Abstract
Background
The pathogenic Clostridia cause neurotoxic, histotoxic and enterotoxic infections in humans and animals. Several Clostridium species have been associated with abomasitis in ruminants. The present study aimed to investigate the frequency, and the presence of virulence genes, of Clostridium perfringens, Paeniclostridium sordellii and Clostridium septicum in lambs and goat kids with hemorrhagic abomasitis.
Results
A total of 38 abomasum samples, collected from lambs and goat kids of 1 week to 1 month of age in different farms located in eastern Turkey between 2021 and 2022, were evaluated by histopathology, culture and PCR. At necropsy, the abomasum of the animals was excessively filled with caseinized content and gas, and the abomasum mucosa was hemorrhagic in varying degrees. In histopathological evaluation, acute necrotizing hemorrhagic inflammation was noted in abomasum samples. The examination of swab samples by culture and PCR revealed that C. perfringens type A was the most frequently detected species (86.84%) either alone or in combination with other Clostridium species. P. sordellii, C. perfringens type F and C. septicum were also harboured in the samples, albeit at low rates. Beta2 toxin gene (cpb2) was found in three of C. perfringens type A positive samples.
Conclusion
It was suggested that vaccination of pregnant animals with toxoid vaccines would be beneficial in terms of protecting newborn animals against Clostridial infections. This study investigated the presence of clostridial toxin genes in abomasal samples for the first time in Turkey.
Similar content being viewed by others
Background
Clostridial diseases are common in livestock worldwide [1, 2]. The toxins produced by Clostridium species cause enteric, neurotoxic or histotoxic diseases in humans and animals [2]. Clostridial abomasitis and enteritis are responsible for extensive morbidity and high mortality rate in especially young ruminants [1]. Clostridial abomasitis is mostly caused by Clostridium perfringens, Paeniclostridium sordellii (previously known as Clostridium sordelllii) and Clostridium septicum species [3,4,5,6,7,8,9,10,11]. C. perfringens is classified into seven types (A-G) based on its capacity to encode six typing toxins, namely alpha, beta, epsilon, iota, enterotoxin and netB [12]. Each toxin type is associated with different enteric disaeases in animals. The beta2 toxin which is a non-typing toxin can be produced by different C. perfringens types [13]. It has been reported that beta2 toxin has a role in the pathogenesis of clostridial diseases [14, 15]. C. perfringens type A is associated with enterotoxemia in lambs (yellow lamb disease), and enteritis or enterotoxemia in cattle, pigs, horses, and goats, abomasitis in ruminants and hemorrhagic canine gastroenteritis [1, 2]. However, C. perfringens type A was mostly detected in cases of abomasitis in calves [5,6,7,8, 16]. C. perfringens type E has also been isolated from calves with abomasitis [17]. In addition to Clostridium species, Sarcinia spp., coccidiosis and copper deficieny have also been reported to be associated with abomasitis in animals [1, 18,19,20,21]. P. sordellii has been described as a cause of gas gangrene in animals [2, 22]. Recently, it has also been associated with abomasitis in lambs [1, 10, 11], enterocolitis in horses [23] and necrotic enteritis in chickens [24]. Lethal toxin (TcsL) and hemorrhagic toxin (TcsH) are mainly responsible for the virulence of P. sordellii [25, 26]. C. septicum causes abomasitis in sheep, known as braxy [2]. C. septicum has been isolated from ruminants with gas gangrene and abomasitis [2,3,4, 27]. It has been reported that alpha toxin is essential for the virulence of C. septicum [28].
Epidemiological investigations may help the development of vaccination programs against clostridial diseases and a better understanding of pathogenicity of Clostridium species. Although many studies have been conducted on the role of Clostridium species in the etiology of abomasitis in calves, there is a paucity of information in terms of etiology of abomasitis in lambs and goat kids. P. sordellii has been isolated from abomasum lesions of lambs in Turkey (11). The isolation of C. perfringens type A from lambs with enteric diseases has also been reported in Turkey and Iran (32, 35). However, to our knowledge, no studies are available concerning the presence of Clostridial toxin genes in abomasal samples in Turkey. This sudy was carried out to investigate the presence of C. perfringens, Clostridium septicum and P. sordellii and their toxin genes in abomasal samples of lambs and goat kids with hemorrhagic abomasitis in Turkey.
