Abstract
Background
Brucella suis is a zoonotic pathogen with a serious impact on public health and the pig industry worldwide. Information regarding B. suis in pigs in Egypt is scarce. This study aimed to investigate the prevalence of B. suis in slaughtered domestic pigs at El-Basatin abattoir in Cairo, Egypt. A total of 1,116 domestic pigs slaughtered in 2020 were sampled for Brucella isolation and identification. Identified Brucella isolates were molecularly confirmed at species, and biovar levels using Bruce ladder PCR and Suis ladder multiplex PCR. Additionally, high-risk practices of 16 abattoir workers (4 veterinarians, 10 butchering and evisceration workers, and 2 scalding workers) were investigated using a pre-piloted structured questionnaire.
Results
Brucella isolates were recovered from 1.3% of examined pigs (n = 14) at consistently low rates (1.1—2.9%) across the year of sampling from February to December 2020. All isolates were confirmed as B. suis biovar (bv) 2. Remarkably, 92.9% (13/14) of isolates showed atypical ability to produce H2S and hence were considered as B. suis bv2 atypical phenotype. The prevalence was higher in males (1.8%) than in females (0.9). However, this difference was not significant (Odds ratio = 1.9; CI 95% 0.7 – 5.7; P = 0.2). No detectable pathological lesions were associated with B. suis bv2 infection in examined pigs. All strains were isolated from cervical lymph nodes, highlighting a potential oral transmission. High-risk practices were recorded among swine abattoir workers in this study: 75% do not wear gloves or disinfect their knives daily, and 18.8% were willing to work with open wound injuries.
Conclusions
To the best of our knowledge, this is the first isolation of B. suis bv2 in Egypt. Detection of H2S producing B. suis bv2 atypical phenotype is alarming as it may result in misinterpretation of these isolates as highly human pathogenic B. suis bv1 in Egypt and possibly elsewhere. Further epidemiological tracing studies are crucial for the detection of the origin of this biovar. Including pigs in the national surveillance program of brucellosis, and an education program for swine abattoir workers about occupational risk of B. suis is a need in Egypt.
Similar content being viewed by others
Background
Brucella suis (B. suis) is the causative agent of porcine brucellosis. Based on preferential infection of different animal hosts, it encompasses five biovars (bv1-5). Biovars 1, 2, and 3 infect domestic pigs, wild boars, and hares, bv 4 is restricted to reindeer and caribou, and bv 5 infects rodents only [1]. Biovar 1 is widely spread in Central and South American pigs, causing several brucellosis outbreaks in pigs, and is linked to zoonotic human infections [2, 3]. Biovars 1 and 3 cause sporadic epidemics in Asia, especially in China [3]. Biovar 4 is endemic to Alaska, Canada, and Northern Russia. It was reported as a rare cause of human disease [4]. On the contrary, biovar 2 is restricted to Europe [5], where both wild boar (Sus scrofa scrofa) and European brown hare (Lepus europaeus) are the only recognized wildlife reservoirs of this biovar [3, 6]. Recently, an emerging increase of biovar 2 brucellosis outbreaks in pigs was reported in Europe. These outbreaks mainly were linked to contact of domestic pigs with wildlife reservoirs [3, 5, 6], import of carrier pigs, or use of infected boar semen [5]. Additionally, spillover infections spread to cattle were reported in several European countries [7, 8]. B. suis bv 2 causes bacteremia, abortion, stillbirths, decreased litter size, weak piglets, infertility, and sometimes chronic focal localization as abscess formation in various organs, metritis in females, orchitis in males, and swollen joints with lameness [3, 9]. B. suis bv 2 outbreaks have been associated with a high economic impact on the pig industry in Europe [5].
Nine human brucellosis cases caused by B. suis bv2 were reported globally; eight were in France [10, 11], and one case was in China [12]. Six out of these eight human cases reported in France had chronic medical conditions that possibly increased their risk of infection [11]. However, under-reporting is expected since most European countries, where biovar 2 is enzootic in wild pigs, do not routinely full type Brucella in human cases [11]. Three of these cases presented with acute brucellosis without focal infection, three cases suffered from arthritis, and two cases were associated with abscesses formation in muscles and liver [11, 12]. One patient died from liver failure [12]. The majority of these cases were linked to frequent exposure to wild boars or their tissues (e.g., hunting, butchering, etc.), which highlights the occupational hazard of this biovar [11].
