Abstract
Reactive oxygen species (ROS) are reactive compounds derived from oxygen. In biological systems, an excessive amount of ROS can cause oxidative damage to biological macromolecules being involved in different diseases. Several assays have been developed in the last 30 years for ROS evaluation. The objective of this article will be to provide an update about the spectrophotometric methods currently used in the assessment of ROS in serum. The chemical basis of four different techniques will be reviewed, and examples of their possible applications will be provided. A particular emphasis about the practical applications of these assays in the dog will be made, but selected information about their use in humans will also be presented for comparative purposes, following a One-Health approach. The information about the spectrophotometric assays presented in this paper should be interpreted with caution once limited information about them is available yet, and further studies should be performed to clarify what they measure and their clinical application. Ideally, when applied to evaluate a sample’s oxidative status, they should be incorporated in a panel of analytes where other oxidants, antioxidants, and biomarkers of inflammation were also included.
Similar content being viewed by others
Background
Oxidants and antioxidants are produced by living organisms in their metabolic activity. The balance between the two is tightly regulated, and it is essential for maintaining cellular and biochemical functions. An unbalance between oxidant production and antioxidants in favour of the former, leading to cellular signalling disruption and chain reactions, is defined as oxidative stress [1].
Oxidants are compounds generated endogenously as a result of aerobic metabolism in physiological conditions [1]. They can have a physiological role since, during inflammation, they are produced by neutrophils and macrophages for the destruction of pathogens; however, if the redox homeostasis is disrupted and oxidants are produced at too high levels, they can produce tissue damage and contribute to disseminating the inflammation [2,3,4].
Antioxidants are natural or synthetic molecules that protect a biological target against oxidative damage. They act by preventing the uncontrolled production of oxidants, intercepting their reactions with biological structures, and repairing the damage caused by oxidative stress. They can be endogenously synthesised, which can be enzymes such as superoxide dismutase, catalase, and the glutathione peroxidase/glutathione reductase system, or non-enzymatic compounds such as peroxiredoxins, ceruloplasmin, ferritin, and albumin. But also there are exogenous or diet-derived antioxidants such as tocopherols, carotenes, ascorbate, and some minerals (e.g., Zn, Mn, Se). Exogenous antioxidants act synergistically with the endogenous ones; however, it has been described that endogenous defences are more protective [2, 5,6,7,8,9].
If the antioxidant system can not counterbalance an excessive production of oxidants, these may indiscriminately target and produce damage to proteins, lipids, polysaccharides, and DNA [2, 10]. These oxidant compounds produced include those derived from the oxygen, called reactive oxygen species (ROS) and those derived from other molecules different from oxygen: reactive nitrogen species (RNS) as nitric oxide and nitric peroxide, reactive carbon species (RCS), and reactive sulphur species (RSS) [11]. This review will focus on ROS compounds, the biomarkers currently most frequently used for evaluating the oxidant status in both animals and humans.
Concept of ROS
ROS is a collective term used to describe oxygen-derived small and reactive molecules. Those include free radicals (molecules containing one or more free electrons), such as superoxide (O2•−), hydroxyl (OH•), peroxyl (ROO•), and alkoxyl (RO•), and nonradicals molecules (with paired electrons) such as singlet oxygen (1O2), hydrogen peroxide (H2O2), organic peroxides (ROOH, hydroperoxides), and ozone (O3), among others (Fig. 1) [4, 12,13,14]. These nonradicals molecules can produce oxidation “per se” or can also be converted into free radicals.
The most important source of ROS in cells is probably the mitochondrial electron-transport chain, but they can also be generated in different cellular locations, such as the endoplasmic reticulum or nucleus. In addition, some ROS such as ROOH can also be formed after the oxidation of different compounds such as lipids, proteins or DNA [15, 16].
The biological lifetime of each ROS is different (Table 1) [17,18,19,20]. For example, although O2•− has a half-life of seconds, ROOH derived from proteins (PrOOH), in the absence of light, heat, reducing agents, and metal ions that can degrade them [21, 22], were stable during 2 h at 37 °C in neutral aqueous solutions [23].
ROS can contribute to different physiological functions, especially in the immune system, such as controlling fibroblast proliferation and differentiation or proper folding and maturation of immunoglobulins [15, 16]. However, as previously stated to oxidant compounds, ROS can become toxic and cause damage to biomolecules when their concentrations are uncontrolled, a situation associated with several diseases in animals and humans [12, 24,25,26,27].
Evaluation of ROS
ROS, particularly the free radical molecules, are difficult to quantify in biological fluids due to their high reactivity [28,29,30]. Most of them persist for only a short time in vivo and cannot be measured directly [11]. Thus, for accurate detection and characterisation of ROS, complex techniques such as electron spin resonance, spin-trapping, or pulse radiolysis should be used [31,32,33]. These techniques can be labour-intensive and time-consuming, and they may also require sophisticated and expensive instrumentation, facts that limit their general use [34].
As an alternative, ROS can be estimated by the products generated during the damage that they can produce to the different biomolecules [11, 35].Some examples of these products are F2-isoprostanes, malondialdehyde (MDA), and ROOH derived from lipids (LOOH) as the phosphatidyl-choline hydroperoxide (PcOOH), that are compounds produced during the lipid damage; or 8-hydroxy-2′-deoxyguanosine produced in case of DNA damage. They can be measured acurately by gas or high-performance liquid chromatography (HPLC) techniques involving post-column chemiluminescence detection, reductive-mode electrochemical, or coupling to a tandem mass spectrometry, although commercially ELISA kits are also available to their estimation [36,37,38,39,40,41,42].
In the two above-described situations, these techniques used are complex and difficult to be used in routine high throughput analysis. Therefore, spectrophotometric assays, which are more simple and easier to set up, have been developed and used to estimate ROS. Possibly the most known assays in this group are the thiobarbituric acid reactive substances (TBARS) or advanced oxidation protein products (AOPP) that evaluate some compounds produced during lipid and protein oxidation, respectively. TBARS is considered an unspecific technique for MDA determination and can produce false increases of MDA generated by the heating step of the assay and also by the interaction with a variety of other compounds, like bile pigments, saturated and unsaturated aldehydes, sucrose, amino acids, and urea [43,44,45,46,47,48,49]. The AOPP assay measures oxidatively modified albumin and di-tyrosine containing cross-linked proteins [50]. Despite their limitations, both assays are still widely used because of their simplicity [11, 51].
