Abstract
Background
We investigated breed and gender variations in the compositions of long-chain (≥ C20) omega-3 polyunsaturated fatty acids (LC omega-3 PUFA), fat melting point (FMP) and intramuscular fat (IMF) contents in biopsy samples of the M. longissimus dorsi muscle of grazing beef cattle. The hypothesis that biopsy compositions of health-beneficial LC omega-3 PUFA, FMP and IMF in a pasture-based production system will vary with breed, was tested. Muscle biopsies were taken from 127 yearling pasture-based Angus, Hereford, and Wagyu heifers and young bulls exclusive to the Australian Bowen Genetics Forest Pastoral breeding stud averaging 12 ± 2.43 months of age and under the same management routine.
Results
Breed had a significant influence on IMF, FMP, and the compositions of oleic acid, α-linolenic acid (ALA), eicosapentaenoic (EPA), docosahexaenoic (DHA), docosapentaenoic (DPA), and total EPA + DHA + DPA in the M. longissimus dorsi muscle biopsies (P ≤ 0.03). The Wagyu breed had the highest (11.1%) and Hereford the lowest (5.9%) IMF (P = 0.03). The reverse trend was observed in FMP values where the Hereford breed had the highest (55 °C), Angus intermediate (46.5 °C), and Wagyu the lowest (33 °C) FMP. The Wagyu and Angus breeds had similar oleic fatty acid (18:1n-9) content, while the Hereford breed had the lowest (P < 0.01). The highest ALA, DPA, total EPA + DHA, total EPA + DHA + DPA and total ALA + EPA + DHA + DPA contents were detected in the Wagyu breed (P ≤ 0.03). The Hereford had similar EPA and DPA contents to the Angus (P ≥ 0.46). Total EPA + DHA + DPA contents in Wagyu, Angus, and Hereford were 28.8, 21.5, and 22.1 mg/100g tissue (P = 0.01), respectively. Sex was an important source of variation that influenced LC omega-3 PUFA composition, FMP and IMF, where yearling heifers had higher IMF (11.9% vs 5.3%), lower FMP (33°C vs 37°C), and higher LC omega-3 PUFA than bulls.
Conclusion
All the results taken together indicate that the Wagyu breed at 28.8 mg/100g tissue, was the closest to meeting the Australia and New Zealand recommended source level threshold of 30 mg/100g tissue of health-beneficial ≥ C20 omega-3 FA content. Since gender was a significant determinant of LC omega-3 PUFA composition, IMF content and FMP, it should be factored into enhancement strategies of healthy meat eating quality traits in grazing cattle. These findings also suggest that the Bowen Genetics Forest Pastoral beef cattle studs are important sources of LC omega-3 PUFA that can be used to cover the deficit in these health claimable fatty acids in Western diets.
Similar content being viewed by others
Background
Consumers perceive consistency in meat eating quality as a critical driver of beef consumption, hence beef producers and processors continuously strive to add value to ruminant-derived meat and meat products to meet this rising market demand [1,2,3,4,5]. Red meat plays a vital role in meeting human dietary requirements, because it provides high quality protein, fat-soluble vitamins, minerals, and essential fatty acids [6, 7]. Red meat is also a source of health-beneficial Long Chain (≥ C20) omega-3 polyunsaturated fatty acids (LC omega-3 PUFA), comprising eicosapentaenoic (EPA, 20:5n-3), docosahexaenoic (DHA, 22:6n-3), and docosapentaenoic (DPA, 22:5n-3) acids. EPA, DHA and DPA are known to positively influence human health with mitigating properties against the prevalence of metabolic, cardiovascular, and chronic diseases [8,9,10], hence the need for striking the right balance between health-beneficial LC omega-3 PUFA composition and intramuscular fat (IMF) content in bovine meat is essential. The research quest for healthy red meat with enhanced culinary properties of sensory organoleptic attributes of taste, aroma, tenderness, juiciness, and health-beneficial LC omega-3 PUFA is on the increase [11,12,13]. However, humans cannot synthesize LC omega-3 PUFA due to their inability to produce Δ12- and Δ15-desaturase enzymes [14, 15]. As a result, humans rely heavily on dietary sources like red meat and seafood to meet their daily dosage of LC omega-3 PUFA [9, 15, 16]. Seafood, comprising mainly oily fish such as mackerel, herrings, sardines, salmon, and tuna, remains a key source (80%) of LC-omega-3 PUFA. However, due to limited access to fish and other seafood products, the focus has shifted to increasing and developing a variety of alternative food sources, especially of animal origin, necessitated by the nutritional and health claims for functional foods rich in LC-omega-PUFA. These include processed and raw foods enriched with LC-omega-3 PUFA from meat, milk, and eggs from livestock fed omega-3 oil rich supplements [17,18,19,20,21,22].
However, over-exploitation of global wild fish stocks threatens the sustainability of seafood as a source of LC omega-3 PUFA [23], while the low availability of seafood in many parts of the world limits consumption [24, 25]. As a result, many consumers do not meet the recommended seafood intake. For instance, Australians consumed 245 g of seafood per week in 2019–2020 [26], which is 135 g below the 375 g recommended by the Food Standards Australia New Zealand [27]. Therefore, red meat will be an important alternative source of LC omega-3 PUFA for Western diets due to preference and culture inculcation [28]. A large contribution of total LC-omega-3 PUFA intake in adult Australians comes from beef and lamb at 28%, compared to poultry and pork at 10% and 4%, respectively [29]. For most Australians, meat is the major contributor of LC-omega-3 PUFA, especially DPA, the predominant PUFA in meat [29,30,31]. Therefore, recognizing the potential significance of meat consumption as a major contributor to dietary intakes of LC-omega-3 PUFA in the Australian diet, this paper sought to explore and unravel the breed variations in LC omega-3 PUFA compositions, IMF and FMP in grazing purebred Angus, Hereford and Wagyu beef cattle.
The beef industry contributes significantly to the Australian economy. Australia produced 1.9 million tonnes of carcass weight in 2021, and was the fourth largest beef and veal exporter after Brazil, India, and the USA [32]. Beef and veal consumption in Australia was 18.1 kg/capita beef carcass weight equivalent in 2020 compared to the global average of 6.3 kg/capita [33]. To meet the market demand while minimizing environmental impacts, the Australian beef production systems target suitable cattle genotypes and high productivity to maximize income and minimize input costs, mainly feed costs, which may exceed 60% of the total production costs [32, 34]. Therefore, beef cattle production in Australia is mainly pasture-based. Grasslands occupy approximately 60% of the land surface, of which beef cattle constitute 78% of the main grazing livestock population [35, 36]. It has been reported that beef production can be sustainable where pastures are the primary feed source [34]. A sustainable beef production system must have a low environmental impact, contribute to food security, maintain biodiversity in the ecosystem, is easily accessible, and economically affordable [34]. A pasture-based production system fits this bill because it provides energy and essential nutrients at approximately half the cost of grain-based feedlot rations, alongside an efficient utilization of beef genotypes for high productivity, maximum income and limited input costs, thus ensuring the most cost-effective means of achieving maximum and efficient beef production sustainably [34, 37]. Pastures generally contain a higher proportion of α-linolenic acid (ALA, 18:3n-3), which is a precursor to the synthesis of the health-beneficial LC omega-3 PUFA [38, 39], than grain-based rations in the feedlot system.
Current research focus in the red meat industry has shifted towards meat eating quality with emphasis now being placed on increasing LC omega-3 PUFA and IMF on one hand, while lowering FMP and saturated fatty acids (SFA) on the other, because SFA serve as precursors for cholesterol and low-density lipoproteins (LDL) [5, 11, 40,41,42,43,44]. Past investigations have suggested that nutritional strategies can be used to increase beneficial LC omega-3 PUFA and IMF contents, and reduce SFA in beef [45]. However, the bioavailability of supplemented omega-3 in the muscle is significantly affected by biohydrogenation in the rumen [46, 47]. Conversely, previous studies have also demonstrated the genetic enhancement of LC omega-3 PUFA in the M. longissimus dorsi muscle of beef cattle through breed selection [48, 49]. Therefore, the genetic selection of beef cattle breeds with greater genetic propensities to synthesize LC omega-3 PUFA offer a more permanent, long-term, and cumulative approach to modifying fatty acid composition in ruminants [50, 51]. Furthermore, there is evidence of regional and production system variations in the fatty acid compositions of Angus, Hereford, Limousin, Longhorn, and Wagyu beef cattle raised in Japan and the USA [40, 42, 43, 52,53,54,55,56]. However, most of these previous beef cattle fatty acid composition studies originated from breeds on diverse diets and of different ages, making it difficult to extrapolate the results and make global comparisons [40, 43].
