Abstract
Background
Control programs were implemented in several countries against bovine viral diarrhea (BVD), one of the most significant cattle diseases worldwide. Most of the programs rely on serological diagnostics in any phase of the program. For the detection of antibodies against BVD virus (BVDV), neutralization tests as well as a variety of (commercially available) ELISAs are used. Here, test systems applied in various laboratories were evaluated in the context of an international interlaboratory proficiency trial. A panel of standardized samples comprising five sera and five milk samples was sent to veterinary diagnostic laboratories (n=51) and test kit manufacturers (n=3).
Results
The ring trial sample panel was investigated by nine commercially available antibody ELISAs as well as by neutralization tests against diverse BVDV-1, BVDV-2 and/or border disease virus (BDV) strains. The negative serum and milk sample as well as a serum collected after BVDV-2 infection were mostly correctly tested regardless of the applied test system. A serum sample obtained from an animal immunized with an inactivated BVDV-1 vaccine tested positive by neutralization tests or by total antibody or Erns-based ELISAs, while all applied NS3-based ELISAs gave negative results. A further serum, containing antibodies against the ovine BDV, reacted positive in all applied BVDV ELISAs, a differentiation between anti-BDV and anti-BVDV antibodies was only enabled by parallel application of neutralization tests against BVDV and BDV isolates. For the BVDV antibody-positive milk samples (n=4), which mimicked prevalences of 20% (n=2) or 50% (n=2), considerable differences in the number of positive results were observed, which mainly depended on the ELISA kit and the sample incubation protocols used. These 4 milk samples tested negative in 43.6%, 50.9%, 3.6% and 56.4%, respectively, of all investigations. Overall, negative results occurred more often, when a short sample incubation protocol instead of an over-night protocol was applied.
Conclusions
While the seronegative samples were correctly evaluated in most cases, there were considerable differences in the number of correct evaluations for the seropositive samples, most notably when pooled milk samples were tested. Hence, thorough validation and careful selection of ELISA tests are necessary, especially when applied during surveillance programs in BVD-free regions.
Similar content being viewed by others
Background
Bovine viral diarrhea (BVD) is one of the most significant cattle diseases worldwide, as it induces major economic losses and represents a substantial issue on animal welfare [1,2,3,4]. The causative agent, bovine viral diarrhea virus (BVDV), is a pestivirus (family Flaviviridae), which exists in the two distinct species BVDV-1 (syn. Pestivirus A) and BVDV-2 (syn. Pestivirus B) [5]. BVDV is closely related to the other two classical pestivirus species border disease virus (BDV, syn. Pestivirus D) and classical swine fever virus (CSFV, syn. Pestivirus C) [5]. During the last years, further, so-called “atypical” pestiviruses have been described [6,7,8,9,10,11], among them HoBi-like viruses (syn. BVDV-3 or Pestivirus H). Hobi-like viruses were originally isolated from fetal calf serum (FCS), infect cattle and could interfere like e.g. the ovine BDV with BVDV diagnostics because of a genetic and antigenic relatedness [12,13,14,15].
The single-stranded positive-sense RNA genome of BVDV encodes four structural proteins, namely the capsid protein C and the envelope glycoproteins Erns (formerly known as E0), E1 and E2, and at least eight non-structural proteins (Npro, p7, NS2, NS3, NS4A, NS4B, NS5A and NS5B) [16]. The resulting polyprotein is co- and post-translationally processed by cellular and viral proteases into the individual proteins [17]. The immunodominant proteins for the induction of antibody responses are Erns, E2 and the non-structural protein NS3 (also referred to as p80) [18, 19]. Neutralizing antibodies are mainly directed against the glycoprotein E2 [16].
Acutely infected, BVDV-naïve cattle show either none or mild to moderate unspecific clinical signs including diarrhea, fever or pneumonia. However, also severe forms characterized by hemorrhagic syndromes and mucosal disease-like lesions may occur, mainly associated with virulent BVDV-2 strains [20,21,22]. The clinical consequences of BVDV infection of naïve pregnant cows depend on the phase of gestation and could result in stillbirth, abortion or congenital malformation. When infection occurs during the first three month of pregnancy, it could lead in a high percentage to the birth of persistently infected (PI), immunotolerant, life-long viremic calves [23,24,25]. As PI animals are unable to develop a specific immunity against the virus strain they are infected with, they shed enormous amounts of BVDV throughout their lives, which makes them the major source for virus perpetuation within individual cattle herds and spread to BVDV-free holdings [26,27,28,29,30].
