Abstract
This study (60 days) was conducted to investigate the ability of diet enriched with Coriandrum sativum powder or its extract to protect Oreochromis niloticus health and survivability at suboptimal temperature (21 ℃). One hundred and twenty (33.14 ± 0.5 g) were divided into four groups; each group has three replicates.. The first control group fed on a basal diet. Second and third groups fed on diet enriched with 30 mg/kg coriander seed powder (CP) and coriander seed ethanolic extract (CE), respectively. The fourth group (OT) fed on diet enriched with 500 mg oxytetracycline/kg diet. The results revealed that CE exhibited a considerable improvement in hematological parameters, hepatic-renal functions, antioxidant status, and immunological markers as well as remarkably increased resistance against Aeromonas veronii. It could be concluded that feeding tilapia CE enriched diet at 30 mg/kg is a recommended strategy to enhance tilapia health and resistance to A. veronii infection reared at 21 ℃.
Similar content being viewed by others
Introduction
Aquaculture makes a considerable contribution to food security, particularly in developing countries [1, 2]. However, this industry has numerous challenges that must be overcome to ensure sustainable aquatic production in the future. Nile tilapia is mainly located in the tropics and subtropics, so low winter temperatures are considered a challenge for the high productivity of tilapia species. The cold months of the year and water temperature fluctuation between the seasons have a negative effect on fish feed intake, metabolism, and growth performance [3], immunity [4], physiological function [5, 6], and antioxidant activity [7]. Additionally, the relationship between fluctuations in water temperature and the incidence of bacterial infection, especially Aeromonas spp., has been recorded in previous studies [8,9,10].
Aeromonus veronii is one of nearly 31 species in the genus Aeromonas [11], and recent studies revealed that it is one of the reasons for economic losses and high mortalities in tilapia fish farms [12,13,14], as well as many other fish species [15,16,17].
Antibiotics are a potent treatment for infectious diseases; in addition, they can be used in a preventative medicated diet to lower mortality and morbidity rates while also promoting growth in farm rearing systems [18]. But unfortunately, antibiotic misuse, particularly in the aquatic ecosystem, has had a number of adverse effects on aquatic organisms, the environment, and public health [19]. The most harmful effects of antibiotic abuse include antibiotic accumulation in the environment over a long period of time, which disturbs the balance of microorganisms and results in the development of antibiotic-resistant bacteria and genes, as well as antibiotic accumulation in various fish tissues [19, 20]. As a result, it has recently become an urgent necessity to find alternatives that boost fish immune efficiency, disease resistance, and are environmentally friendly.
Medicinal plants are considered potential alternatives to antibiotics for preventing fish diseases; not only that, but they can also be used as feed additives, immunostimulants, and alternatives to some expensive imported feed components to reduce the cost of feeding fish [21,22,23,24,25]. A member of the Apiaceae family, coriander (Coriandrum sativum) is an aromatic and therapeutic plant [26]. Coriander seeds have vital components that preserve their pharmacological properties, such as linalool, terpenoids, linoleic acid, vitamin C, and minerals. It is also distinguished by several biological properties, including analgesic, antibacterial, antifungal, and anti-parasitic [26,27,28]. Recent studies have shown that a coriander-enriched diet has an improving effect on fish growth performance and protects them from a variety of pathogenic bacteria [29,30,31]. Furthermore, coriander-enriched diets therapeutic effectiveness against A. veronii was also proven [32]. As well, it acts as an antidote to some heavy metal toxicity [33, 34]. However, according to our information, the effect of a coriander-enriched diet on the growth performance and immune status of tilapia fish at suboptimal temperatures has not been studied.
Therefore, the goal of the current study was to determine the effects of a coriander seed powder or extract-enriched diet on the growth performance, hepatic-renal functions, antioxidant-immune response, and Aeromonas veronii resistance in Oreochromis niloticus at suboptimal temperature.
Materials and methods
Collection and preparation of coriander (Coriandrum sativum)
The coriander seeds gathered for this study were from a local market in Zagazig, Egypt. Before drying for 15 days at 29 ± 2 °C in the shade, the seeds had a thorough water wash. A pestle and mortar were used to grind the dried seeds into a fine powder. A portion of this fine powder was kept at 4 °C in a capped sterile bottle and utilized as a powder (CP). Another fraction of the fine powder was utilized for extraction to produce an ethanolic coriander extract (CE) according to the method reported by Ahmed, Reda [34]. The bioactive substances present in the C. sativum extract were identified using GC–MS analysis (Agilent Technologies, the Central Laboratories Network, National Research Centre, Cairo, Egypt). The findings showed that L-LINALOOL was the primary bioactive molecule, with the highest peak area% (92.52) at 10.107 retention time (RT, min.).
Diet preparation and experimental protocol
According to the National Research Council's recommendations, four experimental diets (Table 1) were formulated to satisfy the nutritional requirements of tilapia fish [35]. The first diet (D1) acted as the basal control diet with no supplements. C. sativum powder (D2) and extract (D3), each at 30 mg/kg diet, were added to the second and third diets as supplements based on the results of a prior study on Nile tilapia by Ahmed, Reda [34]. The fourth (D4) was an antibiotic-supplemented meal that included oxytetracycline at a dosage of 500 mg/kg (Pharma Sweed, Egypt) [36]. One hundred and twenty O. niloticus (33.14 ± 0.5 g), all of which were apparently healthy, were purchased from a private fish farm in El-Abbassa, Sharkia, Egypt. O. niloticus were randomly distributed to four groups of triplicates (10 fish/replica, 30 fish/group), which were acclimated for 2 weeks before the beginning of the experiment. At the beginning of the first week of acclimation, the water's temperature was set to 27- 28 °C. Then the water temperature was gradually lowered for the fish during the second week of acclimatization by one degree each day, reaching 21 °C at the start of the feeding trial [37].
The first control group (CONT) fed on a basal diet (D1). While the 2nd (CP), 3rd (CE), and 4th (OT) groups fed on D2, D3, and D4, respectively for 60 days. The fish in each group were fed 3% biomass through three regular feedings during the day. The average dissolved oxygen (D.O.) concentration was 5 ± 1 mg/L, the pH level was 6.5 ± 0.5, the nitrite concentration was 0.05 mg/L, the nitrate concentration was 9 mg/L, and the ammonium concentration was 0.4 mg/L, which were maintained all over the experimental period. Throughout the trial period, about 25% of the water was replaced daily to maintain its quality.