IMF (also referred to as marbling), is primarily used to evaluate carcass quality in Australia and other developed countries [57,58,59]. IMF is a major determinant of eating quality and contributes to palatability, tenderness, flavour, juiciness and overall liking in beef [60, 61]. IMF is also directly associated with the accumulation of oleic acid (18:1n-9) [62, 63], while beef flavour is positively influenced by the concentration of oleic acid [12, 64, 65]. FMP is an index of fat hardness or softness in beef [61, 66]. Soft fat has a relatively lower melting point than hard fat, and this quality index has implications on beef processing and eating quality [66, 67]. High oleic acid content in beef IMF decreases FMP, leading to softer fat because of the presence of a double bond in its carbon atom nomenclature [67, 68].
In Australia, Hereford, Wagyu, and Angus cattle are increasingly being used to produce beef with high eating quality characteristics such as tenderness, juiciness, flavour and marbling [34, 60, 61]. However, more studies are required to shed more light on the IMF, FMP and LC omega-3 PUFA compositions in the M. longissimus dorsi muscles of Australian pasture-based Angus, Hereford, and Wagyu under the same routine nutritional regime, similar ages and production system for an unbiased comparative analysis of eating quality attributes when the animals are still young and alive. Therefore, the primary objective of this study was to evaluate and compare the compositions of LC omega-3 PUFA, IMF, and FMP in M. longissimus dorsi muscle biopsies of Angus, Hereford, and Wagyu beef cattle in a pasture-based production system. It was hypothesized that the compositions of health-beneficial LC omega-3 PUFA, IMF, and FMP will vary between the muscle biopsies of pasture-fed Angus, Hereford, and Wagyu beef cattle raised under the same production system.
Results
Results from Kruskal–Wallis tests of breed variations in IMF, FMP, and fatty acid profile of the M. longissimus dorsi muscle and test of significance (P < 0.05) in Angus, Hereford, and Wagyu cattle are presented in Table 1, and sex differences are presented in Table 2. Breed had a significant influence on IMF, FMP, and the compositions of oleic acid, ALA, EPA, DHA, DPA, and total EPA + DHA + DPA of M. longissimus dorsi muscle (P ≤ 0.03; Table 1), in which the Wagyu and Angus breeds had the highest and Hereford the lowest IMF (P = 0.03). The reverse trend was observed in FMP values where Hereford cattle had the highest, Angus intermediate, and Wagyu the lowest FMP.
There were variations in fatty acid composition between the cattle breeds (Table 1). The Wagyu and Angus breeds had similar oleic acid (18:1n-9) contents, closely followed by the Hereford (P < 0.01). However, no differences were observed between the breeds in LA (18:2n-6) content. The highest ALA, and DPA were detected in Wagyu (P ≤ 0.03). Hereford had similar EPA and DPA concentrations to Angus (P ≥ 0.46). Total EPA + DHA + DPA content in Wagyu, Angus, and Hereford was 28.8, 21.5, and 22.1 mg/100g tissue (P = 0.01), respectively. At 28.8 mg/100g tissue, the Wagyu was the closest to Australia and New Zealand recommended “source level” threshold of 30 mg/100g tissue of health-beneficial ≥ C20 LC omega-3 PUFA content [69, 70].
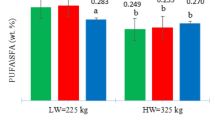
The effect of gender on fatty acid profile, IMF (%), and FMP (oC) of the M. longissimus dorsi muscle of Angus, Hereford and Wagyu cattle are presented in Table 2, where it was demonstrated that sex significantly (P ≤ 0.03) affected IMF, FMP, and the compositions of oleic acid, ALA, DHA, DPA, EPA + DHA, EPA + DHA + DPA, ALA + EPA + DHA + DPA, total saturated fatty acids (tSFA), monounsaturated fatty acids (tMUFA), polyunsaturated fatty acids (tPUFA), and omega-6 (n-6) PUFA. It was also evident that yearling heifers produced more IMF (11.9% vs 5.3%) with lower FMP (33°C vs 37°C) than yearling bulls. Furthermore, yearling heifers had higher contents of oleic acid (930 vs 224 mg/100g), ALA (11.6 vs 9.3 mg/100g), DHA (16.1 vs 12.8 mg/100g), DPA (13.1 vs 10.6 mg/100g), EPA + DHA (29 vs 24 mg/100g), EPA + DHA + DPA (52 vs 38 mg/100g), ALA + EPA + DHA + DPA (853 vs 269 mg/100g), tSFA (1013 vs 276 mg/100g), tMUFA (199 vs 153 mg/100g), tPUFA (135 vs 109 mg/100g), and n-6 PUFA (57 vs 42 mg/100g), than yearling bulls. However, omega-3 (n-3) PUFA (2.13 vs 2.20 mg/100g) and n-6/n-3 ratio (0.48 vs 0.46) were similar regardless of gender. The interaction between breed and sex was a significant (P ≤ 0.05) source of variation in FMP, ALA, EPA, DHA, DPA and EPA + DHA, as demonstrated in Table 3, suggesting that it should be taken into consideration when selecting for health-beneficial LC omega-3 PUFA, FMP and IMF in the M. longissimus dorsi muscle of grazing Angus, Hereford and Wagyu beef cattle.
Pairwise multiple breed comparisons with Bonferroni’s adjusted p-values in Fig. 1 shows differences between the cattle breeds in IMF, FMP, and LC omega-3 PUFA composition in the M. longissimus dorsi muscle. There were differences between Angus and Wagyu in FMP (P < 0.05), ALA (P < 0.01), EPA (P = 0.02), DHA (P = 0.01), DPA (P = 0.01), and total EPA + DHA + DPA (P = 0.01). However, there were no significant differences in oleic acid (P = 0.52) and IMF (P = 0.78) between Angus and Wagyu. There were no differences in ALA (P = 0.94), EPA (P = 0.63), DPA (P = 0.46), and total EPA + DHA + DPA (P = 0.57) compositions between Angus and Hereford. However, differences in IMF (P < 0.01), FMP (P < 0.05), oleic acid (P < 0.01), and DHA (P = 0.02) were observed between Angus and Hereford.
Bonferroni’s adjusted p-values for multiple breed comparisons of intramuscular fat (IMF), fat melting point (FMP), oleic acid (18:1n-9), 18:3n-3 (ALA) and long-chain omega-3 polyunsaturated fatty acids (EPA, DHA, DPA) in the M. longissimus dorsi muscle of Bowen Genetics Forest Pastoral Angus (
 ), Hereford (
), Hereford (
 ) and Wagyu (
) and Wagyu (
 ) cattle. Fatty acids are presented in mg/100g tissue. Significance level set at (P < 0.05)
) cattle. Fatty acids are presented in mg/100g tissue. Significance level set at (P < 0.05)
The correlations between IMF, FMP, oleic acid, LA, ALA, EPA, DHA, DPA, total EPA + DHA, total ALA + EPA + DHA + DPA, and total EPA + DHA + DPA are presented in Fig. 2. Highly positive correlations (P < 0.001) between ALA and oleic acid (0.60), EPA (0.77), DHA (0.57), DPA (0.72), EPA + DHA + DPA (0.74), and total omega-3 (0.94), and medium to low correlations between LA and LC omega-3 PUFA EPA (0.47), DHA (0.28), and DPA (0.46) were observed. Similarly, a positive relationship between IMF and oleic acid was evident (P < 0.01), while the correlations between IMF and FMP, ALA, EPA, DHA, and DPA were negative (Fig. 2B; P < 0.01).
Correlation A and P-value B plots summarizing the relationships between IMF (intramuscular fat), FMP (fat-melting point), and fatty acids of the M. longissimus dorsi muscle. Blue indicates positive correlations, and red shows negative correlations. Total omega-3 = ALA + EPA + DHA + DPA. Fatty acids are presented in mg/100g tissue. (* P < 0.05, ** P < 0.01, *** P < 0.001)
Discussion
Ensuring that all animals were of yearling age, similar body condition and liveweight, grass-fed, kept under the same management, and muscle biopsy samples taken from the same anatomical site minimized the impact of potential confounding phenotypic factors capable of affecting lipid metabolism attributes. The modern consumers' quest for healthy meat products with adequate health-beneficial LC omega-3 PUFA to minimize the risks and burdens of cardiometabolic and cancer-related mortality [71, 72] has been one of the drivers behind meat producers re-aligning their breeding programs to meet this market demand [1]. Although the easiest and fastest means of modifying the fatty acid composition of ruminant livestock is through dietary supplementation, previous studies had demonstrated that this approach is a temporary and non-cumulative measure, with highly variable and contentious results depending on supplement type and a myriad of other factors [8]. The breed selection criterion on the other hand, presents a cumulative and permanent improvement strategy if breed variation in the trait of interest is sufficiently high. The Angus has become very popular among Australian beef producers since the 1990s due to its intrinsic ability for a relatively faster growth rate than the Wagyu, and its intermediate IMF content compared to the Hereford, Australia's traditional beef breed of choice, renowned for its heavy, high-quality carcasses in both grazing and feedlot production systems [73]. The Wagyu breed has a distinctively high IMF content and is a late maturing breed, making it ideal for the long-fed, high-quality, Japanese beef market [73], specifically bred for high marbling carcasses [74]. The selection of beef cattle with great propensity to produce health-beneficial LC omega-3 PUFA may be the primary strategy by which fatty acid composition can be modified permanently.