In addition to TBARS and AOPP, other spectrophotometric assays that have not been so widely studied can also measure ROS molecules, including those produced during oxidative damage.
In this review, the focus will be on these later assays, which have been less studied and used in general. It should be noted that these spectrophotometric techniques have two main general limitations:
-
They do not measure all the ROS molecules, and they are not specific to individual ROS. Therefore, they can just be used to estimate the ROS concentration in the sample [52,53,54].
-
When applied in serum or plasma, the ROS compounds with a short half-life possibly have disappeared from the sample, and these assays probably will only measure the most stable ones, such as H2O2 and ROOH. Therefore, the spectrophotometric assays will estimate the more stable ROS in serum or plasma after blood processing.
Objectives and aspects to cover in this review
The objective of this article will be to provide an update about the spectrophotometric techniques, different to TBARS and AOPP, that can be currently used for the assessment of ROS in serum. To the author’s knowledge, there is a published review of different spectrophotometric assays that can be used in canine serum for the measurement of total antioxidant capacity (TAC) [55]; however, there are no similar reviews about the spectrophotometric evaluation of ROS.
Overall, four different spectrophotometric methods will be presented, and each of them will be described: (1) the chemical basis, (2) their advantages and drawbacks, (3) studies and applications in dogs, and (4) selected information from the human side for comparative purposes. A particular emphasis on the dog will be given in this review; since in this species, there is evidence that different infectious, parasitic, metabolic diseases and other conditions such as stress and ageing are associated with oxidative stress [56,57,58,59,60,61,62]. Therefore there is a growing interest in studying oxidative stress in the dog from a clinical perspective. In addition, this species is gaining importance as an experimental model to study human diseases and biological processes related to oxidative stress [63]. Additionally, we will also provide selected information about reports in humans for comparative purposes, following a One-Health approach. It is expected that this review will be of use for researchers in bioveterinary sciences and could help to better use and interpretation of ROS measurements.
Main text
Total oxidant status measurement based on ferrous ion–o-dianisidine complex (TOS-dianisidine) assay
This assay, also named “total oxidant status”, measures mainly the H2O2 and LOOH [34]. In a dose-response study, the assay gave linear and appropriate responses with H2O2, t-butyl (t-Bu-OOH) and cumene ROOH (Cu-OOH) pure solutions [34]; therefore, it could measure at least these compounds in serum.
The reaction’s basis consists of the oxidation of Fe2+ by ROS of the sample. This yields F3+ and OH•/RO• in an acid reaction mixture containing ferrous sulphate and o-dianisidine diluted in H2SO4 . These Fe3+ can be detected by using the dye xylenol orange (XO; o-cresolsulfonphthalein-3′,3″-bis(methyliminodiacetic acid sodium salt)), which binds Fe3+ forming a complex that absorbs between 540 and 580 nm (Fig. 2) [34, 64, 65]. The TOS-dianisidine assay is commonly calibrated with H2O2, and the results are expressed as μmol/L H2O2.
In this assay, the oxidation reaction rate is enhanced by using glycerol molecules. Besides, the inclusion of o-dianisidine allows a prolonged lifetime of reagents and the prevention of serum proteins’ precipitation during the reaction period, making the assay suitable for routine clinical analysis and easy to adapt to automated analysers [34].
TOS-dianisidine showed adequate stability when serum samples of dogs were stored at − 80 °C for a year [66]. However, it showed low stability with canine samples stored at 25 °C for 24 h, at 4 °C for 72 h and at − 20 °C for a year [66]. In human samples, the serum concentrations were not affected by storage at 4 °C for 1 day or at − 80 °C for 3 months [34].
Advantages and drawbacks
The TOS-dianisidine assay has some advantages [34]:
-
it is quick and easy to perform,
-
it is precise,
-
there are commercially available kits for its measurement,
-
the reagents are easy to prepare, and their lifetime is prolonged,
-
it can be easily automated.
However, the assay presents some drawbacks:
-
haemolysis and bilirubin interfere with the reaction,
-
EDTA inhibited the colour formation,
-
o-dianisidine is a carcinogenic and toxic substance.
Studies in dogs
The results from studies that determined serum TOS-dianisidine in dogs are shown in Table 2. This table shows that TOS values could differ depending on the surgical procedure [67,68,69] and that they decreased after anaesthesia [68, 70]. TOS-dianisidine was increased in dogs with sarcoptic mange, canine monocytic ehrlichiosis, leishmaniosis and anaemia compared to healthy dogs [71,72,73,74]. However, no difference in this assay was observed between different clinical leishmaniosis presentations and before and after treatment against canine leishmaniosis [74, 75], and pneumoperitoneum and hyperbaric oxygen therapy did not produce significant changes [76, 77].
Studies in humans
TOS-dianisidine changes in human patients depending on the surgical procedure [78, 79] and anaesthesia [79]. In addition, their concentrations increased in patients suffering from major endemic zoonoses such as tuberculosis and acute brucellosis [80, 81] and decreased after bacterial meningitis treatment in children [82] (See Additional file 1: Table S1).
Ferric-xylenol orange (FOX) assay
According to previous reports, the FOX assay could measure at least the following four ROS molecules: H2O2, linoleic ROOH (Lo-OOH), t-Bu-OOH and Cu-OOH [83, 84].
The first version of the FOX assay (FOX I) was based on the oxidation of Fe2+ to Fe3+ in acidic solution by ROS compounds present in the sample and its detection by XO (Fig. 3) [64, 65, 85, 86]. The Fe3 + −XO complex sign is read against known concentrations of H2O2 or t-Bu-OOH [85, 87]. The main differences of this assay with TOS-dianisidine are that the o-dianisidine and glycerol compounds are not used here, and that this assay includes incubation periods of 30 min minimum and centrifugation steps [85]. Although sorbitol has been used to stimulate the chain reaction of Fe2+ [84, 85], it should be pointed out that it causes extensive peroxidation of lipid in the FOX assay itself, leading to a false signal [84, 88, 89]. An automatic version of FOX I, in which protein precipitation and centrifugation step are avoided, has been validated [87]. In addition, iron D-gluconate was used instead of ferrous ammonium sulphate to improve the reagents’ stability [87].
As FOX I was not suitable for measuring low levels of LOOH, a second and improved version (FOX II) was developed, which included a butylated hydroxytoluene/methanol system. This allowed a better measurement of ROOH - including LOOH - in plasma samples [90]. The adaptation of the assay to automated analysers improved its use [26, 71, 91, 92]. FOX was stable when serum samples of dogs were stored at − 80 °C for a year [66], and in plasma of humans was stable for at least 1 month when stored at − 20 °C [87]. However, FOX results in serum of dogs increased after storage at 25 °C for 72 h and at − 20 °C for 360 days [66].
