Abstract
Background
Transmission of antimicrobial resistant and virulent Escherichia coli (E. coli) from animal to human has been considered as a public health concern. This study aimed to determine the phylogenetic background and prevalence of diarrheagenic E. coli and antimicrobial resistance in healthy riding-horses in Iran. In this research, the genes related to six main pathotypes of E. coli were screened. Also, genotypic and phenotypic antimicrobial resistance against commonly used antibiotics were studied, then phylo-grouping was performed on all the isolates.
Results
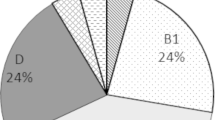
Out of 65 analyzed isolates, 29.23 % (n = 19) were determined as STEC and 6.15 % (n = 4) as potential EPEC. The most prevalent antimicrobial resistance phenotypes were against amoxicillin/clavulanic acid (46.2 %) and ceftriaxone (38.5 %). blaTEM was the most detected resistance gene (98.4 %) among the isolates and 26.15 % of the E. coli isolates were determined as multi-drug resistant (MDR). Three phylo-types including B1 (76.92 %), A (13.85 %) and D (3.08 %) were detected among the isolates.
Conclusions
Due to the close interaction of horses and humans, these findings would place emphasis on the pathogenic and zoonotic potential of the equine strains and may help to design antimicrobial resistance stewardship programs to control the dissemination of virulent and multi-drug resistant E. coli strains in the community.
Similar content being viewed by others
Background
Over time, horses have been bred by humans for meat, leisure and sport. Today, horses are known as important companion animals and have become popular for horse-back riding. Horse-riding clubs are a significant platform for human-horse interaction due to close contact between horses, horse handlers, horse riders and spectators [1]. Horses as companion animals could be considered as a potential reservoir of microbial agents which cause infections and complications in various hosts such as human. Among these microorganisms, some strains of Escherichia coli (E. coli) possess antimicrobial resistance (AMR) and virulence determinants which could be transmitted by direct or indirect contact [2].
A major group of E. coli strains, designated as diarrheagenic E. coli (DEC), cause intestinal infections [3]. According to the pathogenesis of DECs, they are divided into six main pathotypes including enterotoxigenic E. coli (ETEC), enteropathogenic E. coli (EPEC), enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC), diffusely adherent E. coli (DAEC) and Shiga toxin-producing E. coli (STEC) containing a sub-pathotype named enterohemorrhagic E. coli (EHEC) [4]. Animals are usually considered as asymptomatic source of DECs, shedding these strains to the environment.
Many strains of E. coli have intrinsic or/and acquired antimicrobial resistance which should be addressed as a significant threat to public health. Resistant E. coli could be selected among gut microbiota due to use of antimicrobial agents. Epidemiologically, antimicrobial resistant E. coli strains and their AMR determinants may be transferred from animal to human by horizontal transmission of AMR genes or clonal transfer of resistant strains via direct contact, indirect contact and consumption of fecal contaminated food.
Based on phylogenetic assessments, E. coli have been classified into eight phylo-groups including A, B1, B2, C, D, E, F and Escherichia cryptic clade I, by a PCR method [5]. The gut commensal E. coli strains predominantly belong to group A or B1, however, antimicrobial susceptible strains usually belong to the B1 rather than groups A and D [6, 7]. E. coli phylogenetic background is of importance for understanding the relationship between strains, antimicrobial resistance and disease [8].
To the best of our knowledge, there are no comprehensive study on virulence, antimicrobial resistance and phylogenetic analysis of equine E. coli isolates in Middle East. Hence, this study was designed to assess these variables in healthy riding horses in Iran to assess the potential risks of these animals for public health. The results could help to understand the public health risks associated with horse-riding clubs and transmission of antimicrobial resistance and virulence factors of E. coli from horses to humans.
Methods
Sampling and E. coli isolation
In this study, 65 rectal swabs were collected from healthy horses (n = 65) from five riding horse clubs during July to September, 2018, in Kerman province, southeast of Iran. Sterile saline moistened swabs were inserted 10 cm into the rectum and carried to veterinary microbiology laboratory in individual tubes containing Amies transport Medium (Merck, Germany) within 12 h. For E. coli isolation, all samples were cultured on MacConkey agar (Merck, Germany) and incubated at 37°C for 24 h. One presumptive E. coli colony was selected from each sample and confirmed via biochemical tests. One confirmed E. coli isolate from each sample was subjected to phenotypic resistance analysis and genetic assessments.
