Abstract
Background
Phytochemical compounds can modify the rumen microbiome and improve rumen fermentation. This study evaluated the impact of supplementation with tannin and an herbal mixture containing ginger (Zingiber officinale), garlic (Allium sativum), Artemisia (Artemisia vulgaris), and turmeric (Curcuma longa) on the rumen fermentation and microbiota, and histology of rumen tissue of goats. Eighteen Shami male goats were divided into three groups (n = 6): non-supplemented animals fed the basal diet (C, control); animals fed basal diet and supplemented with condensed tannin (T); and animals fed basal diet and supplemented with herbal mixture (HM). Each animal received a basal diet composed of Alfalfa hay and a concentrate feed mixture.
Results
Group HM revealed higher (P < 0.05) rumen pH, total volatile fatty acids (VFA), acetic, propionic, isobutyric, butyric, isovaleric, and valeric. Principal Co-ordinate analysis (PCoA) showed that rumen microbial communities in the control group and supplemented groups were distinct. The supplementation increased (P < 0.05) the relative abundances of phylum Bacteroidota and Proteobacteria and declined (P < 0.05) Firmicutes and Fibrobacterota. Additionally, the dominant genus Prevotella and Rikenellaceae RC9 gut group were increased (P < 0.05) and the family Ruminococcaceae was declined (P < 0.05) due to the supplementation. The supplementation decreased (P < 0.05) the archaeal genus Methanobrevibacter and increased (P < 0.05) Candidatus Methanomethylophilus. Tannin supplementation in T group shortened the rumen papillae.
Conclusions
The results revealed that the herbal mixture might be used to alter the rumen microbiota to improve rumen fermentation.
Similar content being viewed by others
Background
The digestion of plant biomass in the rumen relies on interactions between a complex assemblage of bacteria, archaea, fungi, and protozoa [1,2,3,4]. Rumen bacteria predominate the rumen microbiome and they ferment a wide variety of dietary components such as cellulose, hemicellulose, starch, pectin, and protein. VFA and microbial protein are the main products of rumen fermentation and they provide the host animal with most of energy and protein requirements. Additionally, rumen archaea uses fermentation gases such as hydrogen (H2) and carbon dioxide (CO2) besides methyl group derived from acetic acid, and format to generate methane (CH4) [5]. Methane represents a 2–12% loss of the gross energy feed intake of host animal and it is one of the main reasons for climate change [5, 6]. Rumen methanogens interact with H2 producers and utilizers; therefore, modifying rumen microbial ecosystem is a main target for studies that aim to improve animal efficiency and decrease methane emission [5, 7]. Dietary intervention is the main driver of changes in the rumen microbiome. Consequently, understanding the modifications of rumen microbiome under different diets or feed additives opens the door to designing suitable strategies to improve animal productivity [6]. Phytogenic feed additives are emerging additives to enhance animal’s productivity through the modification of the rumen ecosystem [8, 9]. These additives have been used in the form of extracts, herbal mixtures, or oils.
Herbal plants that are rich in phytochemicals, including ginger (Zingiber officinale), garlic (Allium sativum), artemisia (Artemisia vulgaris), and turmeric (Curcuma longa) [10,11,12,13,14]. Ginger contains several substances such as gingerol and gingerdione [10], and garlic contains several organosulfur compounds, including allicin, diallyl sulfide, γ-glutamylcysteine, and S-allyl cysteine [11, 12]. Furthermore, artemisia contains artemisinin [13], and turmeric contains curcumin and turmerones besides several other substances and oils [14]. Additionally, phytogenic compounds that were commonly observed in ginger, garlic, artemisia, and turmeric, including phenolic compounds, tannins, saponins, and flavonoids [10,11,12,13,14]. Therefore, these plants have been used as growth promoters as well as rumen microbiome modifiers to improve rumen fermentation and promote animal health and performance [10,11,12,13,14,15]. Phytogenic compounds were supplied to farm animals separately or mixed to generate the synergistic effect of the combination. Williamson [16] reported that synergistic interactions between the bioactive compounds of herbal plants are a vital part of their efficacy and total herbal extracts show a better effect than an equivalent dose of an isolated compound. In addition, herbal plants provide essential amino acids, carbohydrates (sucrose and glucose), vitamins, essential oils, and minerals [17,18,19,20]. Several studies applied phytochemical mixtures to modify rumen fermentation in order to improve animal efficiency. Petric et al. [21] noticed that herbal mixture supplementation in lambs affected the bacterial population and the abundance of Ruminococcus and Fibrobacter. In water buffalo, Hassan et al. [22] reported that phytochemicals mixtures increased the relative abundance of phylum Firmicutes and Proteobacteria, while the relative abundances of Bacteroidetes and Spirochaetes besides genus Prevotella were declined. Kholif et al. [8] indicated that herbal mixture increased total VFA and propionic acid besides the relative abundances of propionate-producing bacteria such as family Prevotellaceae and Veillonellaceae, and declined the methane-producing archaea, Methanobacteriaceae. Apart from herbal mixtures, separated phytogenic compounds were used to modify the rumen microbiome. Li et al. [23] noticed that flavonoids supplementation increased rumen acetate, propionate, and total VFAs, and declined the alpha diversity indices in buffalo. Furthermore, flavonoids increased the abundance of Proteobacteria and declined the relative abundances of Actinobacteria, Acidobacteria, Chloroflexi, and Patescibacteria. Salami et al. [24] supplemented lambs with condensed tannins and observed that tannin did not affect rumen fermentation parameters or the bacterial population, while the archaeal population was declined. Moreover, tannin decreased the relative abundances of cellulolytic bacteria, Fibrobacter, and methanogen Methanosphaera. Hassan et al. [25] indicated that Saponins have a modulating effect on rumen fermentation as they declined the population of protozoa and methanogens; therefore, methane production was declined. Rabee et al. [26] analyzed the bacterial community colonized tannin-rich plants in the rumen of camels and noticed that higher Bacteroidetes was observed in lower-tannin plants and the relative abundances of Firmicutes, Proteobacteria, and Tenericutes were higher in tannin-rich plants. Moreover, Fibrobacteria showed sensitivity to tannins and Prevotella showed resistance to some types of tannins. However, studies that compared the effect of separated extracted phytochemicals to mixed compounds or herbal mixtures containing different compounds on rumen fermentation and microbiota are not available.
Shami (Damascus) goat is one of the main goat’s breeds in Egypt that is contributing substantially to meat and milk production in Egypt; however, goats’ production in developing countries is challenged by feeding and health problems [27]. Consequently, safe and available feed additives are required to modulate the rumen fermentation and improve goats’ performance and health. Phytochemicals-rich plants are commonly used in feeding ruminant animals in arid regions [26]. However, the effect of these plants separately or combined in different combinations on rumen ecosystem and animal performance, need to be assessed as different action mechanisms are expected due to the variation in the composition and secondary metabolites [28]. On the other hand, comparative studies that aim to compare the effect of separate phytochemicals to herbal plants containing mixtures of phytochemicals are not available. In addition, no available studies on the effects of phytochemicals on rumen fermentation and the microbiome in Shami goats. Therefore this study aims to investigate the effect of condensed tannin and herbal mixture on the performance, rumen fermentation, rumen microbiota, and rumen histology of goats.
Methods
Ethics
The study, including euthanasia of the experimental animals was conducted under the guidelines of the Animal Care and Use Committee in the Department of Animal and Poultry Production, Desert Research Center, Egypt (Project ID: 43,213). Alexandria University Research Ethics Review Committee, Faculty of Agriculture, University of Alexandria, Egypt approved all experimental procedures (Reference: Alex. Agri. 082305306). All methods and protocols in this study are in compliance with the ARRIVE guidelines.