Growth indices evaluation
To evaluate growth performance, the fish were weighed every two weeks. The final body weight (FBW, g), weight gain (WG, g), daily weight gain (DWG, g), specific growth rate (SGR), and food conversion ratio (FCR) were evaluated [38].
Blood samples collection
At the end of the trial, the fish were anaesthetized using a 100-mg/L benzocaine solution from Al-Nasr Pharmaceutical Chemicals Company. The blood samples were taken from the caudal vessels and placed in clean, sterilized tubes with ethylenediaminetetraacetic acid (EDTA) as an anticoagulant to determine the hematological marker. Additional blood samples were taken and centrifuged for 15 min at 3000 rpm to separate the serum and determine biochemical, antioxidant, and immunological markers.
Hematological markers evaluation
The primary hematologic indices were determined manually using the method given by Groff and Zinkl [39]. A hemocytometer with Natt-dye Herrick's was used to measure the total red blood cell count (RBCs 1012/L). The hematocrit value (Hct; %) of the blood was determined by centrifuging it at 10.000 × g for 5 min. To measure hemoglobin concentration (Hgb; g/dl), the cyanmethemoglobin technique was applied. The following established formulas were utilized to determine mean corpuscular hemoglobin volume (MCV; fl), mean cell hemoglobin (MCH; pg), and mean corpuscular hemoglobin concentration (MCHC; g/dl):
Additional blood samples were obtained and transferred for centrifugation at 3500 rpm for 15 min to obtain blood plasma for the measurement of total protein (TP) [40], albumin (Al) [41], and globulin (Gl) by subtraction of serum albumin from total serum protein. The manual technique described by Dacie and Lewis [42] was used to determine platelets, total leukocytes (WBCs), and differential counts.
Biochemical markers evaluation
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), Urea, and creatinine levels were evaluated using ELISA (enzyme-linked immunosorbent assay, Thermo Fisher Scientific Inc., Egypt) according to commercial kits (MyBiosource Inc., USA) specific instructions [43].
Antioxidant makers evaluation
The blood levels of total antioxidant capacity (TAC, ng/ml), superoxide dismutase (SOD, U/ml), and catalase (CAT, U/ml) were determined in accordance with the manufacturer's instructions using commercial enzyme-linked immunosorbent assay (ELISA) test kits from Cusabio Biotech Co., Ltd.
Non-specific immune markers evaluation
The fish Nitric Oxide Synthase 2 Inducible ELISA Kit (MyBiosource Inc., USA) was used for measuring serum nitric oxide activity (NO, µmol/L) according to the manufacture instructions. According to Ellis [44], serum lysozyme activity was determined based on the lysis of Micrococcus lysodeikticus (Sigma Chemical Co.).
Histopathological marker evaluation
The liver and head kidney of ten fish per group were collected and immediately fixed in a 10% neutral buffered formalin solution for 48 h. After fixation, the specimens were routinely processed for paraffin technique, and 4 µm thick tissue hepatic and renal tissue sections were prepared, stained with hematoxylin and eosin dyes following the protocol described by Suvarna, Layton [45], and investigated microscopically, recording any histological alterations.
Challenge test
The A. veronii (131TF-ID) used in this investigation was isolated from tilapia fries that were naturally infected in Idku, Beheira Governorate, Egypt. Through rRNA gene sequencing, the isolate was identified as A. veronii, and it was recorded in the GenBank database (accession number: MN967136) [14]. The LD50 of A. veronii was previously determined to be 4.3 × 106 CFU/mL [14]. The bacterial stock was kept at -80 °C in tryptic soya broth with 15% glycerol until it was required.
Five fish per replicate were intraperitoneally injected with pathogenic Aeromonas veronii at dosages of 0.2 ml (4.3 × 106, adjusted using McFarland standard tubes) following 60 days of feeding. The challenged fish were monitored for 7 days to record the clinical symptoms, postmortem lesions, and daily mortalities. To reisolate the bacterial strains, samples were obtained from the kidneys and liver of dead and clinically infected fish.
Using the mortalities of all replicates, the relative percentage survival (RPS) was calculated using the formula below [46]:
Animal ethics
The Institutional Animal Care and Use Committee of Zagazig University in Egypt authorized all experimental techniques, and all appropriate institutional guidelines were followed when caring for and utilizing animals in this work (ZU-IACUC/2/F/441/2022).
Fish were euthanized at the end of the experiment using an overdose of buffered benzocaine solution (> 250 mg/L for 10 min), then pithing fish to ensure euthanasia in accordance with the methods of euthanasia listed in the report of the American Veterinary Medical Association.
Statistical analysis
Using IBM® SPSS® Statistical Package for Social Science for Windows (WINSPSS) version 25, the ANOVA one-way followed by Duncan's test at < 0.05 was used to determine the significance of the difference between the tested groups (Chicago, USA). The data were examined for normality using the Shapiro–Wilk W test and homogeneity of variances. The results are shown as the mean and standard error (M ± SE).
Results
Growth performances and survival rate
Table 2 presents the growth performance results. During the feeding trial (60 days). Supplementation with C. sativum extract dramatically enhanced growth indices and exhibited considerably greater FBW, WG, DWG, and SGR when compared to the CONT. FCR was considerably enhanced in CE and CP than in the CONT. C. sativum extract significantly improved the survival rate of fish (96.66%), while the fish fed the control diet showed the lowest survival rate (63.33%).
Hematological indices
The hematological index results are presented in Table 3. There were no significant differences seen in MCV, MCH, MCHC, neutrophils, eosinophils, basophils, and monocytes between the experimental groups. Only a significant decrease was recorded in RBCs, Hgb, platelets, WBCs, and lymphocytes in the OT-treated group in comparison to the CONT group, while there were no significant differences between CONT, CP, and CE. Total protein and albumin levels showed a significant decrease in CP compared with CONT. However, the globulin level showed a significant increase in CE in comparison with the CONT.