Phenotypic antimicrobial resistance assessment
In this study we evaluated antimicrobial resistance of isolates to seven antimicrobial agents which are the drugs of choice in the treatment of equine bacterial infections by disk diffusion method; including amoxicillin/clavulanic acid (20/10 µg), cefazolin (30 µg), ceftriaxone (30 µg), amikacin (30 µg), streptomycin (10 µg), gentamicin (10 µg) and trimethoprim/sulphamethoxazole (1.25/23.7 µg). The horse-specific/human breakpoints have been used to evaluate the results of disk diffusion method; the diameter of growth inhibition zones have been measured and the E. coli isolates were determined as resistant, intermediate and susceptible groups. According to CLSI VET08, human breakpoints are considered to provide zones of inhibition when there are no veterinary breakpoints available for some antimicrobial agents for all animal species [9, 10]. E. coli ATCC 25922 was used for quality control of the test (Table 1).
PCR for antimicrobial resistance, virulence and phylogenetic sequences
Boiling method was used for DNA extraction; a single colony was suspended in 300 µl sterile distilled water, heated up to 98 °C in heating block (Eppendorf, Germany) for 10–15 min, centrifuged in 13,000 rpm for 2 min and the supernatant was stored at -20 °C. The DNA extracts were used as templates in PCR to determine the presence or absence of antimicrobial resistance genes including blaTEM,blaSHV, blaCTX−M, aadA, dhfr1, dhfr5, sul1 and sul2 [11,12,13,14,15] (Table 2).
Six intestinal human pathogenic pathotypes of E. coli were screened by evaluation of stx1, stx2, eae, stII, lt, ipaH, aafII and daaE [16]; EPECs, EIECs, EAECs and DAECs are positive for eae, ipaH, aafII and daaE, respectively. STECs harbor stx1 and/or stx2 genes and ETECs are positive for stII and/or lt (Table 2).
Phylogenetic background of all isolates was determined using the PCR-based method explained by Clermont et al. (2013). In this scheme an E. coli strain could be classified into one of the phylo-types A, B1, B2, C, D, E, F and cryptic clades I to V [5] (Table 2).
All PCR methods were carried out as simplex in 25 µl final reaction volume including 3 µl DNA extract, 0.3 µM of each primer, 12.5 µl 2× Taq DNA Polymerase Master Mix RED (Ampliqon, Denmark) and sterile distilled water up to 25 µl. PCR products were loaded on an 1.5 % electrophoresis agarose gel containing Green Viewer stain (ParsTous, Iran) and imaged by a GelDoc 1000 (Vilber Lourmat, France).
Statistical analysis
For descriptive statistical analysis, all data about presence or absence of studied factors in each strain were imported into SPSS (SPSS 19; IBM) program as binomial variables; prevalence, 95 % confidence level and P value were calculated.
Results
In the present study, sixty five E. coli strains isolated from 65 horses have been evaluated. The most prevalent antimicrobial resistance phenotype was against the two β-lactam antibiotics, amoxicillin/clavulanic acid (46.2 %) and ceftriaxone (38.5 %), and the other antimicrobial resistance (AR) phenotypes were observed in less than 25 % of the E. coli isolates. In agreement with the phenotypic findings, the gene related to β-lactam resistance (blaTEM) was the most detected gene (98.4 %) among the isolates (Table 3).
Twenty-three phenotypic AR patterns were recognized in this study. No significant difference was observed in prevalence of AR patterns (p-value > 0.05); the frequency of the patterns was in the range of 1 to 4. According to studied antimicrobial agents, 26.15 % (n = 17) of the isolates were determined as multi-drug resistant; resistant strains to at least three antimicrobial agents from three different antimicrobial classes are defined as MDR [17]. Most of MDR isolates belonged to B1 phylo-group (12/17; 70.58 %), followed by A (3/17), Unknown (1/17) and D (1/17) phylo-types (Tables 3 and 4).