Due to their crucial role in the spread of BVDV, PI calves are the major target of disease control programs, which are in place in several countries [28, 31,32,33,34,35]. Despite the common goal of virus eradication from the respective cattle population, different approaches were selected for the programs. While the “Scandinavian model” was based on large-scale milk serology, the “Swiss model” was based on the direct antigen or viral genome testing of all animals without serological pre-screening [34, 36]. The latter proved beneficial especially for regions with a high initial virus prevalence and a high level of cattle trading and transport combined with ongoing vaccination campaigns. The centerpiece of the “Swiss approach”, which was also adopted in e.g. Germany and Ireland [31, 37, 38], is the detection of PI animals as early as possible, mainly by ear-notch based testing of every new-born calf for the presence of viral antigen or genome, and their elimination from the respective cattle population. Once all PI animals are removed, non-vaccinated herds become gradually seronegative, allowing for serology-based monitoring of the disease-free status. In those non-vaccinated, BVDV-free herds, bulk milk serology may be used to screen for virus introduction. As an alternative approach, spot-testing of young animals older than 6 months (to avoid the negative influence of maternally derived antibodies acquired by colostrum intake) could be applied [39,40,41,42,43]. The “Scandinavian model” on the other hand, was directly based on large-scale serology to preselect farms with an elevated risk for the presence of PI animals. Thereafter, all animals from herds with high antibody levels were tested individually for virus genome or antigen and the detected PI animals were removed. Finally, an ongoing serological monitoring was established [34]. Hence, in their final phase, both approaches rely on serology-based monitoring of the disease-free status.
For serological diagnostics of previous BVDV infections, neutralization assays represent the gold standard test, as they offer very high sensitivity and specificity. By neutralization tests, antibodies directed against BVDV-1, BVDV-2 and BDV may be differentiated from each other, despite the serological cross-reactivity that exists between these virus species [44]. However, as neutralization tests, which rely on cell-culture systems, are labor-intensive and time-consuming, commercial ELISA tests are used much more frequently during routine diagnostics, as they allow for a more convenient high throughput testing.
During recent studies, varying and sometimes poor sensitivities were observed for BVDV antibody ELISAs [45,46,47]. Here, test systems used for serological BVDV diagnostics in various laboratories have been evaluated in the context of an international interlaboratory proficiency trial. A panel of standardized and blinded samples was sent to veterinary diagnostic laboratories and test kit manufacturers with the request to analyze the samples by methods routinely applied in the respective institution.
Results
Five individual sera (sample IDs 01/21 to 05/51) and one individual (09/21) and four pooled milk samples (06/21 to 08/21 and 10/21) were sent to the participants of the proficiency trial (Table 1). This sample panel was investigated by 51 veterinary diagnostic laboratories and 3 kit manufacturers by using nine commercially available and one in-house antibody ELISA. The applied test systems are listed in Table 2. In some cases, several ELISA tests or sample incubation protocols were used, whereby 71 result sets were generated for the sera and 55 for the milk samples. In addition or alternatively to the analysis by antibody ELISA, the sera were tested in 28 laboratories by the cell-culture based standard microneutralization test against diverse BVDV-1, BVDV-2 and/or BDV strains
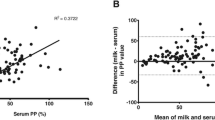
The results generated by the commercial ELISAs are shown in Fig. 1 separately for each test kit.
Results of the commercial BVD antibody ELISAs. Results generated using the respective short incubation protocol are shown in black and results produced by the long sample incubation protocol are depicted in red. Green circles represent results for which the participating laboratory did not indicate the applied protocol. The cut-offs are indicated by horizontal dashed lines (black for short protocol, red for long protocol). When the same cut-off is to be used for both protocols, the line is colored in black. A) Three participants indicated their results in the unit PI%, these results were converted into S/N% for the generation of the figure. Two further participants used another, not further specified unit, these results are not shown
The negative samples (sera 02/21 and 04/21, milk 09/21) were consistently correctly tested negative regardless of the applied test system, with exception of milk sample 09/21 which was tested “positive” in one participating laboratory (Table 2, Figs. 1 and 2). The overall specificity was 99.49% (95% confidence interval [CI] 97.20% to 99.99%). As the participant that tested the sample 09/21 positive did not indicate the unit in the results sheet, an evaluation as to whether the assessment was based on the instructions of the manufacturer and whether it is correct or false was not possible. The serum 01/21, which was taken subsequent to a BVDV-2 infection, tested consistently correctly positive (overall sensitivity for this sample 100.00%, 95% CI 94.94% to 100.00%). However, some discrepancies occurred when analyzing the other antibody-positive samples. The status of the serum 03/21, which originated from an animal immunized with an inactivated BVDV vaccine, was correctly identified as being positive by the neutralization test or by total antibody or E0-based ELISAs, while all applied NS3 (p80)-based ELISAs gave negative results (Table 2, Figs. 1 and 2). The overall sensitivity for this sample when using ELISA systems was 68.27% (95% CI 58.42% to 77.05%), with a sensitivity of 0.00% (95% CI 0.00% to 10.58%) for the NS3 (p80)-based ELISAs and 100.00% (95% CI 90.51% to 100.00%) for total antibody or E0-based ELISAs. The serum 05/21, which was taken after BDV infection, reacted positive in all ELISAs used. A differentiation between BVDV and BDV antibodies was only allowed by parallel application of neutralization tests against BVDV and BDV isolates. When BDV was not included in the virus panel against which the neutralization test was set up, the serum was assessed as BVDV antibody-positive (Fig. 2).