In Egypt, B. suis is an uncommon cause of brucellosis, while B. melitensis bv3 and B. abortus bv1 have been reported in the majority of human and animal brucellosis cases [13,14,15]. Since the first isolation of B. suis in 1942 from aborted sows in upper Egypt [16], only a few recent reports of B. suis in pigs have been published [17, 18]. Pigs are not included in the routine national brucellosis surveillance program, which contributes to the lack of knowledge about the epidemiology of B. suis, its economic impact, or the role of pigs in spreading the infection to other livestock or humans. Pigs were reared in Egypt on intensive large-scale pig farms and small-scale backyard husbandry by garbage collectors in the slums of Cairo and Giza governorates, who raised pigs as the main source of their income [19]. However, in 2009 the General Organization of Veterinary Services (GOVS) ordered the culling of pig populations for fear of transmission of swine influenza virus to humans [19]. Pig slaughtering was permitted again in 2014. Despite the pig rearing ban from 2009 to 2014, garbage collectors managed to smuggle some of their pigs into their homes and reared them hidden from the GOVS. After 2014, the entire pig production stayed with these small-scale breeders, as former large-scale pig farmers refused to reopen their farms in fear of other severe economic losses. In small-scale backyard farms, pigs are fed on organic garbage escaping veterinary supervision and lack biosecurity measures but have contact with other livestock [18, 20]. Notably, on some occasions, pigs of these backyard farms are sold to small breeders in other northern (e.g., Alexandria) and southern (e.g., El-Minia) governorates, which highlights the risk of spreading porcine brucellosis to other livestock and across the country. In support of this assumption, B. suis was recovered from cattle in northern regions [20] and from camels in the southern region of Egypt [21]. Thus, this study was done to investigate the prevalence of B. suis in slaughtered pigs by isolation and molecular identification of its circulating biovars in El-Basatin abattoir and to record the high-risk practices of abattoir workers using a pre-tested questionnaire.
Results
A total of 14 Brucella isolates were recovered from 1,116 slaughtered pigs (1.3%). Using Suis-ladder multiplex PCR, all 14 Brucella isolates were identified as B. suis bv2. The conventional bacteriological examinations showed that 13 isolates were smooth, and one isolate was a rough strain (ID 14, Table 1). Also, all isolates showed the same characteristics, including agglutination with anti-Brucella monospecific anti-A serum (except for the rough strain; ID 14), positive reactions in urease test (fast; within a few minutes), growth without CO2, no growth on thionin (1/25,000) or basic fuchsin dye (20 µg/ml), and lysis by TB phage only at a concentration of 104 × RTD (Table 1). These characteristics are typical for B. suis biovar 2. However, the 13 smooth isolates showed an atypical ability to produce H2S that was only reported in B. suis bv1 (Table 1). Therefore, these isolates were assigned as B. suis bv2a (Table 2). The B. suis bv2 isolates were recovered at consistently low rates (1.1—2.9%) across the year of sampling from February to December 2020 (Table 2). The prevalence and odds of Brucella isolation from males were higher than from females (1.8% vs. 0.9%, respectively; OR = 1.9; CI 95% 0.7 – 5.7). However, these differences were insignificant (P = 0.2) (Table 3). This study had no detectable pathological lesions in slaughtered pigs infected with B. suis bv2. Among the examined lymph nodes samples, B. suis bv2 isolates were only recovered from the cervical lymph node (LN).
This study recorded high-risk practices at high rates among swine abattoir workers (Table 4). For example, none of the workers would wear masks, goggles, or face shields while working, and only 25% would wear gloves. In addition, some workers would work with open hand wounds (18.8%) and smoke while working (37.5%). Finally, only four workers (4 veterinarians, 25%) would disinfect their knives daily.