The isolates have been screened for the presence of 8 virulence genes (VGs) to determine intestinal human diarrheagenic E. coli pathotypes. According to the findings, 29.23 % (19/65) of our isolates were determined as STEC and 6.15 % (4/65) as potential EPEC. Among STECs (n = 19), three virulence gene (VG) profiles were observed including stx1 (15/19; 78.94 %), stx1/stx2 (2/19; 10.52 %) and stx1/eae (2/19; 10.52 %); the latter is similar to some human EHEC gene profiles and stx1 was the most prevalent profile significantly (p-value < 0.0001). Most of VG-positives belonged to B1 phylo-group (20/23; 86.95 %), followed by A (1/23; 4.34 %), D (1/23; 4.34 %) and Unknown (1/23; 4.34 %) phylo-types (Table 5).
Among the resistant VG-positive isolates (n = 14), five isolates were phenotypically multidrug resistant. All of the VG-positives harbored at least one of screened AR genes excluding one; three different AR gene profiles were identified including blaTEM, blaTEM/sulII, blaTEM/blaCTX−M in which blaTEM was the most prevalent significantly (p-value < 0.0001). All VG+/AR-gene+ isolates belonged to B1 except one belonging to A phylo-type (Table 6).
In this study, sixty one E. coli isolates have been distributed among three phylo-types including B1 (76.92 %), A (13.85 %) and D (3.08 %). Four (6.15 %) E. coli isolates could not be classified into the phylo-groups according to Clermont scheme and named as unknown (U). Similarly, B1 was the most prevalent phylo-type in virulent/non-virulent and resistant/non-resistant isolates; no significant difference (p-value > 0.05) has been observed in phylogenic distribution patterns of virulent and resistant isolates (Tables 3 and 4).
Discussion
In the current study, molecular pathotyping of equine E. coli isolates showed that more than one-third of them belonged to one of the diarrheagenic E. coli pathotypes including STEC and EPEC; the most prevalent pathotype was STEC (more than one-fourth of the isolates) followed by potential EPEC (less than 5 %). A few studies have reported the pathotypes in horses which mostly revealed low prevalence of them; Kennedy et al. (2018) in Ireland showed that none of the equine E. coli isolates obtained from 83 fecal samples were STEC or EPEC [18]. In the USA, a very low STEC prevalence, one from 242 equine E. coli isolates, has been reported [19]. Also Pichner et al. (2005) found only one STEC isolate among the 400 screened horse fecal samples in Germany [20]. Hamzeh et al. (2013) and Luna et al. (2018) have been observed STECs in 16.7 % and 11.7 % frequencies, respectively; the sample size in the two latter studies were small (less than 20 horses) [21, 22]. Chandran et al. (2013) have screened E. coli isolates from 11 different host sources and revealed that EPEC had the highest prevalence in horses (50 %) [23]. Despite the low STEC prevalence in horses, exposure to horse feces has been reported as a significant risk factor for clinical cases of STEC infections in humans [24, 25].
STEC pathotypes are usually detected from healthy horses and no clinical features have been observed in these cases. In a research in USA, positive horses for STEC were kept on farms containing ruminants [19]. Generally, ruminants are considered as the primary reservoir of the intestinal pathotypes; equids are not the main source of STEC and EPEC and they are known as spill-over hosts, the secondary species host exposed to the STEC through close contact with ruminants or feeding materials contaminated with ruminant manure [21]. Interestingly, all detected pathotypes of this study belonged to the horses with no history of ruminant direct contact.
Most STECs of this research were only positive for stx1 gene and some possessed stx1 and stx2 simultaneously. It is believed that stx1 and stx2 positives could cause more severe cases of STEC infections in human [26, 27]. The STEC strains which are positive for eae gene could potentially induce the attaching and effacing lesions in human. It has to be considered that the detection of the stx and eae genes is not enough to determine the pathogenicity and virulence of the E. coli strains recovered from animals in the human. Therefore, further phenotypic evaluations in laboratory animal models and intestinal cell lines are considered essential.
The occurrence of antimicrobial resistant E. coli in companion animals has drawn attention as a public health issue in the last decade [28]. Resistance to highly prescribed antimicrobial agents such as betalactames, aminoglycosides and sulphonamides has been studied in equine E. coli strains [29].