Advantages and drawbacks
The FOX assay has as advantages [86, 87]:
-
it can be automated,
-
the FOX II allowed the measurement of LOOH.
On the other hand, the assay also presents some drawbacks [34, 86, 87]:
-
the reagent used in the manual version shows a continuous darkening of the solution, making it stable only for less than 6 h,
-
the assay could require a centrifugation step depending on the version,
-
the ascorbic acid and other compounds that bind Fe3+ through competition with XO (e.g., desferrioxamine, diethylenetriaminepentaacetic acid, ethylenediaminetetraacetic acid) interfere with the reaction,
-
it is influenced by haemolysis,
-
blood collected on EDTA is unsuitable for analysis.
Studies in dogs
FOX results from previous studies can be found in Table 3. FOX was significantly higher in dogs with sarcoptic mange [71], idiopathic inflammatory bowel disease [26] and atopic dermatitis [92] when compared to healthy dogs. However, no difference was observed between dogs with canine monocytic ehrlichiosis and healthy dogs [91].
Studies in humans
FOX was significantly increased in patients with various diseases, including idiopathic dilated cardiomyopathy, epilepsy [93, 94], end-stage renal disease [95], human immune deficiency virus (HIV) [96], hepatitis C [97] and malaria [98]. In addition, it decreased significantly after therapy against HIV [96] and malaria [98]. FOX has also been measured to evaluate the effect of different anaesthetic procedures and in patients with brucellosis and tuberculosis [79,80,81] (See Additional file 1: Table S2).
Reactive oxygen metabolites derived compounds (d-ROMs) assay
The d-ROMs assay measures the ROOH and H2O2, although the exact ROS components that measures have not been described yet [99]. This test is based on Fenton’s reaction, which consists of indirect estimation of total ROOH in a solution test by monitoring N,N-dyethyl-paraphenyldiamine radical cation (DEPPD•−) concentration. This radical cation originates from the diamine oxidation by ROO• and RO• that result from the reaction between peroxides present in the sample and the iron ions (Fe2+, Fe3+) released by the proteins in the acidic medium [100]. Such radicals are then trapped by alchilamine present in the reaction medium [100, 101]. The concentrations of these newly formed radicals (DEPPD•−), which have a pink colour, are measured at 505 nm, and they are directly proportional to the peroxides present in the sample (Fig. 4). The d-ROMs results are expressed in arbitrary units, the Carratelli Units (U. CARR.), which are the difference between absorbances multiplied by 10,000. It has been found that 1 U. CARR. corresponds to 0.08 mg/100 mL H2O2 [99, 100].
The concentrations of d-ROMs were stable in human serum samples when they were stored at 4 °C for 24 h and at − 80 °C for 3 months [102]. However, the validity of this assay has been questioned. Previous studies demonstrated that d-ROMs, in a dose-response study, gave no response with H2O2, t-Bu-OOH and Cu-ROOH pure solutions [34]. In addition, it has been shown that ceruloplasmin is a potential source of the signal detected by the test in serum from different species (mammals and birds), together with other compounds such as iron, albumin, and thiol [103, 104].
Advantages and drawbacks
The advantages are [99, 101, 102, 105]:
-
it is simple, quick, inexpensive, and easy to set up,
-
there are commercially available kits for its measurement,
-
it can be adapted to automated biochemistry analysers.
Nonetheless, it also has some drawbacks [34, 100, 101, 103].
-
the presence of ferroxidase enzyme (ceruloplasmin) in the sample could lead to false higher results. That is an abundant compound in serum, which could increase during inflammation,
-
it is influenced by haemolysis.
Studies in dogs
The studies that have measured d-ROMs in serum samples of dogs are shown in Table 4. Briefly, this table shows that d-ROMs values could change depending on the oestrus cycle phase, after exercise and with antibiotic therapy after surgery [106,107,108,109,110]. D-ROMs decreased after antioxidant diet and increased in dogs with lymphoma and mast cell tumours [111,112,113,114]. On the other hand, differences were observed when dogs with Leishmania were compared with healthy dogs [115].
Studies in humans
Previous studies showed that d-ROMs increased in serum of patients with infections, arthritis, allergies, obesity, cancer, and metabolic disease compared with healthy subjects [101, 116] and that patients with chronic gastritis could have lower d-ROMs when ascorbic acid is supplemented [117]. Also, it was described that sex does not influence their concentrations, but age might affect them [102] (See Additional file 1: Table S3).
Peroxide-activity (POX-act) assay
This assay measures total ROOH [101]. In addition, in previous reports, the POX-Act reacted with H2O2, t-Bu-OOH pure solutions [34], indicating that it should measure at least these molecules in serum or plasma samples.
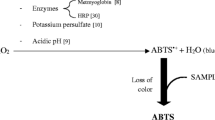
The POX-Act test is based on the oxidation of the chromogen substrate 3,5,3′5’-tetramethylbenzidine (TMB) by the reaction produced between the horseradish peroxidase (HRP) added in the solution and some of the ROS present in the sample (Fig. 5) [118]. Blue coloured products corresponding to the TMB cation free radical (absorbance maximum at 653 nm) are generated [118]. Results are calculated from the standard linear curve using known H2O2 concentrations by subtracting the first absorbance reading from the second [101].
Advantages and drawbacks
The POX-Act system has some advantages [119]:
-
there are commercially available kits for its measurement,
-
it is easy to perform,
-
the TMB oxidation products present high stability at acid pH,
-
the HRP is active over a wide pH range.
On the other hand, it also shows drawbacks such as:
-
incubation of 20 min is needed, which limits automation,
-
there is no available information about its stability during different times and storage conditions.
Studies in dogs
To the author’s knowledge, there are no studies on its use in serum samples of dogs.
Studies in humans
POX-Act was increased in patients after coronary intervention and in those with infections, arthritis, allergies, obesity, and metabolic disease [101, 120]. In addition, oral α-tocopherol supplementation in patients during haemodialysis [121] and rosuvastatin treatment [122] could be related to decreased POX-Act concentrations (See Additional file 1: Table S4).