Discussion
In Egypt, pigs are raised and consumed mainly by Christians and tourists due to religious constrictions on Muslims, the majority of the population. To the best of our knowledge, this is the first report of B. suis bv2 from pigs in Egypt. Despite very early detection of B. suis in sows with reproduction failure in upper Egypt in 1942 [16], records of B. suis detection in livestock in Egypt are very limited. B. suis isolates were previously detected in seven slaughtered pigs from Cairo and Giza governorates [17] and in two cows from Menofia and Beni-Suef governorates [20]. In these two studies, B. suis isolates were typed as biovar 1 (B. suis bv1) either phenotypically [17] or genetically [20]. In another recent study, the DNA of B. suis was detected in the blood of three slaughtered pigs; however, the biovar type was not determined [18]. Remarkably, B. suis bv2 was never reported in Egypt or other counties outside Europe. B. suis bv2 is exclusively reported in Europe, where spillover infections from wild reservoirs have resulted in emerging outbreaks in domestic pig herds in several European countries [3, 5].
Pigs were reared, and pork was consumed all over Egypt in ancient time. Thus, it can be assumed that porcine brucellosis had been present in the past and reintroduced with imported pigs in the recent past or with the import of subclinically infected animals. Therefore, the transmission of Egyptian hares might have resulted as well.
Egypt was self-sufficient in the pig industry as national pig production covers national market needs in the years leading up to 2009. After the 2009 governmental mass culling of more than 300,000 pigs due to the fear of the spread of the swine flu virus [19], the national market needs have been covered by the importation of frozen pork and other pork byproducts but not living pigs. In fact, during the 2009 crisis, the GOVS firmly prohibited the importation of live pigs, banned breeding or keeping pigs across the country, and secured the borders to prevent the potential smuggling of pigs into or out of Egypt. Additionally, there was no official report of live pigs’ importation in the last 20 years. For these reasons, the possible introduction of the B. suis bv2 in Egypt through official imported infected pigs can be ruled out.
Before boar semen importation was officially prohibited in 2009, only a few large-scale pig breeders reported using unofficially imported boar semen to improve the genetic production traits of their pig stocks (personal communication; Airport vet quarantine, Veterinarians working at the pig slaughterhouse, and pig breeders). We could not identify the source of the imported semen as these breeders imported the semen through private companies which are very difficult to trace. Therefore, there is no clue how or when B. suis bv2 strains entered Egypt using official routes.
Pig farming is the main source of income for the garbage collectors living in the slums of Cairo and Giza governorates; hence pig culling left thousands of garbage collectors jobless. Also, the majority of local customers (low-income families) did not buy expensive (and less tasty) imported frozen pork as they relied on cheap local pig meat as the main source of protein [19]. These demanding local needs encouraged many garbage collectors, who succeeded in hiding some of their pigs from culling, to rebuild their small-scale pig farming business. In April 2014, the GOVS permitted the reopening of the pig slaughter section in El-Basatin abattoir, a milestone for pig farmers and the local pig industry. Since then, small-scale pig farmers have been thrilled to increase their pigs’ stocks to cover the rising market needs. This rapid increase in pig numbers (6000 heads in 2014 to 25,000 heads in 2018) over a short period [22] raised concerns about the restocking of the population via illegal routes to improve litter numbers, litter sizes, and meat production performance by the introduction of superior European breeds. Several African countries have imported pigs or boar semen to improve local breeds [23,24,25]. Another scenario is that the potential informal import of pigs or semen from other African countries lacking proper veterinary public health structures is an imminent risk for the pig industry in Egypt.
