Abstract
Bluetongue (BT) is an insect-borne, non-contagious viral disease which affects domestic ruminants including camels and is transmitted by Culicoides spp. Clinical symptoms of BT are typically seen in sheep, although subclinical BT infections are mostly seen in cattle, goats, and camelids. The goal of the present study was to evaluate the sero-prevalence of Bluetongue virus (BTV) in camels from some governorates in Egypt’s southern and northern regions, as well as the infection’s potential risk factors. During 2020–2021, a cross sectional study was conducted to screen presence of anti-BTV antibodies in 400 serum samples, which were collected randomly from camels, examined using competitive enzyme-linked immunosorbent assay (cELISA). The sera of 102 out of 400 camels tested positive for BTV, representing a frequency of 25.5%. Moreover, the odds of sero-positivity were higher among camels living in Aswan (OR = 5.33, 95%CI: 2.35–12.11), especially in females (OR = 2.63, 95%CI = 1.44–4.09) during summer season (OR = 2.40, 95%CI = 1.20–4.81). Furthermore, the probability of getting BTV infection increased when camels were exposed to the insect vectors (OR = 1.63, 95%CI = 0.87–3.09). The high prevalence of BTV in camels in several Egyptian regions highlights the need for more epidemiological investigations of BTV infection in other ruminant species in order to better control BT disease in these regions.
Similar content being viewed by others
Introduction
Bluetongue virus (BTV) is a double-stranded RNA virus that causes bluetongue disease (family Reoviridae, genus Orbivirus) with at least 29 identified serotypes that causes bluetongue in ruminants. The virus is transmitted from one susceptible host to another via different species of Culicoides biting midges. In Africa and southern Europe, C. imicola is the most common vector species [1]. Major susceptible hosts include sheep, goats, cattle, camels, llamas, deer, and antelopes. Clinical signs include fever, salivation, oral mucosa erosions, oedema of the cheeks and lips, erosions in/around nostrils, apathy, dysphagia, coronitis, lameness, conjunctivitis, muscular necrosis, and stiffness in the limbs. The clinical manifestation are severe in sheep, while cattle, goats, camelids, and wildlife typically show moderate symptoms [2].
The disease is classified as “notifiable” because of direct losses (death, reduction of milk production, sterility and/or abortion) and indirect losses (i.e. restrictions on foreign trade, survey and mass vaccination expenses, Control and treatment of vectors) [3,4,5,6,7].
According to Elzein [8], BTV infection is common among camels in Sudan, with a sero-prevalence of 16.6%, whereas Saeed and Aradaib [9] found that sero-prevalence of BTV in camels were extremely high (66.8%) in Khartoum State, Sudan. In Egypt, a few studies have been conducted to examine the overall state of BTV. Khaled et al. [10] recently found that BTV antibodies were common in sheep (23.2%) and goats (10.9%) in the Egyptian governorates of Aswan, Elwadi elgadid, Giza, and Marsa Matrouh. The main cause of BTV introduction into Egypt was thought to be the constant importation of viraemic ruminants or camels, mostly from Sudan [11]. BT is endemic in countries in Sub-Saharan Africa (including Sudan), with significant outbreaks occurring during periods of torrential rain and flooding [12]. Increased uncontrolled transboundary cattle movements could occur from socioeconomic changes, such as changes in meat market pricing, or changes in human population expansion and the resultant increased demand for meat [13]. As a result of all of these factors, the probability of BTV spreading into the Mediterranean basin and the Middle East has increased [14].
The prevalence of BTV infection in Egyptian camels is currently unknown, as well as the risk factors associated with it. Therefore, the goal of the present work was to conduct the first serological investigation of BTV infection and to investigate the associated risk factors in one-humped camels in some Egyptian governorates.