Results
Necropsy and histopathological findings
Necropsies revealed that gross findings were almost similar in all animals. The most striking finding at necropsy was that the abomasums of the animals were excessively dilated with caseinized content and gas, and multifocal black foci were seen in serosal surface (Fig. 1 A). When the abomasum mucosa was opened, it was observed that these black foci were petechial hemorrhages in varying degrees (Fig. 1B). The hemorrhages were quite severe in some animals and were observed to cover the entire abomasum mucosa like a layer, and the abomasal wall was thickened duo to edema (Fig. 1 C). Many erosions and ulcers were observed on the abomasal mucosa after the contents were removed (Fig. 1D). Ecchymotic hemorrhages were also observed in the epicardium and endocardium of the heart in addition to hemorrhages in the abomasum of five lambs and three kids.
Macroscopic view of abomasums. A: Excessively distended abomasum with gas and caseinized contents, and multifocal hemorrhagic foci seen from serosa in a one-week old lamb. B: Multifocal severe petechyial hemorrhages on mucosal surface of the abomasum. C: The entire abomasum mucosa was covered with hemorrhagic content and abomasal wall were thickened duo to edema in a two-week old goat kid. D: Many erosions and ulcers were observed on the abomasal mucosa after the contents were removed in a ten-day old lamb
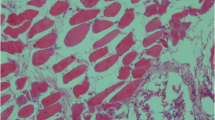
Acute necrotizing hemorrhagic inflammation of abomasum mucosa was observed at microscopic examination. Moderate or severe hyperemie, congestion, edema and hemorrhagies were found in the propria and submucosa (Fig. 2 A). There were erosions (Fig. 2B), some of which extending into submucosa ulcers characterized with epithelial degenerasion/necrosis and desquamation. Mild inflammatory infiltration including neutrophils, lymphocytes and macrophages was seen in the propria mucasa of the abomasums (Fig. 2 C). In addition, many large rod-shaped bacteria were detected in the debris and mucosal surface of abomasum in seven lambs and four kids (Fig. 2D). These bacteria were gram positive in the staining made by gram staining method.
Microscopic views of abomasums. A: Hyperemie and/or congestion (arrows), and hemorrhagies (asterisk) were fonud in the propria mucosa and submucosa of a ten-day old lamb. H&E. 20 X Magnification. B: Mucosal erosion (arrow), and necrotic debris in the lumen of the abomasum (asterisk) in a 15-day old goat kids. H&E. 10 X Magnification. C: Mild numbers of neutrophils, lymphocytes and macrophages infiltrations in the propria mucosa of the abomasum (arrow) in a one-week old lamb. H&E. 20 X Magnification. D: Many large rod-shaped bacteria were found in the debris (arrow) of a two-week old lamb. H&E. 100 X Magnification
Molecular findings
Out of 38 samples, 33 (86.84%) were found positive for C. perfringens type A alone or in combination with other Clostridium species. C. perfringens type F (cpa and cpe gene positive) was detected in two (5.26%) samples. Other types of C. perfringes were not detected in any of the samples. Three samples were positive for cpb2 in addition to cpa. C. perfringens type A and P. sordellii were detected in five (13.15%) samples. Two (5.26%) samples were positive for only P. sordellii and one (2.63%) sample for C. perfringens type A, P. sordellii and C. septicum (Table 1). The genes tcsL and tcsH were not detected in P. sordellii positive samples. One (2.63%) of the samples was found to be negative for either of these bacteria.