For the BVDV antibody-positive milk samples to be tested, there were in some cases considerable differences in the number of correct results, which depended (1) on the applied ELISA kit, and (2) on the used sample incubation protocol (Fig. 1). The milk samples 06/21 and 08/21, which represented an 1:1 mixture of antibody-positive and -negative individual milk samples (Table 1), have been tested 55 times. The sample 06/21 was tested negative by the participants two times (2/55, 3.6%; sensitivity 96.36%, 95% CI 87.47% to 99.56%) and the sample 8/21 was tested negative 31 times (31/55, 56.4%; sensitivity 43.64%, 95% CI 30.30% to 57.68%). For the milk sample 06/21, both false-negative results were produced by using a short sample incubation protocol (Fig. 1). In an additional case, the sample 06/21 was evaluated positive by one participant, although it did not exceed the cut-off as indicated in the instructions of the kit manufacturer (ELISA: Monoscreen AbELISA BVDV (NS3)/blocking). The incorrect negative results for the milk sample 08/21 were generated by either using a short or unknown incubation protocol (Fig. 1; ELISAs: ID Screen® BVD p80 Antibody Competition, BVDV p80 Ab Test, Svanovir® BVDV-Ab Confirmation) or by applying one of the following ELISA kits: Monoscreen AbELISA BVDV (NS3)/blocking (1/1, 100%), BVDV Total Ab Test (16/16, 100%), Svanovir® BVDV-Ab Screening (2/3, 66.7%), PrioCheckTM Ruminant BVD p80 Ab Serum & Milk Kit (2/2, 100%).
Finally, the milk samples 07/21 and 10/21 simulated a herd prevalence of 20% by merging milk samples obtained from 2 seropositive animals with 8 seronegative milk samples (Table 1). The sample 07/21 tested negative 24 times (24/55, 43.6%; sensitivity 56.36%, 95% CI 42.32% to 69.70%), and the sample 10/21 in 28 cases (28/55, 50.9%; sensitivity 49.09%, 95% CI 35.35% to 62.93%). Again, the negative results were related to a short or unknown incubation period (ELISAs: ID Screen® BVD p80 Antibody Competition, BVDV p80 Ab Test, Svanovir® BVDV-Ab Confirmation) or the following ELISA kits (Fig. 1, Table 2): Monoscreen AbELISA BVDV (NS3)/blocking, BVDV Total Ab Test, Svanovir® BVDV-Ab Screening, PrioCheckTM Ruminant BVD p80 Ab Serum & Milk Kit.
Overall, the pooled milk samples were only tested consistently positive when using the ID Screen® BVD p80 Antibody competition or the Svanovir® BVDV-Ab Confirmation ELISA in combination with the respective long sample incubation period (n= 16 and =2, respectively), or the PrioCheckTM Bovine BVDV Ab Plate Kit (n=1).
Discussion
Serological methods are a key component during the surveillance phase of BVDV control programs, when rising seroprevalences are indicative for a new introduction of one or more PI animals into a herd. Besides, serological methods might be applied during purchase investigation to identify so called “Trojan cows”, i.e. pregnant dams infected during the current gestation and therefore at risk for giving birth to a PI calf. In addition to PI calves, “Trojan cows” are a major cause for virus spread into BVDV-free herds [26,27,28,29, 47,48,49] and need to be identified as early as possible in order to separate them during the parturition period from further pregnant cows. Such “Trojan cows” represent a particular challenge when eliminating BVDV from a given area, as the problem becomes visible only after the birth of the PI calf, which might be several months after the purchase of the pregnant dam. Hence, for their early identification serological methods could be beneficial, provided they are applied regularly and sufficient sensitive tests are used.
In this interlaboratory comparison, individual sera obtained from BVDV-infected animals were generally correctly identified by every ELISA format. However, as reported previously [45, 50,51,52,53,54,55] also in this ring trial NS3-based ELISAs showed lower sensitivities for the serum sample obtained from an animal that has been immunized with an inactivated BVDV vaccine. NS3 is produced in large amounts during virus replication in infected animals or after immunization with live vaccines, thereby inducing the production of antibodies against this non-structural protein. In contrast, inactivated vaccines might contain mainly NS3-free BVD virions and do not replicate in the immunized animals. Therefore, the induction of an antibody response against NS3 relies only on the protein load already present in the vaccine [53]. Hence, sera from vaccinated animals might test negative in NS3-based assays, although high titers of antibodies directed against further proteins are measurable. Thus, for herds with animals vaccinated with inactivated vaccines, the application of total antibody or Erns-based ELISAs or of the neutralization test, which predominantly detects antibodies against the envelope glycoprotein E2, is recommended.
