Abstract
The purpose of this study was to perform a histological and biochemical evaluation of the influence of plasma rich in growth factors (PRGF) on muscle regeneration process after a surgically induced grade II muscle laceration. A randomized, single blind, controlled experimental research was conducted including twenty-one adult healthy sheep, randomly divided in three groups (n = 7). A grade II surgical section was performed in the biceps femoris muscle of both hindlimbs. After two days (basal time), intralesional infiltration of autologous PRGF or Saline solution was randomly administered in both hindlimbs. Treatment was repeated once a week. Animal groups were euthanized at 1 (T1), 2 (T2) or 4 (T4) weeks. Histological assessment showed that PRGF intralesional injection induced a significant decrease of inflammatory cells density, significant higher centrally nucleated fibers percentage and significantly smaller fibrotic areas compared to Saline-treated muscles at T1, T2 and T4. Also, lower vascular density, with lower capillaries cross-sectional area, in PRGF group compared to Saline was observed. Biochemical analysis revealed a significant higher expression level of MYOD1, MYF5 and MYOG genes in PRGF groups at T1 compared to Saline treated muscles. At ultrastructural level, PRGF groups presented scarce edema and loss of connective tissue structure, as well as higher mitochondrial density adequately associated to the sarcomere unit in contrast to the Saline group. In conclusion, histological, biochemical, and ultrastructural results showed that PRGF treatment improved muscle regeneration process leading to more mature histological aspect in newly formed muscle tissue after a surgically induced grade II muscle injury.
Similar content being viewed by others
Background
Muscle injuries are common lesion in athletes and workers that causes pain and disability with significant social impact and economic losses [1,2,3]. Grade II muscle injury is a prevalent lesion registered in sport medicine, comprising over 35% of all sports-related injuries, with a high incidence of lesion recurrence after returning to activity [1,2,3,4].
An inflammatory response occurs when muscle fibers are partially severed, and different inflammatory cells are sequentially attracted to the damaged area [5, 6]. This cascade of events facilitates myogenesis via phagocytosis of cellular debris, and release of chemoattractants and growth factors toward the injured muscle [7]. Modulation of the inflammatory response plays a crucial role to achieve a successful muscle healing process [8, 9]. Furthermore, after muscle rupture an angiogenic stimuli is activated in the injured area with the intention of providing the newly formed tissue with oxygen, other nutrients, and blood-derived cells, as well as removing waste-metabolic products [10].
Skeletal muscle tissue presents reparation capacity led by an intrinsic mechanism conducted by a group of undifferentiated reserve cells called satellite cells [10]. In response to injury, these cells differentiate into myoblasts, and form multinucleated myotubes. The newly formed myotubes then fuse with part of the healthy muscle stump [11]. Regenerating myofibers are characterized as centrally nucleated fibers (CNF), so they are considered criteria for muscle regeneration [10, 12, 13]. Likewise, MYOD1, Myf5 and Myogenin (MYOG) are muscle regulatory factors that are up-regulated when satellite cells are activated to proliferate in skeletal muscle repair [12].
The cellular events described during muscle reparation are orchestrated by a pool of growth factors and cytokines, most of which are released by platelets [2, 3, 10]. Platelet rich plasma (PRP) therapies have been used to treat acute muscle injuries in both experimental and clinical scenario [14,15,16,17,18,19]. PRP therapy induces an angiogenic stimulus and these new capillaries allow the transport of oxygen and nutrients to the healing tissue [20]. Furthermore, plasma rich in growth factors (PRGF), a particular type of autologous PRP that contains a moderate platelet concentration (2–3 fold above peripheral blood) with absence of leukocytes and erythrocytes [21], has been successfully proposed to improve muscle healing in clinical practice [2, 22]. Some clinical and experimental results reported after using PRP to manage muscle injuries are quite variable and not conclusive [3, 17].
The purpose of this experimental study is to investigate muscle regeneration properties of intralesional PRGF therapy for surgically induced grade II muscle injury in a sheep model. It was hypothesized that this treatment could modulate inflammation and enhance regeneration properties to restore normal or nearly skeletal muscle tissue.
Methods
Animals
Twenty-one skeletally mature 2- to 3-year-old female sheep, weighing 50–55 kg were included in the study. All sheep used in this study were deemed healthy beforehand and did not suffer from any orthopedic or systemic conditions. Animals were randomly divided into three groups, T1 (n = 7), T2 (n = 7), and T4 (n = 7), according to the time when they were sacrificed along the experiment, at 1 week, 2 weeks, or 4 weeks after treatment, respectively. All procedures were carried out following the guidelines of the European Union (Directive 2010/63/EU) and approved by the Bioethical Committee on Animal Research at Andalucía (Junta de Andalucía, reference 12/09/2018/138). In addition, authors affirm that the research was conducted in accordance with the ARRIVE guidelines.