Discussion
Clostridial abomasitis is characterized by necrosis of abomasal mucosa caused by exotoxins produced by several species of Clostridium genus in the gastrointestinal tract of animals [1]. Overgrowth of Clostridium species within the gastrointestinal tract and subsequent exotoxin release may lead to sudden death in animals under predisposing conditions [2, 29]. Factors such as overfeeding, contamination of pooled colostrum, poor hygiene in farms, changes in diet, mineral deficiencies and formation of trichophytobezoars predispose to abomasal bloat and abomasitis in young ruminants [2]. Although C. septicum is traditionally associated with abomasitis in ruminants, the role of other clostridial agents is not well understood. The present study aimed to investigate the frequency, and the presence of virulence genes, of C. perfringens, P. sordellii and C. septicum in lambs and goat kids with hemorrhagic abomasitis. The vast majority of the 38 abomasal samples were determined to harbour C. perfringens type A. In addition, P. sordellii, C. perfringens type F and C. septicum were detected in the samples, albeit at low rates. Meanwhile, a few samples contained more than one Clostridium species. These findings put forward that C. perfringens type A has an important role in the etiology of hemorrhagic abomasitis in small ruminants. Recently, C. perfringens type A was increasingly isolated from calves with abomasitis [6,7,8]. In the USA, C. perfringens was reported to be present in approximately 67% of calves died of emphysematous abomasitis and abomasal bloat [16]. On the other hand, the number of studies reporting the presence of C. perfringens in abomasitis cases of lambs and goat kids was rather limited. In accordance with our results, C. perfringens type A was detected in 84% of the cases with clostridial enterotoxaemia in lambs and goat kids in Italy [15]. In another study carried out in Pakistan, the majority of the C. perfringens isolates from sheep and goats (healthy and diseased) were characterized as type A (82%) [30]. Likewise, 81% of C. perfringens isolates obtained from domestic livestock were reported to belong to type A in Saudi Arabia [31]. In Turkey, 77% of C. perfringens isolates from lambs suspected of enterotoxemia were genotyped as type A [32]. C. perfringens types B, C and D toxoid vaccines produced in Turkey are used against C. perfringens infections in animals. However, the results of the current study suggest that C. perfringens type A toxoid vaccine should also be considered in the vaccination programs of sheep and goats.
The major toxin of C. perfringens type A is alpha toxin which is a zinc metallophospholipase. High concentrations of this toxin can damage plasma membrans of host cells [2]. It was reported that alpha toxin might contribute to pathogenesis of bovine necro-hemorrhagic enteritis [33]. However, the role of C. perfringens type A in the pathogenesis of enteric disease of animals is still conroversial, as it can be found in the intestine of clinically healthy animals [34]. In recent years, beta2 toxin has frequently been detected in C. perfringens isolates from animals and humans [14, 15, 35]. Also, a significant relationship has been reported between cpb2-positive C. perfringens isolates and diarrhoea in pigs [36]. This toxin has been detected in horses with intestinal disorders, as well [37]. In the present study, although only three of C. perfringens type A positive samples were found to harbour cpb2, it can be suggested that beta2 toxin might act in sinergy with alpha toxin in the pathogenicity of C. perfringens type A [1, 14, 33]. However, it should be underlined that some studies failed to detect cpb2 in C. perfringens type A isolates from either bovine clostridial abomasitis (BCA) or jejunal hemorhage syndrome (JHS) [7, 31].
According to a recently introduced toxin-based typing system, C. perfringens strains containing cpa and cpe genes were classified as type F, while those containing cpa and netB genes were as type G [12]. C. perfringens type F is associated with food poisoning in humans. However, a few enterotoxigenic infections caused by type F strains have been reported in animals [38, 39]. Although the presence of cpe has been reported in a goat with necrotizing enterocolitis [40], the role of enteroxin producing C. perfringens strains in animal diseases is not enlightened [39]. Our results suggested that enterotoxin does not have an important role in hemorrhagic abomasitis cases, but further studies are needed to clarify the pathogenicity of enterotoxin in animal diseases. C. perfringens type G causes necrotic enteritis in poultry [2]. The presence of this type has also been reported in a cow [41]. However, in accordance with many previous studies [7, 14, 42, 43], none of the samples were positive for C. perfringens type G in the present study.