Comparative studies
A few studies have compared different spectrophotometric assays in the same clinical situations. Overall, it could be pointed out that:
-
No correlation among TOS-dianisidine, d-ROMs and POX-Act, was found in humans in between healthy individuals and in osteoarthritis patients [34, 101]. This fact could be due to different factors. One could be because each specific assay could measure compounds that are not measured by the other assays. Besides, different values were obtained when pure solutions of H2O2, t-Bu-OOH and Cu-OOH were tested by the three assays [34], which could indicate that they can have different sensitivity to detect specific compounds. In addition, the different effects that factors such as haemolysis or lipemia could have in the assays might also contribute to these divergences.
-
TOS-dianisidine and FOX showed similar results when dogs with sarcoptic mange were compared with healthy dogs [71]. In the same way, both assays were significantly correlated in human studies [34]. This fact could be explained because they have a similar chemical basis.
-
Dogs with ehrlichiosis did not show significant serum FOX changes when compared with healthy dogs; nevertheless, higher ROS values were found when a luminol-based chemiluminescence assay was used [91].
It is important to highlight that none of these assays can be defined as specific for ROS. In order to gain knowledge about these techniques, several authors recommend the use of integrated panels including various assays to increase the information about their behaviour in the diverse clinical situations [34, 101, 104, 123].
Other techniques that could be potentially used for the estimation of ROS in serum samples
Chemiluminescence techniques can be used for ROS estimation. They are based on detecting a light emission generated during the oxidation reaction between a chemilumigenic compound, such as luminol, and the different ROS present in the sample [124,125,126,127].
The luminol, for example, allows the detection of both extra- and intracellular levels of different ROS such as H2O2, O2•−, and OH• [128]. Although applied to serum or plasma, this technique would not detect the reactive species with short half-lives such as O2•− and OH• and could potentially estimate other more stable compounds such as H2O2. Using a luminol-based chemiluminescence assay, dogs with clinical and subclinical monocytic ehrlichiosis and idiopathic inflammatory bowel disease presented higher ROS concentrations than healthy dogs [26, 91]. Besides, it has been shown that luminol-based chemiluminescence results are stable in canine serum samples stored at 25 °C for 6 h, at 4 °C during 24 h, and for 60 days at − 20 °C and − 80 °C [66]. However, it is not clear which ROS have been measured when this technique was applied in the serum of dogs, and more studies are needed to clarify that.
Future directions
There are some aspects that should be studied in more detail in the future and would allow better use and interpretation of these assays, such as:
-
the different ROS measured by each assay,
-
the clinical value of the different spectrophotometric assays to evaluate ROS in serum.
In addition, further studies comparing the different spectrophotometric assays between them and other biomarkers of oxidative stress such as antioxidants, trace elements, individual oxidants, and inflammation markers in different diseases would be recommended. It would help to gain knowledge about the interpretation of these assays in clinical situations and determine which assay or assays combinations could be more helpful in the management and treatment monitoring in selected diseases.
Conclusions
Spectrophotometric assays can be used to estimate the more stable ROS in serum such as H2O2 and ROOH and provide information about oxidative status. Most of them can set up at the laboratories without the need for high-cost equipment or reagents and, together with data from a set of tests including other markers of oxidative stress, such oxidants and antioxidants, trace elements, and acute-phase proteins , can be potentially used as a tool to help in the identification and monitoring of oxidative stress associated with diseases.
However, these assays have technical drawbacks which should be considered when used. Also, when they are applied in serum or plasma, they can not measure all ROS that the sample initially had; since many of them, due to the high reactivity, have a short half-life and would disappear from the sample during its handling. In addition, studies to determine the different reactive species that each assay measures should be encouraged to make more appropriate their use and clinical interpretation.
Availability of data and materials
Not applicable.
Abbreviations
- 1O2 :
-
singlet oxygen
- AOPP:
-
advanced oxidation protein products
- Cu-OOH:
-
cumene hydroperoxide
- DEPPD•− :
-
N,N-dyethyl-paraphenyldiamine radical cation
- d-ROMs:
-
reactive oxygen metabolites derived compounds
- EDTA:
-
ethylenediaminetetraacetic acid
- FOX:
-
ferric-xylenol orange
- H2O2 :
-
hydrogen peroxide
- H2SO4 :
-
sulfuric acid
- HIV:
-
human immune deficiency virus
- HPLC:
-
high-performance liquid chromatography
- HRP:
-
horseradish peroxidase
- LOOH:
-
hydroperoxides from lipids
- Lo-OOH:
-
linoleic hydroperoxide
- MDA:
-
malondialdehyde
- O2 •− :
-
superoxide radical
- O3 :
-
ozone
- OH• :
-
hydroxyl radical
- PcOOH:
-
phosphatidyl-choline hydroperoxide
- POX-Act:
-
peroxide-activity
- PrOOH:
-
hydroperoxides from proteins
- RCS:
-
reactive carbon species
- RNS:
-
reactive nitrogen species
- ROO• :
-
peroxyl radical
- RO• :
-
alkoxyl radical
- ROOH:
-
organic peroxides or hydroperoxides
- ROS:
-
reactive oxygen species
- RSS:
-
reactive sulphur species
- TAC:
-
total antioxidant capacity
- TBARS:
-
thiobarbituric acid reactive species
- TMB:
-
3,5,3′5’-tetramethylbenzidine
- t-Bu-OOH:
-
t-butyl hydroperoxide
- TOS-dianisidine:
-
total oxidant status method based on ferrous ion–o-dianisidine complex
- U. CARR.:
-
Carratelli Units
- XO:
-
xylenol orange
References
Sies H. Oxidative stress: introductory remarks. In: Oxidative Stress. Elsevier; 1985. p. 1–8. doi:https://doi.org/10.1016/b978-0-12-642760-8.50005-3.
Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;81:291–5.
Kehrer JP, Klotz LO. Free radicals and related reactive species as mediators of tissue injury and disease: implications for health. Crit Rev Toxicol. 2015;45:765–98. https://doi.org/10.3109/10408444.2015.1074159.
Redmond RW, Kochevar IE. Symposium-in-print: singlet oxygen invited review spatially resolved cellular responses to singlet oxygen. Photochem Photobiol. 2006;82:1178–86.
Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev. 2012;70:257–65.
Mirończuk-Chodakowska I, Witkowska AM, Zujko ME. Endogenous non-enzymatic antioxidants in the human body. Adv Med Sci. 2018;63:68–78.
Halliwell B. Free radicals and antioxidants - quo vadis? Trends Pharmacol Sci. 2011;32:125–30. https://doi.org/10.1016/j.tips.2010.12.002.