Animals and diets
This experiment was carried out at Maryout Research Station, Desert Research Center, Alexandria, Egypt. Eighteen male Shami goats (26.66 ± 1.31 kg initial body weight and 11–12 months of age) were selected randomly for this 90-day experiment and divided into three treatments, six animals per treatment. The goats used in this study were the progeny of the goats’ herd of Maryout Research Station, Desert Research Center, Alexandria, Egypt. The three groups received the same basal diet with a 70% concentrates mixture and 30% Alfalfa hay (Medicago sativa) that was formulated to meet the goats’ feeding requirements according to NRC [29]. Goats were housed in shaded pens with free access to water. The first group (C) received the basal diet with no additives, the second group (T) received the basal diet supplemented with commercial Quebracho tannins extract (10 g/head/day), and the third group (HM) received the basal diet supplemented with the herbal mixture (10 g/head/day). The herbal mixture contained 25% ginger (Zingiber officinale), 25% garlic (Allium sativum), 25% artemisia (Artemisia vulgaris), and 25% turmeric (Curcuma longa) that were thoroughly mixed in equal quantities. Animals received the condensed tannin and herbal mixture gradually at 1% of their dry matter (DM) feed intake. Table 1 shows details of the components and chemical compositions of the experimental diet. The weights of the goats were recorded at the beginning and the end of the trial. Drinking water was offered twice a day, the goats were housed in goat pens, and the trial period was 90 days. All experimental additives were mixed with concentrate to confirm full intake. Orts were weighed and feed intake was recorded daily. Diet and orts were sampled weekly and dried in a forced-air oven at 65 o C for 48 h. At the end of the study four animals from every group were slaughtered to conduct the histological evaluation and the rest of animals were released to the goats herd.
Rumen fermentation and predicted methane production
Rumen samples were collected from the animals using a stomach tube and the pH was measured immediately using a pH meter. The rumen contents were filtered through 4 layers of cheesecloth, and rumen fluid samples were used to measure VFA and ammonia as well as DNA extraction to study the microbial community. To analyze ammonia and VFA, 1 mL of rumen fluid samples were transferred to 1.5 mL Eppendorf tubes and acidified with 200 µL of meta-phosphoric acid 25% (w/v). The samples were then stored at -20 °C for later analysis. After thawing, the samples were centrifuged at 30,000×g (15,000 rpm, JA-17 rotor) for 20 min and the resulting supernatant was used for VFA and ammonia determination. For rumen ammonia nitrogen (NH3-N) measurement, 250 µL of supernatant was assessed calorimetrically using an ammonia assay kit (Biodiagnostic, Cairo, Egypt). The remaining supernatants (750 µL) were transferred to GC vials for VFA analysis using a capillary column (TR-FFAP 30 m×0.53 mmI D×0.5 μm) in a Thermo Scientific TRACE 1300 gas chromatography system (Thermo Scientific, Massachusetts, United States). The oven temperature was ramped from 100 to 200 °C at a rate of 10 °C/min, while the injection and flame ionization detector (FID) temperatures were set at 220 and 250 °C, respectively. Nitrogen was used as the carrier gas at a flow rate of 7 ml/min, while hydrogen and make-up gases were set at flow rates of 40 ml/min and 35 ml/min, respectively. The total run time was set at 10 min with calibration done using a standard with known concentrations of VFA. The predicted methane was calculated using the concentration of propionic acid, Methane yield = 316/propionate + 4.4, according to Williams et al. [30].
Histomorphological examination of rumen samples
At the end of the experiment, four goats from each group were selected randomly to slaughter. Animals were fasted for 12 h with ad libitum access to water, transported to Maryout Research Station’s slaughterhouse, and weighed before the slaughter. The slaughter was conducted by an experienced technician by severing the jugular vein with a sharp knife without electrical stimulation. The death of the animals was ensured prior to further processing and sampling. After bleeding, skinning, and eviscerations, rumen autopsy samples from slaughtered goats were collected to conduct the histological examination. The samples were collected and fixed with a 10% neutral buffered formalin solution, then washed, dehydrated in ethyl alcohol of different grades, cleared in methyl benzoate, and embedded in paraffin wax. Blocks were processed using standard procedures, and 5-µm sections were stained with hematoxylin and eosin and examined microscopically [31].
Chemical composition
Dried feeds and orts were ground and analyzed according to the method of AOAC [32] to measure DM, crude Protein (CP), and ether extract (EE). Neutral detergent fiber (NDF) and acid detergent fiber (ADF) were measured using ANKOM 200 Fiber Analyzer (ANKOM Technology, New York, United States) according the method of Van Soest et al. [33]. Total phenolic, total flavonoids, total tannins, and total saponins were determined in the herbal mixture. Total flavonoids were extracted using petroleum ether and 95% ethanol and determined according to the method of Karawaya and Aboutabl [34]. Total phenolic content was determined by the Folin–Ciocalteu according to the method of Kaur and Kapoor [35]. Total saponins were determined using 70% ethanol extraction according to the method of Kurkin and Ryazanova [36]. Total tannins were extracted by boiling in water according to the method of Balbaa [37]. Flavonoids compounds were determined using high-performance liquid chromatography (HPLC) (Thermo Scientific, Massachusetts, United States) using reversed-phase C18 column and 0.05%Trifluoroacetic acid/Acetonitrile (solvent A) and distilled water (solvent B) as a mobile phase [38].
Microbial community
DNA isolation and PCR amplification
Total DNA was extracted from 500 µl of rumen samples. Subsequently, the sample was centrifuged at 13,000 rpm, and the pellets were used in DNA extraction using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The quality and quantity of DNA were assessed using gel electrophoresis and a Nanodrop spectrophotometer 2000 (Thermo Scientific, Massachusetts, United States). The composition and diversity of rumen bacterial and archaeal communities were investigated using amplification of the variable V4 region on 16 S rDNA. The rumen bacterial community was studied by amplification of V4 region by 515 F and 926R primers using the following PCR conditions: 94 °C for 3 min; 35 cycles of 94 °C for 45 s, 50 °C for 60 s, and 72 °C for 90 s; and 72 °C for 10 min. Rumen archaeal community was studied by the amplification of the V4 region using primers Ar915aF (5-AGGAATTGGCGGGGGAGCAC-3) and Ar1386R (5-GCGGTGTGTGCAAGGAGC-3) [6]. The PCR amplification was conducted under the following conditions: 95 °C for 5 min; 30 cycles of 95 °C for 20 s, 55 °C for 15 s, 72 °C for 5 min, and 72 °C for 10 min. PCR amplicons were purified and sequenced using the Illumina MiSeq system (Illumina, California, United States).
Bioinformatics and statistical analyses
The generated paired-end sequence reads were analyzed using the DADA2 pipeline through the R platform [39]. The fastq files of sequence reads were demultiplexed, and their quality was evaluated. Then, the sequences were filtered, trimmed, and dereplicated followed by merging read 1 and read 2 together to get denoised sequences. The chimeras were removed from the denoised sequences to generate Amplicon Sequence Variants (ASVs). Taxonomic assignment of ASVs was performed using the “assignTaxonomy” and “addSpecies” functions, and microbial taxa were identified using the SILVA reference database (version 138). Alpha diversity metrics, including observed ASVs, Chao1, Shannon, and Inverse Simpson, were measured. In addition, the Beta diversity of bacterial and archaeal communities was determined as principal coordinate analysis (PCoA) using Bray–Curtis dissimilarity and figures were created using the phyloseq and ggplot packages. The raw sequence reads were deposited to SRA at https://www.ncbi.nlm.nih.gov/sra/PRJNA1008569.
Statistical analysis
All the data were analyzed using one-way ANOVA in IBM SPSS software v. 20.0 [40] using the Duncan test. And the differences at P < 0.05 were considered statistically significant. Pearson correlation analysis (Heatmap) and Principal component analysis (PCA) were conducted between Relative Growth Rate (RGR), feed intake, predicted methane, rumen fermentation parameters, and relative abundances of dominant bacterial phyla, and dominant bacterial and archaeal genera.