Surgical procedure
Sheep were anaesthetized and both thighs were aseptically prepared for surgery. First, a sonographer performed an ultrasound evaluation to confirm the absence of abnormalities in biceps femoris muscle (edema, structural disorganization and/or fibrosis). In order to standardize the exact point of the surgical lesion, the thickest muscle area to perform the grade II muscle injury was measured mid-point in between the greater trochanter and the proximal aspect of the lateral condyle of the femur. A surgical muscle laceration equivalent to grade IIc injury, consisting of a partial tears of muscle fibers, was performed under ultrasound guidance in the biceps femoris muscle involving 70% of the muscle fibers in all the sheep using a scalpel blade #22 (Aesculap AG, B/Braun, Tuttlingen, Germany) [23]. The magnitude of the grade II muscle rupture was confirmed by ultrasound according to the grading scale suggested by Hamid et al. [23] and Peetrons et al. [24]. Then the fascia lata, subcutaneous and skin layers were sutured in separated planes. The surgical procedure was completed on both thighs. All the animals were operated by the same surgeon. Finally, the wound was covered using sterile dressing. Antibiotic [amoxicillin-clavulanic acid (Synulox®, Zoetis, Madrid, Spain), 10 mg/kg IM] and analgesic [buprenorphine (Buprex® 0,3 mg, Schering-Plough, Madrid, Spain), 0.02 mg/kg/8 h IM] treatment were given for 3 and 5 days, respectively. No anti-inflammatory drug was used anytime. Animals were hospitalized in an indoor stable and were allowed to move freely without splinting.
PRGF preparation
Blood was collected just prior to treatment from the jugular vein of each animal and was divided into four sterile tubes containing 3.8% trisodium citrate. Each 5-mL extraction tube contained 0.5 mL of anticoagulant. Blood was centrifuged at 630 g for 8 min according to the method reported to obtain PRGF (PRGF-Endoret®, BTI, Vitoria, Spain) in sheep [25]. The fraction considered PRGF was the deeper layer of the centrifuged plasma just over the buffy coat and was selected with the use of a pipette to avoid the aspiration of white and red cells. The PRGF fraction volume from each of the four tubes was combined to obtain a total volume of 2 ml per animal.
PRGF treatment injection
Sheep were sedated for treatment injection 48 h after muscle injury. First, hematoma was evacuated from the muscle laceration site using a 21-G needle under ultrasound guidance. PRGF or Saline solution (NaCl 0,9%, BBraun medical, Barcelona, Spain) infiltrations were randomly assigned for each thigh. Platelets contained in PRGF (2 mL) were activated just prior to treatment by adding 50 µL of calcium chloride 10% for 1 mL of plasma ratio, and injected directly into the injured site under ultrasound guidance as previously described in Bubnov et al. [26]. The time delay between blood collection and PRGF application was less than 1 h. Saline solution (2 mL) was injected adding 50 µL of calcium chloride 10% for 1 mL of Saline ratio, under ultrasound guidance. PRGF or Saline solution infiltrations were repeated weekly under ultrasound guidance to complete, one more injection in T2 group, and two more infiltrations in T4 group. The researcher was blinded to the treatment applied on each limb.
Duration of the study
All the animals were humanely sacrificed at 1 week (T1 group), 2 weeks (T2 group), or 4 weeks (T4 group) after the first treatment with PRGF or Saline solution, by using a standard protocol with T61™ (Intervet Schering Plough Animal Health; Salamanca, Spain).
Histological study
After euthanasia, biceps femoris muscles of both thighs were dissected proximal-distally to the damaged area in each animal. Muscle damage area was divided, so one half was used for histochemical evaluation, and the other half was used for ultrastructural and biochemical studies. Samples of the vastus lateralis quadriceps femoris were taken and used as a normal skeletal muscle control (Control group). Samples were oriented keeping muscle fibers along their longitudinal axis.
Histochemical study
For histochemical examination, muscles samples were fixed in 10% neutral buffered formalin and embedded in paraffin. Four µm-thick sections were stained with Hematoxylin and Eosin (HE) and Masson Trichrome with aniline blue (MT) (Bio-Optica, Milan, Italy). Histological images were evaluated using a photomicroscope (Axiophot; Carl-Zeiss, Oberkochen, Germany) with an attached digital camera controller (DS-L1 camera control unit, Nikon).
From HE and MT stained sections, five histological images per slide were randomly taken at 200x magnification and were analyzed with ImageJ software (Java-based image processing program, Oracle corporation, Austin, Texas, USA). HE stained histological images were used to study the inflammatory cells density (cells/mm2), blood vessels density (blood vessels/mm2), blood vessels area (µm2) and percentage of centrally nucleated fibers with respect to total muscle fibers. MT stained histological images were used to assess areas of fibrillar collagen to estimate the percentage of fibrotic areas (FA) development.
Transmission electron microscopy study
For transmission electron microscopy (TEM) evaluation, all the muscle samples were fixed in glutaraldehyde 2% for 48 h at 4 ºC and included in Araldite. Ultrathin sections were stained with uranyl acetate and plumb citrate and evaluated using a transmission electronic microscope Jeol-1400 (JEOL Ltd., Hertfordshire, UK).