Materials and methods
Ethical statement
The study followed the Declaration of Benha University and was approved by the Ethics Committee of the Faculty of Veterinary Medicine (BUFVTM). All procedures were carried out in compliance with the ethical committee of the Faculty of Veterinary Medicine’s standards and regulations. The owners of the camels provided their verbal agreement for the samples to be taken. The study was conducted following ARRIVE guidelines.
Study area
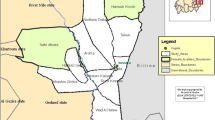
This study was conducted in four governorates: Qalyubia, Kafr ElSheikh, Mersa Matrouh and Aswan which are located at 30°25 N to 31°13 E, 31.1107° N to 30.9388° E, 31°20′N 27°13′E and 24°05′20″N 32°53′59″E, respectively (Fig. 1).
The Köppen-Geiger climate classification for these governorates is BWh, which means “hot desert climate”. In the summer, the weather is very hot. The rainy season lasts 3 months, with yearly rainfall of 100 to 200 mm on average. The average annual temperature is between 20 °C and 45 °C throughout the year, with a relative humidity of approximately 43%. These governorates are one of the country’s major livestock growing zones, with large number of camels in particular. Therefore, the selected samples were representative. Moreover, Egypt shares borders with Sudan from south through Aswan governorate.
Study design and blood sampling
This was a cross-sectional survey done between 2020 and 2021. A total of 400 samples were taken at random from 400 camels at the same time from all governorates under the study: two in the Nile Delta (Qalyubia and Kafr ElSheikh), one in the north portion (Mersa Matrouh), and one in the south (Aswan). The camels from each governorate were selected using simple random sampling. Data regarding to animal’s age (≤2, 2–5 and > 5 years), sex (male, female), season (spring, summer, winter, and autumn), and management risk factors such as herd size (≤50, ≥51), and insect vector (presence or absence) were all collected. Moreover, all of examined camels were non vaccinated against BTV. Blood was collected in plain vacuum tubes (without anticoagulant) and allowed to clot overnight at + 4 °C. After that, the serum was centrifuged for 15 minutes at 2000 rpm and stored at − 20 °C until serological examination.
Serological analysis
A total of 400 serum samples were examined using IDEXX Bluetongue Competition Ab (IDEXX, USA). The procedures followed the manufacturer’s instructions. According to the manufacturer, the IDEXX cELISA kit has 100% specificity and 82.8% sensitivity. The optical density was read singly by AMR-100 reader (AllSheng, China) at 450 nm. When the optical density of the tested sera was less than or equal to 70% of the negative controls’ mean (S/N), they were classified as positive. Tested sera with an optical density more than or equal to 80% of the negative controls’ mean (S/N) were classified as negative, whereas those with an optical density less than 80% but greater than 70% of negative controls’ mean were classified as suspect and those animals were resampled and tested after 1 month.
Statistical analysis
The statistical analysis was performed using SPSS version 24 (SPSS Inc., Chicago, U.S.A.). In a univariable analysis, chi-square test was performed to examine the risk factors’ relationships with BTV seroprevalence. Using multivariable logistic regression, potential risk factors having p-values < 0.2 were further evaluated. For the outcome variable, a multivariable model was performed using logistic regression analysis. Confidence interval (95%CI) and odds ratios (OR) were calculated.
Results
Out of 400 camels tested, 102 (25.5%) were shown to have antibodies to BTV and there were no clinical symptoms of BTV infection in any of camels. BTV sero-positivity had no statistically significant association with age or herd size (P > 0.05). The sero-prevalence rates were significantly different between different localities (P < 0.0001), whereas the highest prevalence rate for BTV was found in Aswan (42.5%), while the lowest prevalence rate was seen in Qalyubia (10.6%), Table 1.