Results
Phytochemical substances in the herbal mixture (HM)
The phytochemical compounds in the HM mixture consisted of total phenolics 60 mg/100 g, total flavonoids 17.63 mg/100 g, total Saponins 48.8 mg/100 g, and total tannins 49 mg/100 g. Flavonoid compounds consisted of Apeginin (20.07% of flavonoids), Rutin (0.18%), Diosmin (5.25%), Kampferol (74.5%).
Feed intake and growth performance
Table 2 presents the relative growth rate (RGR) and feed intake expressed as dry matter intake (DMI), organic matter intake (OMI), crude protein intake (CPI), ether extract intake (EEI), and neutral detergent fiber intake (NDFI) calculated as g/kg 0.75 (kilogram metabolic body weight) as affected by feeding treatments. Feed intake was similar between groups and group HM showed higher numeric RGR without significant differences (Table 2). On the other hand, tannin and herbal mixture intake represented 1% of DM intake.
Rumen fermentation
Rumen pH and ammonia were similar between goat groups (P > 0.05) (Table 3) and the pH was < 6 in group C. The production of total VFA (TVFA) was higher in herbal mixture-supplemented group (HM) and the C group showed the lowest production (P < 0.05) (Table 3). Similar trends were obtained in the proportions of acetic, butyric, and isobutyric (P < 0.05). The values of propionic, valeric, and isovaleric were similar in the experimental groups (Table 3). Based on the results of VFA profile, predicted methane was declined (P < 0.05) due to the supplementation of tannin and herbal mixture.
Analysis of microbial communities
Diversity of bacterial community
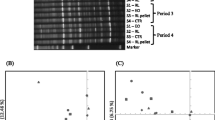
The amplicon sequencing of 16 S rDNA genes revealed a total of 421,540 high-quality non-chimeric sequences read with an average of 25,949 ± 2241 sequences per sample. No significant differences in the number of Amplicon Sequence Variants (ASVs) and alpha diversity indexes, Chao, Shannon, and invsimpson across dietary treatments (Table 4; Fig. 1a). Beta diversity was estimated and visualized for the bacteria community of goats under investigation using principal coordinate analysis (PCoA) based on Bray-Curtis dissimilarity (Fig. 1b). The plot showed that the samples of control groups (C) were separated clearly from the supplemented groups (T and HM).
Alpha diversity indices (a) and Principal coordinates analysis (PCoA) (b) of bacterial community. Alpha diversity indices, including observed species, Chao1, and Shannon as well as PCoA analysis based on Bray-Curtis dissimilarity. The analyses were performed between three goat groups: red circles for the control group (C), blue circles for the tannin-supplemented group (T), and green circles for the herbal mixture-supplemented group (HM)
Composition of the bacterial community
The taxonomic analysis of the bacterial communities in the rumen of goats under study revealed a total of 15 bacterial phyla, out of which one phylum (Synergistota, 0.28%) was detected only in the HM group (Table 5). Bacterial phyla that represented more than 1% of the bacterial community were Bacteroidota (54.62%), Cyanobacteria (1.33%), Firmicutes (40.29%), and Planctomycetota (1.50%). Furthermore, bacterial phyla that represented less than 1% of the bacterial community were Actinobacteriota (0.31%), Chloroflexi (0.15%), Desulfobacterota (0.08%), Elusimicrobiota (0.058%), Fibrobacterota (0.07%), Proteobacteria (0.23), Spirochaetota (0.69%), and Verrucomicrobiota (0.66%) (Table 5). The dietary supplementation affected the relative abundance of six bacteria phyla, including, Actinobacteriota, Bacteroidota, Elusimicrobiota, Firmicutes, Proteobacteria, and Verrucomicrobiota (Table 5).
Phylum Bacteroidota dominated the bacterial community and its relative abundance was increased due to dietary supplementation, whenever the goat group HM showed the highest relative abundance (62.62%) and group C showed the lowest relative abundance (Table 5). This phylum was dominated by family Prevotellaceae, F082, Bacteroidales RF16 group, Muribaculaceae, Bacteroidales BS11 gut group, Rikenellaceae, and Bacteroidales UCG-001. The relative abundance of those families was improved by dietary supplementation (Table 6).
On the genus level, the phylum Bacteroidota is dominated by genus Prevotella, Prevotellaceae UCG-001, and Rikenellaceae RC9 gut group. In addition, genus Alloprevotella was detected only in T and HM groups. Members of phylum Firmicutes were classified into family Ruminococcaceae, Lachnospiraceae, Christensenellaceae, Hungateiclostridiaceae, Anaerovoracaceae, Monoglobaceae, and Streptococcaceae that were declined in groups T and HM (Table 6). In addition to families Oscillospiraceae, Selenomonadaceae, Monoglobaceae, Acidamino:coccaceae, and Acholeplasmataceae whose relative abundances were increased in T and HM supplementation (Table 6).
On the genus level, phylum Firmicutes was affiliated with Lachnospiraceae ND3007 group, Acetitomaculum, Butyrivibrio, Christensenellaceae R-7 group, Saccharofermentans, Mogibacterium, Monoglobus, and Streptococcus that were declined in T and HM. In addition to genus UCG-002, NK4A214 group, UCG-005, Veillonellaceae UCG-001, Succiniclasticum, and Anaeroplasma that were increased in T and HM (Table 6). Furthermore, some genera within Firmicutes were detected only in T and HM such as Marvinbryantia, Papillibacter, and Anaerovibrio. Phylum Planctomycetota was dominated by family Pirellulaceae and genus p-1088-a5 gut group that showed its highest relative abundance in the HM group (Table 6). Phylum Spirochaetota was dominated by family Spirochaetaceae that classified into genus Sphaerochaeta and Treponema which were increased by the supplementation (Table 6).
Diversity of the archaeal community
The amplicon sequencing of archaeal 16 S rDNA genes revealed 178,388 high-quality reads with an average of 11,880 reads per sample. Furthermore, 368 ASVs were detected with an average of 25 ASVs per sample (Table 7) (Fig. 2a). The feeding treatment did not affect the alpha diversity metrics, observed ASVs, Chao, Shannon, and invsimpson (Table 7). Principal coordinate analysis (PCoA) based on Bray-Curtis dissimilarity (Fig. 2b) was used to estimate and visualize the beta diversity of archaeal communities in the rumen of goats. The plot revealed that samples of the C group were separated from the samples of supplemented groups (T and HM).
Alpha diversity indices (a) and Principal coordinates analysis (PCoA) (b) of archaeal community. Alpha diversity indices, including observed species, Chao1, and Shannon as well as PCoA analysis based on Bray-Curtis dissimilarity. The analyses were performed between three goat groups: red circles for the control group (C), blue circles for the tannin-supplemented group (T), and green circles for the herbal mixture-supplemented group (HM)
Composition of archaeal community
The taxonomic analysis of the archaeal community in goat groups showed that the community was affiliated to three phyla, Euryarchaeota, Thermoplasmatota, and Halobacterota (Table 7). Phylum Euryarchaeota was dominated by Methanobacteriaceae, which was classified into genus Methanobrevibacter that was declined by supplementation (P < 0.05), and genus Methanosphaera, which was only detected in the HM group (Table 7). Phylum Thermoplasmatota was dominated by family Methanomethylophilaceae that had two genera, Unclass_Methanomethylophilaceae and Candidatus Methanomethylophilus that showed their highest relative abundances in group HM group (P < 0.05). Phylum Halobacterota was classified into families Halococcaceae and Haloferacaceae which were further classified into genus Halococcus and Natronococcus, respectively. Both Halococcus and Natronococcus were detected only in the HM group (Table 7).
Pearson correlation analysis
Pearson correlation analysis (Fig. 3) revealed several positive and negative relationships. Relative growth rate (RGR) correlated negatively with feed intake (DMI, CPI, and NDFI), rumen ammonia, and methane, and the relative abundances of Rikenellaceae RC9 gut group, and Methanobrevibacter. In addition, RGR correlated positively with total VFA, butyric, and the relative abundances of Prevotella, and Methanomethylophilus. A negative correlation was observed between Methanobrevibacter and total VFA. Methanobrevibacter correlated positively with methane production.