Intramuscular fat and fat melting point
Meat eating quality drives consumer preferences, acceptability, and satisfaction, which all hinge on high IMF and low FMP, as critical drivers of high quality beef consumption. Therefore, beef producers continuously strive to add value to ruminant-derived meat products to meet this rising market demand [74]. In many developed countries, including Australia, IMF is considered as a significant determinant of carcass quality. FMP is associated with the overall palatability of meat [75], because it is an indicator of the degree of saturation in fatty acids, which in turn, impacts meat firmness, processing ease, and consumer appreciation of beef [54, 74]. This study established that the Wagyu breed had the highest IMF (11.1%) and lowest FMP (33.0 °C) in comparison to the Hereford breed with the lowest IMF (5.2%) and highest FMP (55.0 °C). The low FMP of the Wagyu breed in this study agrees with previous reports of Smith et al. [68] and Chung et al. [52] where eight and twelve months old hay-fed Wagyu cattle were reported to have FMP of 35.3 °C and 38.9 °C, respectively. Eight and twelve months old grass-fed Angus cattle had FMP of 37.9 °C and 42.8°C, respectively [52]. Australian Angus x Hereford crossbreds were reported to have FMP of 47.2°C [76], compared to purebred Japanese black steers at 14 months of age with a melting point of 35.5 °C, which decreased to 21.2 °C when they were 28 months old [74].
The main fatty acids found in beef are stearic acid (18:0), palmitic acid (16:0) and oleic acid (18:1), which are known to affect the hardness, softness and FMP of fat [20, 77,78,79]. Soft fat in beef has a low melting point because of its high unsaturated fatty acids content, mainly MUFA and PUFA, whereas hard fat has a high melting point because of its high SFA content [68, 78]. The double bonds in MUFA and PUFA can be more easily broken, resulting in comparatively lower FMP than the more stable SFA single bonds that are more difficult to break, hence resulting in high FMP [61].
The melting points of stearic and palmitic acids are 69.6 °C and 62.9 °C, respectively, hence they are associated with higher FMP [80]. In contrast, oleic acid melts at 13.4 °C, hence its high concentration in beef fat leads to a low FMP [78]. The breed differences in FMP observed in this study may also be due to the stearoyl-CoA desaturase (SCD) enzyme activity and gene expression. The SCD gene encodes the rate-limiting enzyme that catalyzes the synthesis of MUFA from SFA [78]. Chung et al. [81] reported 20% greater SCD activity and 70% more SCD mRNA in Wagyu than in Angus adipose tissues. MUFA constitute soft fat which has a low melting point because it consists of high unsaturated fatty acids content with double bonds that are easy to break under low heat.
Previous studies in Australian and Japanese beef cattle showed significant increases in FMP as the concentration of stearic acid increased in the adipose tissue. Japanese Black cattle raised under Japanese conditions contained only 8% stearic acid of total fatty acids, with an average melting point of 22.8 °C [75]. However, under Australian feedlot management conditions, stearic acid increased to 25% with an average FMP of 45.1 °C [68]. Chung et al. [52] and Wood et al. [79] reported a strong relationship between FMP and stearic acid in addition to demonstrating that different production systems diversely contribute to beef cattle fat deposition, fatty acid composition, and associations with FMP.
A previous report on sensory evaluation found that the IMF content of M. longissimus dorsi of Japanese Black steers ranged from 23.2%—48.6% and significantly increased juiciness, fattiness, and tenderness [82, 83]. In the current study, the IMF was less than the 25%—40% range previously reported in grain-fed, purebred Wagyu in Japan [82, 84, 85], possibly due to dietary and age differences, as the Australian pasture-based Wagyu in the current study, were yearlings fed solely on pasture, hence, the observed lower IMF is expected [73]. It has been reported that grain-fed beef cattle increase their IMF content faster than their pasture-fed counterparts [86]. Therefore, most Wagyu beef cattle aimed at the Japanese market are intensively fed on high energy-dense grains in the feedlot to produce intramuscular lipid concentrations of over 30% [87, 88]. This is because consumers prefer marbling with health-beneficial LC omega-3 PUFA and adequate crude protein [89]. The present results are similar to a previous study conducted in an Australian pasture-based production system where the IMF in Wagyu ranged from 7.8—17.5%, and 5.2—9.9% in Angus [64]. Hereford-sired steers born to Angus, Holstein–Friesian, and Jersey cows had IMF percentages ranging from 2.7% to 5.8% in the New Zealand pasture production system [90]. In Argentina, pasture-fed Angus and Hereford had IMF percentages of 3.1% and 2.4%, respectively [40]. Our results corroborate previous studies that reported pasture-fed beef cattle with lower marbling compared to grain-fed cattle, regardless of breed [86]. The IMF of Angus, Hereford, and Wagyu beef cattle breeds in the current study were all above the 3% threshold required in Australia for classifying beef as high meat eating quality [91, 92].
Linoleic, α-linolenic, oleic acids and their correlations
The current fatty acid composition results demonstrate the dominance of oleic acid in the M. longissimus dorsi muscle of Wagyu and Angus followed by Hereford in that order (Fig. 1 and Table 1). This agrees with other studies that reported similar dominance of oleic acid in the muscles of pasture-fed Wagyu [93], Angus [94], and Hereford x Angus [95]. Positive relationships between fatness and oleic acid in ruminants have also been reported [96, 97]. In the present study, both Angus and Wagyu had greater IMF and oleic acid contents than the Hereford. A direct link exists between low FMP and oleic acid with impacts on the overall palatability and acceptability of red meat by consumers [52, 74]. A low FMP allows the fat to melt in the mouth during consumption and contributes to the unique taste of beef [74, 98]. The type of production system also affects the concentration of oleic acid in the muscle of beef cattle [64]. High and low concentrations of oleic acid in feedlot and grass-fed systems, respectively, were reported by Smith et al. [75].
The concentration of oleic acid in the Wagyu differs from other beef cattle breeds because of its high genetic disposition to increased SCD enzyme activity and gene expression in the adipose and muscle tissues [75], which has been reported to have a very high heritability in Japanese Black cattle [99]. In the current study, the concentration of oleic acid in pasture-based yearling Angus and Wagyu were comparable. This could be explained by the fact that we analysed muscle biopsy samples where the activity of SCD is reported to be low compared to the adipose tissue [100,101,102,103]. Also, it has been previously reported that pasture feeding depresses the activity of SCD and reduces marbling score [81, 104, 105]. Furthermore, although there is a rapid increase in subcutaneous adipocyte volume between birth and weaning, de novo fatty acid biosynthesis is comparatively gradual, slower, and less associated with any significant change in ∆9 desaturase gene expression at the growing stages of yearling calves [75]. Sturdivant et al. [106] reported on the role of SCD in the conversions of stearic, palmitic, and myristic acids to oleic acid in muscle and adipose tissues that support the claim of a genetic basis for oleic acid biosynthesis in ruminants [106]. In this present study, no significant differences were observed in the concentrations of LA in grass-fed Angus, Hereford and Wagyu. However, the Wagyu had nearly twice as much ALA as Angus and Hereford, thus suggesting a possible genetic basis for this variation [59]. Other studies have reported lower LA and ALA values in Angus and Hereford than in the present study [40, 64].