Our phenotypic results revealed that the prevalence of all the resistance phenotypes were less than 50 %. The highest prevalence of antimicrobial resistance has been observed against amoxicillin/clavulanic acid followed by ceftriaxone. Penicillins, sulfonamides and aminoglycosides are amongst the most commonly used antimicrobial classes in equine medicine for various conditions such as respiratory, digestive and pyogenic infections [30]. In a study in South Africa, resistance rate to ceftriaxone and amikacin were similar to the ones found in this work, while the prevalence of resistant isolates to amoxicillin/clavulanic, trimethoprim/sulphamethoxazole and gentamicin were higher than the current study [31]. Fortunately, our resistance rate to trimethoprim/sulphamethoxazole was notably low while other studies worldwide reported higher frequencies. A wide range of resistance against streptomycin and gentamicin in E. coli equine isolates has been reported from various studies, explaining that MDR in this study is rather low (less than 15 %). Variation in prevalence of antimicrobial resistance may be due to evaluation method, sample size, season, antibiotic prescription patterns, microbial population type of gastrointestinal microflora and exposure to antimicrobial resistance determinants [32]. Multidrug resistant bacteria can lead to complicated infections in the susceptible hosts and are addressed as a major public health issue [33]. According to MDR definition, a multidrug resistant bacterium is non-susceptible to at least one antimicrobial agent from three or more different antibiotic categories [17]. In the current study, more than one-fourth of the E. coli isolates and a considerable number of VG-positives were resistant against multiple antibiotics which are highly prescribed in human and equine medicine. This is less than the reported MDR prevalence in Kennedy et al. (2018) and de Lagarde et al. (2020) studies [18, 34]. Dissemination of MDR bacteria may cause the spread of nosocomial and community-acquired infections which could lead to rising antibiotic use, healthcare costs, morbidity and mortality [35].
Two main mechanisms have been proved for MDR; accumulation of several resistance genes by the bacteria and increased expression of resistance genes [36]. The dissemination of antimicrobial resistance is mainly associated with AR genes which are mostly located on mobile genetic elements. Thus, detection of resistance genes in bacteria may help to understand the resistance transmission and improvement of antibiotic-therapy strategies. In this study blaTEM was the most frequent AR gene, identified significantly higher (P < 0.05) than the other genes. Similarly, Johns et al. (2012) and Kennedy et al. (2018) reported high frequency of blaTEM in UK and Ireland respectively, while Gharaibeh et al. (2020) detected the gene only in 15.5 % of equine E. coli isolates in Jordan [18, 37, 38].
The next most prevalent genes were associated with resistance against sulfonamides including sulII (10.7 %) and sulI (1.5 %) which were considerably lower than the 57 % prevalence reported by Kennedy et al. (2018) in Ireland [18]. For the remaining genes, our results are comparable with the study in Jordan; the prevalence of our screened genes were between 0 and 6 % while the frequencies in the Gharaibeh et al. (2020) study were more than 10 % [38]. The diversity in prevalence rates may be due to the use of different methods, genotypic variety of E. coli populations and antibiotic exposure in horse in different countries.
In the current study, phylogenetic assessment of the equine isolates showed no relationship among virulence, resistance and phylogenetic background; resistant/non-resistant and virulent/non-virulent E. coli strains frequently belonged to B1 phylo-group. In agreement with our results, many studies around the world have reported B1 as the predominant phylo-type in equine E. coli isolates [34, 39, 40]. Conversely, Sukmawinata et al. (2019), reported B2 as the most common phylogenetic group among extended-spectrum β-lactamase-producing E. coli isolates from healthy thoroughbred race horses in Japan [41]. Phylo-typing of E. coli strains help to determine the evolutionary relationships among the microorganisms and is a fundamental issue in microbial studies. High dissemination of B1 in all isolate types could be due to various reasons such as nutrition, host species, sex, age, body mass, climate, geographic location and the combination of gut microflora [42].