Biochemical study
Muscle tissue samples stored at -80 ºC were thawed and processed for RNA extraction with TRI reagent (Sigma-Aldrich CO, St Louis, MO, USA). Total RNA was quantified by spectrophotometry (ND-1000, Nanodrop Technologies, Wilmington, DE, USA). Afterwards, total RNA treated with DNAse was used to analyze mRNA expression of MYOD1, MYF5, MYOG and Myostatin (MSTN) genes by RT-PCR using SensiFAST SYBR No-ROX One-Step kit (Bioline Reagents Limited, UK) according to the manufacturer instructions. RT-PCR was performed using a Lightcycler 480 equipment (Roche Molecular Systems, Inc., Indianapolis, IN, USA). Primer sequences are shown in Table 1. The gene expression was calculated following the 2−ΔΔ Ct method using GAPDH as housekeeping gene.
Statistical analysis
The data were statistically evaluated using SPSS 17.0 software (SPSS Inc, Chicago, USA). All variables were analyzed for normality of distribution by the Kolmogorov-Smirnov test. Histochemical variables (inflammatory cells density, blood vessels density, blood vessels area, percentage of CNF, and percentage of FA), and level of expression of MYOD1, MYF5, MYOG and MSTN were expressed as mean ± standard deviation. A mixed general lineal model was performed to analyze each variable with respect to the muscles sample (PRGF group, Saline solution group or Control group). Furthermore, each variable was analyzed with respect to different timepoints (T1, T2 and T4) throughout the study. A one-way ANOVA with post hoc Bonferroni test were used to evaluate differences between groups (Control group vs. PRGF or Saline groups; PRGF group vs. Saline group). Significance level was standardized as p < 0.05.
Results
All animals reached the end of the study without incidents or adverse effects.
Histochemical study
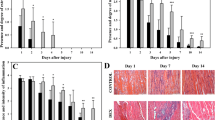
PRGF group, Saline solution group and Control group results are presented in Table 2. Normal skeletal muscle showed absence of inflammatory cells infiltration. Inflammatory cells density increased significantly in both groups treated with PRGF or Saline solution after the surgical injury. Damaged muscles treated with Saline solution showed significant higher inflammatory cells density compared to PRGF-treated group (Fig. 1). This difference was maintained at T1, T2 and T4 (p = 0.001).
Representative histological sections stained with HE from grade II muscle injury in sheep, treated with PRGF (a, c, e) or Saline solution (b, d, f); at 1 (a, b), 2 (c, d) and 4 (e, f) weeks after the surgical muscle damage. Black arrows point at inflammatory cells infiltration; red arrows point at Centrally Nucleated Fibers (CNF). Muscles treated with PRGF showed statistically significant less inflammatory cells infiltration, and higher percentage of CNF than those muscles injected with Saline solution along the study
Regarding vascularization analysis, Control group showed small capillaries distributed through the muscular tissue with low vascular density. After surgical grade II injury of biceps femoris, both PRGF-treated muscles and those injected with Saline solution suffered an important increase in the vascular area, showing significantly larger size capillaries than normal skeletal muscle. PRGF treated muscles exhibited statistically significant smaller vascular area than in those treated with Saline solution at T1 (p = 0.023) and T2 (p = 0.001). Vascular density was significantly increased in both surgically injured muscles injected with PRGF or Saline solution compared with the intact skeletal muscle. All the muscles showed a significant progressive drop in vascular density along the study. PRGF groups exhibited lower vascular density than Saline solution groups with significant differences at T4 (p = 0.019)
CNF were not observed in normal skeletal muscle. After the surgical damage of biceps femoris, a significant progressive increase of the percentage of CNF was registered from T1 to T4 groups in PRGF and Saline solution groups. Comparison between PRGF and Saline treatments revealed that percentage of CNF was significantly higher in muscles injected with PRGF than those treated with Saline solution at T1, T2 and T4. This difference was maintained throughout the experiment (p = 0.001) (Fig. 1)
Histological study showed absence of fibrotic areas in the Control group. During healing process, a significant increase in percentage of FA with respect to the total area were registered in PRGF or Saline solution groups at T1 and T2. Subsequently, this parameter decreased at T4 for both groups. Significantly higher fibrotic areas were described in muscles treated with Saline solution, in comparison with those muscles infiltrated with PRGF. This difference was maintained along the study from T1 to T4 groups (p = 0.002) (Fig. 2).
Representative histological sections stained with MT from grade II muscle injury in sheep, treated with PRGF (a, c, e) or Saline solution (b, d, f); at 1 (a, b), 2 (c, d) and 4 (e, f) weeks after the surgical muscle damage. Statistically significant higher fibrotic areas (blue-stained) were showed in muscles treated with Saline solution than in PRGF-treated muscles
Transmission Electron microscopy study
Normal skeletal muscle showed typical sarcomere structure organized parallel to each other and distributed along the muscle fiber. Injured muscular tissue presented morphology compatible with hypertrophic phenomena, with differences depending on the therapy received throughout the experiment (Fig. 3). At T1 and T2, PRGF treated muscles showed scarce edema and loss of connective tissue structure. In contrast, Saline groups presented diffuse edema with T-tube and endoplasmic reticulum dilated, as well as disorganization in the connective tissue structure. At T4, PRGF-treated muscles exhibited physiologic hypertrophic changes with moderate undulating fibrils, and high mitochondrial density associated to the “Z” band of the sarcomere (Fig. 3a and b). However, Saline-treated muscles revealed pathologic hypertrophic changes with muscle fibrils growing uncoordinatedly non-parallel to the muscle fiber axis, as well as low mitochondrial density randomly distributed along the muscular tissue and many times not associated to the sarcomere (Fig. 3c and d).