The sero-prevalence was significantly (P < 0.0001) higher in females (33.2%) in comparison to males (16.1%), particularly among those infested with insects (29.3%). Moreover, BTV infection was significantly highest (P < 0.0001) during the summer season (37.7%), lowest during the winter season (11.8%), and average during the spring season (16.1%), Table 1. In addition, the median (> 2–5 years) and young ages groups (≤2 years) were more likely to be infected with BTV than older camels while the camels living in herd size (≥51) showed higher sero-positivity than those of small herds (≤50), Table 1.
In the present study, the camels living in Aswan were found more likely to be infected than those in Mersa Matrouh (OR = 5.33, 95%CI: 2.35–12.11, P-value = < 0.0001). Females were approximately three times more likely than males to be infected with BTV (OR = 2.63, 95%CI = 1.44–4.09, P-value = 0.001). Also, the presence of insects on camels increased the probability of BT sero-positivity by 1.63 times when compared to the absence of insects on animals (OR = 1.63, 95%CI = 0.87–3.09), Table 2.
Furthermore, the camels were more likely to contracting BTV infection particularly during summer season (OR = 2.40, 95%CI = 1.20–4.81, P-value = 0.013), Table 2.
Discussion
Bluetongue produces significant financial losses and is a key source of concern for worldwide trade [15]. The severity of BTV infection ranges from completely subclinical to clinical, with the majority of infected camels showing no symptoms at all [16]. Furthermore, the global prevalence and form of BTV infection have altered dramatically in recent past. Individual BTV serotypes distribution is not consistent over the world, and it has changed dramatically as a result of climate change [17].
As a result of the high frequency of outbreaks among wild and domestic ruminants in formerly BTV-free areas, BTV has become a major veterinary issue for dairy producers, wildlife managers, and veterinary diagnosticians [18, 19]. However, in the Middle East and East Central Africa, including Egypt, relatively little information about BTV epidemiology is available. Moreover, absence of control measures for the disease (eg. vaccination, entomological or serological surveillance) in Egypt increase the possibility of disease spreading. We performed the present study to investigate the prevalence of BTV infection and its related risk factors among camels in certain governorates representing the north and south of Egypt, in order to progress beyond current knowledge of the disease’s epidemiology.
BTV sero-prevalence was found to be 25.5% overall in this work. Because Egypt does not have a BTV vaccination program, this sero-prevalence of BTV in some governorates could be attributed to spontaneous camel infection. BTV sero-positivity has been reported in dromedaries in numerous studies in different countries. The sero-prevalence rate of BTV among camels in this work lie in the same range that reported in previous study in Saudi Arabia (25.7%) by Yousef et al. [20]. Moreover, previous epidemiological studies found higher sero-prevalence for BTV in Sudan (78.6%) [21], Iran (93.5%) [22] and Turkey (88%) [23]. However, the overall prevalence of this study was greater than the previous reported rate in camels in the Kassala region in Sudan, with a prevalence of 4.3% [8].
In addition, the sero-prevalence was varied between different localities in the present study where the highest prevalence rate was found in South. This may be due to the co-existence of other species like as cattle, sheep, and goats with camel herds, as well as the favorable climatic circumstances that allow vectors to thrive in these areas [24,25,26,27,28,29,30].
In comparison, the Qalyubia governorate had the lowest infection rate (10%), this could be due to camels being separated from other animals, as well as the large camel population in this area [21]. It’s critical to stress the importance of the quantity and dispersion of Culicoides vector populations, as well as the composition of host species, climatic circumstances, and virus strains, may all influence sero-prevalence rates between countries [6, 31,32,33,34].
There was a significant relationship between BTV sero-positivity and sex when sex was considered a risk factor, with females having higher sero-prevalence than males. A similar findings were reported by Mahmoud et al. [35] in Libya.
Contrary to the present findings, Abraheem et al. [36] found equal sero-prevalence rate in both sexes with no significant disparity. The disparity in the present work could be related to disparities in male and female sample sizes, as well as variances in husbandry procedures [21, 36].