Brenneisen P, Steinbrenner H, Sies H. Selenium, oxidative stress, and health aspects. Mol Asp Med. 2005;26:256–67.
Mailloux RJ. Mitochondrial antioxidants and the maintenance of cellular hydrogen peroxide levels. Oxidative Med Cell Longev. 2018;2018:1–10.
Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–44. https://doi.org/10.1113/jphysiol.2003.049478.
Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–55. https://doi.org/10.1038/sj.bjp.0705776.
Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;86:715–48.
Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem. 2015;97:55–74. https://doi.org/10.1016/j.ejmech.2015.04.040.
Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313.
Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21:363–83. https://doi.org/10.1038/s41580-020-0230-3.
Brand MD. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic Biol Med. 2016;100:14–31. https://doi.org/10.1016/j.freeradbiomed.2016.04.001.
Gebicki JM. Protein hydroperoxides as new reactive oxygen species. Redox Rep. 1997;3:99–110.
Cadet J, Di Mascio P. Peroxides in biological systems. PATAI'S Chem Funct Groups. 2009.
Sies H. Oxidative stress: from basic research to clinical application. Am J Med. 1991;91:S31–8.
Girotti AW. Action in biological systems. J Lipid Res. 1998;39:1529–42.
Fu S, Gebicki S, Jessup W, Gebicki JM, Dean RT. Biological fate of amino acid, peptide and protein hydroperoxides. Biochem J. 1995;311:821–7.
Simpson JA, Narita S, Gieseg S, Gebicki S, Gebickit JM, Dean RT. Long-lived reactive species free-radical-damaged proteins. Biochem J. 1992;282:621–4.
Headlam HA, Davies MJ. Cell-mediated reduction of protein and peptide hydroperoxides to reactive free radicals. Free Radic Biol Med. 2003;34:44–55.
Valko M, Morris H, Mazúr M, Rapta P, Bilton RF. Oxygen free radical generating mechanisms in the colon: do the semiquinones of vitamin K play a role in the aetiology of colon cancer? Biochim Biophys Acta - Gen Subj. 2001;1527:161–6.
Fan Q, Chen M, Fang X, Lau WB, Xue L, Zhao L, et al. Aging might augment reactive oxygen species (ROS) formation and affect reactive nitrogen species (RNS) level after myocardial ischemia/reperfusion in both humans and rats. Age (Omaha). 2013;35:1017–26.
Rubio CP, Martínez-Subiela S, Hernández-Ruiz J, Tvarijonaviciute A, Cerón JJ, Allenspach K. Serum biomarkers of oxidative stress in dogs with idiopathic inflammatory bowel disease. Vet J. 2017;221:56–61. https://doi.org/10.1016/j.tvjl.2017.02.003.
Woolcock AD, Serpa PBS, Santos AP, Christian JA, Moore GE. Reactive oxygen species, glutathione, and vitamin E concentrations in dogs with hemolytic or nonhemolytic anemia. J Vet Intern Med. 2020;34:2357–64.
Arteel GE. Leveraging oxidative stress questions in vivo: implications and limitations. Arch Biochem Biophys. 2016;595:40–5. https://doi.org/10.1016/j.abb.2015.11.009.
Dikalov SI, Harrison DG. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxidants Redox Signal. 2014;20:372–82.
Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. 2014;224:164–75. https://doi.org/10.1016/j.cbi.2014.10.016.
Rice-Evans CA, Diplock AT, Symons MCR. Techniques in free radical research. Lab Tech Biochem Mol Biol. 1991;22:1–278.
Frischer T, Pullwitt A, Kühr J, Meinert R, Haschke N, Studnicka M, et al. Aromatic hydroxylation in nasal lavage fluid following ambient ozone exposure. Free Radic Biol Med. 1997;22:201–7.
Utsumi H, Yamada KI. In vivo electron spin resonance-computed tomography/nitroxyl probe technique for non-invasive analysis of oxidative injuries. Arch Biochem Biophys. 2003;416:1–8.
Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–11. https://doi.org/10.1016/j.clinbiochem.2005.08.008.
Yuen JWM, Benzie IFF. Hydrogen peroxide in urine as a potential biomarker of whole body oxidative stress. Free Radic Res. 2003;37:1209–13. https://doi.org/10.1080/10715760310001616032.
Klawitter J, Haschke M, Shokati T, Klawitter J, Christians U. Quantification of 15-F2t-isoprostane in human plasma and urine: results from enzyme-linked immunoassay and liquid chromatography/tandem mass spectrometry cannot be compared. Rapid Commun Mass Spectrom. 2011;25:463–8.
Milne GL, Gao B, Terry ES, Zackert WE, Sanchez SC. Measurement of F2-isoprostanes and isofurans using gas chromatography-mass spectrometry. Free Radic Biol Med. 2013;59:36–44. https://doi.org/10.1016/j.freeradbiomed.2012.09.030.
Suttnar J, Čermák J, Dyr JE. Solid-phase extraction in malondialdehyde analysis. Anal Biochem. 1997;249:20–3.
Valavanidis A, Vlachogianni T, Fiotakis C. 8-Hydroxy-2′ -deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Heal - Part C Environ Carcinog Ecotoxicol Rev. 2009;27:120–39.
Spickett CM, Rennie N, Winter H, Zambonin L, Landi L, Jerlich A, et al. Detection of phospholipid oxidation in oxidatively stressed cells by reversed-phase HPLC coupled with positive-ionization electroscopy MS. Biochem J. 2001;355:449–57.
Ravanat JL, Duretz B, Guiller A, Douki T, Cadet J. Isotope dilution high-performance liquid chromatography-electrospray tandem mass spectrometry assay for the measurement of 8-oxo-7,8-dihydro-2′-deoxyguanosine in biological samples. J Chromatogr B Biomed Appl. 1998;715:349–56.
Miyamoto S, Ronsein GE, Prado FM, Uemi M, Corrêa TC, Toma IN, et al. Biological hydroperoxides and singlet molecular oxygen generation. IUBMB Life. 2007;59:322–31.
Wasowicz W, Neve J, Peretz A. Optimized steps in fluorometric determination of thiobarbituric acid- reactive substances in serum: importance of extraction pH and influence of sample preservation and storage. Clin Chem. 1993;39:2522–6.
Jentzsch AM, Bachmann H, Fürst P, Biesalski HK. Improved analysis of malondialdehyde in human body fluids. Free Radic Biol Med. 1996;20:251–6.