Biochemical parameters
While there were no significant changes between CONT, CP, and CE, there was only a significant increase in ALT, AST, ALP (Fig. 1), urea, and creatinine (Fig. 2) in the OT group in comparison to the CONT.
Alanine transaminase (ALT, U/L) (A), Aspartate transaminase (AST, U/L) (B) and alkaline phosphatase (ALP, U/L) (C) of Oreochromis niloticus fed on Coriandrum sativum seed powder or its extract-enriched diets for 60 days at suboptimal temperature. Values are presented as the mean ± SE for three samples/replicate (n = 9/group). The bars with different superscripts (a and b) are significantly different (P < 0.05, one-way ANOVA). CONT (control group): Fish fed with normal diet for 60 days at suboptimal temperature. CP: Fish fed with diet supplemented with Coriandrum sativum seed powder (CP) at 30 mg/kg for 60 days at suboptimal temperature. CE: Fish fed with diet supplemented with Coriandrum sativum seed extract (CE) at 30 mg/kg for 60 days at suboptimal temperature. OT: Fish fed with diet supplemented with 500 mg oxytetracycline/kg diet for 60 days at suboptimal temperature
Urea (mg/dl) (A) and creatinine (mg/dl) (B) of Oreochromis niloticus fed on Coriandrum sativum seed powder or its extract-enriched diets for 60 days at suboptimal temperature. Values are presented as the mean ± SE for three samples/replicate (n = 9/group). The bars with different superscripts (a and b) are significantly different (P < 0.05, one-way ANOVA). CONT (control group): Fish fed with normal diet for 60 days at suboptimal temperature. CP: Fish fed with diet supplemented with Coriandrum sativum seed powder (CP) at 30 mg/kg for 60 days at suboptimal temperature. CE: Fish fed with diet supplemented with Coriandrum sativum seed extract (CE) at 30 mg/kg for 60 days at suboptimal temperature. OT: Fish fed with diet supplemented with 500 mg oxytetracycline/kg diet for 60 days at suboptimal temperature
Oxidant/antioxidant status
The TAC indicated a substantial drop in the OT group when compared to the CONT group, while there were no appreciable changes between CONT, CP, and CE. On the other hand, SOD and catalase activity significantly decreased in the OT group compared to the CONT group; they dramatically increased in the CE group (Fig. 3).
Total antioxidant capacity (ng/ml) (A), Superoxide dismutase (u/ml) (B), and Catalase (u/ml) (C) of Oreochromis niloticus fed on Coriandrum sativum seed powder or its extract-enriched diets for 60 days at suboptimal temperature. Values are presented as the mean ± SE for three samples/replicate (n = 9/group). The bars with different superscripts (a, b and c) are significantly different (P < 0.05, one-way ANOVA). CONT (control group): Fish fed with normal diet for 60 days at suboptimal temperature. CP: Fish fed with diet supplemented with Coriandrum sativum seed powder (CP) at 30 mg/kg for 60 days at suboptimal temperature. CE: Fish fed with diet supplemented with Coriandrum sativum seed extract (CE) at 30 mg/kg for 60 days at suboptimal temperature. OT: Fish fed with diet supplemented with 500 mg oxytetracycline/kg diet for 60 days at suboptimal temperature
Immunological parameters
The nitric oxide and lysozyme activities were markedly enhanced in the CE group than in CONT, whereas there were no notable differences between the CONT, CP, and OT groups (Fig. 4).
Nitric oxide (µmol/L) (A) and Lysozyme activity (ng/ml) (B) of Oreochromis niloticus fed on Coriandrum sativum seed powder or its extract-enriched diets for 60 days at suboptimal temperature. Values are presented as the mean ± SE for three samples/replicate (n = 9/group). The bars with different superscripts (a, b and c) are significantly different (P < 0.05, one-way ANOVA). CONT (control group): Fish fed with normal diet for 60 days at suboptimal temperature. CP: Fish fed with diet supplemented with Coriandrum sativum seed powder (CP) at 30 mg/kg for 60 days at suboptimal temperature. CE: Fish fed with diet supplemented with Coriandrum sativum seed extract (CE) at 30 mg/kg for 60 days at suboptimal temperature. OT: Fish fed with diet supplemented with 500 mg oxytetracycline/kg diet for 60 days at suboptimal temperature
Histopathological findings
Normal histology was seen in all examined hepatic tissue sections of the CONT group (Fig. 5A), CP, and CE groups. Yet marked decrease in the lipoidal cytoplasmic vacuolations, associated with the presence of few numbers of lymphocytes particularly around the exocrine pancreas, besides, slight proliferations of the MMCs were observed in the hepatic tissue sections of the CP (Fig. 5B). These alterations were more pronounced in the CE group (Fig. 5C). Treatment with oxytetracycline induced variable degenerative, necrotic, and circulatory alterations in most specimens including increased cytoplasmic vacuolation, single-cell necrosis, and vascular congestion. Additionally, a few tissue sections of the OT group exhibited focal lytic necrosis infiltrated with numerous extravasated erythrocytes (Fig. 5D).
A-D Representative photomicrograph of H&E-stained (10x) hepatic tissue sections showing a normal histological picture in the control fish (A). A marked decrease in the lipoidal cytoplasmic vacuolations is seen in the hepatic tissue sections of the CP (B), and CE (C) groups. A focal lytic necrotic area containing numerous extravasated erythrocytes (ellipse) is seen in the OT (D) group. E–H Representative photomicrograph of H&E-stained (40x) renal tissue sections showing normal histological pictures in the control fish (E), CP (F), and CE (G) groups. Tubular vacuolation (red arrowhead), tubular necrosis (yellow arrowhead), glomerular atrophy (black arrowhead), glomerular necrosis (black arrow), and vascular congestion (red arrow) are seen in the OT (H) group
The kidneys of the CONT (Fig. 5E), CP (Fig. 5F), and CE (Fig. 5G) groups showed normal histological pictures without any variations between these groups. Controversy, most tissue sections of the OT fish manifested various degenerative changes such as tubular vacuolations and necrosis, cast formation, glomerular atrophy, and necrosis, and congestion of the interstitial vasculatures (Fig. 5H).