Conclusions
In this study, a significant number of equine isolates belonged to one of the diarrheagenic E. coli pathotypes including STEC and potential EPEC, in which the most prevalent pathotype was STEC. Equids are known as spill-over hosts when exposed to the STEC through close contact with ruminants or feeding materials contaminated with ruminant manure. All detected pathotypes of this study belonged to the horses with no history of ruminant contact, which indicates that horses may take a role as a potential reservoir in spreading virulent pathotypes. Moreover, some VG-positive isolates were recognized as MDR. All the sampled animals in this study were riding horses with close contact to human including horse riders, club personnel and spectators. This represents the pathogenic and zoonotic potential of the equine strains in human medicine and would place emphasis on the design of antimicrobial resistance stewardship programs to control the dissemination of virulent and multi-drug resistant E. coli strains in the community.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AK:
-
Amikacin
- AMC:
-
Amoxicillin/clavulanic acid
- AMR:
-
Antimicrobial resistance
- arpA :
-
Ankyrin repeat protein A
- chuA :
-
E. coli heme-utilization gene A
- CRO:
-
Ceftriaxone
- CZ:
-
Cefazolin
- DAEC:
-
Diffusely adherent E. coli
- DEC:
-
Diarrheagenic E. coli
- eae :
-
Escherichia coli attaching and effacing gene
- EAEC:
-
Enteroaggregative E. coli
- EHEC:
-
Enterohemorrhagic E. coli
- EHECs:
-
Enterohemorrhagic Escherichia coli strains
- EIEC:
-
Enteroinvasive E. coli
- EPEC:
-
Enteropathogenic E. coli
- ETEC:
-
Enterotoxigenic E. coli
- GN:
-
Gentamicin
- PCR:
-
Polymerase chain reaction
- S:
-
Streptomycin
- SPSS:
-
Statistical Package for the Social Sciences
- STEC:
-
Shiga toxin-producing E. coli
- STECs:
-
Shiga toxin-producing Escherichia coli strains
- Stx:
-
Shiga toxin
- SXT:
-
Trimethoprim-sulphamethoxazole
- trpA:
-
Tryptophan synthase alpha
- TspE4.C2:
-
An anonymous DNA fragment in E. coli
- VG:
-
Virulence gene
- yjaA :
-
E. coli K12 gene
References
Hausberger M, Roche H, Henry S, Visser EK. A review of the human–horse relationship. Appl Anim Behav Sci. 2008;109:1–24.
Lyimo B, Buza J, Subbiah M, Smith W, Call DR. Comparison of antibiotic resistant Escherichia coli obtained from drinking water sources in northern Tanzania: a cross-sectional study. BMC Microbiol. 2016;16:254.
Kagambega A, Martikainen O, Siitonen A, Traore AS, Barro N, Haukka K. Prevalence of diarrheagenic Escherichia coli virulence genes in the feces of slaughtered cattle, chickens, and pigs in Burkina Faso. MicrobiologyOpen. 2012;1:276–84.
Derakhshan S, Farhadifar F, Roshani D, Ahmadi A, Haghi F. Study on the presence of resistant diarrheagenic pathotypes in Escherichia coli isolated from patients with urinary tract infection. Gastroenterol Hepatol from bed to bench. 2019;12:348.
Clermont O, Christenson JK, Denamur E, Gordon DM. The Clermont Escherichia coli phylo-typing method revisited: Improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep. 2013;5:58–65.
Cristea VC, Gheorghe I, Czobor Barbu I, Popa LI, Ispas B, Grigore GA, et al. Snapshot of phylogenetic groups, virulence, and resistance markers in Escherichia coli uropathogenic strains isolated from outpatients with urinary tract infections in Bucharest, Romania. Biomed Res Int. 2019; 5712371. doi: https://doi.org/10.1155/2019/5712371
Bukh AS, Schønheyder HC, Emmersen JMG, Søgaard M, Bastholm S, Roslev P. Escherichia coli phylogenetic groups are associated with site of infection and level of antibiotic resistance in community-acquired bacteraemia: a 10 year population-based study in Denmark. J Antimicrob Chemother. 2009;64:163–8.
Yılmaz EŞ, Aslantaş Ö. Phylogenetic group/subgroups distributions, virulence factors, and antimicrobial susceptibility of Escherichia coli strains from urinary tract infections in hatay. Rev Soc Bras Med Trop. 2020;53.
CLSI. Performance Standards for antimicrobial susceptibility testing (CLSI supplement M100S). 26th edition. Wayne: PA: Clinical and Laboratory Standards Institute; 2016.
CLSI. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals (CLSI supplement VET08). 4th edition. Wayne: PA: Clinical and Laboratory Standards Institute; 2018.
Olesen I, Hasman H, Møller Aarestrup F. Prevalence of β-lactamases among ampicillin-resistant Escherichia coli and Salmonella isolated from food animals in Denmark. Microb drug Resist. 2004;10:334–40.
Weill F-X, Demartin M, Tandé D, Espié E, Rakotoarivony I, Grimont PAD. SHV-12-like extended-spectrum-β-lactamase-producing strains of Salmonella enterica serotypes Babelsberg and Enteritidis isolated in France among infants adopted from Mali. J Clin Microbiol. 2004;42:2432–7.