Heatmap based on Pearson correlation. The correlation was conducted between the RGR, feed intake, rumen fermentation parameters, predicted methane production, and relative abundances of dominant ruminal bacteria and archaea of goats fed different diets. The black boxed ellipses indicate to significant correlations at P < 0.05
Effect of the dietary supplementation on performance, rumen fermentation and relative abundance of rumen bacteria and archaea
Principal component analysis (PCA) (Fig. 4) was conducted based on RGR, feed intake, methane, rumen fermentation parameters, and the relative abundance of dominant bacteria and archaea. The PCA plot showed that the samples were separated into three groups. The important parameters that drove the differences between the samples were RGR, total VFA, acetic, the relative abundances of Firmicutes, Prevotella, and Ruminococcaceae.
Principal component analysis (PCA). The PCA was conducted using the data of the RGR,feed intake,rumen fermentation parameters, predicted methane production, and relative abundances of dominant ruminal bacteria and archaea of goats fed different diets. Black dots are for group C, blue triangles are for group T, and red squares are for group HM
Histological examination of the rumen
Examination of the rumen sector from group C and HM showed a normal appearance of ruminal papillae and musclosa; while, shortness of ruminal papillae, slight degeneration, and atrophy were detected in group TT (Fig. 5).
The histology of goat rumen papillae as affected by tannin and herbal supplementation. C: refers to rumen papillae of the control group and showing normal ruminal papillae, intact mucosa, sub mucosa and musclosa (H&E, X10); T refers to ruminal papillae of the tannin-supplemented group and showing degeneration and atrophy of ruminal papillae, shortness of papillae and rupture (yellow arrows); HM refers to papillae of the herbal mixture-supplemented group and showing normal histological appearance
Discussion
Animal performance
Phytogenic substances such as phenolics, flavonoids, Saponins, and tannins are promising modifiers for the rumen microbial community to improve rumen fermentation and animal performance [21, 23], which highlights the herbal mixture used in the current study as it contains a variety of phytochemicals that provide the synergistic effect to the animal [41,42,43]. Previous studies [28, 44, 45] on herbal mixtures reported no changes in animal feed intake, which support the current findings. In addition, Reynolds et al. [46] included tannin-rich ground pine bark in the diets of meat goats and observed no changes in feed intake and daily gain. Glasscock et al. [47] reported that herbal plants mixture did not affect DMI or RGR in goats. In contrast, Waghorn et al. [48] reported that high tannin concentration in animal diets depressed the digestibility and declined animal feed intake. In the current study, the concentration of condensed tannin was 1% of DM feed intake and did not affect feed intake or RGR negatively, which indicates that tannins concentration is in the suitable range. This speculation is supported by Min et al. [49] who suggested that 2–4% tannin based on DM is suitable to maintain favorable growth performance and rumen fermentation. Orzuna-Orzuna et al. [45] explained that flavonoids supplementation modulates and improves rumen fermentation and metabolism to improve animal performance and health in ruminants.
Rumen fermentation parameters
Rumen pH was > 6 in group T and HM, which supports the fiber degradation, and VFA production in the rumen [50], which could explain higher VFA production in T and HM [44, 45]. Li et al. [23] supplemented lactating buffalo with flavonoids and noticed improvements in the VFAs’ production, which support the current findings. The higher acetic acid in supplemented groups was also indicated in cattle supplemented with ensiled mulberry leaves [51]. Razo Ortiz et al. [28] explained that polyherbal mixtures stimulated the propionic and butyric acids-producing bacteria such as Prevotella and Butyrivibrio which improve the VFA production, and this explanation is supported by the correlation between rumen bacteria and rumen fermentation parameters (Fig. 3).
Bacterial community
The improvements in the rumen fermentation parameters were consistent with variations in the composition of microbial communities due to the supplementation, which agrees with previous studies [44, 52]. A large proportion of the bacterial community was affiliated with the members of phylum Bacteroidota and Firmicutes, which agrees with previous studies on rumen bacteria of goats [45, 53, 54]. The supplementation increased the members of Bacteroidota and Proteobacteria and declined the members of Firmicutes and Actinobacteriota (Table 5). Li et al. [23] noticed that flavonoids supplementation in lactating buffalo increased Bacteroidota, Firmicutes, and Proteobacteria, but declined Actinobacteriota. On the other hand, Rabee et al. [26] reported that phylum Fibrobacteria is sensitive to phenolic compounds, which explains its decline in the supplemented groups (T, HM). The members of Bacteriodota degrade wide range of substrates including cellulose, pectin, and soluble polysaccharides and unclassified members of this phylum are specialized in lignocellulose degradation [26]. The main members of this phylum, Prevotella and Rikenellaceae RC9 gut group, were higher in supplemented groups (T and HM) (Table 6), which is supported by previous studies [23, 52, 55].
Genus Prevotella dominated the bacterial communities in several ruminant species and it digests wide range of substrates such as hemicellulose and protein [56]. Furthermore, this genus is the main propionate producers in the rumen [57], which explains the positive correlation between Prevotella and propionic (Fig. 3). Furthermore, Prevotella was associated with high efficient cattle [58], which support the correlation between RGR and genus Prevotella. Li et al. [23] reported higher Prevotella in Buffalo supplemented with Flavonides, which supports the current finding. Genus Rikenellaceae RC9 gut group, the second dominant genus in phylum Bacteriodota, has an important role in fiber degradation in the rumen [59, 60]. The increment in the Prevotella and Rikenellaceae RC9 gut group is a positive point for the supplementation, which is supported by the positive correlation between RGR and Prevotella, Bacteriodota, total VFA, and propionic (Fig. 3).
On the other hand, the prevalence of Prevotella and RC9_gut_group in the rumen of goats supplemented with tannin and herbal mixture highlights the resistance of these genera to phytochemicals [26]. Genus Alloprevotella is well adapted to tannin and other plant secondary metabolites, which explains its presence in group T and HM [26]. Hassan et al. [25] explained that essential oils of Origanum vulgare, garlic and peppermint decreased the abundance of Firmicutes and methane production, while increasing Bacteroidetes, which support the current findings. Family Ruminococcaceae, the dominant family in Firmicutes, includes cellulolytic bacteria and is sensitive to different types of tannins, which could justify the decline in the relative abundance of this family in the rumen of the supplemented goats [26, 61, 62]. The relative abundance of the genus Succiniclasticum was improved by supplementation. This genus converts succinate to propionate [63]. Previous studies [64, 65] explained that cattle with higher propionate-producing rumen bacteria such as Succiniclasticum and Prevotella and lower abundance of genus Mogibacterium had lower methane emission and higher feed efficiency. This finding highlights the importance of phytogenic supplementation and explains the positive correlation between RGR, rumen fermentation parameters and Prevotella.
Rumen archaea
Dietary intervention is the main determiner of rumen archaea by affecting hydrogen producers and utilizers that affect the available growth substrates for methanogens [59]. Consequently, variations in the composition and relative abundances of rumen methanogens due to tannin and herbal mixture supplementation are explained. The archaeal community was dominated by the genus Methanobrevibacter and Candidatus Methanomethylophilus, which agrees with the results on goats [66]. Previous studies [65, 67, 68] reported that phytochemicals such as tannin and flavonoids declined rumen methanogens and methane production and improved feed efficiency, which supports the decline in predicted methane production and Methanobrevibacter in the current study.
Methanobrevibacter, the main methane producer in the rumen, uses H2 besides other substrates to produce methane [69, 70]. Arndt et al. [71] reported that high-efficiency dairy cow exhibited lower methane emissions, and lower relative abundance of Methanobrevibacter, which highlights the benefits of phytogenic supplementation and explains the negative correlation between RGR, Methanobrevibacter, and methane production (Fig. 3). A similar conclusion was obtained by Zhou et al. [72] who explained that Methanobrevibacter uses acetate as a substrate for CH4 production, which leads to energy loss in the form of methane and decline in animal feed efficiency.