However, the risk assessment found no link between sero-prevalence and the age of the animal or the size of the herd. The findings are directly in line with previous findings reported in camels in Sudan [21, 36,37,38].
The results of the present study confirmed that the highest sero-prevalence of BTV was more common among herd ≥51when compared by ones ≤50, which are in accordance with findings reported by Saeed and Aradaib [9]. This could indicate that camels are more susceptible to infection when raised larger herds or in contact with more susceptible species such as cattle, sheep, and goats [39].
According to the findings, BTV sero-prevalence increased significantly during the summer season as compared to other seasons, especially in areas with a high insect population. This is consistent with previous findings of Elmahi et al. [21]. Such findings could be attributed to high density of Culicoides in these areas [18, 40], which plays an important role in transmission of BTV and its multiplication increased significantly during summer [38, 41, 42].
Conclusion
The BTV antibodies have been detected in camels from Egypt. Moreover, the multivariable logistic regression analysis confirmed that the BTV seropositivity is strongly associated with locality, female sex, during summer season and in presence of insects. It is suggested that biting Culicoides midges engaged in BTV transmission be monitored using entomological methods. Additionally, studies on the ecology and epidemiology of BT in Egypt should be conducted in order to better predict the disease.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Change history
08 March 2023
Incorrect Funding note.
References
Legisa D, Gonzalez F, De Stefano G, Pereda A, Santos MD. Phylogenetic analysis of bluetongue virus serotype 4 field isolates from Argentina. J Gen Virol. 2013;94:652–62.
Elbers AR, Backx A, Mintiens K, Gerbier G, Staubach C, Hendrickx G, et al. Field observations during the bluetongue serotype 8 epidemic in 2006: II. Morbidity and mortality rate, case fatality and clinical recovery in sheep and cattle in the Netherlands. Prev Vet Med. 2008;87:31–40.
Coetzee P, Van Vuuren M, Stokstad M, Myrmel M, Venter EH. Bluetongue virus genetic and phenotypic diversity: towards identifying the molecular determinants that influence virulence and transmission potential. Vet Microbiol. 2012;161:1–12.
Wilson AJ, Mellor PS. Bluetongue in Europe: past, present and future. Philos Trans R Soc B Biol Sci. 2009;364:2669–81.
Selim A, Abdelhady A. The first detection of anti-West Nile virus antibody in domestic ruminants in Egypt. Trop Anim Health Prod. 2020;52:3147–51.
Selim A, Abdelrahman A, Thiéry R, Sidi-Boumedine K. Molecular typing of Coxiella burnetii from sheep in Egypt. Comp Immunol Microbiol Infect Dis. 2019;67:101353.
Selim A, Ali A-F, Moustafa SM, Ramadan E. Molecular and serological data supporting the role of Q fever in abortions of sheep and goats in northern Egypt. Microb Pathog. 2018;125:272–5.
Elzein EA. Bluetongue in camels: a serological survey of the one-humped camel (Camelus dromedarius) in the Sudan. Rev Elev Med Vet Pays Trop. 1985;38:438–42.
Saeed SI, Aradaib IE. A survey of bluetongue virus antibodies and associate risk factors among camels in Khartoum State, Sudan; 2017.
Khaled SM, Goda MA, Arnaout FK, Galila EM, Salem SA. Prevalence of blue tongue virus antibodies and associated risk factros among sheep and goat in Egypt GOAT IN EGYPT. Eur J Pharm Med Res. 2019;6:180–6.
Napp S, Chevalier V, Busquets N, Calistri P, Casal J, Attia M, et al. Understanding the legal trade of cattle and camels and the derived risk of Rift Valley fever introduction into and transmission within Egypt. Plos Negl Trop Dis. 2018;12:e0006143.
Kamal SA. Observations on rift valley fever virus and vaccines in Egypt. Virol J. 2011;8:1–9.