Biochemical study
Level of expression of myogenic genes MYOD1, MYF5 and MYOG, as well as the inhibitor gene MSTN, from intact skeletal muscle and injured muscles treated with PRGF and Saline solution are described in Table 3. MYOD1, MYF5 and MYOG genes showed significantly enhanced level of expression in the damaged muscles with respect to the Control group along the experiment. At T1, muscles treated with PRGF showed significantly higher level of expression of MYOD1, MYF5 and MYOG, in comparison with the group of muscles injected with Saline solution (p = 0.025; p = 0.03; p = 0.025, respectively). However, on later stage of muscle healing at weeks 2 and 4, no differences were registered between the PRGF and Saline groups. Regarding MSTN, as inhibitor gene of muscle regeneration, no statistical differences were registered in the comparison either between injured muscles and Control group, or between PRGF and Saline groups throughout the experiment.
Discussion
Muscle injuries are one of most frequently reported lesions related to the sports world [3]. Acceleration of the muscle regeneration with no appearance of recurrences is the main objective of an effective therapeutic strategy [10]. On this line, platelet-rich therapies have been demonstrated to enhance the healing of musculoskeletal injuries through the growth factors and cytokines pool released from platelets and plasma [27].
PRGF is an autologous treatment that accelerates tissue regeneration and reduces the recovery period, improving the quality of tissue repaired with the aim of decreasing relapse incidence [28,29,30]. There are few publications with the use of PRGF in the treatment of grade II muscle injuries [2, 22]. To our knowledge, PRGF has not been employed in any experimental setting to evaluate its effect on muscle regeneration after a surgical grade II injury on the biceps femoris in a sheep model.
In the present study, the anatomical references considered and ultrasound guidance used were useful to locate the thickest muscle area to precisely determine the surgical lesions site. Furthermore, the grade II muscle injuries in the biceps femoris muscle was accurately standardized using an ultrasound muscle injury grading scale previously reported [23]. Ultrasonography was used to visualize the scalpel blade during the procedure, and also to assess that a grade IIc muscle injury had been performed after surgery. Subsequently, according to these criteria an accurate, homogeneous, and repeatable sheep model of grade II biceps femoris muscle injury was developed to evaluate the effect of PRGF therapy on muscle regeneration. In the present study, a tear of 70% of muscle fibers was performed in order to create a demanding muscle damage to assess PRGF therapy in comparison with the saline group and a control muscle. Similarly, PRP treatment with rehabilitation program has been successfully utilized to manage similar size of muscle tears in people [23].
When muscle fibers are partially severed, inflammatory phenomena occurs and different inflammatory cells are sequentially attracted to the damaged area [5, 6]. Several growth factors contained in PRP contribute to the modulation and subsequent resolution of inflammation after muscle tears [2, 31]. In this study, PRGF groups showed a significant decrease in inflammatory cells density compared with Saline-treated muscles in this study. This finding suggested an anti-inflammatory effect of PRGF on muscle repair since the first week of the regeneration process. However, Borrione et al. [32] described an early increase in inflammatory cells infiltration in muscles treated with PRP in contrast to the untreated group, after a surgically created grade II muscle injury at days 2 and 5. In addition, Delos et al. [15] registered no differences between PRP and Saline treatments in inflammatory cells density after muscle contusion at days 1, 4, 7, 10, and 14. Both experimental studies [15, 32] used a platelet concentration in PRP of at least 4-fold above peripheral blood. Although, excessive platelet concentration in the PRP could lead to non-desired effect in tissue regeneration process [33,34,35]. Furthermore, Delos et al. [15] included the leukocytes layer in their PRP. Therefore, leukocytes could interfere with the beneficial effects that some growth factors have on modulating the inflammatory response due to alteration of the growth factor release profile, as well as contributing to the increase of proinflammatory cytokines [6, 36, 37]. The anti-inflammatory effect of PRGF has been previously reported in other tissues [27, 28, 38].
The restoration of vascular supply is prerequisite for subsequent morphological and functional recovery of the injured muscle [10]. In the present study, smaller size capillaries and lower vascular density were observed in PRGF-treated muscles in comparison with Saline group updating that PRGF therapy modulated neovascularization phenomena. However, Borrione et al. [32] described no significant effect of PRP on blood vessels density and blood vessels diameter in damaged muscles with respect to the untreated group.