The possible explanations for Methanobrevibacter decline is the low hydrogen availability [70]. This speculation is supported by the increase of Succiniclasticum and Prevotella, which use hydrogen to produce propionate [64]. A similar conclusion was obtained by Hassan et al. [25] who explained that essential oils of Origanum vulgare, garlic, and peppermint increased the propionate-producing bacterial families such as Succinivibrioanceae, which decreases the available hydrogen for methanogenesis. Another explanation, phytochemicals have antimicrobial properties against rumen protozoa, the main hydrogen providers to methanogens [44]. A similar conclusion was obtained by Li et al. [23] on buffalo supplemented with flavonoides.
Previous studies [73, 74] reported that saponins improved animal performance by suppressing rumen methanogens and methane production. Aboagye and Beauchemin [75] and Tawab et al. [76] explained that phytochemicals disrupt the cell wall of methanogens causing toxicity. Moreover, Szulc et al. [77] indicated that polyphenols inhibit the populations and/or activity of methanogens by changing the rumen environment (pH value) and the toxic effect on methanogens. Furthermore, previous studies [25, 78] reported that the relative abundance of Methanobrevibacter was decreased by chestnut tannin supplementation and essential oils. Candidatus Methanomethylophilus is an H2-dependent methylotrophic methanogen and derives its energy from the metabolism of methanol and methylamine [79, 80]. Previous studies [81,82,83] indicated that higher relative abundance of Candidatus Methanomethylophilus was associated with improved feed efficiency, and average daily gain, and lower methane emission in sheep and steers, which supports the positive correlation between RGR, rumen fermentation parameters, and the relative abundance of Candidatus Methanomethylophilus (Fig. 3). These findings highlight the tannin and herbal mixture as suitable additives to improve feed efficiency and decreases methane emissions. The increase in the relative abundance of Candidatus Methanomethylophilus could be a result of the availability of methanol and methylamine in the rumen, which are produced via the fermentation of pectin by pectinolytic Lachnospiraceae and Spirochaetaceae [84]. Li et al. [85] reported that a higher abundance of Candidatus Methanomethylophilus was noted in the rumen of Sika Deer fed a high concentration of tannin-rich oak leaves. Choi et al. [86] reported that Candidatus Methanomethylophilus was enriched in the rumen of goats supplemented with Pinus koraiensis which is rich in essential oils.
Histology of the rumen papillae
Previous studies [87, 88] indicated that dietary intervention affects the morphology of rumen papillae. The normal appearance of rumen papillae was noted in the control and herbal mixture-supplemented goat groups. A similar finding was observed by Petric et al. [21] on lambs supplemented by an herbal mixture. Tannin supplementation in the T group shortened the rumen papillae, which agrees with Hill [89] on Impala grazed on Combretum imberbe with high-tannin content [90]. This conclusion was confirmed by Brown et al. [91] who noted the atrophy of the rumen papillae in sheep-fed acacia. Redoy et al. [44] explained that increased VFA production in the rumen might increase the size of rumen papillae. Ghandour [92] found that tannins in Acacia hay had a negative effect on histological changes on rumen of sheep as there was a focal inflammatory cells infiltration and edemas in the lamina propria.
Conclusion
Herbal mixture contains ginger, garlic, Artemisia, and turmeric improved the relative abundance of fiber-degrading bacteria, and decreased the major methane-producing archaea. Consequently, the rumen fermentation parameters were improved with balanced rumen pH and the morphology of rumen papillae was not affected negatively compared to tannin group (T). Therefore, it can be concluded that the herbal mixture in this study could be used as feed additive to alter rumen microbiota and improve rumen fermentation.
Data availability
The datasets generated and/or analyzed during the current study are available in SRA at https://www.ncbi.nlm.nih.gov/sra/PRJNA1008569.
Abbreviations
- ADF:
-
Acid detergent fiber
- NH3-N:
-
Ammonia nitrogen
- ASV:
-
Amplicon Sequence Variants
- CO2:
-
Carbon dioxide
- CT:
-
Condensed tannins
- CP:
-
Crude Protein
- CPI:
-
Crude protein intake
- DM:
-
Dry matter
- DMI:
-
Dry matter intake
- EE:
-
Ether extract
- EEI:
-
Ether extract intake
- FID:
-
Flame ionization detector
- HPLC:
-
High-performance liquid chromatography
- H2:
-
Hydrogen
- CH4:
-
Methane
- NDF:
-
Neutral detergent fiber
- NDFI:
-
Neutral detergent fiber
- OMI:
-
Organic matter intake
- PCA:
-
Principal component analysis
- PCoA:
-
Principal Co-ordinate analysis
- RGR:
-
Relative Growth Rate
- VFA:
-
Volatile fatty acids
References
Coleman GS, Laurie JI, Bailey JE, Holdgate SA. The cultivation of cellulolytic protozoaisolated from the rumen. J Gen Microbiol. 1976;95(1):144–50. https://doi.org/10.1099/00221287-95-1-144.
Devillard E, Newbold CJ, Scott KP, Forano E, Wallace RJ, Jouany JP, Flint HJ. A xylanase produced by the rumen anaerobic protozoan Polyplastron multivesiculatum shows close sequence similarity to family 11 xylanases from Gram-positive bacteria. FEMS Microbiol Lett. 1999;181(1):145–52. https://doi.org/10.1111/j.1574-6968.1999.tb08837.x.
Béra-Maillet C, Devillard E, Cezette M, Jouany J, Forano E. Xylanases and carboxymethylcellulases of the rumen protozoa Polyplastron Multivesiculatum, Eudiplodinium maggii and entodinium sp. FEMS Microbiol Lett. 2005;244(1):149–56. https://doi.org/10.1016/j.femsle.2005.01.035.
Orpin CG, Joblin KN. Rumen anaerobic fungi. In: Hobson PN, Stewart CS, editors. The Rumen Microbial Ecosystem. London: Blackie Academic and Professional; 1997. pp. 140–84.
Park T, Mao H, Yu Z. Inhibition of Rumen Protozoa by specific inhibitors of Lysozyme and Peptidases in vitro. Front Microbiol. 2019;10:2822. https://doi.org/10.3389/fmicb.2019.02822.
Rabee AE, Kewan KZ, El Shaer HM, Lamara M, Sabra EA. Effect of olive and date palm by-products on rumen methanogenic community in Barki sheep. AIMS Microbiol. 2022;8(1):26–41. https://doi.org/10.3934/microbiol.2022003.
Patra A, Park T, Kim M, Yu Z. Rumen Methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. J Anim Sci Biotechnol. 2017;8:13. https://doi.org/10.1186/s40104-017-0145-9.
Kholif AE, Anele UY, Patra AK, Varadyova Z, Editorial. The Use of Phytogenic Feed Additives to Enhance Productivity and Health in ruminants. Front Vet Sci. 2021;8:685262. https://doi.org/10.3389/fvets.2021.685262.
Abd El-hamid IS, Rabee AE, Ghandour MM, Mohammed RS, Sallam AM. Influence of phytochemicals on haemato-biochemical parameters, oxidative status, semen characteristics and histological changes in Damascus Goat bucks under Heat stress conditions. Adv Anim Vet Sci. 2023;11(1):112–12.
Al-Khalaifah H, Al-Nasser A, Al-Surrayai T, Sultan H, Al-Attal D, Al-Kandari R, Al-Saleem H, Al-Holi A, Dashti F. Effect of Ginger powder on production performance, antioxidant status, Hematological Parameters, digestibility, and plasma cholesterol content in broiler chickens. Anim MDPI. 2022;12(7):901. https://doi.org/10.3390/ani12070901.
Chen J, Wang F, Yin Y, Ma X. The nutritional applications of garlic (Allium sativum) as natural feed additives in animals. PeerJ. 2021;9:e11934. https://doi.org/10.7717/peerj.11934.