Eicosapentaenoic, docosahexaenoic and docosapentaenoic acids
The highest DPA and EPA + DHA + DPA LC omega-3 PUFA contents were in Wagyu, except for EPA and DHA, where no clear differences were observed between Wagyu, Angus and Hereford. The levels of EPA, DHA, and DPA observed in the current study are similar to those reported by Frank et al. [64] and Bermingham et al. [93]. Other reports have shown that LC omega-3 PUFA concentrations are higher in pasture-finished than feedlot-finished production systems [107,108,109], primarily due to the high concentration of ALA in pastures [19, 110]. This suggests that the pasture-based production system could provide a cheap and long-term solution for producing beef with consistent meat eating quality and improved health-promoting LC omega-3 PUFA. This result will sit very well with health-conscious consumers who are willing to pay a premium for healthy beef [1, 16]. At 28.8 mg/100g, the Wagyu was closest to the Australia and New Zealand recommended “source level” threshold of 30 mg/100g tissue of health-beneficial LC omega-3 fatty acids content [69]. Although seafood is the richest dietary source of LC omega-3 PUFA [111], over-exploitation of fish stocks, individual preference, and cultural inculcation limit the use of seafood as a sustainable source of LC omega-3 PUFA [23,24,25]. In Australia, the recommended total DHA + EPA + DPA levels are 90 and 160 mg/day for women and men, respectively [69]. Since beef and veal consumption is estimated at 49.5 g/day [33], the cattle breeds evaluated in this study can provide approximately 12–16% of the recommended DHA, EPA and DPA intake for women and 7–9% for men. Therefore, red meat will continue to be an important source of LC omega-3 PUFA [28] that may help meet the dietary deficit caused by low consumption of seafood. Although an increase in muscle PUFA composition can potentially reduce meat oxidative stability [112], the storage of vacuum-packaged meat at 1.5 ± 0.8 °C has been reported to maintain the oxidative stability of striploin muscle samples obtained from Australian grass-fed cattle for up to 20 weeks [113], hence, consumers can access beef with high composition of the health claimable PUFA at a reduced risk of lipid oxidation.
The Wagyu breed has a fatty acid profile that is different from other cattle breeds, because of its comparatively higher genetic predisposition to produce more MUFA than other bovine breeds [49, 53, 63, 74, 98, 114,115,116]. The heritability of MUFA and PUFA in Wagyu cattle has also been reported to be very high at 0.68 and 0.47, respectively [99]. Breed affects the concentration of unsaturated fatty acid in beef cattle by influencing the activities and expressions of lipogenic genes such as the SCD, fatty acid binding protein (FABP) and fatty acid synthase (FAS) [8, 52, 86]. Therefore, the summation differences in total EPA + DHA + DPA seen in Table 1 could be as a result of variation in genetic predisposition between the breeds, because we ensured that all potential confounding phenotypic factors capable of affecting lipid metabolism were minimized in the present study. The pasture feeding of beef cattle also influences the concentrations of linoleic and α-linolenic acids [8]. Previous studies have shown that the duodenal flow of α-linolenic acid (a precursor for the de novo synthesis long-chain polyunsaturated fatty acids) in pasture-fed cattle increases, while that of oleic acid simultaneously decreases due to a decrease in the activity of SCD [117, 118]. Therefore, the genetic selection of beef cattle breeds in a pasture-based system with greater genetic propensities to synthesize LC omega-3 PUFA may be the solution for a permanent, sustainable, long-term, and cumulative approach to modifying fatty acid composition in ruminants.
Gender and interactions with breed
The composition of fatty acids and IMF in the skeletal muscle of cattle is markedly affected by gender [119]. The main effect of gender in the present study was observed for IMF, FMP, the compositions of ALA, DHA, DPA, EPA + DHA, EPA + DHA + DPA and ALA + EPA + DHA + DPA, where yearling heifers performed better than yearling bulls in all categories. These data are consistent with the previous results showing that heifers have significantly higher fat content and the composition of fatty acids compared to intact bulls [120]. A study conducted by Carrilho et al., [121] found that castrated bulls produced more IMF and the composition of oleic acid and DPA than intact bulls. Judge et al., [122] found that meat quality of intact bulls was inferior to that of both castrated bulls and heifers with comparable results between the latter two genders, suggesting that the effect could be a result of differences in sex hormones [123] between the intact bulls and heifers. A study by Zhang et al., [124] found that testosterone levels were significantly reduced in castrated bulls, whereas the IMF content and triglycerides (TGs) were significantly increased compared to intact bulls. The effect of hormones, testosterone and oestrogen, in muscle tissues has been well documented [125]. Oestrogen plays a major role in the regulation of energy metabolism pathway, including glycolysis, fatty acid β-oxidation synthesis and glucose transportation [126]. Testosterone on the other hand, downregulates the activities of glycerol-3-phosphate dehydrogenase and adiponectin secretion, an adipose-specific secretory protein, in the differentiation of bovine intramuscular adipocytes [127, 128]. A previous study found that castrated Korean cattle expressed the lipogenic genes acetyl-CoA carboxylase and fatty acid synthase, but suppressed the expressions of the lipolytic genes adipose triglyceride lipase and monoglyceride lipase in the M. longissimus dorsi [129]. The results in the present study suggest that gender contributes to an improved meat quality through the regulation of hormones and lipogenic genes to increase lipid uptake and lipogenesis, and should be a considered strategy for improving the composition of LC omega-3 PUFA, IMF and FMP in Angus, Hereford and Wagyu in a pasture-based production system. The significant interaction between breed and sex observed in FMP, ALA, EPA, DHA, DPA and EPA + DHA (Table 3) suggest that hormonal differences between genders would vary between breeds and this interaction should be taken into consideration when selecting for FMP, IMF and health-beneficial LC omega-3 PUFA in the M. longissimus dorsi muscle of grazing Angus, Hereford and Wagyu beef cattle.
Conclusion
Taken together, the outcomes of the current study conclusively demonstrate that in a pasture-based production system, breed, sex and their interactions were significant sources of variation in IMF, FMP and LC omega-3 PUFA content in yearling bulls and heifers of the same age and routine management. Yearling heifers produced more IMF with lower FMP, and higher LC omega-3 PUFA content than yearling bulls. At 28.8 mg/100g tissue, the Wagyu breed produced M. longissimus dorsi muscle with the highest LC omega-3 PUFA content, and was the closest to attaining the Australia and New Zealand recommended “source level” threshold of 30 mg/100g tissue. These findings also suggest that the Bowen Genetics Forest Pastoral beef cattle studs are well positioned in the international research context to improve and provide important alternative sources of LC omega-3 PUFA that can be used to cover the deficit in the health claimable fatty acids in Western diets.
Methods
The reporting in the manuscript follows the recommendations of Kilkenny et al. [130] in the ARRIVE guidelines (2.0 Essential 10 list for animal research). The use of animals and protocols performed in this study were approved by the James Cook University Animal Ethics Committee and were conducted in accordance with the Animal Care and Protection Act for the Australian Code for the Care and Use of Animals for Scientific Purposes (Ethics Approval Number A2724). The owners of the farm consented to the use of their herds as experimental animals for this study.
Animals and management
In this on-farm study, 30 Angus (14 males, 16 females), 22 Hereford (19 males, 3 females), and 75 Wagyu (25 males, 50 females) beef cattle, progeny of 15 sires in total, comprising 5 sires from each breed, raised, and maintained as self-replacing purebred herds at Bowen Genetics Forest Pastoral stud, Barraba, New South Wales, Australia, were utilized. As opposed to an on-station experimental design where equal numbers of animals are possible, this was an on-farm experiment with variable number of available cattle, hence an unequal distribution of sample sizes between breeds. The animals grazed with their dams until they were weaned at 6–8 months and pasture-fed on ryegrass, kept in the same property but separated by breed and sex into six herds. The six paddocks were monitored regularly to ensure sufficient ryegrass pasture allowance. At sampling, the cattle were 12 ± 2.43 months old, of similar body condition scores (3.0–3.5) and 450–455 kg liveweight.
Muscle biopsy sampling, fatty acid, intramuscular fat, and fat-melting point analyses
The M. longissimus dorsi biopsy samples were obtained from the interface of the 12th and 13th ribs, and the sampling procedure was similar to that described previously by Malau-Aduli et al. [51], Pewan et al. [131], and Otto et al. [132], while the laboratory procedures for the determination of fatty acid profile, IMF, and FMP have been extensively described in our previous publications [22, 50, 57]. Briefly, the hair from around the 12th and 13th ribs of the experimental animals kept in a crush was clipped. A disinfectant, alcohol/chlorohexidine solution was applied at the surgical site. About 20 mL of a local anaesthetic, lignocaine, was injected at the surgical site before 2-5g of muscle biopsy was collected with a sterilized scalpel. The incision site was stitched, and antibiotics administered to prevent infection. One gram of the muscle biopsy sample was homogenised, transferred to a labelled 50 mL plastic tube containing 20 mL of CHCl3:MeOH (2:1) solvent and 5 mL of 10% KCl to analyse for IMF content. The extracted IMF was placed in an oven at 100 °C for about 1–2 min to melt. The melted IMF was sucked into a thin capillary tube using air suction and gradually heated in a test tube containing cold water and a bulb thermometer to estimate the FMP. A single-phase overnight extraction technique utilizing CHCl3:MeOH:H2O (1:2:0.8 v/v) was used to extract total lipids from the remaining 1 g of un-homogenized muscle tissue samples, followed by phase separation with the addition of CHCl3:Saline-Milli-Q H2O (1:1 v/v). The lipids were separated into classes by thin-layer chromatography (TLC). An aliquot of the lipid extract was utilized for transmethylation with MeOH:CHCl3:HCl (10:1:1 v/v). Fatty acid methyl esters (FAME) were extracted thrice using hexane (4:1 v/v). An internal standard (C19:0) of a known concentration was added in a 1500 μL vial encompassing the extracted FAME. The fatty acids content was calculated as follows: FA mg/100 g = (Total lipid) × (LCF [0.912]) × ([%FA]/100) × 1000, where 0.912 was the lipid conversion factor (LCF).