Aeromonas veronii resistance
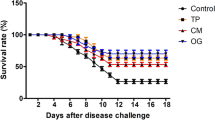
After 60 days of the feeding trial, O. niloticus challenged with A. veronii showed detachment of the scales, destruction of fin rays with fin rot, hemorrhage at the base of fins, especially the pectoral fin, congestion of the gill cover with excessive mucus secretion, and eye turbidity (Fig. 6A and B). The cumulative mortality rate was elevated in the control group (66.66%), followed by the CP (46.66%), OT (33.33%), and CE (33.33%) groups (Fig. 6C). The highest RPS was recorded with the CE group, while there was no significant difference between the CP and OT groups (Fig. 6D).
A and B Main symptom of fish from control group (Fish fed with normal diet for 60 days at suboptimal temperature then challenged with Aeromonas veronii) showing detached scales (yellow arrow), fin rot (black arrow), hemorrhage at the base of pectoral fin (green star), high mucus secretion (black star), eye turbidity (white circle), congestion of the operculum (red rectangle). C and D Cumulative mortality rate and Relative percentage survival of Oreochromis niloticus fed on Coriandrum sativum seed powder or its extract-enriched diets for 60 days at suboptimal temperature and challenged with pathogenic Aeromonas veronii for 7 days. CONT (control group): Fish fed with normal diet for 60 days at suboptimal temperature. CP: Fish fed with diet supplemented with Coriandrum sativum seed powder (CP) at 30 mg/kg for 60 days at suboptimal temperature. CE: Fish fed with diet supplemented with Coriandrum sativum seed extract (CE) at 30 mg/kg for 60 days at suboptimal temperature. OT: Fish fed with diet supplemented with 500 mg oxytetracycline/kg diet for 60 days at suboptimal temperature
Discussion
The negative impact of suboptimal and cold temperatures on tilapia health has been shown in previous studies [4,5,6, 47]. The objective of the current study was to determine how C. sativum affected O. niloticus reared at suboptimal temperatures in terms of growth, health condition, survival rate, and resistance to A. veronii.
About 25–32 °C is the ideal temperature range for tilapia to grow and develop normally [48]. This study exhibited that CE was able to improve the growth performance and survival rate of tilapia fish raised at suboptimal temperatures compared to the CONT group. The results of Farsani, Hoseinifar [31] have shown the enhancement of Oncorhynchus mykiss growth performances when fed diets supplemented with CE at a rate of 2% for 8 weeks. Also, the dietary administration of 10–20 g coriander seed powder/kg for 150 days has improved the growth performance and survival rate of Dicentrarchus labrax [49]. The enhancement of the growth performance could be related to (i) the ability of coriander to reduce counts of pathogenic intestinal bacteria that compete for nutrients by their antibacterial effect [49, 50]. (ii) The antioxidant constituents in CE prevent intestinal mucosal damage and enhance absorption [51, 52]. (iii) The ability of CE to stimulate bile and digestive enzyme secretion, which improves the digestion process [50].
O. niloticus is extremely sensitive to any drop in the ambient temperature, which causes poor growth and massive mortality [53, 54]. The current study demonstrated an improvement in the survival rate of fish fed a diet supplemented with CE (96.66%) compared to those fed a basal diet (63.33%) during the experimental period under suboptimal temperature conditions. This suggests that CE may benefit tilapia under low-temperature stress. This finding could be related to the richness of CE with phenolic (especially linalool) and flavonoid compounds [29, 51, 55], which protect fish from the oxidative stress resulting from the suboptimal temperature. According to the data we have gathered, this study is considered the first to demonstrate the beneficial effect of coriander extract on the survival rate of tilapia reared under suboptimal temperature conditions.
In aquaculture, hematological studies are used to assess the health and welfare of fish. These analyses are thought to be a good indicator of a variety of environmental parameters, such as infections, diet, and water quality [56]. RBCs, Hgb, platelets, WBCs, and lymphocytes of the OT-treated group in the current study were significantly lower than those of the CONT group, while there was no significant difference between CONT and the other experimental group. According to earlier research, fish exposed to antibiotics may have a variety of hematological alterations, including an increase or decrease in leukocyte count and red blood cell parameters (RBC, Ht, and Hb). Similar to our results, Omoregie and Oyebanji [57] found that oxytetracycline-induced significant reductions in leukocyte, erythrocyte, thrombocyte, hematocrit, and hemoglobin values of O. niloticus after being fed on diets incorporating varying concentrations of the drug (5.00, 2.50, 1.25, and 0.63%) for 8 weeks indicated an anemic response. On the other hand, the globulin level indicated a substantial increase in the CE group as compared to the CONT group in the current study. Globulin is a group of serum proteins (e.g., enzymes, complement, and immunoglobulin) formed by liver and plasma cells [58]. Therefore, as reported by Ola and Sofolahan [59], the increase in globulin levels in the CE group may be attributable to the presence of linalool and phenolic compounds, which in turn improve liver functions and hematological markers.
The liver is primarily responsible for the metabolism of antibiotics, while the kidneys are responsible for their active form of elimination. The results of the current study showed that the renal and liver functions of the OT group were disturbed, as evidenced by elevated levels of urea, creatinine, AST, ALT, and other compounds. Notably, oxytetracycline induced similar disturbances in the liver and kidney function of O. niloticus at 500 mg/kg of diet for 14 days [60] and 100 mg/kg of diet for 84 days [61] and O. mykiss at 2.5 g/kg of diet for 14 days [62]. Other types of antibiotics have also been shown to have a similar effect on liver and kidney function. For example, gentamicin injection at a dose of 36 mg/kg caused significant disruption in the trunk kidney and liver of O. niloticus and raised ALT and AST levels [63]. On the other hand, the addition of coriander, whether as an extract or powder, did not negatively impact the liver or kidney activities in the present study. This may be due to active coriander seed ingredients like linalool, linoleic acid, vitamin C, terpenoids, and minerals, which maintain the physiological functions of the liver and kidneys [50, 64,65,66].