Only 11 of 54 African countries conducted targeted surveillance for Brucella in pigs (2007–2020) [26]. Accordingly, B. suis is believed to be widespread yet underreported in Africa [3, 27]. Moreover, B. suis spillover infections have been reported from atypical hosts such as cattle [20, 28] and rodents [27], indicating well-established B. suis reservoirs in Africa. B. suis bv2 may have entered Africa by pig/semen import and become established, but unidentified, among domestic pig breed or wildlife reservoirs a long time ago. Cross-border live animal or semen trade, either formal or informal, is very common in Africa, and control of animal movement across borders is very limited [23]. Egypt shares borders and makes trades with Sudan, a country with cross-border trade with Uganda, Rwanda, and Kenya. These countries imported boar semen from Europe in the past [23]. As the transmission of infections across the border from Sudan to Egypt is not unfamiliar, and recently B. suis DNA detection (unknown biovar) in camels’ sera in a southern governorate of Egypt was attributed to possible transboundary transmission of B. suis infection from Sudanese pig farms [21]. The assumed import of brucellosis via this trade route is also supported by an informal report (possibly through the private sector) stating that Egypt imported 32 pigs from Sudan in 2016 [29].
Whatever the potential routes of entry of B. suis bv2 infection to Egypt are, our results suggest that this introduction is not recent. We could detect B. suis bv2 all over the year of our sampling and at a relatively persistent low prevalence (1.1%—2.1% from February to October 2020). This highlights that B. suis bv2 infection is established in several pig herds in Egypt. Further genetic studies e.g., whole-genome sequence analysis, may add information on the origin of these isolates and help to trace their sources.
Remarkably, 13 of the detected B. suis bv2 isolates showed atypical ability to produce H2S that was only reported in B. suis bv1 [30]. This is a critical finding since H2S production is used to differentiate B. suis bv1 from other B. suis biovars (negative on H2S production); hence, mis- or under-reporting of these variants of B. suis bv2 is expected in Egypt and possibly elsewhere. These findings recommend routine use of molecular confirmation for both species and biovar in routine identification of Brucella isolates. B. suis isolates with atypical phenotype were previously reported, mostly for dye resistance [2, 31]. This finding, however, points to a single source of infection, e.g., active trade of semen or pigs. Whole-genome sequencing will facilitate a better understanding of this finding as well.
None of the positive pigs for B. suis bv2 showed visible pathological lesions in this study. This could be attributed to the young age of slaughtered animals, as most of the pigs were five months old; young animals rarely demonstrate clinical signs of B. suis infection [3, 32]. Some studies suggested that the long incubation period of the disease and/or the lack of sexual maturity were the reasons for the lack of clinical signs in younger pigs [3, 32]. In some instances, even mature pigs may show no clinical signs when infected with B. suis [3, 33]. There was no significant difference in prevalence between males and females (P = 0.2). This finding also can be explained by the age of the slaughtered pigs: most animals were not sexually mature. Differences are more evident in mature animals, as B. suis tends to localize more frequently and for a longer time in reproductive tissues of boars than in female ones [3]. All B. suis bv2 isolates were recovered from cervical LN. The cervical LNs are selective niches for localization of Brucella spp. in case of oral infection; Brucella strains may persist for up to two months in these lymph nodes after infection [34]. This highlights that oral exposure via feed (or environment) contaminated by reproductive discharges of infected sows was the predominant intra-herd transmission route among this study's slaughter pigs. This is expected since these young immature animals were reared for fattening, not breeding. Also, the majority of these animals did not reach sexual maturity, so venereal transmission and localization in genital lymph nodes are deemed unlikely.
The B. suis infection is an occupational risk for abattoir workers [35]. Human clinical cases caused by B. suis bv2 were reported from Europe [10, 11] and elsewhere [12]. The majority of these cases had a high risk of infection as they were often exposed to infection because of their occupation (e.g., skinning and gutting hunted wild boar) [11]. Furthermore, most abattoir workers in this study did not wear masks or gloves or disinfect their knives (75—100%). This highlights the potential high risk of infection among abattoir workers in the study region for brucellosis caused by B. suis or other Brucella spp. found in pigs.