In terms of sample materials, milk is a convenient to collect and cost-effective alternative to serum, given that sampling of sera and analyses of herds by spot testing was the biggest cost driver during the transition from ear notch-based testing to serological surveillance in Switzerland [56]. When compared to the testing of individual sera, the investigation of bulk milk in dairy herds has the advantage that it can be performed more frequently at lower costs. However, lower sensitivities of commercial ELISAs have been reported for milk as sample matrix [46, 54, 57]. Therefore, bulk milk samples would most likely score only positive when a sufficient proportion of cows contributing to the pool seroconverted. This poorer diagnostic sensitivity could be at least partially decreased by routinely using the long incubation protocol of the ELISAs and by using the best performing test systems. As demonstrated in this proficiency trial and previously observed during a study comparing diverse commercial BVDV antibody ELISAs [45], the long incubation protocol often resulted in an increased diagnostic sensitivity compared to the short protocol of the respective test. Therefore, the standard application of a long-term incubation protocol is strongly recommended for the analysis of (bulk) milk samples. Nevertheless, independent of the used test and protocol, regular bulk milk analyses offer the possibility to compare current data to historical results, thereby identifying an increase (or decrease in case of competitive ELISAs) of the S/P% (S/N%) or % inhibition values, which might be indicative for a BVDV infection in the respective herd [58, 59].
It was previously reported that besides the incubation protocol and antigen used for ELISA plate coating, the ELISA format could influence the sensitivity of the respective test. An evaluation of 16 commercial antibody ELISAs suggested that competitive ELISAs show a lower diagnostic sensitivity than indirect tests also for milk samples [45]. Interestingly, the kits that performed best for pooled milk samples in this interlaboratory proficiency trial belong to both categories, as the best performance of all kits, which were used in more than one laboratory, for this sample matrix was achieved by the competitive ID Screen® BVD p80 Antibody Competition ELISA and by the indirect Svanovir® BVDV-Ab Confirmation ELISA, given that the respective long sample incubation protocol was applied.
In addition to the diagnostic sensitivity, the specificity is a key characteristic of diagnostic test systems. In the context of pestiviruses, the serological cross-reactivity of different virus species, e.g. between BVDV and BVD, represents unfortunately a major issue. As shown by the results of the serum sample 05/21, none of the currently applied ELISA test is able to differentiate anti-BVDV from anti-BDV antibodies. BVDV and BDV are closely related and both viruses may be transmitted between cattle and small ruminants, predominantly sheep, when those species are kept together [56, 60]. In Switzerland, for instance, up to 10% of all pestivirus antibody-positive cattle sera were reactive to BDV rather than to BVDV [61, 62]. However, disease control programs are generally restricted to BVDV in Bovidae. To correctly attribute antibodies to one of the virus species, thereby avoiding restrictions and costs for farms in case of BDV instead of BVDV infections, labor-intensive and costly neutralization assays using different virus strains, preferentially adjusted to the epidemiolocal situation in the respective area, are necessary [36].
Conclusions
The presented interlaboratory proficiency trial for serological BVD diagnostics revealed, dependent on the test system and incubation period, considerable differences in the number of correct evaluations for BVDV-seropositive samples, most notably when considering the results obtained for pooled milk samples. Therefore, thorough validation and careful selection of the best performing ELISA tests is highly recommended, especially for laboratories analyzing samples in the context of the surveillance phase of eradication programs or in order to identify pregnant dams at risk for the birth of a PI calf. Here, in the context of an interlaboratory proficiency trial, the best performance for pooled milk samples of all kits, which were used in more than one laboratory, was achieved by the ID Screen® BVD p80 Antibody Competition and Svanovir® BVDV-Ab Confirmation ELISAs performed using the long sample incubation protocol.
Methods
Five sera and five milk samples were sent to the participants, which were asked to investigate these samples with the methods and test systems routinely used in their laboratory. The sera comprised two cattle samples seronegative against pestiviruses (IDs 02/21 and 04/21), a sample taken from a cattle immunized with the inactivated BVDV-1 vaccine Bovilis® BVD-MD (MSD Tiergesundheit, Haar, Germany) (03/21), and sera obtained after experimental infection with BVDV-2 (01/21) or BDV (05/21), respectively. While all sera represented individual samples, the milk samples were prepared to mimic bulk tank milk with seroprevalences of 50% (06/21 and 08/21) and 20% (07/21 and 10/21), respectively. For that, four seropositive milk samples were merged with four negative milk samples or two seropositive with eight negative milk samples. The remaining milk sample (09/10) was seronegative UHT milk. With exception of the long-life milk, corresponding sera were available for all milk samples and they were tested for BVDV-specific antibodies by a standard microneutralization test [63] against BVDV-1 strain NADL. The resulting neutralizing titers are given in Table 1.