Batchelor M, Hopkins K, Threlfall EJ, Clifton-Hadley FA, Stallwood AD, Davies RH, et al. blaCTX–M genes in clinical Salmonella isolates recovered from humans in England and Wales from 1992 to 2003. Antimicrob Agents Chemother. 2005;49:1319–22.
Van TTH, Chin J, Chapman T, Tran LT, Coloe PJ. Safety of raw meat and shellfish in Vietnam: an analysis of Escherichia coli isolations for antibiotic resistance and virulence genes. Int J Food Microbiol. 2008;124:217–23.
Kerrn MB, Klemmensen T, Frimodt-Möller N, Espersen F. Susceptibility of Danish Escherichia coli strains isolated from urinary tract infections and bacteraemia, and distribution of sul genes conferring sulphonamide resistance. J Antimicrob Chemother. 2002;50:513–6.
Vidal M, Kruger E, Durán C, Lagos R, Levine M, Prado V, et al. Single multiplex PCR assay to identify simultaneously the six categories of diarrheagenic Escherichia coli associated with enteric infections. J Clin Microbiol. 2005;43:5362–5.
Sweeney MT, Lubbers B V, Schwarz S, Watts JL. Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens. J Antimicrob Chemother. 2018;73:1460–3.
Kennedy CA, Walsh C, Karczmarczyk M, O’Brien S, Akasheh N, Quirke M, et al. Multi-drug resistant Escherichia coli in diarrhoeagenic foals: Pulsotyping, phylotyping, serotyping, antibiotic resistance and virulence profiling. Vet Microbiol. 2018;223:144–52. doi:https://doi.org/10.1016/j.vetmic.2018.08.009.
Lengacher B, Kline TR, Harpster L, Williams ML, Lejeune JT. Low prevalence of Escherichia coli O157:H7 in horses in Ohio, USA. J Food Prot. 2010;73:2089–92.
Pichner R, Sander A, Steinrück H, Gareis M. Occurrence of Salmonella spp. and Shiga toxin-producing Escherichia coli (STEC) in horse faeces and horse meat products. Berl Munch Tierarztl Wochenschr. 2005;118:321–5.
Luna S, Krishnasamy V, Saw L, Smith L, Wagner J, Weigand J, et al. Outbreak of E. coli O157:H7 infections associated with exposure to animal manure in a rural community — Arizona and Utah, June-July 2017. Morb Mortal Wkly Rep. 2018;67:659–62.
Hamzah AM, Mohammed Hussein A, Mahmoud Khalef J. Isolation of Escherichia coli O157:H7 strain from fecal samples of zoo animal. Sci World J. 2013; 843968. https://doi.org/10.1155/2013/843968
Chandran A, Mazumder A. Prevalence of diarrhea-associated virulence genes and genetic diversity in Escherichia coli isolates from fecal material of various animal hosts. Appl Environ Microbiol. 2013;79:7371–80.
Byrne L, Jenkins C, Launders N, Elson R, Adak GK. The epidemiology, microbiology and clinical impact of Shiga toxin-producing Escherichia coli in England, 2009–2012. Epidemiol Infect. 2015;143:3475–87.
Chalmers RM, Salmon RL, Willshaw GA, Cheasty T, Looker N, Davies I, et al. Vero-cytotoxin-producing Escherichia coli O157 in a farmer handling horses. Lancet. 1997;349:1816.
Friedrich AW, Bielaszewska M, Zhang W-L, Pulz M, Kuczius T, Ammon A, et al. Escherichia coli harboring Shiga toxin 2 gene variants: Frequency and association with clinical symptoms. J Infect Dis. 2002;185:74–84. doi:https://doi.org/10.1086/338115.
Vu-Khac H, Cornick NA. Prevalence and genetic profiles of Shiga toxin-producing Escherichia coli strains isolated from buffaloes, cattle, and goats in central Vietnam. Vet Microbiol. 2008;126:356–63. doi:https://doi.org/10.1016/j.vetmic.2007.07.023.
Ewers C, Grobbel M, Stamm I, Kopp PA, Diehl I, Semmler T, et al. Emergence of human pandemic O25:H4-ST131 CTX-M-15 extended-spectrum-β-lactamase-producing Escherichia coli among companion animals. J Antimicrob Chemother. 2010;65:651–60.