Mansfield KL, Banyard AC, McElhinney L, Johnson N, Horton DL, Hernández-Triana LM, et al. Rift Valley fever virus: a review of diagnosis and vaccination, and implications for emergence in Europe. Vaccine. 2015;33:5520–31.
Chevalier V, Pépin M, Plee L, Lancelot R. Rift Valley fever-a threat for Europe? Eurosurveillance. 2010;15:19506.
Spedicato M, Carmine I, Teodori L, Leone A, Casaccia C, Di Gennaro A, et al. Transplacental transmission of the Italian bluetongue virus serotype 2 in sheep. Vet Ital. 2019;55:131–41.
Constable PD, Hinchcliff KW, Done SH, Grünberg W. Veterinary medicine-e-book: a textbook of the diseases of cattle, horses, sheep, pigs and goats: Elsevier Health Sciences; 2016.
Maclachlan NJ. Bluetongue: history, global epidemiology, and pathogenesis. Prev Vet Med. 2011;102:107–11.
Lundervold M, Milner-Gulland E, O’callaghan C, Hamblin C. First evidence of bluetongue virus in Kazakhstan. Vet Microbiol. 2003;92:281–7.
Saegerman C, Berkvens D, Mellor PS. Bluetongue epidemiology in the European Union. Emerg Infect Dis. 2008;14:539.
Yousef MR, Al-Eesa AA, Al-Blowi MH. High seroprevalence of bluetongue virus antibodies in sheep, goats, cattle and camel in different districts of Saudi Arabia. Vet World. 2012;5:389–93.
Elmahi MM, Hussien MO, Karrar ARE, Elhassan AM, El Hussein ARM. Sero-epidemiological survey of bluetongue disease in one-humped camel (Camelus dromedarius) in Kassala State, Eastern Sudan. Irish Vet J. 2021;74:1–7.
Shoorijeh SJ, Ramin A, Maclachlan NJ, Osburn B, Tamadon A, Behzadi M, et al. High seroprevalence of bluetongue virus infection in sheep flocks in West Azerbaijan. Iran Comp Immunol Microbiol Infect Dis. 2010;33:243–7.
Gür S. A serologic investigation of blue tongue virus (BTV) in cattle, sheep and gazella subgutturosa subgutturosa in southeastern Turkey. Trop Anim Health Prod. 2008;40:217–21.
Mozaffari AA, Sakhaee E, Khalili M, Ardakani AP. High seroprevalence of bluetongue virus (BTV) antibodies in camel in Yazd province of Iran. J Camel Pract Res. 2013;20:171–3.
Rymaszewska A, Grenda S. Bacteria of the genus Anaplasma–characteristics of Anaplasma and their vectors: a review. Vet Med. 2008;53:573–84.
Selim A, Megahed AA, Kandeel S, Abdelhady A. Risk factor analysis of bovine leukemia virus infection in dairy cattle in Egypt. Comp Immunol Microbiol Infect Dis. 2020;72:101517.
Selim A, Radwan A, Arnaout F. Seroprevalence and molecular characterization of West Nile virus in Egypt. Comp Immunol Microbiol Infect Dis. 2020;71:101473.
Selim A, Radwan A, Arnaout F, Khater H. The recent update of the situation of West Nile fever among equids in Egypt after three decades of missing information. Pak Vet J. 2020;40:390–3.
Elhaig MM, Selim A, Mandour AS, Schulz C, Hoffmann B. Prevalence and molecular characterization of peste des petits ruminants virus from Ismailia and Suez, northeastern Egypt, 2014–2016. Small Rumin Res. 2018;169:94–8.
Reisberg K, Selim AM, Gaede W. Simultaneous detection of Chlamydia spp., Coxiella burnetii, and Neospora caninum in abortion material of ruminants by multiplex real-time polymerase chain reaction. J Vet Diagn Investig. 2013;25:614–9.