Aliquots of 1ml were prepared of all serum and milk samples in 2-ml injection bottles (Zscheile & Klinger GmbH, Hamburg, Germany). Thereafter, the samples were lyophilized and the injection bottles were sealed with rubber plug and flanged caps (Zscheile & Klinger GmbH, Hamburg, Germany). The aliquots were stored at 4°C until sent to the participating institutions.
The ring test sample panel was investigated by a total of 51 veterinary diagnostic laboratories from 15 countries (Austria, Belgium, Denmark, France, Germany, Ireland, Israel, Italy, Latvia, Lithuania, the Netherlands, Poland, Russia, Switzerland, United Kingdom) and three manufacturers of commercial ELISA kits. The following commercial antibody ELISA kits were used by the participating laboratories: Monoscreen AbELISA BVDV (E0)/blocking (Erns (E0)-based; Bio-X Diagnostics S.A., Rochefort, Belgium), Monoscreen AbELISA BVDV (NS3)/blocking (NS3 (p80)-based; Bio-X Diagnostics S.A.), ID Screen® BVD p80 Antibody Competition (NS3 (p80)-based; Innovative Diagnostics, Grabels, France), BVDV Total Ab Test (configured by immobilizing BVDV antigen on the plates; IDEXX, Westbrook, United States), BVDV p80 Ab Test (NS3 (p80)-based; IDEXX), Svanovir® BVDV-Ab Screening (plates coated with non-infectious BVDV antigen; SVANOVA, Uppsala, Sweden), Svanovir® BVDV-Ab Confirmation (plates coated with non-infectious BVDV antigen; SVANOVA), PrioCheckTM Ruminant BVD p80 Ab Serum & Milk Kit (NS3 (p80)-based; Thermo Fisher Scientific, Waltham, United States), PrioCheckTM Bovine BVDV Ab Plate Kit (NS3 (p80)-based; Thermo Fisher Scientific). In one laboratory, an in-house ELISA was applied. For some of the commercial kits two distinct sample incubation protocols are proposed in the kit manual (short incubation protocol: kit-dependent 1 or 2 hours; long incubation protocol: kit-dependent 12 up to 20 hours). Both protocols were applied by the participants. Furthermore, the sera were tested in 28 laboratories by the cell-culture based standard microneutralization test against diverse BVDV-1, BVDV-2 and/or BDV strains.
The sensitivities and specificities as mentioned in the Results section were calculated by using the free statistical calculator MedCalc (MedCalc Software, Ostend, Belgium).
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Abbreviations
- BDV:
-
border disease virus
- BVD:
-
bovine viral diarrhea
- BVDV:
-
bovine viral diarrhea virus
- cp:
-
cytopathic
- CSFV:
-
classical swine fever virus
- FCS:
-
fetal calf serum
- ncp:
-
non-cytopathic
- PI:
-
persistently infected
References
Houe H. Economic impact of BVDV infection in dairies. Biologicals. 2003;31(2):137–43.
Richter V, Lebl K, Baumgartner W, Obritzhauser W, Kasbohrer A, Pinior B. A systematic worldwide review of the direct monetary losses in cattle due to bovine viral diarrhoea virus infection. Vet J. 2017;220:80–7.
Lindberg A, Brownlie J, Gunn GJ, Houe H, Moennig V, Saatkamp HW, Sandvik T, Valle PS. The control of bovine viral diarrhoea virus in Europe: today and in the future. Rev Sci Tech. 2006;25(3):961–79.
Pinior B, Garcia S, Minviel JJ, Raboisson D. Epidemiological factors and mitigation measures influencing production losses in cattle due to bovine viral diarrhoea virus infection: A meta-analysis. Transbound Emerg Dis. 2019;66(6):2426–39.
ICTV: Genus: Pestivirus. Available from: https://talk.ictvonline.org/ictv-reports/ictv_online_report/positive-sense-rna-viruses/w/flaviviridae/361/genus-pestivirus 2019
Kirkland PD, Frost MJ, Finlaison DS, King KR, Ridpath JF, Gu X. Identification of a novel virus in pigs - Bungowannah virus: a possible new species of pestivirus. Virus research. 2007;129(1):26–34.
Vilcek S, Ridpath JF, Van Campen H, Cavender JL, Warg J. Characterization of a novel pestivirus originating from a pronghorn antelope. Virus research. 2005;108(1–2):187–93.
Becher P, Fischer N, Grundhoff A, Stalder H, Schweizer M, Postel A. Complete genome sequence of bovine pestivirus strain PG-2, a second member of the tentative pestivirus species Giraffe. Genome announcements. 2014;2(3):e00376-14.