Ding H, Ao C, Zhang X. Potential use of garlic products in ruminant feeding: a review. Anim Nutr. 2023. https://doi.org/10.1016/j.aninu.2023.04.011.
Cherian G, Orr A, Burke IC, Pan W. Feeding Artemisia annua alters digesta pH and muscle lipid oxidation products in broiler chickens. Poult Sci. 2013;92(4):1085–90. https://doi.org/10.3382/ps.2012-02752.
Nm J, Joseph A, Maliakel B, Im K. Dietary addition of a standardized extract of turmeric (TurmaFEEDTM) improves growth performance and carcass quality of broilers. J Anim Sci Technol. 2018;60:8. https://doi.org/10.1186/s40781-018-0167-7.
Lillehoj H, Liu Y, Calsamiglia S, Fernandez-Miyakawa ME, Chi F, Cravens RL, Oh S, Gay CG. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet Res. 2018;49(1):76. https://doi.org/10.1186/s13567-018-0562-6.
Williamson EM. Synergy and other interactions in phytomedicines. Phytomedicine. 2001;8(5):401–9. https://doi.org/10.1078/0944-7113-00060.
Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;1(2):125–9. https://doi.org/10.1016/S1286-4579(99)80003-3.
Focke M, Feld A, Lichtenthaler HK. Allicin, a naturally occurring antibiotic from garlic, specifically inhibits acetylCoA synthetase. FEBS Lett. 1990;261(1):106–8. https://doi.org/10.1016/0014-5793(90)80647-2.
de Lima RMT, Dos Reis AC, de Menezes APM, Santos JVO, Filho J, Ferreira JRO, Melo-Cavalcante AAC. Protective and therapeutic potential of ginger (Zingiber offici‐nale) extract and [6]‐gingerol in cancer: a comprehensive review. Phytother Res. 2018;321885–907. https://doi.org/10.1002/ptr.6134.
Sorokina M, Steinbeck C. Review on natural products databases. J Cheminformatics. 2020;1220. https://doi.org/10.1186/s13321-020-00424-9.
Petric D, Mrav ˇ cáková D, Kucková K, ˇ Kišidayová S, Cieslak A, Szumacher-Strabel M, Huang H, Kolodziejski P, Lukomska A, Slusarczyk S. Cobanová K and ˇ Váradyová Z. Impact of Zinc and/or Herbal mixture on Ruminal Fermentation, Microbiota, and histopathology in lambs. Front Vet Sci. 2021;8:630971. https://doi.org/10.3389/fvets.2021.630971.
Hassan F, Ebeid HM, Tang Z, Li M, Peng L, Peng K, Liang X, Yang C. A mixed Phytogenic modulates the Rumen Bacteria Composition and Milk Fatty Acid Profile of Water Buffaloes. Front Vet Sci. 2020a;7:569. https://doi.org/10.3389/fvets.2020.00569.
Li M, Hassan F, Peng L, Xie H, Liang X, Huang J, Huang F, Guo Y, Yang C. Mulberry flavonoids modulate rumen bacteria to alter fermentation kinetics in water buffalo. PeerJ. 2022;10:e14309. https://doi.org/10.7717/peerj.14309.
Salami SA, Valenti B, Bella M, O’Grady MN, Luciano G, Kerry JP, Jones E, Priolo A, Newbold CJ. Characterisation of the ruminal fermentation and microbiome in lambs supplemented with hydrolysable and condensed tannins. FEMS Microbiol Ecol. 2018;94(5). https://doi.org/10.1093/femsec/fiy061.
Hassan F, Arshad MA, Ebeid HM, Rehman MS, Khan MS, Shahid S, Yang C. Phytogenic additives can modulate Rumen Microbiome to Mediate Fermentation kinetics and methanogenesis through exploiting Diet–Microbe Interaction. Front Vet Sci. 2020b;7:575801. https://doi.org/10.3389/fvets.2020.575801.
Rabee AE, Abd El Rahman T, Lamara M. Changes in the bacterial community colonizing extracted and non-extracted tannin-rich plants in the rumen of dromedary camels. PLoS ONE. 2023;18(3):e0282889. https://doi.org/10.1371/journal.pone.0282889.
Sallam AM, Abou-Souliman I, Reyer H, Wimmers K, Rabee AE. New insights into the genetic predisposition of brucellosis and its effect on the gut and vaginal microbiota in goats. Sci Rep. 2023;13(1):20086. https://doi.org/10.1038/s41598-023-46997-x.
Razo Ortiz PB, Mendoza Martínez GD, Silva GV, Osorio Teran AI, González Sánchez JF, de la Hernández García P. Torre Hérnandez ME, Espinosa Ayala E. Polyherbal feed additive for lambs: effects on performance, blood biochemistry and biometry. J Appl Anim Res. 2020;48:419–24.
National Research Council, NRC. Nutrient requirements of small ruminants: Sheep, goats, cervids, and New World camelids. Washington DC: The National Academies Press; 2007.
Williams SRO, Hannah MC, Jacobs JL, Wales WJ, Moate PJ. Volatile fatty acids in ruminal fluid can be used to predict methane yield of dairy cows. Animals. 2019;9(12):1006.
Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. 7th Edition, Churchill Livingstone of Elsevier, Philadelphia. 2013; 172–186.
AOAC. Association of Official Analytical Chemists International Official Methods of Analysis. 16th Edition, AOAC, Arlington. 1997.
Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fibre, neutral detergent Fibre and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–97.
Karawaya MS, Aboutabl EA. Phytoconstituents of Tabernaemontana coronaria Jac. Q. Willd and Dichotoma roxb growing in Egypt. Part IV: the flavonoids. Bull Fac Pharm Cairo Univ. 1982;XXI 1:41–9.
Kaur C, Kapoor HC. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Technol. 2002;37:153–61.
Kurkin VA, Ryazanova TK. Quantitative determination of total saponins in Aralia Mandshurica plant raw material. Pharm Chem J. 2018;52(5):455–8.
Balbaa SI. In Chemistry of Crude Drugs. Laboratory Manual. Faculty of Pharmacy, Cairo University. 1986. pp:195.
Biswas N, Balac P, Narlakanti KS, Haque E, Hassan M. (2013). Identification of Phenolic Compounds in Processed Cranberries by HPLC Method. J. Nutr. Food Sci. 2013; 3:1.
Callahan B, McMurdie P, Rosen M, Han AW, Johnson AJ, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–3.
SPSS. Statistical package for social science Release 15, SPSS INC, Chicago. USA. 1999.
Mravčáková D, Váradyová Z, Kopčáková A,Čobanová K, Grešáková L, Kišidayová S, Babják M, Dolinská MU, Dvorožňáková E, Königová A, Vadlejch J, Cieslak A, Ślusarczyk S, Várady M. Natural chemotherapeutic alternatives for controlling of haemonchosis in sheep. BMC Vet Res. 2019;15:302. https://doi.org/10.1186/s12917-019-2050-2.
Chanjula P, Wungsintaweekul J, Chiarawipa R, Phesatcha K, Suntara C, Prachumchai R, Pakdeechanuan P, Cherdthong A. Effects of supplementing finishing goats with Mitragyna speciosa (Korth) Havil Leaves Powder on growth performance, Hematological Parameters, Carcass Composition, and Meat Quality. Anim MDPI. 2022;12(13):1637. https://doi.org/10.3390/ani12131637.
Ku-Vera JC, Jiménez-Ocampo R, Valencia-Salazar SS, Montoya-Flores MD, Molina-Botero IC, Arango J, Gómez-Bravo CA. Aguilar-Pérez CF and Solorio-Sánchez FJ. Role of secondary plant metabolites on Enteric Methane Mitigation in ruminants. Front Vet Sci. 2020;7:584. https://doi.org/10.3389/fvets.2020.00584.