Although P. sordellii is known to cause gas gangrene in animals [2], it has also been isolated from lambs with abomasitis [10, 11, 44]. In the current study, P. sordellii was detected as the second most common bacterial species after C. perfringens type A. Lethal and hemorrhagic toxins (TcsL and TcsH) are considered to be the main virulence factors of P. sordellii. These toxins are members of the Large Clostridial Cytotoxin (LCC) family [45, 46]. Although at low rates, the presence of LCC genes in P.sordellii strains isolated from clinical cases including horses with enterocolitis has been demonstrated in the UK, USA and Australia [23, 46]. However, this study failed to show these genes in P. sordellii isolates. Similarly, Zerrouki et al. [47] reported that P. sordellii isolates originated from a hospital environment in Algeria did not harbour either TcsL or TcsH toxins. The absence of these genes in our isolates was not surprising due to the facts that the number of isolates was small (n = 8) and that the majority of P.sordellii strains do not encode LCC genes [46]. It is therefore possible that additional virulence factors can contribute to the pathogenicity of P. sordellii. However, it is known that nontoxigenic strains of P.sordelli can cause the disease, albeit at low severity [48, 49].
Clostridium septicum is considered as the etiological agent of braxy in sheep [2]. It has been determined in lambs and calves with suppurative abomasitis [4, 9]. Acute haemorrhagic abomasitis due to C. septicum has also been reported in experimental sheep [3]. However, the results of this study suggested that C. septicum was not the primary agent of abomasitis in lambs and goat kids in Turkey. It is known that small ruminants are mostly vaccinated against braxy in Turkey. Therefore, the detection of this agent in only one sample in the present study may be attributed to passive immunity in newborns. Similar results have also been reported elsewhere. For instance, C. septicum was not detected from lambs aged between 2 and 5 weeks with abomasal haemorrhage and ulcers in Norway [10].
Other bacterial agents such as Clostridium fallax have rarely been reported in abomasitis cases of lambs [10, 20]. Recently, Sarcinia genus bacteria have also isolated from lambs and calves with abomasitis [10, 19, 20]. In the present study, the samples were not analyzed for either C. fallax or Sarcinia spp. However, the organisms consistent with Sarcinia spp. were not observed on the surface or within the abomasum wall in the histopathological examination.
Conclusion
The results of this study showed that C. perfringens type A was the most common species followed by P. sordellii in lambs and goat kids with hemorrhagic abomasitis in eastern Turkey. Interestingly, C. septicum was detected at the lowest rate in the abomasal samples, probably due to widespread vaccination of mothers. Also, the present study provided data concerning clostridial toxin genes in abomasum samples for the first time in Turkey. Further comprehensive studies are needed to have a better understanding of the pathogenic mechanisms of Clostridium species in abomasitis of small ruminants.
Methods
Sampling
The study material consisted of 21 lambs and 17 goat kids aged between 1 week and 1 month belonging to 30 different farms located in Eastern Turkey, which were submitted to the Pathology Department of Firat University between 2021 and 2022. Sudden onset of weakness, inability to suck the mother, reluctance to move or rise, swelling in the abdomen and death within 2–3 h to 1 day were recorded as general complaints in animals that were born healthy and received sufficient amount of colostrum. The mortality rate of the offsprings in the abovementioned age groups was informed to be around 15–20% in the flocks.
Necropsy and histopathological examination
Systematic necropsies were performed, tissue samples were taken from all animals, and fixed in 10% buffered neutral formalin solution. After routine procedures, the prepared paraffin blocks were cut into 3 μm thick, stained with haematoxylin and eosin (H&E), Brown and Brenn method for gram staining, and were evaluated by light microscopy.
Bacteriological culture
The swab samples were cultured onto 5% blood agar and incubated in an anaerobic jar for 48 h at 37 °C. Following Gram staining, suspected colonies were subcultured into cooked meat medium and incubated for 48 h at 37 °C in anaerobic conditions. Anaerobic media were supplied with anaerobic gas kits (Anaerocult A, Merck).