Conclusions
This is the first report of isolation and molecular confirmation of B. suis bv2 in domesticated pigs in Egypt. Scenarios about possible introduction via informal or illegal smuggling of living pigs or via boar semen need further studies, including whole-genome sequence (WGS) analysis. The detection of B. suis bv 2 at consistently low rates across the year suggests a long-established reservoir rather than a recent introduction of foreign strains. Detection of H2S producing B. suis bv2 atypical phenotype is alarming as it may result in misinterpretation of these isolates as highly human pathogenic B. suis bv1 in Egypt and possibly elsewhere. Thus, the use of the molecular approach for B. suis biovar identification should be mandatory to detect these atypical phenotype isolates if stable genetic markers can be identified. Finally, the study showed that pigs reared in slums could be a neglected source for spillover infections to humans or other animals with B. suis and possibly other Brucella species in Egypt. This finding necessitates immediate and regular surveillance of these small-scale pig herds by GOVS authorities for early detection and control of B. suis biovars spread in Egypt.
Materials and methods
Study area
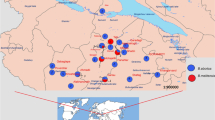
Pig samples were collected from slaughtered pigs in El-Basatin abattoir in the southern region of Cairo governorate. The GOVS permitted pig slaughtering in El-Basatin abattoir in April 2014 after the official banning of pig rearing and slaughtering in 2009. All pigs sent for slaughtering in El-Basatin abattoir were raised in the slums of Cairo and Giza governorates by garbage collectors as one of their main sources of income. In these slums, pigs are reared in backyard pens containing 50 to 100 pigs. Five large slums in Cairo and Giza governorates are currently raising pigs. The Cairo governorate slums include Manshiet Nassr, Cairo's largest pig farming slum, in the western Cairo region (30.0362°N, 31.2783°E); Ezbet El Nakhl (30.1393°N, 31.3244°E) in eastern Cairo region; and 15 May city (29.8579°N, 31.3885°E) in southern Cairo region. The Giza governorate slums included Al-Baragel (30.0767°N, 31.1593°E); and Ard El Lewa (30.0544°N, 31.1854°E) (Fig. 1). Most pigs reared for fattening are slaughtered at the age of 5 months.
Sample collection
The minimum sample size was calculated to be 246 pigs using Win Episcope 2.0. Criteria for sample size calculation included a confidence level of 95%, an accepted error of 5%, a population size of 30,000 (around 25,000 pigs were slaughtered in 2018; [22], and a minimum expected prevalence of 20% (The prevalence of animal brucellosis in Egypt is between 0.2% and 20%; [15]. A total of 1,116 slaughtered pigs were randomly selected from 19 February to 10 December 2020. The pigs from various herds are collected together in abattoir pens before slaughtering without any identification. Most of these animals are bought by pig merchants who collected them from different small-scale farmers before shipping them to the abattoir. For this reason, it was impossible to trace back sampled slaughtered pigs to their origin herds. Cervical lymph nodes (LN), inguinal LN, and supra-mammary LN were collected for each pig. In addition, the gender and any gross pathological lesions of chosen pigs' carcasses were recorded. All samples were labeled and transferred on ice to the Reference Laboratory of Brucellosis Research at the Animal Health Research Institute (AHRI), Giza, for Brucella isolation and identification.
Isolation of brucellae and identification of species and biovars
Swaps of macerated lymph nodes were cultured on trypticase soy agar (Oxoid LTD, Basingstoke, UK) supplemented with Brucella selective supplement (Oxoid LTD, Basingstoke, UK). The culture plates were incubated with and without a 5–10% CO2 atmosphere at 37 °C and examined for Brucella growth for two weeks.
Phenotypic characterization of the Brucella isolates was conducted as previously described [30] in terms of colony morphology, urease, catalase, oxidase, nitrate reduction, lactose fermentation, and reaction to acriflavine (1/1,000 solution) to detect dissociation and ensure smoothness of colonies (genus identification). All suspected Brucella isolates were sent to the Institute of Bacterial Infections and Zoonoses (IBIZ, Jena, Germany) for molecular confirmation of B. suis isolates at the species and the biovar levels. In addition, Matrix-Assisted Laser Desorption/Ionization (MALDI-TOF–MS) was performed as previously described [36] for rapid Brucella genus identification. A safe genus identification' was defined as a MALDI log score between 2.000 and 2.290. Furthermore, the requirement for CO2 at initial culture, H2S production, growth in the presence of dyes (thionine at 1/25,000 concentration; basic fuchsin at 1/50,000; safranin O dye 100 µg/ml), agglutination with A, M, and R monospecific anti-sera, and lysis by Tbilisi (Tb) phage using both routine test dilution (RTD) and 104 × RTD and Izatnagar (Iz) phage were done for conventional identification of Brucella species and biovar.