Knight JA, Pieper RK, McClellan L. Specificity of the thiobarbituric acid reaction: its use in studies of lipid peroxidation. Clin Chem. 1988;34:2433–8.
Tug T, Karatas F, Terzi SM, Ozdemir N. Comparison of serum malondialdehyde levels determined by two different methods in patients with COPD: HPLC or TBARS methods. Lab Med. 2005;36:41–4.
Seljeskog E, Hervig T, Mansoor MA. A novel HPLC method for the measurement of thiobarbituric acid reactive substances (TBARS). A comparison with a commercially available kit. Clin Biochem. 2006;39:947–54.
Nair V, Cooper CS, Vietti DE, Turner GA. The chemistry of lipid peroxidation metabolites: crosslinking reactions of malondialdehyde. Lipids. 1986;21:6–10.
Buege JA, Aust SD. Biomembranes - Part C: Biological Oxidations. Elsevier. 1978. https://doi.org/10.1016/S0076-6879(78)52032-6.
Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–13. https://doi.org/10.1038/ki.1996.186.
Valli A, Suliman ME, Meert N, Vanholder R, Lindholm B, Stenvinkel P, et al. Overestimation of advanced oxidation protein products in uremic plasma due to presence of triglycerides and other endogenous factors. Clin Chim Acta. 2007;379:87–94.
Dizdar EA, Uras N, Oguz S, Erdeve O, Sari FN, Aydemir C, et al. Total antioxidant capacity and total oxidant status after surfactant treatment in preterm infants with respiratory distress syndrome. Ann Clin Biochem. 2011;48:462–7.
Huang Q, Feng J, Wu R, Yang Y, Dai C, Li J, et al. Total oxidant/antioxidant status in sera of patients with esophageal cancer. Med Sci Monit. 2017;23:3789–94.
Köksal H, Kurban S, Doğru O. Total oxidant status, total antioxidant status, and paraoxonase activity in acute appendicitis. Ulus Travma Acil Cerrahi Derg. 2015;21:139–42.
Rubio CP, Hernández-Ruiz J, Martinez-Subiela S, Tvarijonaviciute A, Ceron JJ. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: an update. BMC Vet Res. 2016;12:166. https://doi.org/10.1186/s12917-016-0792-7.
Verk B, Nemec Svete A, Salobir J, Rezar V, Domanjko PA. Markers of oxidative stress in dogs with heart failure. J Vet Diagnostic Investig. 2017;29:636–44.
Panda D, Patra RC, Nandi S, Swarup D. Oxidative stress indices in gastroenteritis in dogs with canine parvoviral infection. Res Vet Sci. 2009;86:36–42. https://doi.org/10.1016/j.rvsc.2008.05.008.
Kiral F, Karagenc T, Pasa S, Yenisey C, Seyrek K. Dogs with Hepatozoon canis respond to the oxidative stress by increased production of glutathione and nitric oxide. Vet Parasitol. 2005;131:15–21. https://doi.org/10.1016/j.vetpar.2005.04.017.
Alexander JE, Colyer A, Haydock RM, Hayek MG, Park J. Understanding how dogs age: longitudinal analysis of markers of inflammation, immune function, and oxidative stress. J Gerontol - Ser A. 2018;73:720–8.
Ryad NM, Ramadan ES, Salem NY, Saleh IAE-S. Oxidative biomarkers and lipid alterations in euthyroid and hypothyroid dogs. Comp Clin Path. 2021;1–6. doi:https://doi.org/10.1007/s00580-021-03219-y.
Passantino A, Quartarone V, Pediliggeri MC, Rizzo M, Piccione G. Possible application of oxidative stress parameters for the evaluation of animal welfare in sheltered dogs subjected to different environmental and health conditions. J Vet Behav. 2014;9:290–4. https://doi.org/10.1016/J.JVEB.2014.06.009.
Vannucchi CI, Jordao AA, Vannucchi H. Antioxidant compounds and oxidative stress in female dogs during pregnancy. Res Vet Sci. 2007;83:188–93. https://doi.org/10.1016/j.rvsc.2006.12.009.
Head E, Rofina J, Zicker S. Oxidative stress, aging, and central nervous system disease in the canine model of human brain aging. Vet Clin North Am Small Anim Pract. 2008;38:167–78.
Jiang ZY, Woollard ACS, Wolff SP. Hydrogen peroxide production during experimental protein glycation. FEBS Lett. 1990;268:69–71.
Gupta BL. Microdetermination techniques for H2O2 in irradiated solutions. Microchem J. 1973;18:363–74.
Rubio CP, Tvarijonaviciute A, Caldin M, Hernández-Ruiz J, Cerón JJ, Martínez-Subiela S, et al. Stability of biomarkers of oxidative stress in canine serum. Res Vet Sci. 2018;121:85–93.
Lee JY, Won HS, Hwang HK, Jeong SM, Kim MC. Evaluation of the systemic oxidative stress status during major orthopedic surgery in dogs: a clinical study. J Vet Clin. 2013;30:1–4.
Pârvu AE, Ţălu Ş, Taulescu MA, Bota A, Cătoi F, Crăciun C, et al. Fractal analysis of ibuprofen effect on experimental dog peri-implantitis. Implant Dent. 2014;23:295–304. https://doi.org/10.1097/ID.0000000000000065.
Lee JY, Kim MC. Comparison of oxidative stress status in dogs undergoing laparoscopic and open ovariectomy. J Vet Med Sci. 2014;76:273–6. https://doi.org/10.1292/jvms.13-0062.
Lee JY. Oxidative stress due to anesthesia and surgical trauma and comparison of the effects of propofol and thiopental in dogs. J Vet Med Sci. 2012;74:663–5. https://doi.org/10.1292/jvms.11-0221.
Camkerten I, Sahin T, Borazan G, Gokcen A, Erel O, Das A. Evaluation of blood oxidant/antioxidant balance in dogs with sarcoptic mange. Vet Parasitol. 2009;161:106–9.
Gultekin M, Ural K, Pasa S, Balikci C, Asici GSE. Oxidative status and lipid profile in mono- and co-infection with canine monocytic ehrlichiosis. Med Weter. 2017;73:797–801.
Gultekin M, Voyvoda H. Evaluation of oxidative status in dogs with anemia. Med Weter. 2017;73:496–9. https://doi.org/10.21521/mw.5754.