Muscle regeneration have been evaluated in several experiments using CNF counting as histological criteria [13, 15]. Our results showed that PRGF treatment induced higher muscle regeneration stimulus after a grade II muscle injury due to a significant higher percentage of CNF than Saline-infiltrated muscles. Nevertheless, Delos et al. using a PRP that included platelet concentration superior to 4 times the whole blood level reported no effect of the therapy on muscle reparation [15]. Therefore, moderate platelet concentration included in PRGF could enhance the muscle regeneration.
During muscle repair process, connective tissue occupies the gap created in between the ruptured myofibers [10]. The production of connective tissue and subsequent scar formation at the site of muscle injury is considered one of the most important pathological steps in muscle healing, and therefore it is extremely associated to relapses [10, 39]. Thus, with the aim to reduce the fibrotic scar, novel therapies are needed to deal with grade II muscle injuries. In the present study, after surgical grade II muscle injury, MT staining revealed significantly smaller fibrotic areas in the group of muscles treated with PRGF with respect to Saline-treated muscles. In contrast, Delos et al. [15] and Cunha et al. [40], reported that damaged muscles injected with PRP showed no difference in fibrotic areas compared to control group. These authors used a PRP that contained a platelet concentration superior to 4 times above peripheral blood, higher than the PRGF platelet concentration (2–3 folds) utilized in our study. TGF-β1 is the growth factor that initiates the production of fibrosis-related proteins, as well as down-regulating the expression of myogenic proteins [41, 42]. Several studies have demonstrated that fibrotic tissue in regenerated muscle could be prevented using either a TGF-β1 neutralization antibody or an antifibrotic agent, thereby improving the recovery of strength in the injured muscle, as well as promoting muscle regeneration [13, 34, 43]. Consequently, in contrast to our experiment, excessive TGF-β1 may disguise the positive effect that other growth factors have on muscle fibrosis and regeneration.
The hypertrophic reaction described in all the surgically damaged muscles is associated to the activation of satellite cells and the beginning of its division, with the aim of regenerating the injured muscle [44]. The ultra-structural evaluation in this study revealed that during the first 2 weeks PRGF therapy induced better connective tissue structure and less edema than Saline group. Therefore, these findings are correlated with minor inflammatory cells infiltration described in the HE stained sections. Besides that, at week 4 of study, PRGF was associated with less undulated muscle fibrils, which was indicative of physiologic and controlled regeneration process [44]. Moreover, PRGF treated muscles group showed higher mitochondrial density located more efficiently attached to the “Z” band of the sarcomere. Mitochondrial organelles are in charge of oxidative metabolism, which constitutes the principal energy pathway in the mature skeletal myofibers [10, 45]. It may suggest that muscles injected with PRGF presented higher metabolism rate to accelerate the regeneration process in comparison with those injected with Saline solution.
At biochemical level, the expression of muscle regulatory factors becomes objective criteria that have been utilized to evaluate muscle regeneration [16, 18]. In concordance with previous studies [16, 18], our results showed that PRGF-treated muscles described statistically higher level of expression of MYOD1, MYF5 and MYOG in the precocious stage of muscle regeneration at one week after grade II muscle rupture. This finding, together with statistically higher percentage of CNF previously described in the group of muscles treated with PRGF, suggests that PRGF therapy enhances the regeneration stimulus of muscle, accelerating the repair process, and subsequently it may shorten the convalescence period after grade II muscle injury. On the other hand, MSTN level of expression did not show statistical differences with respect to the PRGF of Saline solution therapy along the study. Thereby, muscle regeneration process is not being inhibited despite PRGF o Saline solution injection.
Several types of PRP have been used in clinical setting with the goal of accelerating the muscle regeneration process, as well as avoiding fibrotic tissue scar at the end of muscle healing procedure [17, 19, 22, 26, 46,47,48,49,50,51,52]. Protocols to obtain PRP, its final composition, and the method to assess its clinical effect on muscle regeneration are different from one study to the other. Subsequently, clinical results with the use of PRP, as general concept of platelet rich concentrate, are extremely variable and non-consistent in grade II muscle injuries. Hamilton et al. [17] evaluated the efficacy of PRP application in reducing time to return to sport after a grade II muscle injury in athletes. They recorded no benefits of a single dose of PRP injection over an intensive rehabilitation program with MRI evaluation [17]. Martinez-Zapata et al. [50] reported that a single dose of PRP injection did not significantly improve the time to completely heal a grade II muscle rupture compared to the control group. Both clinical studies used a PRP which contained 5–9 fold platelet concentration above peripheral blood [17, 50]. Besides that, Hamilton et al. [17] included 5-fold leukocytes concentration above peripheral blood in the PRP composition. Excessive platelet concentration and consequently unbalanced concentrations of certain growth factors such as TGF-β1 are associated to pathologically newly formed muscle tissue with the presence of fibrotic scar [13, 34, 43]. Moreover, leukocytes incorporated to the PRP composition are rich in metalloproteases that destroy growth factors and promote inflammatory phenomena [6, 37]. In addition, a single dose of PRP was injected in a grade II muscle injury in both clinical studies [17, 50]. However, 3 weekly infiltrations were performed in our experimental model. Thus, it would reproduce a moderate supraphysiological stimulus maintained throughout the muscle regeneration. Likewise, PRGF has been successfully used in clinical setting [2, 22]. Loo et al.[22] described 3 weekly intralesional injections of PRGF under ultrasound guidance for adductor longus tear treatment. The patient reported good pain relief, likely associated with reduced inflammation, and was able to get back to competition in 4 weeks [22]. The absence of leukocytes, and moderate platelet concentration described in the PRGF may induce a balanced stimulus on the muscle regeneration. In consequence, PRGF could accelerate the muscle regeneration process and enhance the quality of newly formed muscle tissue, with the presence of less fibrotic scar after a grade II muscle rupture in clinical setting.