DNA extraction
DNA extraction was carried out from the bacterial culture in the cooked meat medium. For this, 300 µL of bacterial cultures were transferred to Eppendorf tubes. Each sample was treated with 300 µl TNES buffer (20mM Tris, 150 mM NaCl, 10 mM EDTA, 0.2% SDS) and 6 µl proteinase K (20 mg/ml), and then inactivated at 56 °C for 2 h. After the mixture was boiled for 10 min., 600 µL phenol-chloroform-isoamyl alcohol was added. The mixture was shaken vigorously for 5 min and centrifuged at 11600x g for 10 min. The upper phase was transferred to another Eppendorf tube and 3 M sodium acetate (0.1 volume) and ethanol (2.5 volume) were added. The mixture was vortexed and kept at -20 °C overnight. The mixture was then centrifuged at 11600x g for 10 min, and supernatant was removed. The pellet was washed with 70% ethanol and centrifuged at 11.600 g for 5 min. Finally, the pellet was dried for 45 min and suspended in 50 µL distilled water.
Polymerase chain reaction (PCR)
C. perfringens NCTC 8239 (cpa+ and cpe+), C. perfringens NCTC 13,110 (cpa+, cpb+ cpb2 and etx+), C. perfringens CCUG 44,727 (cpa+ and itx+), C. septicum genomic DNA and P. sordellii genomic DNA were used as positive controls. A multiplex PCR was performed for the detection of alpha (cpa), beta (cpb), epsilon (etx), iota (itx), enterotoxin (cpe) and beta2 (cpb2) toxin genes of C. perfringens in a thermal cycler (Techne, Staffordshire, UK), in a total reaction volume of 25 µL containing 12.5 µL 2X AmpMasterTaq master mix (AmpMasterTMGeneAll Biotechnology, Cambiol, Cambridge, Cat No: 541 − 010), 2.5 µL of DNA, and 1 µL of each specific primer (10 µM). The amplified products were electrophoresed in a 1.5% agarose gel containing 10 µL ethidium bromide solution. Amplified products were visualized and photographed under UV light. PCR products with the molecular sizes of approximately 402 bp, 196 bp, 567 bp, 655 bp, 293 and 506 bp were considered positive for cpa, cpb, cpb2, etx, itx and cpe genes, respectively. A single PCR was employed for the detection of the C. perfringens necrotic enteritis B-like toxin (netB) gene. A primer pair specific for the flagellin gene (fliC) was used for the identification of C. septicum by a single PCR. The C. septicum positive sample was also screened for the presence of alpha toxin (Hemolysin) gene. For the identification of P. sordellii, the samples were tested with PCR as previously described [50]. The primers of JRP4589 and JRP4590 were used to amplify a fragment of approximately 470 bp, representing an internal region of sdlO gene. PCR was also performed to determine the presence of the lethal toxin (tcsl) and hemorrhagic toxin (tcsh) genes of P. sordellii as previously described [29]. The primers used in this study were listed in Table 2.
Availability of data and materials
The data supporting our findings are contained within the manuscript.
Abbreviations
- HE:
-
Hematoxylin-eosin
- PCR:
-
Polymerase chain reaction
References
Simpson KM, Callan RJ, Van Metre DC. Clostridial abomasitis and enteritis in ruminants. Vet Clin Food Anim. 2018;34:155–84.
Uzal FA, Songer JG, Prescott JF, Popoff MR. Clostridial Diseases of Animals. Ames IA: Willey and Blackwell; 2016.
Ellis TM, Rowe JB, Lloyd JM. Acute abomasitis due to Clostridium septicum infection in experimental sheep. Aust Vet J. 1983;60:308–9.
Eustis SL, Bergeland ME. Suppurative abomasitis associated with Clostridium septicum infection. J Am Vet Med Assoc. 1981;178:732–34.
Roeder BL, Chengappa MM, Nagaraja TG. Isolation of Clostridium perfringens from neonatal calves with ruminal and abomasal tympany, abomasitis, and abomasal ulcerration. J Am Vet Assoc. 1987;190:1550–55.
Songer JG, Miskimins DW. Clostridial abomasitis in calves: case report and review of the literature. Anaerobe. 2005;11:290–94.