Gultekin M, Paşa S, Ural K, Balıkçı C, Ekren Aşıcı GS, Gültekin G. Oxidative status and lipid profile among dogs at different stages of visceral Leishmaniasis. Turkiye parazitolojii Derg. 2017;41:183–7.
Rubio CP, Martinez-Subiela S, Tvarijonaviciute A, Hernández-Ruiz J, Pardo-Marin L, Segarra S, et al. Changes in serum biomarkers of oxidative stress after treatment for canine leishmaniosis in sick dogs. Comp Immunol Microbiol Infect Dis. 2016;49:51–7.
Lee JY, Choi SH. Evaluation of total oxidant and antioxidant status in dogs under different CO2 pneumoperitoneum conditions. Acta Vet Scand. 2015;57:23. https://doi.org/10.1186/s13028-015-0113-3.
Gautier A, Graff EC, Bacek L, Fish EJ, White A, Palmer L, et al. Effects of ovariohysterectomy and hyperbaric oxygen therapy on systemic inflammation and oxidation in dogs. Front Vet Sci. 2020;6:506.
Koksal H, Kurban S. Total oxidant status, total antioxidant status, and paraoxonase and arylesterase activities during laparoscopic cholecystectomy. Clinics. 2010;65:285–90. https://doi.org/10.1590/S1807-59322010000300008.
Yalcin S, Aydoğan H, Yuce HH, Kucuk A, Karahan MA, Vural M, et al. Effects of sevoflurane and desflurane on oxidative stress during general anesthesia for elective cesarean section. Wien Klin Wochenschr. 2013;125:467–73.
Esen R, Aslan M, Kucukoglu ME, Cıkman A, Yakan U, Sunnetcioglu M, et al. Serum paraoxonase activity, total thiols levels, and oxidative status in patients with acute brucellosis. Wien Klin Wochenschr. 2015;127:427–33.
Selek S, Cosar N, Kocyigit A, Erel O, Aksoy N, Gencer M, et al. PON1 activity and total oxidant status in patients with active pulmonary tuberculosis. Clin Biochem. 2008;41:140–4.
Aycicek A, Iscan A, Erel O, Akcali M, Ocak AR. Oxidant and antioxidant parameters in the treatment of meningitis. Pediatr Neurol. 2007;37:117–20.
Jiang ZY, Hunt JV, Wolff SP. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem. 1992;202:384–9.
Gay C, Collins J, Gebicki JM. Hydroperoxide assay with the ferric-xylenol orange complex. Anal Biochem. 1999;273:149–55.
Wolff SP. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994;233(C):182–9.
Nourooz-Zadeh J, Tajaddini-Sarmadi J, Wolff SP. Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol orange assay in conjunction with triphenylphosphine. Anal Biochem. 1994;220:403–9.
Arab K, Steghens J-P. Plasma lipid hydroperoxides measurement by an automated xylenol orange method. Anal Biochem. 2004;325:158–63. https://doi.org/10.1016/j.ab.2003.10.022.
Gay C, Gebicki JM. A critical evaluation of the effect of sorbitol on the ferric-xylenol orange hydroperoxide assay. Anal Biochem. 2000;284:217–20.
Jiang ZY, Woollard ACS, Wolff SP. Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids. 1991;26:853–6.
Nourooz-Zadeh J, Tajaddini-Sarmadi J, McCarthy S, John Betteridge D, Wolff SP. Elevated levels of authentic plasma hydroperoxides in IDDM. Diabetes. 1995;44:1054–8.
Rubio CP, Yilmaz Z, Martínez-Subiela S, Kocaturk M, Hernández-Ruiz J, Yalcin E, et al. Serum antioxidant capacity and oxidative damage in clinical and subclinical canine ehrlichiosis. Res Vet Sci. 2017;115:301–6.
Almela RM, Rubio CP, Cerón JJ, Ansón A, Tichy A, Mayer U. Selected serum oxidative stress biomarkers in dogs with non-food-induced and food-induced atopic dermatitis. Vet Dermatol. 2018;29:229–e82.
Demirbag R, Yilmaz R, Erel O, Gultekin U, Asci D, Elbasan Z. The relationship between potency of oxidative stress and severity of dilated cardiomyopathy. Can J Cardiol. 2005;21:851–5.
Aycicek A, Iscan A. The effects of carbamazepine, valproic acid and phenobarbital on the oxidative and antioxidative balance in epileptic children. Eur Neurol. 2007;57:65–9.
Horoz M, Aslan M, Koylu AO, Bolukbas C, Bolukbas FF, Selek S, et al. The relationship between leptin level and oxidative status parameters in hemodialysis patients. Artif Organs. 2009;33:81–5.
Nsonwu-Anyanwu A, Ighodalo E, King D, Elochukwu A, Jeremiah S, Solomon O, et al. Biomarkers of oxidative stress in HIV seropositive individuals on highly active antiretroviral therapy. React Oxyg Species. 2017;3:1–11.
Horoz M, Bolukbas C, Bolukbas FF, Aslan M, Koylu AO, Selek S, et al. Oxidative stress in hepatitis C infected end-stage renal disease subjects. BMC Infect Dis. 2006;6:1–7. https://doi.org/10.1186/1471-2334-6-114.
Nsonwu-Anyanwu AC, Osuoha UO, Nsonwu MC, Usoro CAO. Antimalaria therapy and changes in oxidative stress indices in falciparum malaria infection in Calabar metropolis, Nigeria. Trop J Pharm Res. 2019;18:2431–7.
Cesarone MR, Belcaro G, Carratelli M, Cornelli U, De Sanctis MT, Incandela L, et al. A simple test to monitor oxidative stress. Int Angiol. 1999;18:127–30.
Alberti A, Bolognini L, Macciantelli D, Caratelli M. The radical cation of N,N-diethyl-para-paraphenylendiamine: A possible indicator of oxidative stress in biological samples. Res Chem Intermed. 2000;26:253–67. https://doi.org/10.1163/156856700X00769.
Lindschinger M, Nadlinger K, Adelwöhrer N, Holweg K, Wögerbauer M, Birkmayre J, et al. Oxidative stress: potential of distinct peroxide determination systems. Clin Chem Lab Med. 2004;42:907–14.
Vassalle C, Boni C, Di Cecco P, Ndreu R, Zucchelli GC. Automation and validation of a fast method for the assessment of in vivo oxidative stress levels. Clin Chem Lab Med. 2006;44:1372–5.
Kilk K, Meitern R, Härmson O, Soomets U, Hõrak P. Assessment of oxidative stress in serum by d-ROMs test. Free Radic Res. 2014;48:883–9.