There are several limitations to this study. First, ultrasonographic follow-up was not evaluated to check the muscle repair stage on ultrasound-imaging. The goal of the present study was to evaluate the histological and genomic effect of PRGF on muscle regeneration process. Therefore, ultrasound technique was only used as guidance to intralesional infiltration of PRGF or Saline solution, as previously described [26]. Second, functional testing was not performed. Since ovine specie is a prey in the wild animal world, they do not show any pain in weight bearing unless the muscular damage is severe. In this line, visual analogue scale or similar would not provide adequate results on the pain the animal was experiencing. Nevertheless, electromyography is a standardized test that would have addressed objective measurements about the functionality of the damaged muscle [53]. We will strongly consider this technique for future study to elucidate the effect of PRGF on the time to return to total functionality in a grade II muscle tear. Third, a different skeletal muscle, vastus lateralis quadriceps femoris, was used as control muscle in the histological analysis. However, an identical histological aspect was found compared to biceps femoris and accordingly, for ethical reasons we decided not to include more animals in the study.
In conclusion, PRGF intralesional treatment produced a significant decrease in the inflammatory cell density, with lower vascular density and blood vessel area, in comparison with the Saline-treated muscles. PRGF stimulated a significant higher percentage of CNF, and also the expression of myogenic genes (MYOD1, MYF5 and MYOG) was significantly higher in PRGF-treated muscles at the early stage of muscle regeneration. Furthermore, PRGF treatment leaded to newly formed muscle tissue with significant smaller fibrotic areas along the study, in contrast with Saline-treated muscles. These findings suggested an anti-inflammatory effect of PRGF on muscle repair, with an enhanced muscle regeneration stimulus, and improved repair quality after a surgically induced grade II muscle injury.
Data Availability
All data are provided in the main manuscript file and supplementary material along with raw data file in respective section.
References
Ekstrand J, Hagglund M, Walden M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med. 2011;39:1226–32.
Sanchez M, Anitua E, Delgado D, Sanchez P, Orive G, Padilla S. Muscle repair: platelet-rich plasma derivates as a bridge from spontaneity to intervention. Injury. 2014;45(Suppl 4):7–14.
Andia I, Abate M. Platelet-rich plasma in the treatment of skeletal muscle injuries. Expert Opin Biol Ther. 2015;15:987–99.
Croisier JL. Factors associated with recurrent hamstring injuries. Sports Med. 2004;34:681–95.
Tidball JG. Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc. 1995;27:1022–32.
Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–53.
Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, Authier FJ, Dreyfus PA, Gherardi RK. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol. 2003;163:1133–43.
Chazaud B, Brigitte M, Yacoub-Youssef H, Arnold L, Gherardi R, Sonnet C, Lafuste P, Chretien F. Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc Sport Sci Rev. 2009;37:18–22.
Segawa M, Fukada S, Yamamoto Y, Yahagi H, Kanematsu M, Sato M, Ito T, Uezumi A, Hayashi S, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Yamamoto H. Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Exp Cell Res. 2008;314:3232–44.
Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33:745–64.
Hurme T, Kalimo H. Activation of myogenic precursor cells after muscle injury. Med Sci Sports Exerc. 1992;24:197–205.
Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–38.
Terada S, Ota S, Kobayashi M, Kobayashi T, Mifune Y, Takayama K, Witt M, Vadala G, Oyster N, Otsuka T, Fu FH, Huard J. Use of an antifibrotic agent improves the effect of platelet-rich plasma on muscle healing after injury. J Bone Joint Surg Am. 2013;95:980–8.
Borrione P, Gianfrancesco AD, Pereira MT, Pigozzi F. Platelet-rich plasma in muscle healing. Am J Phys Med Rehabil. 2010;89:854–61.
Delos D, Leineweber MJ, Chaudhury S, Alzoobaee S, Gao Y, Rodeo SA. The effect of platelet-rich plasma on muscle contusion healing in a rat model. Am J Sports Med. 2014;42:2067–74.
Dimauro I, Grasso L, Fittipaldi S, Fantini C, Mercatelli N, Racca S, Geuna S, Di Gianfrancesco A, Caporossi D, Pigozzi F, Borrione P. Platelet-rich plasma and skeletal muscle healing: a molecular analysis of the early phases of the regeneration process in an experimental animal model. PLoS ONE. 2014;9:e102993.