Schlegel BJ, Nowell VJ, Parreira VR, Soltes G, Prescott JF. Toxin-associated and other genes in Clostridium perfringens type a isolates from bovine clostridial abomasitis (BCA) and jejunal hemorrhage syndrome (JHS). Can J Vet Res. 2012;76:248–54.
Manteca C, Jauniaux T, Daube G, Czaplicki G, Mainil JG. Isolation of. from three neonatal calves with haemorrhagic abomasitis Revue Med Vet. 2001;152:637–39.
Sanford SE. Braxy-like abomasitis in a calf. Can Vet J. 1992;33:676–7.
Vatn S, Tranulis MA, Hofshagen N. Sarcina-like bacteria, Clostridium fallax and Clostridium sordellii in lambs with abomasal bloat, hemorrhage and ulcers. J Comp Path. 2000;122:193–200.
Akan M, Sareyyupoglu B, Oncel T, Tel OY, Ilhan Z, Cantekin Z. Isolation of. from abomasum lesions of lambs in Turkey Ankara Univ Vet Fak Derg. 2008;55:103–6.
Rood JI, Adams V, Lacey J, Lyras D, McClane BA, Melville SB, et al. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe. 2018;53:5–10.
Gibert M, Jolivet-Reynaud C, Popoff MR. Beta2 toxin, a novel toxin produced by Clostridium perfringens. Gene. 1997;203:65–73.
Forti K, Ferroni L, Pellegrini M, Cruciani D, Giuseppe AD, Crotti S, et al. Molecular characterization of Clostridium perfringens strains isolated in Italy. Toxins. 2020;12:1–20.
Greco G, Madio A, Buonavoglia D, Totaro M, Corrente M, Martella V, et al. Clostridium perfringens toxin-types in lambs and kids affected with gastroenteric pathologies in Italy. Vet J. 2005;170:346–50.
Van Kruiningen HJ, Nyaoke CA, Sidor IF, Fabis JJ, Hinckley LS, Lindell KA. Clostridial abomasal disease in Connecticut dairy calves. Can Vet J. 2009;50:857–60.
Songer JG, Miskimmins DW. Type E enteritis in calves: two cases and a brief review of the literature. Anaerobe. 2004;10:239–42.
Maratea KA, Miller MA. Abomasal coccidiosis associated with proliferative abomasitis in a sheep. J Vet Diagn Invest. 2007;19:118–21.
Panciera RJ, Boileau MJ, Douglas LS. Tympany, acidosis, and mural emphysema of the stomach in calves: report of cases and experimental induction. J Vet Diagn Invest. 2007;19:392–95.
Filho RVL, Bianchi MV, Fredo G, de Oliveira EC, Laisse CJM, Driemeier D, et al. Emphysematous abomasitis in a lamb by bacteria of the. genus in Southern Brazil Ciênc Rural. 2016;46:300–3.
DeBey BM, Blanchard PC, Durfee PT. Abomasal bloat associated with Sarcina-like bacteria in goat kids. J Am Med Assoc. 1996;2009:1468–69.
Sacco SC, Ortega J, Navarro MA, Fresneda KC, Anderson M, Woods LW, et al. Associated gas gangrene in 8 horses, 1998–2019. J Vet Diagn Invest. 2020;32:32:246–51.
Nyaoke AC, Navarro MA, Fresneda K, Diab SS, Moore J, Lyras D, et al. Paeniclostridium (Clostridium) sordelliiassociated enterocolitis in 7 horses. J Vet Diagn Invest. 2020;32:239–45.
Rimoldi AE, Uzal F, Chin RP, Palombo EA, Awad M, Lyras D, et al. Necrotic enteritis in chickens associated with Clostridium sordellii. Avian Dis. 2015;59:447–51.
Unger-Torroledo L, Straub R, Lehmann AD, Graber F, Stahl C, Frey J, et al. Lethal toxin of Clostridium sordellii is associated with fatal equine atypical myopathy. Vet Microbiol. 2010;144:487–92.
Vidor C, Awad M, Lyras D. Antibiotic resistance, virulence factors and genetics of Clostridium sordellii. Res Microbiol. 2015;166:368–74.