Saminathan M, Singh KP, Khorajiya JH, Dinesh M, Vineetha S, Maity M, et al. An updated review on bluetongue virus: epidemiology, pathobiology, and advances in diagnosis and control with special reference to India. Vet Q. 2020;40:258–321.
Selim A, Manaa EA, Alanazi AD, Alyousif MS. Seroprevalence, risk factors and molecular identification of bovine leukemia virus in Egyptian cattle. Animals. 2021;11:319.
Selim A, Marawan MA, Ali A-F, Manaa E, AbouelGhaut HA. Seroprevalence of bovine leukemia virus in cattle, buffalo, and camel in Egypt. Trop Anim Health Prod. 2020;52:1207–10.
Selim A, Attia K, Ramadan E, Hafez YM, Salman A. Seroprevalence and molecular characterization of Brucella species in naturally infected cattle and sheep. Prev Vet Med. 2019;171:104756.
Mahmoud AS, Savini G, Spedicato M, Monaco F, Carmine I, Lorusso A, et al. Exploiting serological data to understand the epidemiology of bluetongue virus serotypes circulating in Libya. Vet Med Sci. 2019;5:79–86.
Abraheem HH, Elhassan AM, Hussien MO, Enan KA, Musa AB, El Hussein ARM. A survey of bluetongue infection and associated risk factors among the one-humped camel (Camelus dromedaries) in Gadarif state, eastern Sudan. Vet Med Int. 2021;2021:1–5.
Selim A, Yang E, Rousset E, ThiÚry R, Sidi-Boumedine K. Characterization of Coxiella burnetii strains from ruminants in a galleria mellonella host-based model. New Microbes New Infect. 2018;24:8–13.
Selim AM, Elhaig MM, Moawed SA, El-Nahas E. Modeling the potential risk factors of bovine viral diarrhea prevalence in Egypt using univariable and multivariable logistic regression analyses. Vet World. 2018;11:259.
Elmahi M. Serosurveillance and molecular detection of bluetongue virus in domestic ruminants in Kassala state. Sudan: University of Khartoum, Kassala, Sudan; 2018.
Selim A, Ali A-F, Ramadan E. Prevalence and molecular epidemiology of Johne’s disease in Egyptian cattle. Acta Trop. 2019;195:1–5.
Foxi C, Delrio G, Falchi G, Marche MG, Satta G, Ruiu L. Role of different Culicoides vectors (Diptera: Ceratopogonidae) in bluetongue virus transmission and overwintering in Sardinia (Italy). Parasit Vectors. 2016;9:1–13.
Selim AM, Elhaig MM, Gaede W. Development of multiplex real-time PCR assay for the detection of Brucella spp., Leptospira spp. and Campylobacter foetus. Vet Ital. 2014;50:75.
Acknowledgements
The authors extend their appreciation to Researchers Supporting Project number (RSP-2021/369), King Saud University, Riyadh, Saudi Arabia.
Funding
This research was funded by King Saud University, Riyadh, Saudi Arabia, grant number RSP-2021/369.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, formal analysis, investigation, resources, data curation, writing-original draft preparation, A.S., R.A., I.K. and K.A.; writing-review and editing, A.S., R.A., I.K., F.A. and K.A.; project administration, A.S., F.A. and K.A.; funding acquisition, A.S., R.A. and I.K. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study followed the Declaration of Benha University and was approved by the Ethics Committee of the Faculty of Veterinary Medicine (BUFVTM). All procedures were carried out in compliance with the ethical committee of the Faculty of Veterinary Medicine’s standards and regulations. The owners of the camels provided their verbal agreement for the samples to be taken. The study was conducting following ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
There are no conflicts of interest declared by the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Selim, A., Alsubki, R.A., Albohairy, F.M. et al. A survey of bluetongue infection in one-humped camels (Camelus Dromedarius); seroprevalence and risk factors analysis. BMC Vet Res 18, 322 (2022). https://doi.org/10.1186/s12917-022-03421-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-022-03421-2