Statistical analysis
The R statistical software version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria) was used to perform data analysis. Summary statistics were initially computed to scrutinize the data for entry errors and normality testing. The data were found to be non-normally distributed, hence, a non-parametric approach was applied. Descriptive summary statistics of fatty acid composition, IMF, and FMP were presented as medians and inter-quartile ranges. Breed, sex and sire within breed differences in fatty acid composition were statistically tested using the Kruskal–Wallis test and Wilcoxon tests (Mann–Whitney) with Bonferroni’s adjusted P-value across groups. The relationships between IMF, FMP, and fatty acids were evaluated using the Spearman correlation analysis. Statistical inference was set at a P < 0.05 level of significance. Furthermore, the effect of breed, sex and their interactions on fatty acid composition were tested using linear regression models.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to contractual confidentiality clause obligation, but are available from the corresponding author on reasonable request.
Abbreviations
- LC omega-3PUFA:
-
Long-chain omega-3 polyunsaturated fatty acids
- FMP:
-
Fat-melting point
- IMF:
-
Intramuscular fat
- LA:
-
Linoleic acid
- ALA:
-
α-Linolenic acid
- EPA:
-
Eicosapentaenoic
- DHA:
-
Docosahexaenoic
- DPA:
-
Docosapentaenoic
- tSFA:
-
Total saturated fatty acids
- tMUFA:
-
Total monounsaturated fatty acids
- tPUFA:
-
Total polyunsaturated fatty acids
- n-6:
-
Omega-6 fatty acids
- n-3:
-
Omega-3 fatty acids
- ARRIVE:
-
Animal Research: Reporting of In Vivo Experiments
- CHCl3:
-
Chloroform
- MeOH:
-
Methanol
- TLC:
-
Thin-layer chromatography
- FAME:
-
Fatty acid methyl esters
- LCF:
-
Lipid conversion factor
- KCL:
-
Potassium chloride
- H2O:
-
Water
- FA:
-
Fatty acid
References
Henchion MM, McCarthy M, Resconi VC. Beef quality attributes: a systematic review of consumer perspectives. Meat Sci. 2017;128:1–7.
Felderhoff C, Lyford C, Malaga J, Polkinghorne R, Brooks C, Garmyn A, et al. Beef quality preferences: factors driving consumer satisfaction. Foods. 2020;9:289.
de Nazaré Santos Torres R, Bertoco JPA, Arruda MCG, de Melo Coelho L, Paschoaloto JR, Ezequiel JMB, et al. The effect of dietary inclusion of crude glycerin on performance, ruminal fermentation, meat quality and fatty acid profile of beef cattle: Meta-analysis. Res Vet Sci. 2021;140:171–84.
Hoa V-B, Song D-H, Seol K-H, Kang S-M, Kim H-W, Kim J-H, et al. Coating with chitosan containing lauric acid (C12:0) significantly extends the shelf-life of aerobically – packaged beef steaks during refrigerated storage. Meat Sci. 2022;184:108696.
Pogorzelski G, Pogorzelska-Nowicka E, Pogorzelski P, Półtorak A, Hocquette J-F, Wierzbicka A. Towards an integration of pre- and post-slaughter factors affecting the eating quality of beef. Livest Sci. 2022;255:104795.
Indurain G, Beriain MJ, Sarries MV, Insausti K. Effect of weight at slaughter and breed on beef intramuscular lipid classes and fatty acid profile. Animal. 2010;4:1771–80.
Williams P. Nutritional composition of red meat. Nutr Dietet. 2007;64.
Nogoy KMC, Sun B, Shin S, Lee Y, Zi Li X, Choi SH, et al. Fatty acid composition of grain- and grass-fed beef and their nutritional value and health implication. Food Sci Anim Resour. 2022;42:18–33.
Calder PC. Docosahexaenoic acid. Ann Nutr Metab. 2016;69(Suppl. 1):8–21.
Calder PC. Very long chain omega-3 (n-3) fatty acids and human health. Eur J Lipid Sci Technol. 2014;116:1280–300.
Patel A, Desai SS, Mane VK, Enman J, Rova U, Christakopoulos P, et al. Futuristic food fortification with a balanced ratio of dietary ω-3/ω-6 omega fatty acids for the prevention of lifestyle diseases. Trends Food Sci Technol. 2022;120:140–53.
Garmyn A. Consumer preferences and acceptance of meat products. Foods. 2020;9:708.
Liu J, Ellies-Oury M-P, Chriki S, Legrand I, Pogorzelski G, Wierzbicki J, et al. Contributions of tenderness, juiciness and flavor liking to overall liking of beef in Europe. Meat Sci. 2020;168:108190.
Lee J, Lee H, Kang S, Park W. Fatty acid desaturases, polyunsaturated fatty acid regulation, and biotechnological advances. Nutrients. 2016;8:23.
Calder PC, Yaqoob P. Omega-3 polyunsaturated fatty acids and human health outcomes. BioFactors. 2009;35:266–72.
Byelashov OA, Sinclair AJ, Kaur G. Dietary sources, current intakes, and nutritional role of omega-3 docosapentaenoic acid. Lipid Technol. 2015;27:79–82.
Nguyen QV, Le VH, Nguyen DV, Malau-Aduli BS, Nichols PD, Malau-Aduli AEO. Supplementing grazing dairy ewes with plant-derived oil and rumen-protected EPA+DHA pellets enhances health-beneficial n−3 long-chain polyunsaturated fatty acids in sheep milk. Eur J Lipid Sci Technol. 2018;120(6):1700256.
Van Le H, Nguyen DV, Vu Nguyen Q, Malau-Aduli BS, Nichols PD, Malau-Aduli AEO. Fatty acid profiles of muscle, liver, heart and kidney of Australian prime lambs fed different polyunsaturated fatty acids enriched pellets in a feedlot system. Sci Rep. 2019;9:1238.
Le H, Nguyen QV, Nguyen DV, Otto JR, Malau-Aduli BS, Nichols PD, Malau-Aduli AEO. Enhanced omega-3 polyunsaturated fatty acid contents in muscle and edible organs of Australian prime lambs grazing lucerne and cocksfoot pastures. Nutrients. 2018;10:1985.
Mwangi FW, Blignaut DJ, Charmley E, Gardiner CP, Malau-Aduli BS, Kinobe RT, Malau-Aduli AEO. Lipid metabolism, carcass characteristics and Longissimus dorsi muscle fatty acid composition of tropical crossbred beef cattle in response to Desmanthus spp. forage backgrounding. Metabolites. 2021;11:804.
Otto JR, Malau-Aduli BS, Nichols PD, Malau-Aduli AEO. Influence of supplementing pasture-based primiparous Holstein- Friesian dairy cows with crude degummed canola oil on milk fatty acid composition. J Nutr Therapeut. 2014;3:55–66.
Pewan SB, Otto JR, Kinobe RT, Adegboye OA, Malau-Aduli AEO. Nutritional enhancement of health beneficial omega-3 long-chain polyunsaturated fatty acids in the muscle, liver, kidney, and heart of Tattykeel Australian White MARGRA lambs fed pellets fortified with omega-3 oil in a feedlot system. Biology. 2021;10:912.
Nichols PD, Petrie J, Singh S. Long-chain omega-3 oils –an update on sustainable sources. Nutrients. 2010;2:572–85.
Kennedy ET, Luo H, Ausman LM. Cost implications of alternative sources of (n-3) fatty acid consumption in the United States. J Nutr. 2012;142:605S-609S.
Walker R, Decker EA, McClements DJ. Development of food-grade nanoemulsions and emulsions for delivery of omega-3 fatty acids: opportunities and obstacles in the food industry. Food Funct. 2015;6:41–54.
Tuynman H, Dylewski M. Australian fisheries and aquaculture statistics 2021, Fisheries Research and Development Corporation. Canberra. https://daff.ent.sirsidynix.net.au/client/en_AU/search/asset/1034361/0. 2022.
FSANZ. Food Standards Australia New Zealand. Advice on fish consumption . https://www.foodstandards.gov.au/Pages/default.aspx. 2013.