Molecular confirmation of B. suis and its biovars
The conventionally identified Brucella isolates were molecularly confirmed at the genus level using IR-1 and IR-2 primers as previously described [37]. The Brucella species were identified using AMOS-PCR [37] and Bruce-Ladder multiplex PCR [38]. The B. suis biovar was confirmed using Suis-ladder multiplex PCR [39].
Defining high-risk practices of swine abattoir workers
High-risk practices of 16 abattoir workers (4 veterinarians, 10 butchering and evisceration workers, and 2 scalding workers) were investigated using a pre-piloted structured questionnaire. The abattoir workers' practices included questions about using personal protective equipment (PPE), working with open wounds on their hands, eating or smoking while working, hand washing habits, and routine disinfection of knives.
Statistical analysis
The Univariate regression analysis was carried out using SPSS v19 (IBM, Armonk, NY). A significant association was considered at P ≤ 0.05.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- AHRI:
-
Animal Health Research Institute
- Biovar:
-
Bv
- Brucella:
-
B
- CI 95%:
-
95% Confidence intervals
- FAOSTAT:
-
The Food and Agriculture Organization Corporate Statistical Database
- GOVS:
-
General Organization of Veterinary Services
- IBIZ:
-
Institute of Bacterial Infections and Zoonoses
- Iz:
-
Izatnagar phage
- LN:
-
Lymph node
- MALDI-TOF–MS:
-
Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry
- OR:
-
Odds ratio
- PCR:
-
Polymerase chain reaction
- PPE:
-
Personal protective equipment
- Prevalence:
-
P
- RTD:
-
Routine test dilution
- Tb:
-
Tbilisi phage
- WGS:
-
Whole Genome Sequencing
References
García-Yoldi D, Le Fleche P, De Miguel MJ, Muñoz PM, Blasco JM, Cvetnic Z, et al. Comparison of multiple-locus variable-number tandem-repeat analysis with other PCR-based methods for typing Brucella suis isolates. J Clin Microbiol. 2007;45(12):4070–2.
Lucero NE, Ayala SM, Escobar GJ, Jacob NR. Brucella isolated in humans and animals in Latin America from 1968 to 2006. Epidemiol Infect. 2008;136(4):496–503.
Olsen S, Tatum F. Swine brucellosis: current perspectives. Vet Med: Res Rep. 2016;8:1–12.
Corbel MMJ. Brucellosis in humans and animals. WHO Library catalogue in publication Data. 2006;1–88. Available: https://www.who.int/publications/i/item/9789241547130.
Muñoz PM, Mick V, Sacchini L, Janowicz A, de Miguel MJ, Cherfa MA, et al. Phylogeography and epidemiology of Brucella suis biovar 2 in wildlife and domestic swine. Vet Microbiol. 2019;233(March):68–77.
De Massis F, Zilli K, Di DG, Nuvoloni R, Pelini S, Sacchini L, et al. Distribution of Brucella field strains isolated from livestock, wildlife populations, and humans in Italy from 2007 to 2015. PLoS One. 2019;14(3):1–16.
Fretin D, Mori M, Czaplicki G, Quinet C, Maquet B, Godfroid J, et al. Unexpected Brucella suis biovar 2 infection in a dairy cow, Belgium. Emerg Infect Dis. 2013;19(12):2053–4.
Szulowski K, Iwaniak W, Weiner M, Złotnicka J. Brucella suis biovar 2 isolations from cattle in Poland. Ann Agric Environ Med. 2013;20(4):672–5.
Meirelles-Bartoli RB, Mathias LA, Samartino LE. Brucellosis due to Brucella suis in a swine herd associated with a human clinical case in the State of São Paulo, Brazil. Trop Anim Health Prod. 2012;44(7):1575–9.