Exposure to suboptimal temperatures leads to the production of reactive oxygen species (ROS). Excessive ROS generation impairs physiological processes, damages DNA, induces cellular apoptosis, and suppresses the immune system [7, 37, 47]. In the current study, TAC, SOD, and catalase activity significantly decreased in the OT group compared to the CONT group. Previous studies have proven that OT overdose is linked to decreased tissue antioxidant indicators, damages mitochondria, impairs the respiratory chain, and peroxides the lipids in membranes [67,68,69]. The results are in line with previous reports regarding Limbu, Zhang [70], who recorded that hepatic SOD and GST activities decreased and MDA level and AST activity increased in O. niloticus fed on a diet supplemented with 80 mg/kg of oxytetracycline for 35 days, which indicates oxidative damage. On the other hand, the group that was fed a diet containing CE in the present study was distinguished by an increase in SOD and catalase activity due to the richness of CE in phenolic compounds, the most important of which is linalool [29, 71]. Strong antioxidant properties found in linalool enable it to scavenge ROS produced by a variety of stressors [72, 73]. Similar to our results, Das (2023) reported a significant increase in the serum SOD activity of O. niloticus in the coriander oil-treated groups compared to the control after 60 days. These findings clarify the reasons for the improvement of hematological and biochemical parameters in the CE group in the present study.
Fish immune systems are weakened by the suboptimal temperature, which also interferes with vital physiological and biochemical processes [4, 37]. In the current study, at the suboptimal temperature, the nitric oxide and lysozyme activities were markedly enhanced in the CE group compared to the CONT group. Different functional components, including phenolic compounds, notably linalool, flavonoids, and alkaloids, give C. sativum its pharmacological, immunostimulant, and antioxidant properties [50, 74]. Similarly, the respiratory burst, myeloperoxidase, lysozyme, and antiprotease activities of O. niloticus fed a diet supplemented with 1.5 and 2% coriander oil for 60 days exhibited significant increases compared to the control [29]. Ashry, Habiba [49] recorded a significant increase in the lysozyme and phagocytic activities of Dicentrarchus labrax fed 10 and 20 g of coriander seed powder per kg of diet for 150 days. Moreover, O. mykiss fed a diet enriched with 2% coriander seed extract for 8 weeks showed significant improvements in lysozyme and alternative complement activities [31].
Histopathology is a well-known and strong method for identifying changes in the normal condition of live tissues and potentially their causative agent that cannot be noticed by the naked eye [75]. In the present study, histopathological abnormalities were only observed in the liver and renal tissues of fish in the OT group. This confirms the earlier findings in this study regarding disturbances in hepatic and renal function, as well as antioxidant activity in the OT group. Abraham, Roy [76] observed histological changes in hepatic and renal tissues of O. niloticus treated with oxytetracycline at 80 mg/kg biomass/day that progressed from mild to moderate on day 10. These results are similar to those of Islam, Rasul [77] for Barbonymus gonionotus, Krupesha Sharma, Sumithra [78] for Trachinotus blochii, Solanki, Avunje [79] for Cirrhinus mrigala, and Rodrigues, Antunes [80] for O. mykiss, despite differences in the type of fish, the dose of oxytetracycline, and the duration of exposure. On the other hand, the recent study conducted by Yigit and Kocaayan [81] confirmed that no pathological findings were observed in any organ (gill, liver, or kidney) of O. mykiss in the coriander group at the different concentrations (20, 50, 70, 100, 150, 200, 300, 400, 500, and 600 mg L−1). Several studies also supported the idea that C. sativum protects liver and kidney cell tissues from oxidative damage brought on by various stressors [29, 82, 83].
Fish exposure to suboptimal temperatures has negative effects on their physiological functions and immune response, as well as impairing their ability to resist pathogens and thus compromising their overall health [4]. In the present study, CE enhanced tilapia resistance to A. veronii and increased RPS. This is in line with the results of Das, Pradhan [29], who recorded an increase in the survival rate of O. niloticus challenged with A. hydrophila and fed on diets supplemented with coriander oil in a dose-dependent manner compared to the control group. Similar to this, O. mykiss fed on a diet supplemented with CE at 2% and challenged with Y. ruckeri exhibited the lowest mortality rate (40%), compared to the control group (60%) [31]. Linalool and other bioactive components, which can destroy the bacterial cell membrane and result in cell death, may be responsible for the antibacterial effect of CE and the enhancement of survival rates [32, 84]. In addition, the foregoing results in the current study indicated an enhancement in the antioxidant capacity and immune status of fish fed on diets enriched with CE, so they were more resistant to A. veronii infection. These findings are supported by previous reports that have proven the immunostimulant effect of the CE to enhance the resistance of various types of fish against pathogens [29,30,31].
Conclusion
These findings demonstrated that dietary supplementation with C. sativum extract at 30 mg/kg can lessen the immunosuppressive effects of suboptimal temperature in O. niloticus and increase resistance to A. veronii infection.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Pradeepkiran JA. Aquaculture role in global food security with nutritional value: a review. Transl Anim Sci. 2019;3(2):903–10.
Jiang Q, Bhattarai N, Pahlow M, Xu Z. Environmental sustainability and footprints of global aquaculture. Resour Conserv Recycl. 2022;180:106183.
El Asely AM, Reda RM, Salah AS, Mahmoud MA, Dawood MAO. Overall performances of Nile tilapia (Oreochromis niloticus) associated with using vegetable oil sources under suboptimal temperature. Aquac Nutr. 2020;26(4):1154–63.
Abram QH, Dixon B, Katzenback BA. Impacts of low temperature on the Teleost immune system. Biology. 2017;6(4):1–15.
Panase P, Saenphet S, Saenphet K. Biochemical and physiological responses of Nile tilapia Oreochromis Niloticus Lin subjected to cold shock of water temperature. Aquac Rep. 2018;11:17–23.
da Silva BC, Pereira A, Marchiori NC, Mariguele KH, Massago H, Klabunde GHF. Cold tolerance and performance of selected Nile tilapia for suboptimal temperatures. Aquac Res. 2021;52(3):1071–7.
Nobrega RO, Dafre AL, Corrêa CF, Mattioni B, Batista RO, Pettigrew JE, et al. Oxidative damage in Nile tilapia, Oreochromis niloticus, is mainly induced by water temperature variation rather than Aurantiochytrium sp. meal dietary supplementation. Fish Physiol Biochem. 2022;48(1):85–99.