Kronberg SL, Scholljegerdes EJ, Maddock RJ, Barceló-Coblijn G, Murphy EJ. Rump and shoulder muscles from grass and linseed fed cattle as important sources of n-3 fatty acids for beef consumers. Eur J Lipid Sci Technol. 2017;119(7):1600390.
Howe P, Meyer B, Record S, Baghurst K. Dietary intake of long-chain ω-3 polyunsaturated fatty acids: Contribution of meat sources. Nutr. 2006;22:47–53.
Howe PRC, Meyer BJ, Record S, Baghurst K. Contribution of red meat to very long chain omega-3 fatty acid (VLC ω3) intake. Asia Pacific J Clin Nutr. 2003;12(Suppl):S27.
Mann NJ, Ponnampalam EN, Yep Y, Sinclair AJ. Feeding regimes affect fatty acid composition in Australian beef cattle. Asia Pacific J Clin Nutr. 2003;12:42–42.
MLA. State of the industry report: The Australian red meat and livestock industry 2022 . https://www.mla.com.au/globalassets/mla-corporate/prices--markets/documents/trends--analysis/soti-report/2879-mla-state-of-industry-report-2022_d6_low-res_spreads.pdf. 2022.
OECD. OECD Data. Meat consumption . https://data.oecd.org/agroutput/meat-consumption.htm. 2021.
Greenwood PL. Review: An overview of beef production from pasture and feedlot globally, as demand for beef and the need for sustainable practices increase. Animal. 2021;15:100295.
Earl J. Grazing and pasture management and utilisation in Australia. In: Cottle D, Kahn L, editors. Beef Cattle Production and Trade. 1st ed. Collingwood, Victoria, Australia: CSIRO; 2014. p. 339–80.
Kemp DR, Michalk DI. Introduction. In pasture management technology for the 21st century. Melbourne, Victoria, Australia: CSIRO; 1994.
Hynd PI. Growing and finishing beef cattle at pasture and in feedlots. In: Cottle D, Khan L, editors. Beef cattle production and trade. Eds. Collingwood, Australia: CSIRO Publishing; 2014. p. 381–400.
Clapham WM, Foster JG, Neel JPS, Fedders JM. Fatty acid composition of traditional and novel forages. J Agric Food Chem. 2005;53:10068–73.
Casey NH, van Niekerk WA, Spreeth EB. Fatty acid composition of subcutaneous fat of sheep grazed on eight different pastures. Meat Sci. 1988;23:55–63.
Papaleo Mazzucco J, Goszczynski DE, Ripoli MV, Melucci LM, Pardo AM, Colatto E, et al. Growth, carcass and meat quality traits in beef from Angus, Hereford and cross-breed grazing steers, and their association with SNPs in genes related to fat deposition metabolism. Meat Sci. 2016;114:121–9.
Giuffrida-Mendoza M, de Moreno LA, Huerta-Leidenz N, Uzcátegui-Bracho S, Valero-Leal K, Romero S, et al. Cholesterol and fatty acid composition of longissimus thoracis from water buffalo (Bubalus bubalis) and Brahman-influenced cattle raised under savannah conditions. Meat Sci. 2015;106:44–9.
de Freitas AK, Lobato JFP, Cardoso LL, Tarouco JU, Vieira RM, Dillenburg DR, et al. Nutritional composition of the meat of Hereford and Braford steers finished on pastures or in a feedlot in southern Brazil. Meat Sci. 2014;96:353–60.
Huuskonen A, Jansson S, Honkavaara M, Tuomisto L, Kauppinen R, Joki-Tokola E. Meat colour, fatty acid profile and carcass characteristics of Hereford bulls finished on grazed pasture or grass silage-based diets with similar concentrate allowance. Livest Sci. 2010;131:125–9.
Muchenje V, Hugo A, Dzama K, Chimonyo M, Strydom PE, Raats JG. Cholesterol levels and fatty acid profiles of beef from three cattle breeds raised on natural pasture. J Food Comp Anal. 2009;22:354–8.
Scollan N, Hocquette J-F, Nuernberg K, Dannenberger D, Richardson I, Moloney A. Innovations in beef production systems that enhance the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 2006;74:17–33.
Buccioni A, Decandia M, Minieri S, Molle G, Cabiddu A. Lipid metabolism in the rumen: New insights on lipolysis and biohydrogenation with an emphasis on the role of endogenous plant factors. Anim Feed Sci Technol. 2012;174:1–25.
Lourenço M, Ramos-Morales E, Wallace RJ. The role of microbes in rumen lipolysis and biohydrogenation and their manipulation. Animal. 2010;4:1008–23.
Nürnberg K, Ender B, Papstein H-J, Wegner J, Ender K, Nürnberg G. Effects of growth and breed on the fatty acid composition of the muscle lipids in cattle. Zeitschrift Lebensmitteluntersuchung und -Forschung A. 1999;208:332–5.
Zembayashi M, Nishimura K, Lunt DK, Smith SB. Effect of breed type and sex on the fatty acid composition of subcutaneous and intramuscular lipids of finishing steers and heifers. J Anim Sci. 1995;73:3325–32.
Malau-Aduli AEO, Holman BWB, Kashani A, Nichols PD. Sire breed and sex effects on the fatty acid composition and content of heart, kidney, liver, adipose and muscle tissues of purebred and first-cross prime lambs. Anim Prod Sci. 2016;56:2122.
Malau-Aduli AEO, Siebert BD, Bottema CD, Pitchford WS. Breed comparison of the fatty acid composition of muscle phospholipids in Jersey and Limousin cattle. J Anim Sci. 1998;76:766.
Chung KY, Lunt DK, Choi CB, Chae SH, Rhoades RD, Adams TH, et al. Lipid characteristics of subcutaneous adipose tissue and M. longissimus thoracis of Angus and Wagyu steers fed to US and Japanese endpoints. Meat Sci. 2006;73:432–41.
Oka A, Iwaki F, Dohgo T, Ohtagaki S, Noda M, Shiozaki T, et al. Genetic effects on fatty acid composition of carcass fat of Japanese Black Wagyu steers. J Anim Sci. 2002;80:1005–11.
Perry D, Nicholls PJ, Thompson JM. The effect of sire breed on the melting point and fatty acid composition of subcutaneous fat in steers. J Anim Sci. 1998;76:87.
Boylston TD, Morgan SA, Johnson KA, Busboom JR, Wright RW, Reeves JJ. Lipid content and composition of wagyu and domestic breeds of beef. J Agric Food Chem. 1995;43:1202–7.
Eichhorn JM, Coleman LJ, Wakayama EJ, Blomquist GJ, Bailey CM, Jenkins TG. Effects of breed type and restricted versus ad libitum feeding on fatty acid composition and cholesterol content of muscle and adipose tissue from mature bovine females. J Anim Sci. 1986;63:781–94.
Malau-Aduli AEO, Edriss MA, Siebert BD, Bottema CDK, Pitchford WS. Breed differences and genetic parameters for melting point, marbling score and fatty acid composition of lot-fed cattle. J Anim Physiol Anim Nutr. 2000;83:95–105.
Stewart SM, Lauridsen T, Toft H, Pethick DW, Gardner GE, McGilchrist P, et al. Objective grading of eye muscle area, intramuscular fat and marbling in Australian beef and lamb. Meat Sci. 2021;181:108358.
Carvalho VV, Smith SB. Slip points of subcutaneous adipose tissue lipids do not predict beef marbling score or percent intramuscular lipid. Meat Sci. 2018;139:201–6.
Mwangi FW, Charmley E, Gardiner CP, Malau-Aduli BS, Kinobe RT, Malau-Aduli AEO. Diet and genetics influence beef cattle performance and meat quality characteristics. Foods. 2019;8:648.
Pewan SB, Otto JR, Huerlimann R, Budd AM, Mwangi FW, Edmunds RC, et al. Genetics of omega-3 long-chain polyunsaturated fatty acid metabolism and meat eating quality in Tattykeel Australian White lambs. Genes. 2020;11:587.
Brooks MA, Choi CW, Lunt DK, Kawachi H, Smith SB. Subcutaneous and intramuscular adipose tissue stearoyl-coenzyme A desaturase gene expression and fatty acid composition in calf- and yearling-fed Angus steers. J Anim Sci. 2011;89:2556–70.
Piao S, Okura T, Irie M. On-site evaluation of Wagyu beef carcasses based on the monounsaturated, oleic, and saturated fatty acid composition using a handheld fiber-optic near-infrared spectrometer. Meat Sci. 2018;137:258–64.
Frank D, Ball A, Hughes J, Krishnamurthy R, Piyasiri U, Stark J, et al. Sensory and flavor chemistry characteristics of Australian beef: Influence of intramuscular fat, feed, and breed. J Agric Food Chem. 2016;64:4299–311.