Teyssou R, Morvan J, Leleu JP, Roumegou P, Goullin B, Carteron B. A propos d’un cas de brucellose humaine a Brucella suis biovar 2. Medecine et Maladies Infectieuses. 1989;19(3):160–1.
Mailles A, Ogielska M, Kemiche F, Garin-Bastuji B, Brieu N, Burnusus Z, et al. Brucella suis biovar 2 infection in humans in France: emerging infection or better recognition? Epidemiol Infect. 2017;145(13):2711–6.
Paton NI, Tee NW, Vu CK, Teo TP. Visceral abscesses due to Brucella suis infection in a retired pig farmer. Clin Infect Dis. 2001;32(8):129–30.
Wareth G, Hikal A, Refai M, Melzer F, Roesler U, Neubauer H. Animal brucellosis in Egypt. J Infect Dev Ctries. 2014;8(11):1365–73.
Abdel-Hamid NH, El-bauomy E, Ghobashy HM, Shehata AAE. Genetic variation of Brucella isolates at strain level in Egypt. Vet Med Sci. 2020;6:421–32.
Abdel-Hamid NH, Ghobashy HM, Beleta EI, Elbauomy EM, Ismail RI, Nagati SF, et al. Risk factors and Molecular genotyping of Brucella melitensis strains recovered from humans and their owned cattle in Upper Egypt. One Health. 2021;13:100281.
Zaki R. Brucella infection among ewes, camels and pigs in Egypt. J Comp Pathol Ther. 1948;58(2):145–51.
Ibrahim S. Studies on swine brucellosis in Egypt. J Egypt Vet Med Assoc. 1996;56:1–12.
Khan AU, Melzer F, El-Soally SAGE, Elschner MC, Mohamed SA, Ahmed MAS, et al. Serological and molecular identification of brucella spp. In pigs from Cairo and Giza governorates. Egypt Pathogens. 2019;8(4):1–7.
Fahmi W, Sutton K. Cairo’s contested garbage: Sustainable solid waste management and the Zabaleen’s right to the city. Sustainability. 2010;2(6):1765–83.
Menshawy AMS, Perez-Sancho M, Garcia-Seco T, Hosein HI, García N, Martinez I, et al. Assessment of genetic diversity of zoonotic Brucella spp. Recovered from livestock in Egypt using multiple locus VNTR analysis. BioMed Res Int. 2014;2014:353876.
Khan AU, Sayour AE, Melzer F, El-soally SAGE, Elschner MC, Shell WS, et al. Seroprevalence and molecular identification of brucella spp. In camels in Egypt. Microorganisms. 2020;8(7):1–14.
CAPMAS. Annual Bulletin statistics livestock in 2018. 2020 [cited 1 Aug 2021]. Available from: https://www.capmas.gov.eg/Pages/Publications.aspx?page_id=5104&Year=23567
FAO. The Second Report on the State of the World’s Animal Genetic Resources for Food and Agriculture. 2015. Available from: http://www.fao.org/3/a-i4787e/index.html
Krüger DA, van Marle-Koster E, Theron HE. Comparison of on-farm progeny performances from local and imported boar semen used in the South African pig industry. S Afr J Anim Sci. 2017;47(5):688–96.
Vincent N, Kugonza DR, Hirwa CD. Success drivers of pig artificial insemination based on imported fresh semen. Int J Livest Prod. 2018;9(6):102–7.
OIE-WAHIS. Surveillance and control measures. 2021 [cited 12 Dec 2021]. Available from: https://wahis.oie.int/#/dashboards/control-measure-dashboard
McDermott JJ, Arimi SM. Brucellosis in sub-Saharan Africa: epidemiology, control and impact. Vet Microbiol. 2002;90(1–4):111–34.
Ledwaba B, Mafofo J, Van Heerden H. Genome sequences of Brucella abortus and Brucella suis strains isolated from bovine in Zimbabwe. Genome Announc. 2014;2(5):6–7.
UN Comtrade Database. United Nations Statistics Division [Internet]. 2016. Available from: https://comtrade.un.org/data/
Alton G, Jones L, Angus R, Verger J. Techniques for the brucellosis laboratory. Paris: Institut National De La Recherche Agronomique (INRA) Publications; 1988.