Redoy MRA, Shuvo AAS, Cheng L, Al-Mamun M. Effect of herbal supplementation on growth, immunity, rumen histology, serum antioxidants and meat quality of sheep. Animal: An International Journal of Animal Bioscience. 2020;14(11):2433–41. https://doi.org/10.1017/S1751731120001196.
Orzuna-Orzuna JF, Dorantes-Iturbide G, Lara-Bueno A, Mendoza-Martínez GD, Miranda-Romero LA, López-Ordaz R, Hernández-García PA. Productive performance, Carcass Traits, and meat quality in finishing lambs supplemented with a Polyherbal mixture. Agriculture. 2021;11:942. https://doi.org/10.3390/agriculture11100942.
Reynolds D, Min BR, Gurung N, McElhenney W, Lee JH, Solaiman S, Bolden-Tiller O. Influence of tannin-rich pine bark supplementation in the grain mixes for meat goats: growth performance, blood metabolites, and carcass characteristics. Anim Nutr. 2020;6(1):85–91. https://doi.org/10.1016/j.aninu.2019.09.003.
Glasscock JL, Whitney TR, Navarro JR, Angle SG, Holmes AR, Stewart WC, Scholljegerds EJ. Substituting ground woody plants for cottonseed hulls in kid goat feedlot diets: growth performance and blood chemistry. J Anim Sci. 2018;96:2851e60.
Waghorn GC, Shelton TD, McNabb WC, McCutcheon SN. Effects of condensed tannins in Lotus pedunculatus on its nutritive value for sheep. 2. Nitrogeneous aspects. J Agric Sci. 1994;123:109e19.
Min BR, Solaiman S, Gurung N, Behrends J, Eun JS, Taha E, Rose J. Effects of pine bark supplementation on performance, rumen fermentation, and carcass characteristics of Kiko crossbred male goats. J Anim Sci. 2012;90:3556e67. https://doi.org/10.2527/jas.2011-4931.
Wanapat M, Gunun P, Anantasook N, Kang S. Changes of rumen pH, fermentation and microbial population as influenced by different ratios of roughage (rice straw) to concentrate in dairy steers. J Agric Sci. 2014;152(4):675–85. https://doi.org/10.1017/S0021859613000658.
Zhou Z, Zhou B, Ren L, Meng Q. Effect of ensiled mulberry leaves and sun-dried mulberry fruit pomace on finishing steer growth performance, blood biochemical parameters, and carcass characteristics. PLoS ONE. 2014;9(1):e85406. https://doi.org/10.1371/journal.pone.0085406.
Lan W, Yang C. Ruminal methane production: associated microorganisms and the potential of applying hydrogen-utilizing bacteria for mitigation. Sci Total Environ. 2019;654:1270–83.
Giger-Reverdin S, Domange C, Broudiscou LP, Sauvant D, Berthelot V. Rumen function in goats, an example of adaptive capacity. J Dairy Res. 2020;87(1):45–51. https://doi.org/10.1017/S0022029920000060.
Luo T, Li Y, Zhang W, Liu J, Shi H. Rumen and fecal microbiota profiles associated with immunity of young and adult goats. Front Immunol. 2022;13:978402. https://doi.org/10.3389/fimmu.2022.978402.
Wei H, Ding L, Wang X, Yan Q, Jiang C, Hu C, Wang G, Zhou Y, Henkin Z, Degen AA. Astragalus root extract improved average daily gain, immunity, antioxidant status and ruminal microbiota of early weaned yak calves. J Sci Food Agric. 2020;101:82–90.
Matsui H, Ogata K, Tajima K, Nakamura M, Nagamine T, Aminov RI, Benno Y. Phenotypic characterization of polysaccharidases produced by four Prevotella type strains. Curr Microbiol. 2000;41:45–9. https://doi.org/10.1007/s0028400.
Betancur-Murillo CL, Aguilar-Marín SB, Jovel J, Prevotella. A key player in Ruminal Metabolism. Microorganisms. 2022;11(1):1. https://doi.org/10.3390/microorganisms11010001.
Brooke CG, Najafi N, Dykier KC, Hess M. Prevotella copri, a potential indicator for high feed efficiency in western steers. Anim Sci J. 2019;90(5):696–701. https://doi.org/10.1111/asj.13197.
Rabee AE, Forster R, Elekwachi C, Sabra E, Lamara M. Comparative analysis of the metabolically active microbial communities in the rumen of dromedary camels under different feeding systems using total rRNA sequencing. PeerJ. 2020;8:e10184.
Huang C, Ge F, Yao X, Guo X, Bao P, Ma X, Wu X, Chu M, Yan P, Liang C. Microbiome and Metabolomics reveal the effects of different Feeding systems on the growth and Ruminal Development of Yaks. Front Microbiol. 2021; 12.
Mannelli F, Daghio M, Alves SP, Bessa RJB, Minieri S, Giovannetti L, Conte G, Mele M, Messini A, Rapaccini S, Viti C, Buccioni A. Effects of Chestnut Tannin Extract, Vescalagin and gallic acid on the Dimethyl acetals Profile and Microbial Community Composition in Rumen Liquor: an in Vitro Study. Microorganisms. 2019;7:202. https://doi.org/10.3390/microorganisms7070202.
Vasta V, Daghio M, Cappucci A, Buccioni A, Serra A, Viti C, Mele M. Invited review: plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: experimental evidence and methodological approaches. J Dairy Sci. 2019;102:3781–804. https://doi.org/10.3168/jds.2018-14985.
Van Gylswyk NO. Succiniclasticum ruminis gen. nov. sp. nov., a rumen bacterium converting succinate to propionate as sole energy yielding mechanism. Int J Syst Bacteriol. 1995;45:297–300. https://doi.org/10.1099/00207713-45-2-297.
Shabat SK, Sasson G, Doron-Faigenboim A, Durman T, Yaacoby S, Berg Miller ME, White BA, Shterzer N, Mizrahi I. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J. 2016;10:2958–72.
Wallace RJ, Rooke JA, McKain N, Duthie CA, Hyslop JJ, Ross DW, Waterhouse A, Watson M, Roehe R. The rumen microbial metagenome associated with high methane production in cattle. BMC Genom. 2015;16:839. https://doi.org/10.1186/s12864-015-2032-0.
Guo J, Li P, Liu S, Miao B, Zeng B, Jiang Y, Li L, Wang L, Chen Y, Zhang H. Characterization of the Rumen Microbiota and volatile fatty acid profiles of weaned Goat kids under shrub-grassland grazing and indoor feeding. Anim MDPI. 2020;10(2):176. https://doi.org/10.3390/ani10020176.
Patra AK, Kamra DN, Agarwal N. Effects of extracts of spices on rumen methanogenesis, enzyme activities and fermentation of feeds in vitro. J Sci Food Agric. 2010;90(3):511–20. https://doi.org/10.1002/jsfa.3849.
Zhou M, Hernandez-Sanabria E. Characterization of variation in Rumen methanogenic communities under different dietary and host feed efficiency conditions, as determined by PCR-denaturing gradient gel electrophoresis analysis. Appl Environ Microbiol. 2010;76:3776–86.
Jeyanathan J, Kirs M, Ronimus RS, Hoskin SO, Janssen PH. Methanogen community structure in the rumens of farmed sheep, cattle and red deer fed different diets: Rumen methanogen community. FEMS Microbiol Ecol. 2011;76:311–26. https://doi.org/10.1111/j.1574-6941.2011.01056.x.
Tapio I, Snelling TJ, Strozzi F, Wallace RJ. The ruminal microbiome associated with methane emissions from ruminant livestock. J Anim Sci Biotechnol. 2017;8:7. https://doi.org/10.1186/s40104-017-0141-0.
Arndt C, Powell JM, Aguerre MJ, Crump PM, Wattiaux MA. Feed conversion efficiency in dairy cows: repeatability, variation in digestion and metabolism of energy and nitrogen, and ruminal methanogens. J Dairy Sci. 2015;98(6):3938–50. https://doi.org/10.3168/jds.2014-8449.