Maalej S, Denis M, Dukan S. Temperature and growth-phase effects on Aeromonas hydrophila survival in natural seawater microcosms: role of protein synthesis and nucleic acid content on viable but temporarily nonculturable response. Microbiology. 2004;150(1):181–7.
Hassan MA, Noureldin EA, Mahmoud MA, Fita NA. Molecular identification and epizootiology of Aeromonas veronii Infection among farmed Oreochromis niloticus in Eastern Province, KSA. Egypt J Aquat Res. 2017;43(2):161–7.
Edberg SC, Browne FA, Allen MJ. Issues for Microbial Regulation: Aeromonas as a model. Crit Rev Microbiol. 2007;33(1):89–100.
Chen P-L, Lamy B, Ko W-C. Aeromonas dhakensis, an increasingly recognized human pathogen. Front Microbiol. 2016;7:793.
Abd El Latif AM, Elabd H, Amin A, Eldeen AIN, Shaheen AA. High mortalities caused by Aeromonas veronii: identification, pathogenicity, and histopathologicalstudies in Oreochromis niloticus. Aquac Int. 2019;27(6):1725–37.
Amal MNA, Koh CB, Nurliyana M, Suhaiba M, Nor-Amalina Z, Santha S, et al. A case of natural co-infection of Tilapia Lake Virus and Aeromonas veronii in a Malaysian red hybrid tilapia (Oreochromis niloticus×O. mossambicus) farm experiencing high mortality. Aquaculture. 2018;485:12–6.
Reda RM, El-Murr A, Abd Elhakim Y, El-Shahat W. Aeromonas veronii detection in Egyptian fish farms with summer tilapia mortality outbreaks and the role of formic acid in limiting its spread. Aquac Res. 2022;53(3):940–56.
Cai S-H, Wu Z-H, Jian J-C, Lu Y-S, Tang J-F. Characterization of pathogenic Aeromonas veronii Bv. Veronii associated with ulcerative syndrome from Chinese longsnout catfish (Leiocassis longirostris Günther). Braz J Microbiol. 2012;43:382–8.
Chandrarathna HPSU, Nikapitiya C, Dananjaya SHS, Wijerathne CUB, Wimalasena SHMP, Kwun HJ, et al. Outcome of co-infection with opportunistic and multidrug resistant Aeromonas hydrophila and A. Veronii in zebrafish: identification, characterization, pathogenicity and immune responses. Fish Shellfish Immunol. 2018;80:573–81.
Chen F, Sun J, Han Z, Yang X, Xian JA, Lv A, et al. Isolation, identification and characteristics of Aeromonas veronii from Diseased Crucian Carp (Carassius auratus Gibelio). Front Microbiol. 2019;10:2742.
Tiseo K, Huber L, Gilbert M, Robinson TP, Van Boeckel TP. Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiot. 2020;9(12):918.
Hossain A, Habibullah-Al-Mamun M, Nagano I, Masunaga S, Kitazawa D, Matsuda H. Antibiotics, antibiotic-resistant bacteria, and resistance genes in aquaculture: risks, current concern, and future thinking. Environ Sci Pollut Res. 2022;29(8):11054–75.
Romero J, Feijoó CG, Navarrete P. Antibiotics in aquaculture–use, abuse and alternatives. In: Carvalho E, editor. Health and environment in aquaculture. 159. Europe: InTech 2012. p. 159 – 98.
Jeyavani J, Sibiya A, Sivakamavalli J, Divya M, Preetham E, Vaseeharan B, et al. Phytotherapy and combined nanoformulations as a promising disease management in aquaculture: a review. Aquac Int. 2022;30(2):1–16.
Citarasu T. Herbal biomedicines: a new opportunity for aquaculture industry. Aquac Int. 2010;18(3):403–14.
Jadaun JS, Chownk M, Bose SK, Kumari S, Sangwan NS. Food and vegetables as source of Phytoactives for Immunomodulation. In: Sangwan NS, Farag MA, Modolo LV, editors. Plants and Phytomolecules for Immunomodulation: recent trends and advances. Singapore: Springer Nature Singapore; 2022. p. 439–68.
Van Hai N. The use of medicinal plants as immunostimulants in aquaculture: a review. Aquaculture. 2015;446:88–96.
Nasr MAF, Reda RM, Ismail TA, Moustafa A. Growth, hemato-biochemical parameters, body composition, and myostatin gene expression of Clarias gariepinus fed by replacing fishmeal with plant protein. Animals. 2021;11(3): 889.
Prachayasittikul V, Prachayasittikul S, Ruchirawat S, Prachayasittikul V. Coriander (Coriandrum sativum): a promising functional food toward the well-being. Food Res Int. 2018;105:305–23.
Bankar R, Kumar A, Puri S, Ali I, Sharma A, Khan IA. Chemical composition and antimicrobial activity of essential oil from seed of Coriandrum sativum L. Anal Chem Lett. 2011;1(2):189–93.
Bhuiyan MNI, Begum J, Sultana M. Chemical composition of leaf and seed essential oil of Coriandrum sativum L. from Bangladesh. Bangladesh J Pharmacol. 2009;4(2):150–3.
Das S, Pradhan C, Pillai D. Dietary coriander (Coriandrum sativum L) oil improves antioxidant and anti-inflammatory activity, innate immune responses and resistance to Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2023;132:108486.
Innocent BX, fathima MSA. Dhanalakshmi. Studies on the immouostimulant activity of Coriandrum sativum and resistance to Aeromonas hydrophila in Catla catla. J Appl Pharm Sci. 2011(Issue):132–5.
Farsani MN, Hoseinifar SH, Rashidian G, Farsani HG, Ashouri G, Van Doan H. Dietary effects of Coriandrum sativum extract on growth performance, physiological and innate immune responses and resistance of rainbow trout (Oncorhynchus mykiss) against Yersinia ruckeri. Fish Shellfish Immunol. 2019;91:233–40.
Said AA, Reda RM, Metwally MMM, Abd El-Hady HM. Therapeutic efficacy of coriander (Coriandrum sativum) enriched diets in Oreochromis niloticus: effect on hepatic-renal functions, the antioxidant-immune response and resistance to Aeromonas veronii. Fish Physiol Biochem. 2023;49(4):687–709.