Corbel MJ, Thomas TLEL, Garcia-Carillo C. Taxonomic studies on some atypical strains of Brucella suis. Br Vet J. 1984;140(1):34–43.
Aparicio ED. Epidemiology of brucellosis in domestic animals caused by Brucella melitensis, Brucella suis and Brucella abortus. OIE Revue Scientifique et Technique. 2013;32(1):43–60.
Shome R, Kalleshamurthy T, Natesan K, Jayaprakash KR, Byrareddy K, Mohandoss N, et al. Serological and molecular analysis for brucellosis in selected swine herds from Southern India. J Infect Public Health. 2019;12(2):247–51.
Von Bargen K, Gagnaire A, Arce-Gorvel V, De Bovis B, Baudimont F, Chasson L, et al. Cervical lymph nodes as a selective niche for brucella during oral infections. PLoS One. 2015;10(4):1–26.
Young EJ. An overview of human brucellosis. Clin Infect Dis. 1995;21(2):283–90.
Karger A, Melzer F, Timke M, Bettin B, Kostrzewa M, Nöckler K, et al. Interlaboratory comparison of intact-cell matrix-assisted laser desorption ionization-time of flight mass spectrometry results for identification and differentiation of Brucella spp. J Clin Microbiol. 2013;51(9):3123–6.
Bricker BJ, Halling SM. Differentiation of Brucella abortus bv. 1, 2, and 4, Brucella melitensis, Brucella ovis, and Brucella suis bv. 1 by PCR. J Clin Microbiol. 1994;32(11):2660–6.
López-Goñi I, García-Yoldi D, Marín CM, De Miguel MJ, Muñoz PM, Blasco JM, et al. Evaluation of a multiplex PCR assay (Bruce-ladder) for molecular typing of all Brucella species, including the vaccine strains. J Clin Microbiol. 2008;46(10):3484–7.
López-Goñi I, García-Yoldi D, Marín CM, de Miguel MJ, Barquero-Calvo E, Guzmán-Verri C, et al. New Bruce-ladder multiplex PCR assay for the biovar typing of Brucella suis and the discrimination of Brucella suis and Brucella canis. Vet Microbiol. 2011;154(1–2):152–5.
Acknowledgements
The authors would like to thank the General Organization for Veterinary Services and AHRI directors for giving permission and facilitating samples collections from the El-Basatin abattoir in this study.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work received no external fund.
Author information
Authors and Affiliations
Contributions
Conceptualization, W.E., N.H.A.; methodology: W.E., N.H.A., M.E.R.H., E.I.M.B., M.M., F.M., and G.W.; data analysis: W.E., N.H.A.; and G.W.; data visualization: W.E., N.H.A.; data curation: W.E., N.H.A., M.E.R.H., E.I.M.B., M.M.; G.W. and H.N. manuscript draft writing: W.E., N.H.A.; manuscript review and editing: W.E., N.H.A., M.E.R.H., M.M., E.I.M.B., F.M., G.W. and H.N. All authors have agreed to publish the manuscript in its current format. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study protocol was approved by the Research Ethics Committee for Experimental and Clinical Studies, Animal Health Research Institute, and aligned with the Helsinki Declaration and World Health Organization guidelines. The written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Table 1. Detailed identification of Brucella suis biovar 2 recovered from cervical lymph nodes of slaughtered pigs at El-Basatin abattoir, Egypt during 2020 using MALDI-TOFMS, classical bacteriological typing, AMOS-PCR, and B. suis ladder PCR. Supplementary Table 2. Pre-piloted structured questionnaire template used to collect data regarding abattoir workers' high-risk practices.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Elmonir, W., Abdel-Hamid, N.H., Hamdy, M.E.R. et al. Isolation and molecular confirmation of Brucella suis biovar 2 from slaughtered pigs: an unanticipated biovar from domestic pigs in Egypt. BMC Vet Res 18, 224 (2022). https://doi.org/10.1186/s12917-022-03332-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-022-03332-2