Zhou M, Hernandez-Sanabria E, Guan LL. Assessment of the microbial ecology of ruminal methanogens in cattle with different feed efficiencies. Appl Environ Microbiol. 2019;75:6524–33.
Mao HL, Wang JK, Zhou YY, Liu JX. Effects of addition of tea saponins and soybean oil on methane production, fermentation and microbial population in the rumen of growing lambs. Livest Sci. 2010;129:56–62.
Liu Y, Ma T, Chen D, Zhang N, Si B, Deng K, Tu Y, Diao Q. Effects of tea saponin supplementation on nutrient digestibility, methanogenesis, and ruminal microbial flora in Dorper crossbred ewe. Animals. 2019;9:29.
Aboagye IA, Beauchemin KA. Potential of Molecular Weight and structure of tannins to reduce methane emissions from ruminants: a review. Anim MDPI. 2019;9(11):856. https://doi.org/10.3390/ani9110856.
Tawab AMAE, Khattab MSA, Hadhoud FI, Shaaban MM. Effect of mixture of herbal plants on ruminal fermentation, degradability and gas production. Acta Sci - Anim Sci. 2021;43:e48549. https://doi.org/10.4025/actascianimsci.v43i1.48549.
Szulc P, Mravčáková D, Szumacher-Strabel M, Váradyová Z, Várady M, Čobanová K, Syahrulawal L, Patra AK, Cieslak A. Ruminal fermentation, microbial population and lipid metabolism in gastrointestinal nematode-infected lambs fed a diet supplemented with herbal mixtures. PLoS ONE. 2020;16(4):e0231516. https://doi.org/10.1371/journal.pone.0231516.
Szumacher-Strabel M, Cieślak A. Potential of phytofactors to mitigate rumen ammonia and methane production. J Anim Feed Sci. 2010;19(3):319–37. https://doi.org/10.22358/jafs/66296/2010.
Borrel G, Harris HM, Tottey W, Mihajlovski A, Parisot N, Peyretaillade E, Peyret P, Gribaldo S, O’Toole PW, Brugère JF. Genome sequence of Candidatus Methanomethylophilus Alvus Mx1201, a methanogenic archaeon from the human gut belonging to a seventh order of methanogens. J Bacteriol. 2012;194(24):6944–5. https://doi.org/10.1128/JB.01867-12.
Noel SJ, Højberg O, Urich T, Poulsen M. Draft genome sequence of Candidatus Methanomethylophilus sp. 1R26, enriched from bovine rumen, a methanogenic archaeon belonging to the methanomassiliicoccales order. Genome Announc. 2016;4:e01734–15.
Auffret MD, Stewart R, Dewhurst RJ, Duthie CA, Rooke JA, Wallace RJ, Freeman TC, Snelling TJ, Watson M, Roehe R, Identification. Comparison, and validation of Robust Rumen Microbial biomarkers for methane emissions using diverse Bos Taurus breeds and basal diets. Front Microbiol. 2018;8:2642. https://doi.org/10.3389/fmicb.2017.02642.
Martínez-Álvaro M, Auffret MD, Stewart RD, Dewhurst RJ, Duthie CA, Rooke JA, Wallace RJ, Shih B, Freeman TC, Watson M, Roehe R. Identification of Complex Rumen Microbiome Interaction within Diverse Functional niches as mechanisms affecting the variation of methane emissions in bovine. Front Microbiol. 2020;11:659. https://doi.org/10.3389/fmicb.2020.00659.
McLoughlin S, Spillane C, Campion FP, Claffey N, Sosa CC, McNicholas Y, Smith PE, Diskin MG, Waters SM. Breed and ruminal fraction effects on bacterial and archaeal community composition in sheep. Sci Rep. 2023;13(1):3336. https://doi.org/10.1038/s41598-023-28909-1.
Bharanidharan R, Lee CH, Thirugnanasambantham K, Ibidhi R, Woo YW, Lee H-G, Kim JG, Kim KH. Feeding systems and host breeds influence Ruminal Fermentation, methane production, microbial diversity and Metagenomic Gene Abundance. Front Microbiol. 2021;12:701081. https://doi.org/10.3389/fmicb.2021.701081.
Li Z, Wright AD, Liu H, Fan Z, Yang F, Zhang Z, Li G. Response of the Rumen Microbiota of Sika Deer (Cervus nippon) Fed Different Concentrations of Tannin Rich Plants. PLoS ONE. 2015;10(5):e0123481. https://doi.org/10.1371/journal.pone.0123481.
Choi Y, Lee SJ, Kim HS, Eom JS, Jo SU, Guan LL, Seo J, Park T, Lee Y, Lee SS, Lee SS. Oral administration of Pinus koraiensis cone essential oil reduces rumen methane emission by altering the rumen microbial composition and functions in Korean native goat (Capra hircus coreanae). Front Vet Sci. 2023;10:1168237. https://doi.org/10.3389/fvets.2023.1168237.
Wang B, Wang D, Wu X, Cai J, Liu M, Huang X, Wu J, Liu J, Guan L. Effects of dietary physical or nutritional factors on morphology of rumen papillae and transcriptome changes in lactating dairy cows based on three different forage-based diets. BMC Genomics. 2017;18(1):353. https://doi.org/10.1186/s12864-017-3726-2.
Novak TE, Rodriguez-Zas SL, Southey BR, Starkey JD, Stockler RM, Alfaro GF, Moisá SJ. Jersey steer ruminal papillae histology and nutrigenomics with diet changes. J Anim Physiol Anim Nutr. 2019;103(6):1694–707. https://doi.org/10.1111/jpn.13189.
Hill RH. Effect of Dietary extremes on Impala (Aepyceros melampus) Rumen Epimural Flora. Appl Environ Microbiol. 1982;44(1):198–202. https://doi.org/10.1128/aem.44.1.198-202.1982.
Salih EYA, Julkunen-Tiitto R, Luukkanen O, Fahmi MKM, Fyhrquist P. Hydrolyzable tannins (ellagitannins), flavonoids, pentacyclic triterpenes and their glycosides in antimycobacterial extracts of the ethnopharmacologically selected Sudanese medicinal plant Combretum Hartmannianum Schweinf. Biomed Pharmacotherapy = Biomedecine Pharmacotherapie. 2021;144:112264. https://doi.org/10.1016/j.biopha.2021.112264.
Brown DL, Yimegnuhal A, Odenyo AA, McCrabb G. Acacia angustissima Intoxication of Menz lambs requires two components. J Appl Res Veterinary Med. 2005;3(1):13–9.
Ghandour MM. Reducing the Adverse Effects of Anti-Nutritional Factors in Some Desert Plants on Small Ruminants. Ph D., Thesis. Fac. Agric. Cairo Univ. Egypt. 137 p. 2015.
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
AER: Conceptualization, Experimental operation, Data curation, Visualization, Investigation, Writing-Original draft preparation. MMG: Experimental operation, Data curation. AS: Software, Visualization, Investigation. EAE: Data curation, Investigation. RM: Data curation, Visualization, Validation. ES: Data curation, Visualization, Investigation. AA: Experimental operation, Data curation. DMM: Investigation, Validation, Methodology. AAH: Data curation, Visualization, Software. ORH: Data curation, Supervision. All authors have written, reviewed, read, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study, including euthanasia of the experimental animals was conducted under the guidelines of the Animal Care and Use Committee in the Department of Animal and Poultry Production, Desert Research Center, Egypt (Project ID: 43,213). Alexandria University Research Ethics Review Committee, Faculty of Agriculture, University of Alexandria, Egypt approved all experimental procedures (Reference: Alex. Agri. 082305306). All methods and protocols in this study are in compliance with the ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rabee, A.E., Mohamed M. Ghandour, M., Sallam, A. et al. Rumen fermentation and microbiota in Shami goats fed on condensed tannins or herbal mixture. BMC Vet Res 20, 35 (2024). https://doi.org/10.1186/s12917-024-03887-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-024-03887-2