps.2009-00215. PMID: 19762865.
Kromann S, Olsen RH, Bojesen AM, Jensen HE, Thøfner I. Development of an aerogenous Escherichia coli infection model in adult broiler breeders. Sci Rep. 2021;11(1):19556.
Lis M, Szczypka M, Suszko A, Switała M, Obmińska-Mrukowicz B. The effects of florfenicol on lymphocyte subsets and humoral immune response in mice. Pol J Vet Sci. 2011;14(2):191–8. https://doi.org/10.2478/v10181-011-0029-4.
Lumeij JT, Chapter 28 - Avian Clinical Biochemistry, Editor(s): J. Jerry Kaneko, John W. Harvey, Michael L. Bruss, Clinical Biochemistry of Domestic Animals (Sixth Edition), Academic Press, 2008, Pages 839–872, ISBN 9780123704917, https://doi.org/10.1016/B978-0-12-370491-7.00030-1.
Meng FL, Liu KH, Shen YS, Li PX, Wang TL, Zhao YR, Liu SD, Liu MD, Gang WA. Florfenicol can inhibit chick growth and lead to immunosuppression1. J Integr Agric. 2023. https://doi.org/10.1016/j.jia.2023.11.040.
Nakamura K, Imada Y, Maeda M. Lymphocytic depletion of bursa of Fabricius and thymus in chickens inoculated with Escherichia coli. Vet Pathol. 1986;23(6):712–7. https://doi.org/10.1177/030098588602300610.
Nolan LK, Vaillancourt JP, Barbieri NL, Logue CM. “Colibacillosis,” In: Swayne DE, ed Diseases of Poultry. Hoboken, NJ: Wiley-Blackwell (2020). p. 770–830. https://doi.org/10.1002/9781119371199.ch18.
Saberi A, Mosleh N, Shomali T, Naziri Z. A Comparative Study on Two Infection Models of Colibacillosis in Broilers: Clinical Features, Pathogenesis, and Response to Therapy. Avian Dis. 2023;67(3):261–8.
Sharma V, Jakhar KK, Nehra V, Kumar S. Biochemical studies in experimentally Escherichia coli infected broiler chicken supplemented with neem (Azadirachta indica) leaf extract. Vet World. 2015;8(11):1340–5. https://doi.org/10.14202/vetworld.2015.1340-1345.
Thrall MA, Weiser G, Allison RW, Campbell TW. Veterinary Hematology and Clinical Chemistry. John Wiley & Sons; 2012.
Trif E, Cerbu C, Olah D, Zăblău SD, Spînu M, Potârniche AV, Pall E, Brudașcă F. Old Antibiotics Can Learn New Ways: A Systematic Review of Florfenicol Use in Veterinary Medicine and Future Perspectives Using Nanotechnology. Animals (Basel). 2023;13(10):1695. https://doi.org/10.3390/ani13101695.
Usman S, Anjum A, Usman M, Imran MS, Ali M, Moustafa M, et al. Antibiotic resistance pattern and pathological features of avian pathogenic Escherichia coli O78:K80 in chickens. Braz J Biol. 2024;84:e257179.
Voigt GL, Swist SL. Hematology Techniques and Concepts for Veterinary Technicians. John Wiley & Sons; 2011.
Wigley P, Kaiser P. Avian cytokines in health and disease. Brazilian Journal of Poultry Science. 2003;5:1–4.
Wu Z, Hu T, Rothwell L, Vervelde L, Kaiser P, Boulton K, Nolan MJ, Tomley FM, Blake DP, Hume DA. Analysis of the function of IL-10 in chickens using specific neutralising antibodies and a sensitive capture ELISA. Dev Comp Immunol. 2016;63:206–12. https://doi.org/10.1016/j.dci.2016.04.016.
Zhang X, Song Y, Ci X, An N, Fan J, Cui J, Deng X. Effects of florfenicol on early cytokine responses and survival in murine endotoxemia. Int Immunopharmacol. 2008;8(7):982–8. https://doi.org/10.1016/j.intimp.2008.02.015.
Acknowledgements
None.
Funding
This work was supported by the Shiraz University under Grant number 1GCB3M163773.
Author information
Authors and Affiliations
Contributions
T. Sh and N. Mosleh conceptualization, data analysis, supervision, T. Sh prepared the draft, Z. Gh, S. Nazifi and A. Kh. T data acquisition and methodology, all authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures used in this study were approved by the Shiraz University, School of Veterinary Medicine ethical committee and were compatible with Directive 2010/63/EU on the protection of animals used for scientific purposes.
Consent for publication
Not applicable.
Competing interests
The authors report there are no competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ghahramani, Z., Mosleh, N., Shomali, T. et al. A study on selected responses and immune structures of broiler chickens with experimental colibacillosis with or without florfenicol administration. BMC Vet Res 20, 371 (2024). https://doi.org/10.1186/s12917-024-04232-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-024-04232-3