Jung E-Y, Hwang Y-H, Joo S-T. The relationship between chemical compositions, meat quality, and palatability of 10 primal cuts from Hanwoo steers. Korean J Food Sci Anim Resour. 2016;36:145–51.
Flakemore AR, McEvoy PD, Balogun RO, Malau-Aduli BS, Nichols PD, Malau-Aduli AEO. Degummed crude canola oil supplementation affects fat depot melting points in purebred and first-cross Merino sheep. Anim Vet Sci. 2014;2:75–80.
Smith SB, Smith DR, Lunt DK. Chemical and physical characteristics of meat/adipose tissue. In: Encyclopaedia of Meat Sciences. Elsevier; 2004. p. 225–38.
Smith SB, Yang A, Larsen TW, Tume RK. Positional analysis of triacylglycerols from bovine adipose tissue lipids varying in degree of unsaturation. Lipids. 1998;33:197–207.
National Health and Medical Research Council (Dept. of Health C and A. Nutrient Reference Values for Australia and New Zealand . https://www.nhmrc.gov.au/about-us/publications/nutrient-reference-values-australia-and-new-zealand-including-recommended-dietary-intakes#block-views-block-file-attachments-content-block-1. 2006.
Clayton E. Graham Centre Monograph No. 4: Long-chain omega-3 polyunsaturated fatty acids in ruminant nutrition: benefits to animals and humans. Charles Sturt University, Wagga Wagga, New South Wales, Australia; 2014.
Al-Shaar L, Satija A, Wang DD, Rimm EB, Smith-Warner SA, Stampfer MJ, et al. Red meat intake and risk of coronary heart disease among US men: prospective cohort study. BMJ. 2020;371:m4141.
Jo G, Oh H, Singh GM, Park D, Shin M-J. Impact of dietary risk factors on cardiometabolic and cancer mortality burden among Korean adults: results from nationally representative repeated cross-sectional surveys 1998–2016. Nutr Res Pract. 2020;14:384.
Campbell MA, King BJ, Allworth MB. The southern Australian beef industry. In: Cottle D, Kahn L, editors. Beef Cattle Production and Trade. 1st ed. Collingwood, Victoria, Australia: CSIRO; 2014. p. 185–204.
Motoyama M, Sasaki K, Watanabe A. Wagyu and the factors contributing to its beef quality: a Japanese industry overview. Meat Sci. 2016;120:10–8.
Smith SB, Lunt DK, Chung KY, Choi CB, Tume RK, Zembayashi M. Adiposity, fatty acid composition, and delta-9 desaturase activity during growth in beef cattle. Anim Sci J. 2006;77:478–86.
Yang A, Larsen TW, Powell VH, Tume RK. A comparison of fat composition of Japanese and long-term grain-fed Australian steers. Meat Sci. 1999;51:1–9.
Dinh TTN, To KV, Schilling MW. Fatty acid composition of meat animals as flavor precursors. Meat Musc Biol. 2021;5.
Turk SN, Smith SB. Carcass fatty acid mapping. Meat Sci. 2009;81:658–63.
Wood JD, Richardson RI, Nute GR, Fisher AV, Campo MM, Kasapidou E, et al. Effects of fatty acids on meat quality: A review. Meat Sci. 2004;66:21–32.
Smith SB. Marbling and its nutritional impact on risk factors for cardiovascular disease. Korean J Food Sci Anim Resour. 2016;36:435–44.
Chung KY, Lunt DK, Kawachi H, Yano H, Smith SB. Lipogenesis and stearoyl-CoA desaturase gene expression and enzyme activity in adipose tissue of short- and long-fed Angus and Wagyu steers fed corn- or hay-based diets. J Anim Sci. 2007;85:380–7.
Lida F, Saitou K, Kawamura T, Yamaguchi S, Nishimura T. Effect of fat content on sensory characteristics of marbled beef from Japanese Black steers. Anim Sci J. 2015;86:707–15.
Okumura T, Saito K, Sakuma H, Nade T, Nakayama S, Fujita K, et al. Intramuscular fat deposition in principal muscles from twenty-four to thirty months of age using identical twins of Japanese Black steers. J Anim Sci. 2007;85:1902–7.
Cameron PJ, Zembayashi M, Lunt DK, Mitsuhashi T, Mitsumoto M, Ozawa S, et al. Relationship between Japanese beef marbling standard and intramuscular lipid in the M. longissimus thoracis of Japanese Black and American Wagyu Cattle. Meat Sci. 1994;38:361–4.
Ueda Y, Watanabe A, Higuchi M, Shingu H, Kushibiki S, Shinoda M. Effects of intramuscular fat deposition on the beef traits of Japanese Black steers (Wagyu). Anim Sci J. 2007;78:189–94.
Smith SB, Gill CA, Lunt DK, Brooks MA. Regulation of fat and fatty acid composition in beef cattle. Asian-Aust J Anim Sci. 2009;22:1225–33.
Smith SB, Zembayashi M, Lunt DK, Sanders JO, Gilbert CD. Carcass traits and microsatellite distributions in offspring of sires from three geographical regions of Japan. J Anim Sci. 2001;79:3041.
Zembayashi M, Lunt DK, Smith SB. Dietary tea reduces the iron content of beef. Meat Sci. 1999;53:221–6.
Joo S-T, Hwang Y-H, Frank D. Characteristics of Hanwoo cattle and health implications of consuming highly marbled Hanwoo beef. Meat Sci. 2017;132:45–51.
Coleman LW, Hickson RE, Schreurs NM, Martin NP, Kenyon PR, Lopez-Villalobos N, et al. Carcass characteristics and meat quality of Hereford sired steers born to beef-cross-dairy and Angus breeding cows. Meat Sci. 2016;121:403–8.
Australian Brahman Breeders’ Association Limited. What do we know about getting fat into muscle? Beef CRC Bulletin. http://www.brahman.com.au/technical_information/meatScience/fatIntoMuscle.html. 2007.
Flakemore RA, Balogun RO, McEvoy PD, Malau-Aduli BS, Nichols PD, Malau-Aduli AEO. Genetic variation in intramuscular fat of prime lambs supplemented with varying concentrations of degummed crude canola oil. Int J Nutr Food Sci. 2014;3:203.
Bermingham EN, Reis MG, Subbaraj AK, Cameron-Smith D, Fraser K, Jonker A, et al. Distribution of fatty acids and phospholipids in different table cuts and co-products from New Zealand pasture-fed Wagyu-dairy cross beef cattle. Meat Sci. 2018;140:26–37.
Purchas RW, Knight TW, Busboom JR. The effect of production system and age on concentrations of fatty acids in intramuscular fat of the longissimus and triceps brachii muscles of Angus-cross heifers. Meat Sci. 2005;70:597–603.
Knight TW, Knowles S, Death AF, West J, Agnew M, Morris CA, et al. Factors affecting the variation in fatty acid concentrations in lean beef from grass-fed cattle in New Zealand and the implications for human health. New Zealand J Agric Res. 2003;46:83–95.
De la Fuente J, Díaz MT, Álvarez I, Oliver MA, Fonti FM, Sañudo C, et al. Fatty acid and vitamin E composition of intramuscular fat in cattle reared in different production systems. Meat Sci. 2009;82:331–7.
Raes K, De Smet S, Demeyer D. Effect of dietary fatty acids on incorporation of long chain polyunsaturated fatty acids and conjugated linoleic acid in lamb, beef and pork meat: A review. Anim Feed Sci Technol. 2004;113:199–221.
May SG, Sturdivant CA, Lunt DK, Miller RK, Smith SB. Comparison of sensory characteristics and fatty acid composition between Wagyu crossbred and Angus steers. Meat Sci. 1993;35:289–98.
Nogi T, Honda T, Mukai F, Okagaki T, Oyama K. Heritabilities and genetic correlations of fatty acid compositions in longissimus muscle lipid with carcass traits in Japanese Black cattle. J Anim Sci. 2011;89:615–21.
Cameron PJ, Rogers M, Oman J, May SG, Lunt DK, Smith SB. Stearoyl coenzyme A desaturase enzyme activity and mRNA levels are not different in subcutaneous adipose tissue from Angus and American Wagyu steers. J Anim Sci. 1994;72:2624–8.
Chang JHP, Lunt DK, Smith SB. Fatty acid composition and fatty acid elongase and stearoyl-CoA desaturase activities in tissues of steers fed high oleate sunflower seed. J Nutr. 1992;122:2074–80.
St John LC, Lunt DK, Smith SB. Fatty acid elongation and desaturation enzyme activities of bovine liver and subcutaneous adipose tissue microsomes. J Anim Sci. 1991;69:1064.