Hamilton B, Tol JL, Almusa E, Boukarroum S, Eirale C, Farooq A, Whiteley R, Chalabi H. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomised controlled trial. Br J Sports Med. 2015;49:943–50.
Hammond JW, Hinton RY, Curl LA, Muriel JM, Lovering RM. Use of autologous platelet-rich plasma to treat muscle strain injuries. Am J Sports Med. 2009;37:1135–42.
Wetzel RJ, Patel RM, Terry MA. Platelet-rich plasma as an effective treatment for proximal hamstring injuries. Orthopedics. 2013;36:e64–70.
Lyras DN, Kazakos K, Verettas D, Polychronidis A, Tryfonidis M, Botaitis S, Agrogiannis G, Simopoulos C, Kokka A, Patsouris E. The influence of platelet-rich plasma on angiogenesis during the early phase of tendon healing. Foot Ankle Int. 2009;30:1101–6.
Anitua E, Prado R, Sánchez M, Orive G. Platelet-Rich Plasma: Preparation and Formulation. Oper Tech Orthop. 2012;22:25–32.
Loo WL, Lee DY, Soon MY. Plasma rich in growth factors to treat adductor longus tear. Ann Acad Med Singapore. 2009;38:733–4.
Hamid MSA, Mohamed Ali MR, Yusof A, George J. Platelet-rich plasma (PRP): an adjuvant to hasten hamstring muscle recovery. A randomized controlled trial protocol (ISCRTN66528592). BMC Musculoskelet Disord. 2012;13:138.
Peetrons P. Ultrasound of muscles. EurRadiol. 2002;12:35–43.
Anitua E, Sanchez M, Nurden AT, Zalduendo M, de la Fuente M, Orive G, Azofra J, Andia I. Autologous fibrin matrices: a potential source of biological mediators that modulate tendon cell activities. J Biomed Mater Res A. 2006;77:285–93.
Bubnov R, Yevseenko V, Semeniv I. Ultrasound guided injections of platelets rich plasma for muscle injury in professional athletes. Comparative study. Med Ultrason. 2013;15:101–5.
Sanchez M, Anitua E, Orive G, Mujika I, Andia I. Platelet-rich therapies in the treatment of orthopaedic sport injuries. Sports Med. 2009;39:345–54.
Fernandez-Sarmiento JA, Dominguez JM, Granados MM, Morgaz J, Navarrete R, Carrillo JM, Gomez-Villamandos RJ, Munoz-Rascon P, Martin de Las Mulas J, Millan Y, Garcia-Balletbo M, Cugat R. Histological study of the influence of plasma rich in growth factors (PRGF) on the healing of divided Achilles tendons in sheep. J Bone Joint Surg Am. 2013;95:246–55.
Sanchez M, Anitua E, Azofra J, Andia I, Padilla S, Mujika I. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35:245–51.
Seijas R, Rius M, Ares O, Garcia-Balletbo M, Serra I, Cugat R. Healing of donor site in bone-tendon-bone ACL reconstruction accelerated with plasma rich in growth factors: a randomized clinical trial. Knee Surg Sports Traumatol Arthrosc. 2015;23:991–7.
Bentzinger CF, Wang YX, Rudnicki MA. (2012) Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol 4.
Borrione P, Grasso L, Chierto E, Geuna S, Racca S, Abbadessa G, Ronchi G, Faiola F, Di Gianfrancesco A, Pigozzi F. Experimental model for the study of the effects of platelet-rich plasma on the early phases of muscle healing. Blood Transfus. 2014;12(Suppl 1):s221–8.
Boswell SG, Schnabel LV, Mohammed HO, Sundman EA, Minas T, Fortier LA. Increasing platelet concentrations in leukocyte-reduced platelet-rich plasma decrease collagen gene synthesis in tendons. Am J Sports Med. 2014;42:42–9.
Li H, Hicks JJ, Wang L, Oyster N, Philippon MJ, Hurwitz S, Hogan MV, Huard J. Customized platelet-rich plasma with transforming growth factor beta1 neutralization antibody to reduce fibrosis in skeletal muscle. Biomaterials. 2016;87:147–56.20.
Liu D, Black BL, Derynck R. TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev. 2001;15:2950–66.
Anitua E, Zalduendo MM, Prado R, Alkhraisat MH, Orive G. Morphogen and proinflammatory cytokine release kinetics from PRGF-Endoret fibrin scaffolds: evaluation of the effect of leukocyte inclusion. J Biomed Mater Res A. 2015;103:1011–20.
Pizza FX, McLoughlin TJ, McGregor SJ, Calomeni EP, Gunning WT. Neutrophils injure cultured skeletal myotubes. Am J Physiol Cell Physiol. 2001;281:C335–41.
Aguilar-García D, Fernández-Sarmiento JA, Granados MdM, Morgaz J, Navarrete R, Carrillo JM, Vilar JM, Cugat R, Domínguez JM. Effect of plasma rich in growth factors on the early phase of healing of surgically severed Achilles tendon in sheep: histological study. J Appl Anim Res. 2018;46:471–8.