Lindschinger M, Wonisch W. POX-act assay and d-ROMs test - what are the facts? Clin Chem Lab Med. 2006;44:121–2.
Trotti R, Carratelli M, Barbieri M. Performance and clinical application of a new, fast method for the detection of hydroperoxides in serum. Panminerva Med. 2002;44:37–40.
Mutinati M, Spedicato M, Manca R, Trisolini C, Minoia G, Rizzo A, et al. Influence of antibiotic therapy on serum levels of reactive oxygen species in ovariectomized bitches. J Vet Pharmacol Ther. 2008;31:18–21.
Pasquini A, Luchetti E, Marchetti V, Cardini G, Iorio EL. Analytical performances of d-ROMs test and BAP test in canine plasma. Definition of the normal range in healthy Labrador dogs. Vet Res Commun. 2008;32:137–43. https://doi.org/10.1007/s11259-007-9014-x.
Rizzo A, Roscino MT, Minoia G, Trisolini C, Spedicato M, Mutinati M, et al. Serum levels of reactive oxygen species (ROS) in the bitch. Immunopharmacol Immunotoxicol. 2009;31:310–3.
Pasquini A, Luchetti E, Cardini G. Evaluation of oxidative stress in hunting dogs during exercise. Res Vet Sci. 2010;89:120–3. https://doi.org/10.1016/j.rvsc.2010.01.004.
Chiofalo B, Fazio E, Lombardi P, Cucinotta S, Mastellone V, Di Rosa AR, et al. Effects of dietary protein and fat concentrations on hormonal and oxidative blood stress biomarkers in guide dogs during training. J Vet Behav. 2020;37:86–92.
Sechi S, Fiore F, Chiavolelli F, Dimauro C, Nudda A, Cocco R. Oxidative stress and food supplementation with antioxidants in therapy dogs. Can J Vet Res. 2017;81:206–16.
Shinohara Y, Oyama A, Usui T, Sasaki K. Possible anti-oxidative effects of long-term administration of juzen-taiho-to in dogs. J Vet Med Sci. 2019;81:1616–20.
Pasquini A, Gavazza A, Biagi G, Lubas G. Oxidative stress in lymphoma: similarities and differences between dog and human. Comp Clin Path. 2013;24:69–73. https://doi.org/10.1007/s00580-013-1856-8.
Finotello R, Pasquini A, Meucci V, Lippi I, Rota A, Guidi G, et al. Redox status evaluation in dogs affected by mast cell tumour. Vet Comp Oncol. 2014;12:120–9.
Paltrinieri S, Ravicini S, Rossi G, Roura X. Serum concentrations of the derivatives of reactive oxygen metabolites (d-ROMs) in dogs with leishmaniosis. Vet J. 2010;186:393–5.
Mantovani G, Macciò A, Madeddu C, Mura L, Massa E, Gramignano G, et al. Reactive oxygen species, antioxidant mechanisms and serum cytokine levels in cancer patients: impact of an antioxidant treatment. J Cell Mol Med. 2002;6:570–82.
Sasazuki S, Hayashi T, Nakachi K, Sasaki S, Tsubono Y, Okubo S, et al. Protective effect of vitamin C on oxidative stress: a randomized controlled trial. Int J Vitam Nutr Res. 2008;78:121–8.
Josephy D, Eling T, Mason R. The horseradish peroxidase-catalyzed oxidation of 3,5,3′,5′- Tetramethylbenzidine. J Biol Chem. 1982;257:3669–75.
Tatzber F, Griebenow S, Wonisch W, Winkler R. Dual method for the determination of peroxidase activity and total peroxides-iodide leads to a significant increase of peroxidase activity in human sera. Anal Biochem. 2003;316:147–53. https://doi.org/10.1016/S0003-2697(02)00652-8.
Kochiadakis GE, Arfanakis DA, Marketou ME, Skalidis EI, Igoumenidis NE, Nikitovic D, et al. Oxidative stress changes after stent implantation: a randomized comparative study of sirolimus-eluting and bare metal stents. Int J Cardiol. 2010;142:33–7.
Roob JM, Khoschsorur G, Tiran A, Horina JH, Holzer H, Winklhofer-Roob BM. Vitamin E attenuates oxidative stress induced by intravenous iron in patients on hemodialysis. J Am Soc Nephrol. 2000;11:539–49.
Resch U, Tatzber F, Budinsky A, Sinzinger H. Reduction of oxidative stress and modulation of autoantibodies against modified low-density lipoprotein after rosuvastatin therapy. Br J Clin Pharmacol. 2006;61:262–74.
Lichtenberg D, Pinchuk I, Weber D. Oxidative stress, as assayed by a single test, cannot be used as a diagnostic tool. BioFactors. 2018;44:222–3.
Curtin JF, Donovan M, Cotter TG. Regulation and measurement of oxidative stress in apoptosis. J Immunol Methods. 2002;265:49–72.
Liochev SI, Fridovich I. Lucigenin as mediator of superoxide production: revisited. Free Radic Biol Med. 1998;25:926–8.
Allen RC, Stjernholm RL, Steele RH. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972;47:679–84.
Easmon CSF, Cole PJ, Williams AJ, Hastings M. The measurement of opsonic and phagocytic function by luminol-dependent chemiluminescence. Immunology. 1980;41:67–74.
Bedouhène S, Moulti-Mati F, Hurtado-Nedelec M, Dang PM-C, El-Benna J. Luminol-amplified chemiluminescence detects mainly superoxide anion produced by human neutrophils. Am J Blood Res. 2017;7:41–8.
Acknowledgements
Not applicable.
Funding
This work was supported by the Seneca Foundation of Murcia Region (19,894/GERM/15). The funding bodies had no role in the preparation of this manuscript or the decision to publish it.
Author information
Authors and Affiliations
Contributions
CPR and JJC have participated in conceptualization and manuscript preparation and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Results of studies in which the spectrophotometric assays were applied in human serum samples. In this file, the studies in humans cited in the manuscript are described in more detail, with the subjects studied and results observed for each assay. They are in tables that are named S1 to S4 according to their order in the text.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rubio, C.P., Cerón, J.J. Spectrophotometric assays for evaluation of Reactive Oxygen Species (ROS) in serum: general concepts and applications in dogs and humans. BMC Vet Res 17, 226 (2021). https://doi.org/10.1186/s12917-021-02924-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-021-02924-8