Gazioğlu A, Karagülle B, Yüksel H, Açık MN, Keçeci H, Dörtbudak MB, et al. Sudden death due to gas gangrene caused by Clostridium septicum in goats. BMC Vet Res. 2018;14:1–6.
Kennedy CL, Krejany EO, O’Connor JR, Awad MM, Boyd RL, Emmins JJ, et al. The alpha-toxin of Clostridium septicum is essential for virulence. Mol Microbiol. 2005;57:1357–66.
Uzal FA. Diagnosis of Clostridium perfringens intestinal infections in sheep and goats. Anaerobe. 2004;10:135–43.
Mohiuddin M, Iqbal Z, Siddique A, Liao S, Salamat MKF, Qi N, et al. Prevalence, genotypic and phenotypic characterization and antibiotic resistance profile of Clostridium perfringens type A and D isolated from feces of sheep (Ovis aries) and goats (Capra hircus) in Punjab, Pakistan. Toxins. 2020;12:1–14.
Omer SA, Al-Olayan EM, Babiker SEH, Aljulaifi MZ, Alagaili AN, Mohammed OB. Genotyping of Clostridium perfringens isolates from domestic livestock in Saudi Arabia. Biomed Res Int. 2020;2020:9035341. https://doi.org/10.1155/2020/9035341.
Hadimli HH, Erganis O, Sayın Z, Aras Z. Toxinotyping of Clostridium perfringens isolates by ELISA and PCR from lambs suspected of enterotoxemia. Turk J Vet Anim Sci. 2012;36:409–15.
Goossens E, Valgaeren BR, Pardon B, Haesebrouck F, Ducatelle R, Deprez PR, et al. Rethinking the role of alpha toxin in. Rev bovine necro-haemorrhagic enteritis Vet Res. 2017;48:1–17.
Uzal FA, Hostetter J, Plattner B. The alimentary system, in sixth ed. In: Maxie MG, editor. Jubb Kennedy and Palmer’s Pathology of domestic animals, vol. 2. Elsevier: St. Louis MO; 2016. p. 1–260.
Jabbari AR, Tekyei F, Esmaelizad M, Pilehchian Langroudi R. Occurrence of Beta2 toxigenic Clostridium perfringens isolates with different toxin types in Iran. Arch Razi Inst. 2012;67:133–37.
Waters M, Savoie A, Garmory HS, Bueschel D, Popoff MR, Songer JG, et al. Genotyping and phenotyping of beta2-toxigenic Clostridium perfringens fecal isolates associated with gastrointestinal diseases in piglets. J Clin Microbiol. 2003;41:3584–91.
Herholz C, Miserez R, Nicolet J, Frey J, Popoff M, Gibert M, et al. Prevalence of β2-toxigenic Clostridium perfringens in horses with intestinal disorders. J Clin Microbiol. 1999;37:358–61.
Bueschel D, Walker R, Woods L, Kokai-Kun J, McClane B, Songer JG. Enterotoxigenic Clostridium perfringens type a necrotic enteritis in a foal. J Am Vet Med. 1998;213:1305–7.
Busch K, Suchodolski JS, Kühner KA, Minamoto Y, Steiner JM, Mueller RS, et al. Clostridium perfringens enterotoxin and Clostridium difficile toxin A/B do not play a role in acute haemorrhagic diarrhoea syndrome in dogs. Vet Rec. 2015;176:253.
Miyawaka MEF, Saputo J, St Leger J, Puschmer B, Fisher DJ, McClane BA, et al. Necrotizing enterocolitis and death in a goat kid associated with enterotoxin (CPE)-producing Clostridium perfringens type A. can. Vet J. 2018;48:1266–69.
Smyth JA, Martin TG. Disease producing capability of netB positive isolates of C. perfringens recovered from normal chickens and a cow, and netB positive and negative isolates from chickens with necrotic enteritis. Vet Microbiol. 2010;146:76–84.
Anju K, Karthik K, Divya V, Priyadharshini MLM, Sharma RK, Manoharan S. Toxinotyping and molecular characterization of antimicrobial resistance in Clostridium perfringens isolated from different sources of livestock and poultry. Anaerobe. 2021;67:1–8.