Garg K, Corona BT, Walters TJ. Therapeutic strategies for preventing skeletal muscle fibrosis after injury. Front Pharmacol. 2015;6:87.
Cunha RC, Francisco JC, Cardoso MA, Matos LF, Lino D, Simeoni RB, Pereira G, Irioda AC, Simeoni PR, Guarita-Souza LC, Carvalho KA. Effect of platelet-rich plasma therapy associated with exercise training in musculoskeletal healing in rats. Transpl Proc. 2014;46:1879–81.
Li WQ, Qureshi HY, Liacini A, Dehnade F, Zafarullah M. Transforming growth factor Beta1 induction of tissue inhibitor of metalloproteinases 3 in articular chondrocytes is mediated by reactive oxygen species. Free Radic Biol Med. 2004;37:196–207.
Serrano AL, Mann CJ, Vidal B, Ardite E, Perdiguero E, Munoz-Canoves P. Cellular and molecular mechanisms regulating fibrosis in skeletal muscle repair and disease. Curr Top Dev Biol. 2011;96:167–201.
Fukushima K, Badlani N, Usas A, Riano F, Fu F, Huard J. The use of an antifibrosis agent to improve muscle recovery after laceration. Am J Sports Med. 2001;29:394–402.
Eurell J. Muscle. In: Eurell J, Frappier B, editors. Dellmann´s Textbook of Veterinary Histology. 6th ed.: Blackwell Publishing; 2006. pp. 79–90.
Ashmore CR, Doerr L. Oxidative metabolism in skeletal muscle of normal and dystrophic chicks. Biochem Med. 1970;4:246–59.
Sanchez MAE, Andia I. (2005) Application of Autologous Growth Factors on Skeletal Muscle Healing. In: 2nd World Congress on Regenerative Medicine, Leipzig, Germany, May 18–20.
MS AH, Mohamed Ali MR, Yusof A, George J, Lee LP. Platelet-rich plasma injections for the treatment of hamstring injuries: a randomized controlled trial. Am J Sports Med. 2014;42:2410–8.
Bernuzzi G, Petraglia F, Pedrini MF, De Filippo M, Pogliacomi F, Verdano MA, Costantino C. Use of platelet-rich plasma in the care of sports injuries: our experience with ultrasound-guided injection. Blood Transfus. 2014;12(Suppl 1):s229–34.
Else B, Fau - Emel TJ, Emel Tj Fau - Kern T, Kern T. Fau - Cavanagh LE, Cavanagh Le Fau - Allen TW, Allen TW Peroneus Longus Rupture at Its Origin Managed With Platelet Rich Plasma.
Martinez-Zapata MJ, Orozco L, Balius R, Soler R, Bosch A, Rodas G, Til L, Peirau X, Urrutia G, Gich I, Bonfill X. Efficacy of autologous platelet-rich plasma for the treatment of muscle rupture with haematoma: a multicentre, randomised, double-blind, placebo-controlled clinical trial. Blood Transfus. 2016;14:245–54.
Rossi LA, Molina Romoli AR, Bertona Altieri BA, Burgos Flor JA, Scordo WE, Elizondo CM. Does platelet-rich plasma decrease time to return to sports in acute muscle tear? A randomized controlled trial. Knee Surg Sports Traumatol Arthrosc; 2016.
Zanon G, Combi F, Combi A, Perticarini L, Sammarchi L, Benazzo F. Platelet-rich plasma in the treatment of acute hamstring injuries in professional football players. Joints. 2016;4:17–23.
Krechina EK, Lisovskaia VT, Pogabalo IV. [ElectroMYOGraphic evaluation of functional status of temporal muscles and mastication muscles in patients with close position of frontal teeth in cases of different occlusion]. Stomatologiia (Mosk). 2010;89:69–71.
Acknowledgements
One of the authors (DAG) was the recipient of a grant from the Ministry of Education, Culture and Sport of Spanish Government (FPU Program AP2012-1787).
Funding
This study was funded by the García-Cugat Foundation for Biomedical Research.
Author information
Authors and Affiliations
Contributions
JMDP designed the experiments and amended the manuscript. DAG, JMDP, MMGM, PMR, RNC, JMCP and RCB carried out the surgical and anesthetic procedures. JMR and JAFS completed the statistical analysis and prepared the figures. YMR and DAG performed the histological and ultrastructural study. JMC did the biochemical analysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The present study was carried out following the guidelines of the European Union (Directive 2010/63/EU), ARRIVE guidelines and approved by the Bioethical Committee on Animal Research at Andalucía (Junta de Andalucía, reference 12/09/2018/138).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Aguilar-García, D., Fernández-Sarmiento, J.A., del Mar Granados Machuca, M. et al. Histological and biochemical evaluation of plasma rich in growth factors treatment for grade II muscle injuries in sheep. BMC Vet Res 18, 400 (2022). https://doi.org/10.1186/s12917-022-03491-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-022-03491-2