Firth C, Bhat M, Firth MA, Williams SH, Frye MJ, Simmonds P, Conte JM, Ng J, Garcia J, Bhuva NP, et al. Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York City. MBio. 2014;5(5):e01933-01914.
Jo WK, van Elk C, van de Bildt M, van Run P, Petry M, Jesse ST, Jung K, Ludlow M, Kuiken T, Osterhaus A. An evolutionary divergent pestivirus lacking the N(pro) gene systemically infects a whale species. Emerging microbes & infections. 2019;8(1):1383–92.
Wu Z, Ren X, Yang L, Hu Y, Yang J, He G, Zhang J, Dong J, Sun L, Du J, et al. Virome analysis for identification of novel mammalian viruses in bat species from Chinese provinces. J Virol. 2012;86(20):10999–1012.
Schirrmeier H, Strebelow G, Depner K, Hoffmann B, Beer M. Genetic and antigenic characterization of an atypical pestivirus isolate, a putative member of a novel pestivirus species. J Gen Virol. 2004;85(Pt 12):3647–52.
Bauermann FV, Ridpath JF, Dargatz DA. A serosurvey for ruminant pestivirus exposure conducted using cattle sera collected for brucellosis surveillance in the United States. J Vet Diagn Invest. 2017;29(1):76–82.
Decaro N, Lucente MS, Mari V, Sciarretta R, Pinto P, Buonavoglia D, Martella V, Buonavoglia C. Hobi-like pestivirus in aborted bovine fetuses. J Clin Microbiol. 2012;50(2):509–12.
Mosena ACS, Cibulski SP, Weber MN, Silveira S, Silva MS, Mayer FQ, Roehe PM, Canal CW. Genomic and antigenic relationships between two ’HoBi’-like strains and other members of the Pestivirus genus. Arch Virol. 2017;162(10):3025–34.
Tautz N, Tews BA, Meyers G. The molecular biology of pestiviruses. Adv Virus Res. 2015;93:47–160.
Lindenbach BD, Murray CL, Thiel HJ, Rice CM: Flaviviridae. In: In: Knipe, DM, Howley, PM (Eds), Fields Virology Lippincott Williams and Wilkins, Philadelphia, PA. edn.; 2013: 712.
Donis RO, Dubovi EJ. Glycoproteins of bovine viral diarrhoea-mucosal disease virus in infected bovine cells. J Gen Virol. 1987;68(Pt 6):1607–16.
Weiland E, Ahl R, Stark R, Weiland F, Thiel HJ. A second envelope glycoprotein mediates neutralization of a pestivirus, hog cholera virus. J Virol. 1992;66(6):3677–82.
Brodersen BW. Bovine viral diarrhea virus infections: manifestations of infection and recent advances in understanding pathogenesis and control. Veterinary pathology. 2014;51(2):453–64.
Ridpath JF. Practical significance of heterogeneity among BVDV strains: Impact of biotype and genotype on U.S. control programs. Preventive Veterinary Medicine. 2005;72(1–2):17–30.
Lanyon SR, Hill FI, Reichel MP, Brownlie J. Bovine viral diarrhoea: pathogenesis and diagnosis. Vet J. 2014;199(2):201–9.
Baker JC. The clinical manifestations of bovine viral diarrhea infection. The Veterinary clinics of North America Food animal practice. 1995;11(3):425–45.
Brownlie J. The pathogenesis of bovine virus diarrhoea virus infections. Rev Sci Tech. 1990;9(1):43–59.
Brock KV. The persistence of bovine viral diarrhea virus. Biologicals. 2003;31(2):133–5.
Ezanno P, Fourichon C, Seegers H. Influence of herd structure and type of virus introduction on the spread of bovine viral diarrhoea virus (BVDV) within a dairy herd. Veterinary research. 2008;39(5):39.
Bitsch V, Hansen KE, Ronsholt L. Experiences from the Danish programme for eradication of bovine virus diarrhoea (BVD) 1994–1998 with special reference to legislation and causes of infection. Veterinary microbiology. 2000;77(1–2):137–43.
Moennig V, Becher P. Control of bovine viral diarrhea. Pathogens (Basel, Switzerland). 2018;7(1):29.
Akagami M, Seki S, Kashima Y, Yamashita K, Oya S, Fujii Y, Takayasu M, Yaguchi Y, Suzuki A, Ono Y, et al. Risk factors associated with the within-farm transmission of bovine viral diarrhea virus and the incidence of persistently infected cattle on dairy farms from Ibaraki prefecture of Japan. Res Vet Sci. 2020;129:187–92.
Tråvén M, Alenius S, Fossum C, Larsson B. Primary bovine viral diarrhoea virus infection in calves following direct contact with a persistently viraemic calf. Zentralbl Veterinarmed B. 1991;38(6):453–62.