Maddox TW, Clegg PD, Williams NJ, Pinchbeck GL. Antimicrobial resistance in bacteria from horses: Epidemiology of antimicrobial resistance. Equine Vet J. 2015;47:756–65.
Schnepf A, Bienert-Zeit A, Ertugrul H, Wagels R, Werner N, Hartmann M, et al. Antimicrobial usage in horses: the use of electronic data, data curation, and first results. Front Vet Sci. 2020;7:216. doi: https://doi.org/10.3389/fvets.2020.00216.
Chipangura JK, Chetty T, Kgoete M, Naidoo V. Prevalence of antimicrobial resistance from bacterial culture and susceptibility records from horse samples in South Africa. Prev Vet Med. 2017;148:37–43. doi:https://doi.org/10.1016/j.prevetmed.2017.10.004.
Manyi-Loh C, Mamphweli S, Meyer E, Okoh A. Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules. 2018;23:795.
Fair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem. 2014;6:25–64.
de Lagarde M, Larrieu C, Praud K, Sall G, Fairbrother JM, Schouler C. Spread of multidrug-resistant IncHI1 plasmids carrying ESBL gene blaCTX–M–1 and metabolism operon of prebiotic oligosaccharides in commensal Escherichia coli from healthy horses, France. Int J Antimicrob Agents. 2020;55(6):105936. doi:https://doi.org/10.1016/j.ijantimicag.2020.105936.
Van Duin D, Paterson DL. Multidrug-resistant bacteria in the community: trends and lessons learned. Infect Dis Clin. 2016;30:377–90.
Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem. 2009;78:119–46.
Johns I, Verheyen K, Good L, Rycroft A. Antimicrobial resistance in faecal Escherichia coli isolates from horses treated with antimicrobials: A longitudinal study in hospitalised and non-hospitalised horses. Vet Microbiol. 2012;159:381–9.
Gharaibeh MH, Abutarbush SM, Mustafa FG, Lafi SQ, Halaiqa MS. Identification of risk factors associated with antimicrobial resistance in equine fecal Escherichia coli isolates. Infect Genet Evol. 2020;83:104317.
Elias L, Gillis DC, Gurrola-Rodriguez T, Jeon JH, Lee JH, Kim TY, et al. The occurrence and characterization of extended-spectrum-beta-lactamase-producing Escherichia coli isolated from clinical diagnostic specimens of equine origin. Animals. 2020;10:1–14.
Johnson JR, Johnston BD, Delavari P, Thuras P, Clabots C, Sadowsky MJ. Phylogenetic backgrounds and virulence-associated traits of Escherichia coli isolates from surface waters and diverse animals in Minnesota and Wisconsin. Appl Environ Microbiol. 2017;83(24): e01329-17.
Sukmawinata E, Sato W, Mitoma S, Kanda T, Kusano K, Kambayashi Y, et al. Extended-spectrum β-lactamase-producing Escherichia coli isolated from healthy thoroughbred racehorses in Japan. J Equine Sci. 2019;30:47–53.
Touchon M, Perrin A, de Sousa JAM, Vangchhia B, Burn S, O’Brien CL, et al. Phylogenetic background and habitat drive the genetic diversification of Escherichia coli. Plos Genet. 2020;16:e1008866.
Acknowledgements
The authors would like to express their gratitude to all horse owners and riding horse club managers of Kerman for their kind participation in this research.
Funding
There was no financial support for this research.
Author information
Authors and Affiliations
Contributions
MJ, MB and RG designed the study and analyzed the data; PR and FH performed the main experiments; MJ wrote and drafted the manuscript; MA, HA, MAB, SS and NA performed the complementary experiments and English edition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental protocols were approved by the committee for ethics in biomedical research in Veterinary Faculty of Shahid Bahonar University of Kerman, Iran. Also, all methods were carried out in accordance with relevant guidelines and regulations presented by Iran National Committee for Ethics in Biomedical Research. We obtained informed consent from the horse owners and riding horse clubs owners for using animals for sample collection by qualified persons.
Consent for publication
Not applicable.
Competing interests
All authors declare that the authors have no competing interests as defined by BMC, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Reshadi, P., Heydari, F., Ghanbarpour, R. et al. Molecular characterization and antimicrobial resistance of potentially human‐pathogenic Escherichia coli strains isolated from riding horses. BMC Vet Res 17, 131 (2021). https://doi.org/10.1186/s12917-021-02832-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-021-02832-x