Ren H, Jia H, Kim S, Maita M, Sato S, Yasui M, et al. Effect of Chinese parsley Coriandrum sativum and chitosan on inhibiting the accumulation of cadmium in cultured rainbow trout Oncorhynchus mykiss. Fish Sci. 2006;72(2):263–9.
Ahmed SA, Reda RM, ElHady M. Immunomodulation by Coriandrum sativum seeds (coriander) and its ameliorative effect on lead-induced immunotoxicity in Nile tilapia (Oreochromis niloticus L). Aquac Res. 2020;51(3):1077–88.
NRC (National Research Council). Nutrient requirements of fish and shrimp. Washington: National Academies Press; 2011.
Hashem NMA, El-Son MAM, Ateya AI, Saleh RM. Impact of lactoferrin supplementation on oxidative stress, gene expression and immunity dysfunction induced by Aeromonas veronii in Nile tilapia (Oreochromis niloticus). Aquac Res. 2022;53(6):2392–407.
Reda RM, El Asely A, Salah AS, Mahmoud MA. Replacement of dietary fish oil with plant oils improves the immunological responses and the antioxidant status in Oreochromis niloticus exposed to suboptimal temperature. Fish Physiol Biochem. 2020;46(6):2181–96.
Reda RM, Selim KM. Evaluation of Bacillus amyloliquefaciens on the growth performance, intestinal morphology, hematology and body composition of Nile tilapia, Oreochromis niloticus. Aquac Int. 2015;23(1):203–17.
Groff JM, Zinkl JG. Hematology and Clinical Chemistry of Cyprinid Fish: common carp and goldfish. Vet Clin North Am Exot Anim Pract. 1999;2(3):741–76.
Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177(2):751–66.
Weichsebum T. Method for determination of albumin in serum blood. J Amer J Clin Pathol. 1946;16:40.
Dacie JV, Lewis SM. Practical Haematology, 9th edn. (ed. by S.M. Lewis, B.J. Bain & I. Bates). London: Churchill Livingstone, Elsevier; 2001.
Wu AHB. Tietz Clinical Guide to Laboratory tests. E-Book: Elsevier Health Sciences; 2006.
Ellis AE. Lysozyme assays. Techniques in fish Immunology. 1990;1:101–3.
Suvarna KS, Layton C, Bancroft JD. Bancroft’s Theory and Practice of Histological Techniques E-Book. 8 ed. Elsevier Health Sciences; 2018.
Amend DF. Potency testing of fish vaccines. Fish Biologics: Serodiagnostics and Vaccines. 1981:447–54.
Dellagostin EN, Martins AWS, Blödorn EB, Silveira R, Komninou TL, Varela Junior ER. Chronic cold exposure modulates genes related to feeding and immune system in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2022;128:269–78.
El-Sayed A-FM, Kawanna M. Optimum water temperature boosts the growth performance of Nile tilapia (Oreochromis niloticus) fry reared in a recycling system. Aquac Res. 2008;39(6):670–2.
Ashry AM, Habiba MM, Desouky MG, El-Zayat AM, Moonmanee T, Van Doan H, et al. The effects of coriander (Coriandrum sativum) seeds on the growth performance, growth hormone, antibacterial capacity, and immune response of European sea bass (Dicentrarchus labrax). Ann Anim Sci. 2022;22(4):1273–80.
Laribi B, Kouki K, M’Hamdi M, Bettaieb T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia. 2015;103:9–26.
Msaada K, Jemia MB, Salem N, Bachrouch O, Sriti J, Tammar S, et al. Antioxidant activity of methanolic extracts from three coriander (Coriandrum sativum L.) fruit varieties. Arab J Chem. 2017;10:3176-S3183.
Nadeem M, Anjum FM, Khan MI, Tehseen S, El-Ghorab A, Sultan JI. Nutritional and medicinal aspects of coriander (Coriandrum sativum L.). Br Food J. 2013;115(5):743–55.
El-Sayed A-FM. Tilapia culture. CABI publishing; 2006.
Charo-Karisa H, Rezk MA, Bovenhuis H, Komen H. Heritability of cold tolerance in Nile tilapia, Oreochromis niloticus, juveniles. Aquaculture. 2005;249(1):115–23.
Dakhlaoui S, Wannes WA, Sari H, Hmida MB, Frouja O, Limam H, et al. Combined effect of essential oils from lavender (Lavandula officinalis L.) Aerial Parts and Coriander (Coriandrum sativum L.) seeds on Antioxidant, Anti-diabetic, anti-cancer and anti-inflammatory activities. J Essent Oil-Bear. 2022;25(1):188–99.
Witeska M, Kondera E, Ługowska K, Bojarski B. Hematological methods in fish – not only for beginners. Aquaculture. 2022;547:737498.
Omoregie E, Oyebanji SM. Oxytetracycline-Induced blood disorder in Juvenile Nile Tilapia Oreochromis Niloticus (Trewavas). J World Aquac Soc. 2002;33(3):377–82.
Busher JT. Serum albumin and globulin. In: Walker HKHW, Hurst JW, editors. Clinical methods: the history, physical, laboratory examinations. 3 3. Boston: Butterworths; 1990. p. 497–9.
Ola OS, Sofolahan TA. A monoterpene antioxidant, linalool, mitigates benzene-induced oxidative toxicities on hematology and liver of male rats. Egypt J Basic appl sci. 2021;8(1):39–53.
El-Adawy M, El-Aziz MA, El-Shazly K, Ali NG, El-Magd MA. Dietary propionic acid enhances antibacterial and immunomodulatory effects of oxytetracycline on Nile tilapia, Oreochromis niloticus. Environ Sci Pollut Res. 2018;25(34):34200–11.
Reda RM, Ibrahim R, Ahmed E-NG, El-Bouhy Z. Effect of oxytetracycline and florfenicol as growth promoters on the health status of cultured Oreochromis niloticus. Egypt J Aquat Res. 2013;39(4):241–8.
Hoseini SM, Yousefi M. Beneficial effects of thyme (Thymus vulgaris) extract on oxytetracycline-induced stress response, immunosuppression, oxidative stress and enzymatic changes in rainbow trout (Oncorhynchus mykiss). Aquac Nut. 2019;25(2):298–309.