Wernike K, Gethmann J, Schirrmeier H, Schröder R, Conraths FJ, Beer M. Six years (2011–2016) of mandatory nationwide bovine viral diarrhea control in Germany - A success story. Pathogens (Basel, Switzerland). 2017;6(4):50.
Moennig V, Houe H, Lindberg A. BVD control in Europe: current status and perspectives. Anim Health Res Rev. 2005;6(1):63–74.
Stahl K, Alenius S. BVDV control and eradication in Europe - an update. The Japanese journal of veterinary research. 2012;60(Suppl):S31-39.
Moennig V, Becher P. Pestivirus control programs: how far have we come and where are we going? Anim Health Res Rev. 2015;16(1):83–7.
Hodnik JJ, Acinger-Rogić Ž, Alishani M, Autio T, Balseiro A, Berezowski J, Carmo LP, Chaligiannis I, Conrady B, Costa L, et al. Overview of cattle diseases listed under category C, D or E in the Animal Health Law for which control programmes are in place within Europe. Frontiers in veterinary science. 2021;8: 688078.
Schweizer M, Stalder H, Haslebacher A, Grisiger M, Schwermer H, Di Labio E. Eradication of bovine viral diarrhoea (BVD) in cattle in Switzerland: Lessons taught by the complex biology of the virus. Frontiers in veterinary science. 2021;8: 702730.
Graham DA, Lynch M, Coughlan S, Doherty ML, O’Neill R, Sammin D, O’Flaherty J. Development and review of the voluntary phase of a national BVD eradication programme in Ireland. The Veterinary record. 2014;174(3):67.
Clegg TA, Graham DA, O’Sullivan P, McGrath G, More SJ. Temporal trends in the retention of BVD+ calves and associated animal and herd-level risk factors during the compulsory eradication programme in Ireland. Prev Vet Med. 2016;134:128–38.
Booth RE, Brownlie J. Comparison of bulk milk antibody and youngstock serology screens for determining herd status for Bovine Viral Diarrhoea Virus. BMC Vet Res. 2016;12(1):177.
Houe H. Bovine virus diarrhea virus - Detection of Danish dairy herds with persistently infected animals by means of a screening-test of 10 young stock. Prev Vet Med. 1994;19(3–4):241–8.
Pritchard G: Milk antibody testing in cattle. In Practice 2001, 23(9):542-+.
Houe H. Serological analysis of a small herd sample to predict presence or absence of animals persistently infected with bovine viral diarrhoea virus (BVDV) in dairy herds. Res Vet Sci. 1992;53(3):320–3.
Valle PS, Wayne Martin S, Skjerve E. A Bayesian approach to estimating the performance of a bovine virus diarrhoea virus (BVDV) antibody ELISA bulk-tank milk test. Prev Vet Med. 2001;50(1–2):71–87.
Ridpath JF, Fulton RW, Kirkland PD, Neill JD. Prevalence and antigenic differences observed between Bovine viral diarrhea virus subgenotypes isolated from cattle in Australia and feedlots in the southwestern United States. J Vet Diagn Invest. 2010;22(2):184–91.
Hanon JB, De Baere M, De la Ferte C, Roelandt S, Van der Stede Y, Cay B. Evaluation of 16 commercial antibody ELISAs for the detection of bovine viral diarrhea virus-specific antibodies in serum and milk using well-characterized sample panels. J Vet Diagn Invest. 2017;29(6):833–43.
Wernike K, Beer M. Diagnostics in the context of an eradication program: Results of the German bovine viral diarrhea proficiency trial. Veterinary microbiology. 2019;239: 108452.
Albrecht K, Linder M, Heinrich A, Höche J, Beer M, Gaede W, Wernike K. Re-introduction of bovine viral diarrhea virus in a disease-free region: Impact on the affected cattle herd and diagnostic implications. Pathogens (Basel, Switzerland). 2021;10(3):360.
Reardon F, Graham DA, Clegg TA, Tratalos JA, O’Sullivan P, More SJ. Quantifying the role of Trojan dams in the between-herd spread of bovine viral diarrhoea virus (BVDv) in Ireland. Prev Vet Med. 2018;152:65–73.
Van Duijn L, Santman-Berends I, Biesheuvel M, Mars J, Waldeck F, van Schaik G. Why test purchased cattle in BVDV control programs? Frontiers in veterinary science. 2021;8: 686257.
González AM, Arnaiz I, Yus E, Eiras C, Sanjuán M, Diéguez FJ. Evaluation of long-term antibody responses to two inactivated bovine viral diarrhoea virus (BVDV) vaccines. Vet J. 2014;199(3):424–8.
Alvarez M, Donate J, Makoschey B. Antibody responses against non-structural protein 3 of bovine viral diarrhoea virus in milk and serum samples from animals immunised with an inactivated vaccine. Vet J. 2012;191(3):371–6.