Wu K, Feng H. Prevalence, toxin-typing and antimicrobial susceptibility of Clostridium perfringens in sheep with different feeding modes from Gansu and Qinghai provinces, China. Anaerobe. 2022;73:1–6.
Lewis CJ, Naylor R. Sudden death in sheep associated with Clostridium sordellii. Vet Rec. 1998;142:417–21.
Just I, Gerhard R. Large clostridial cytotoxins. Rev Physiol Biochem Pharmacol. 2004;152:23–47.
Couchman EC, Browne HP, Dunn M, Lawley TD, Songer JG, Hall V, et al. Clostridium sordellii genome analysis reveals plasmid localized toxin genes encoded within pathogenicity loci. BMC Genomics. 2015;16:1–13.
Zerrouki H, Rebiahi S, Elhabiri Y, Fatmi A, Baron SA, Pagnier I, et al. Prevalence and antimicrobial resistance of Paeniclostridium sordellii in hospital settings. Antibiotics. 2022;11:1–14.
Walk ST, Jain R, Trivedi I, Grossman S, Newton DW, Thelen T, et al. Non-toxigenic Clostridium sordellii: Clinical and microbiological features of a case of cholangitis-associated bacteremia. Anaerobe. 2011;17:252-56.009.
Hao Y, Senn T, Opp JS, Young VB, Thiele T, Srinivas G. Lethal toxin is a critical determinant of rapid mortality in rodent models of Clostridium sordellii endometritis. Anaerobe. 2010;16:155–60.
Chean R, Kotsanas D, Francis MJ, Palombo EA, Jadhav SR, Awad MM, et al. Comparing the identification of Clostridium spp. by two matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry platforms to 16S rRNA PCR sequencing as a reference standard: a detailed analysis of age of culture and sample preparation. Anaerobe 30. 2014;30:85–9.
Yoo HS, Lee SU, Park KY, Park YH. Molecular typing and epidemiological survey of prevalence of Clostridium perfringens types by multiplex PCR. J Clin Microbiol. 1997;35:228–32.
Meer RR, Songer JG. Multiplex polymerase chain reaction assay for genotyping Clostridium perfringens. Am J Vet Res. 1997;58:702–5.
Baums CG, Schotte U, Amtsberg G, Goethe R. Diagnostic multiplex PCR for toxin genotyping of Clostridium perfringens isolates. Vet Microbiol. 2004;100:11–6.
Garmory HS, Chanter N, French NP, Bueschel D, Songer JG, Titball RW. Occurrence of C. perfringens β2-toxin amongst animals, determined using genotyping and subtyping PCR assays. Epidemiol Infect. 2000;124:61–7.
Keyburn AL, Boyce JD, Vaz PB, Bannam TL, Ford ME, Parker D, et al. NetB, a new toxin that is associated with avian necrotic enteritis caused by. Clostridium perfringens PLoS Pathog. 2008;4:1–26.
Acknowledgements
Not applicable.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
HK and HO contributed to the conception, design, molecular analysis and writing of the study. Burcu K, HEB and EA performed microbiological and molecular analysis. BC conributed interpretation of the data and writing. NT, AC, Burak K, CAI and EE contributed to necropsy, sampling and histopathological examination. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experiments were performed in accordance with relevant guidelines and regulations. All methods were reported in accordance with ARRIVE guidelines (https://arriveguidelines.org) for the reporting of animal experiments.The approval for the study was obtained from Animal Experiments Local Ethics Committee of Firat University (Approval no. 2022/16). The samples were taken from dead animals and owner consent was obtained for the animals used for the post-mortem examinations.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kalender, H., Öngör, H., Timurkaan, N. et al. Detection and molecular characterization of Clostridium perfringens, Paeniclostridium sordellii and Clostridium septicum from lambs and goat kids with hemorrhagic abomasitis in Turkey. BMC Vet Res 19, 8 (2023). https://doi.org/10.1186/s12917-023-03569-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-023-03569-5