Chen C-Y, Wooster GA, Bowser PR. Comparative blood chemistry and histopathology of tilapia infected with Vibrio vulnificus or Streptococcus iniae or exposed to carbon tetrachloride, gentamicin, or copper sulfate. Aquaculture. 2004;239(1):421–43.
Coşkuner Y, Karababa E. Physical properties of coriander seeds (Coriandrum sativum L). J Food Eng. 2007;80(2):408–16.
Önder A. Coriander and its phytoconstituents for the beneficial effects. Potential of essential oils. 2018:165–85.
Samojlik I, Lakic N, Mimica-Dukic N, Đaković-Švajcer K, Bozin B. Antioxidant and hepatoprotective potential of essential oils of coriander (Coriandrum sativum L.) and caraway (Carum carvi L.)(Apiaceae). J Agric Food Chem. 2010;58(15):8848–53.
Pari L, Gnanasoundari M. Influence of Naringenin on Oxytetracycline mediated oxidative damage in Rat Liver. Basic Clin Pharmacol Toxicol. 2006;98(5):456–61.
Gnanasoundari M, Pari L. Impact of Naringenin on oxytetracycline-mediated oxidative damage in kidney of rats. Ren Fail. 2006;28(7):599–605.
Bojarski B, Kot B, Witeska M. Antibacterials in aquatic environment and their toxicity to fish. Pharmaceuticals. 2020;13(8):189.
Limbu SM, Zhang H, Luo Y, Chen L-Q, Zhang M, Du Z-Y. High carbohydrate diet partially protects Nile tilapia (Oreochromis niloticus) from oxytetracycline-induced side effects. Environ Pollut. 2020;256:113508.
Duarte A, Luís Â, Oleastro M, Domingues FC. Antioxidant properties of coriander essential oil and linalool and their potential to control Campylobacter spp. Food Control. 2016;61:115–22.
Sabogal-Guáqueta AM, Hobbie F, Keerthi A, Oun A, Kortholt A, Boddeke E, et al. Linalool attenuates oxidative stress and mitochondrial dysfunction mediated by glutamate and NMDA toxicity. Biomed Pharmacother. 2019;118:109295.
Azırak S, Özgöçmen M. Linalool prevents kidney damage by inhibiting rifampicin-induced oxidative stress and apoptosis. J Tissue Cell. 2023;82:102097.
Momin AH, Acharya SS, Gajjar AV. Coriandrum sativum-review of advances in phytopharmacology. Int J Pharm Sci Res. 2012;3(5):1233.
Costa PM. The handbook of histopathological practices in aquatic environments: guide to Histology for Environmental Toxicology. Amsterdam: Elsevier Science; 2017.
Abraham TJ, Roy A, Julinta RB, Singha J, Patil PK. Dietary therapeutic dose of oxytetracycline influences histopathological alterations in Nile tilapia Oreochromis Niloticus (L). Aquac Res. 2021;52(11):5925–30.
Islam M, Rasul M, Kashem M, Hossain M, Liza A, Sayeed M, et al. Effect of oxytetracycline on Thai silver barb (Barbonymus gonionotus) and on it’s culture environment. J Fish Aquat Sci. 2015;10(5):323.
Krupesha Sharma SR, Sumithra TG, Gangadharan S, Shahansha A, Sanil NK, Ashok Kumar K, et al. Evaluation of biosafety and tissue residue of oxytetracycline in juvenile snubnose pompano, Trachinotus blochii along with in vitro efficacy against fish pathogens. Aquaculture. 2021;533:736184.
Solanki HG, Avunje S, Ananda Raja R, Trangadia BJ, Verma A, Vanza JG, et al. Evaluation of safety and withdrawal period of orally administered Oxytetracycline (OTC) in advance fingerlings of Cirrhinus mrigala (Hamilton, 1822). Aquaculture. 2022;554:738167.
Rodrigues S, Antunes SC, Nunes B, Correia AT. Histological alterations in gills and liver of rainbow trout (Oncorhynchus mykiss) after exposure to the antibiotic oxytetracycline. Environ Toxicol Pharmacol. 2017;53:164–76.
Yigit NO, Kocaayan H. Efficiency of thyme (Origanum onites) and coriander (Coriandrum sativum) essential oils on anesthesia and histopathology of rainbow trout (Oncorhynchus mykiss). Aquaculture. 2023;562:738813.
Islam MR, Jahid MA, Hasan I. Health-promoting activities of coriander seed extracts. Handbook of coriander (Coriandrum sativum). Boca Raton: CRC Press; 2023. p. 517–30.
Nicula M, Dumitrescu G, Pacala N, Stef L, Tulcan C, Dragomirescu M, et al. Garlic, cilantro and chlorella’s effect on kidney histoarchitecture changes in Cd-intoxicated Prussian carp (Carassius gibelio). Scientific Papers: Animal Science & Biotechnologies/Lucrari Stiintifice: Zootehnie si Biotehnologii. 2016;49(1):168–77.
Silva F, Ferreira S, Queiroz JA, Domingues FC. Coriander (Coriandrum sativum L.) essential oil: its antibacterial activity and mode of action evaluated by flow cytometry. J Med Microbiol. 2011;60(10):1479–86.
Acknowledgements
The authors are grateful to the Aquatic Animal Medicine Department, Faculty of Veterinary Medicine, Zagazig University, for providing the facilities that were needed for this study to be completed.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Ahmed Abdou Said, Rasha M. Reda, Mohamed M. M. Metwally, Heba M. Abd El-Hady were involved in conceptualization, data curation, formal analysis, investigation, methodology, resources, validation, visualization, roles/writing original draft, writing review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Animal Care and Use Committee of Zagazig University in Egypt authorized all experimental techniques, and all appropriate institutional guidelines were followed when caring for and utilizing animals in this work (ZU-IACUC/2/F/441/2022).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Said, A.A., Reda, R.M., Metwally, M.M.M. et al. The contribution of Coriandrum sativum in enhancing Oreochromis niloticus rearing at sub-optimal temperatures: effect on growth, health status, survival rate, and resistance to Aeromons Veronii. BMC Vet Res 19, 254 (2023). https://doi.org/10.1186/s12917-023-03809-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-023-03809-8