Graham DA, German A, Mawhinney K, Goodall EA. Antibody responses of naive cattle to two inactivated bovine viral diarrhoea virus vaccines, measured by indirect and blocking ELISAS and virus neutralisation. The Veterinary record. 2003;152(26):795–800.
Raue R, Harmeyer SS, Nanjiani IA. Antibody responses to inactivated vaccines and natural infection in cattle using bovine viral diarrhoea virus ELISA kits: assessment of potential to differentiate infected and vaccinated animals. Vet J. 2011;187(3):330–4.
Hanon JB, De Baere M, de la Ferte C, Roelandt S, Guillot G, Van der Stede Y, Cay B. Serological monitoring on milk and serum samples in a BVD eradication program: A field study in Belgium showing antibody ELISA performances and epidemiological aspects. Prev Vet Med. 2018;160:136–44.
Makoschey B, Sonnemans D, Bielsa JM, Franken P, Mars M, Santos L, Alvarez M. Evaluation of the induction of NS3 specific BVDV antibodies using a commercial inactivated BVDV vaccine in immunization and challenge trials. Vaccine. 2007;25(32):6140–5.
Braun U, Hilbe M, Peterhans E, Schweizer M. Border disease in cattle Vet J. 2019;246:12–20.
Foddai A, Enøe C, Stockmarr A, Krogh K, Uttenthal Å. Challenges for bovine viral diarrhoea virus antibody detection in bulk milk by antibody enzyme-linked immunosorbent assays due to changes in milk production levels. Acta Vet Scand. 2015;57(1):32.
Beaudeau F, Assié S, Seegers H, Belloc C, Sellal E, Joly A. Assessing the within-herd prevalence of cows antibody-positive to bovine viral diarrhoea virus with a blocking ELISA on bulk tank milk. The Veterinary record. 2001;149(8):236–40.
Lanyon SR, McCoy R, Bergman E, Reichel MP. Milk as a diagnostic sample for a commercially available ELISA to identify bovine viral diarrhoea (BVD) antibodies in dairy herds. Aust Vet J. 2014;92(7):269–73.
Braun U, Bachofen C, Buchi R, Hassig M, Peterhans E. Infection of cattle with border disease virus by sheep on communal alpine pastures. Schweizer Archiv fur Tierheilkunde. 2013;155(2):123–8.
Huser AF, Schär JG, Bachofen C, de Martin E, Portmann J, Stalder H, Schweizer M. Benefit of bovine viral diarrhoea (BVD) eradication in cattle on pestivirus seroprevalence in sheep. Frontiers in veterinary science. 2021;8: 681559.
Kaiser V, Nebel L, Schupbach-Regula G, Zanoni RG, Schweizer M. Influence of border disease virus (BDV) on serological surveillance within the bovine virus diarrhea (BVD) eradication program in Switzerland. BMC Vet Res. 2017;13(1):21.
Friedrich-Loeffler-Institut: Official collection of test methods for bovine viral diarrhea. Available online: https://www.openagrar.de/receive/openagrar_mods_00056479. 2021.
Acknowledgements
We thank Bianka Hillmann for excellent technical assistance, Juliane Rieger and Christina Ries for the help in sample preparation and especially all the diagnostic laboratories for their efforts in participating in this BVD ring trial.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was financially supported by the Animal Disease Funds (Tierseuchenkassen) of the German federal states Lower Saxony, Thuringia, Hesse, Rhineland-Palatinate, North Rhine-Westphalia and by the Ministry of Energy, Agriculture, the Environment, Nature and Digitalization of the German federal state Schleswig-Holstein. The funders played no role in the design, analysis and reporting of the study.
Author information
Authors and Affiliations
Contributions
Conceptualization, K.W. and M.B.; methodology, K.W.; formal analysis, K.W.; writing—original draft preparation, K.W.; writing—review and editing, M.B.; visualization, K.W.; funding acquisition, K.W. and M.B. Both authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The BVDV-1 antibody-positive serum originated from a vaccination study, which was evaluated by a state ethics commission and approved by the competent authority (State Office for Agriculture, Food Safety and Fisheries of Mecklenburg-Vorpommern, Rostock, Germany; permission number 7221.3-1.1-075/10). The BVDV-2 and BDV antibody-positive sera were generated in a study aiming at the production of diagnostic antisera (permission number LVL-MV 310-4/7221.3-2.1-015/01). The milk samples were taken by the responsible farm veterinarians in the context of the health monitoring program of the respective farms, no ethics approval was necessary to use these specimens.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wernike, K., Beer, M. International proficiency trial for bovine viral diarrhea virus (BVDV) antibody detection: limitations of milk serology. BMC Vet Res 18, 168 (2022). https://doi.org/10.1186/s12917-022-03265-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-022-03265-w