Archibeque SL, Lunt DK, Gilbert CD, Tume RK, Smith SB. Fatty acid indices of stearoyl-CoA desaturase do not reflect actual stearoyl-CoA desaturase enzyme activities in adipose tissues of beef steers finished with corn-, flaxseed-, or sorghum-based diets. J Anim Sci. 2005;83:1153–66.
Lunt DK, Chung KY, Choi CB, Smith SB. Production characteristics and carcass quality of Angus and Wagyu steers fed to US and Japanese endpoints. J Anim Vet Adv. 2005;4:949–53.
Duckett SK, Pratt SL, Pavan E. Corn oil or corn grain supplementation to steers grazing endophyte-free tall fescue. II. Effects on subcutaneous fatty acid content and lipogenic gene expression. J Anim Sci. 2009;87:1120–8.
Sturdivant CA, Lunt DK, Smith GC, Smith SB. Fatty acid composition of subcutaneous and intramuscular adipose tissues and M. longissimus dorsi of Wagyu cattle. Meat Sci. 1992;32:449–58.
Duckett SK, Wagner DG, Yates LD, Dolezal HG, May SG. Effects of time on feed on beef nutrient composition. J Anim Sci. 1993;71:2079–88.
Varela A, Oliete B, Moreno T, Portela C, Monserrrat L, Carballo JA, et al. Effect of pasture finishing on the meat characteristics and intramuscular fatty acid profile of steers of the Rubia Gallega breed. Meat Sci. 2004;67:515–22.
Enser M, Hallett KG, Hewett B, Fursey GAJ, Wood JD, Harrington G. Fatty acid content and composition of UK beef and lamb muscle in relation to production system and implications for human nutrition. Meat Sci. 1998;49:329–41.
Garton GA. Fatty acid composition of the lipids of pasture grasses. Nature. 1960;187:511–2.
Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, et al. n−3 fatty acids from fish or fish-oil supplements, but not α-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: A systematic review. Am J Clin Nutr. 2006;84:5–17.
Kearns M, Ponnampalam EN, Jacquier J-C, Grasso S, Boland TM, Sheridan H, et al. Can botanically-diverse pastures positively impact the nutritional and antioxidant composition of ruminant meat? – Invited review. Meat Sci. 2023;197:109055.
Holman BWB, Bailes KL, Kerr MJ, Hopkins DL. Point of purchase fatty acid profile, oxidative status and quality of vacuum-packaged grass-fed Australian beef held chilled for up to 12 weeks. Meat Sci. 2019;158:107878.
Louis F, Furuhashi M, Yoshinuma H, Takeuchi S, Matsusaki M. Mimicking Wagyu beef fat in cultured meat: Progress in edible bovine adipose tissue production with controllable fatty acid composition. Mater Today Bio. 2023;21:100720.
Sakuma H, Kobayashi E. Excellent meat quality in Wagyu beef and its genetic analysis. J Japan Assoc Odor Environ. 2011;42:276–84.
Suzuki K, Yokota S, Shiora H, Shimazu T, Iida F. Effect of meat grade, gender of the animal, and fatty acid content on the eating quality of Japanese black beef meat determined using testing panel. Nihon Chikusan Gakkaiho. 2013;84:375–82.
Kucuk O, Hess BW, Ludden PA, Rule DC. Effect of forage:concentrate ratio on ruminal digestion and duodenal flow of fatty acids in ewes. J Anim Sci. 2001;79:2233.
Waters SM, Kelly JP, O’Boyle P, Moloney AP, Kenny DA. Effect of level and duration of dietary n-3 polyunsaturated fatty acid supplementation on the transcriptional regulation of Δ9-desaturase in muscle of beef cattle. J Anim Sci. 2009;87:244–52.
Nguyen DV, Nguyen OC, Malau-Aduli AEO. Main regulatory factors of marbling level in beef cattle. Vet Anim Sci. 2021;14:100219.
Zhang YY, Zan LS, Wang HB, Xin YP, Adoligbe CM, Ujan JA. Effect of sex on meat quality characteristics of Qinchuan cattle. Afr J Biotechnol. 2010;9:4504–9.
de Oliveira CARRILHO C, Fernando KU, MOLETTA JL, de MENEZES LF. Effect of age and sexual condition on the fatty acid profile of intramuscular fat of cattle finished in feedlot. Food Sci Technol. 2023;43.
Judge MM, Conroy S, Hegarty PJ, Cromie AR, Fanning R, Kelly D, et al. Eating quality of the longissimus thoracis muscle in beef cattle – Contributing factors to the underlying variability and associations with performance traits. Meat Sci. 2021;172:108371.
Picard B, Gagaoua M, Al Jammas M, Bonnet M. Beef tenderness and intramuscular fat proteomic biomarkers: Effect of gender and rearing practices. J Proteomics. 2019;200:1–10.
Zhang Y-Y, Wang H-B, Wang Y-N, Wang H-C, Zhang S, Hong J-Y, et al. Transcriptome analysis of mRNA and microRNAs in intramuscular fat tissues of castrated and intact male Chinese Qinchuan cattle. PLoS ONE. 2017;12:e0185961.
Critchlow AJ, Hiam D, Williams R, Scott D, Lamon S. The role of estrogen in female skeletal muscle aging: A systematic review. Maturitas. 2023;178: 107844.
Xu Y, López M. Central regulation of energy metabolism by estrogens. Mol Metab. 2018;15:104–15.
Oh YS, Cho SB, Baek KH, Choi CB. Effects of testosterone, 17β-estradiol, and progesterone on the differentiation of bovine intramuscular adipocytes. Asian-Aust J Anim Sci. 2005;18:1589–93.
Baharun A, Said S, Arifiantini RI, Karja NWK. Correlation between age, testosterone and adiponectin concentrations, and sperm abnormalities in Simmental bulls. Vet World. 2021;14(8):2124–30.
Bong JJ, Jeong JY, Rajasekar P, Cho YM, Kwon EG, Kim HC, et al. Differential expression of genes associated with lipid metabolism in longissimus dorsi of Korean bulls and steers. Meat Sci. 2012;91:284–93.
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412.
Pewan SB, Otto JR, Kinobe RT, Adegboye OA, Malau-Aduli AEO. MARGRA lamb eating quality and human health-promoting omega-3 long-chain polyunsaturated fatty acid profiles of Tattykeel Australian White sheep: Linebreeding and gender effects. Antioxidants. 2020;9:1118.
Otto JR, Mwangi FW, Pewan SB, Adegboye OA, Malau-Aduli AEO. Lipogenic gene single nucleotide polymorphic DNA markers associated with intramuscular fat, fat melting point, and health-beneficial omega-3 long-chain polyunsaturated fatty acids in Australian pasture-based Bowen Genetics Forest Pastoral Angus, Hereford, and Wagyu beef cattle. Genes. 2022;13:1411.
Acknowledgements
The authors gratefully acknowledge Bowen Genetics Forest Pastoral, Barraba, New South Wales, Australia for access to herd, farm resources, and research support; the Innovation Connections grant of the Australian Commonwealth Department of Industry for research funding and the Commonwealth Scientific and Industrial Research Organization Marine & Atmosphere Hobart, Tasmania, for fatty acid analysis. We would also like to appreciate the support of the College of Public Health, Medical and Veterinary Sciences, James Cook University, Townsville, Queensland, Australia.
Funding
This research was funded by the Innovations Connections Grant of the Australian Commonwealth Department of Industry and Innovation, Bowen Genetics Forest Pastoral Co Pty Ltd., grant number ICG001520, awarded to the first named author. The funding body played no part in the design, analysis and reporting of the study.
Author information
Authors and Affiliations
Contributions
This work was carried out in collaboration between all authors. Conceptualization, AEOM-A and JRO. Methodology, AEOM-A and JRO. Validation, AEOM-A. Formal analysis, AEOM-A, JRO and OAA. Investigation, JRO, SBP and FWM. Resources, AEOM-A. Data curation, JRO, SBP and FWM. Writing and original draft preparation, JRO. Writing, review and editing, AEOM-A, OAA, SBP and FWM. Supervision, AEOM-A. Project administration, AEOM-A. Funding acquisition, AEOM-A. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the James Cook University Animal Ethics Committee (Approval Number A2724, issued on the 20th of November 2020) and the Australian code of practice for the care and use of animals for scientific purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Otto, J.R., Mwangi, F.W., Pewan, S.B. et al. Muscle biopsy long-chain omega-3 polyunsaturated fatty acid compositions, IMF and FMP in Australian pasture-based Bowen Genetics Forest Pastoral Angus, Hereford, and Wagyu Beef Cattle. BMC Vet Res 20, 95 (2024). https://doi.org/10.1186/s12917-024-03906-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-024-03906-2