Abstract
Background
Vibriosis is an important bacterial disease of cultured marine fishes worldwide. However, information on the virulence and antibiotic resistance of Vibrio spp. isolated from fish are scarce. This study investigates the distribution of virulence associated genes and antibiotic resistance patterns of Vibrio spp. isolated from cage-cultured marine fishes in Malaysia.
Results
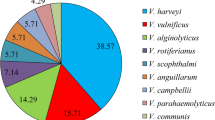
A total of 63 Vibrio spp. isolated from 62 cultured marine fishes in various geographical regions in Peninsular Malaysia were analysed. Forty-two of the isolates (66.7%) were positive for all chiA, luxR and vhpA, the virulence genes produced by pathogenic V. harveyi. A total of 62 Vibrio isolates (98%) had tlh gene of V. parahaemolyticus, while flaC gene of V. anguillarum was detected in 43 of isolates (68%). Other virulence genes, including tdh, trh, hlyA and toxRvc were absent from any of the isolates. Multiple antibiotic resistance (MAR) was exhibited in all strains of Harveyi clade, particularly against ampicillin, penicillin, polypeptides, cephems and streptomycin. The MAR index ranged between 0.06 and 0.56, and 75% of the isolates have MAR index of higher than 0.20. Host species and geographical origin showed no correlation with the presence of virulence genes and the antibiotic resistance patterns of Vibrio spp.
Conclusions
The study indicates that majority of Vibrio spp. isolated from cultured marine fishes possess virulence genes, but were not associated with human pathogen. However, the antibiotics resistance is a real concern and warrants ongoing surveillance. These findings represent an updated knowledge on the risk of Vibrio spp. to human health, and also provides valuable insight on alternative approaches to combat vibriosis in cultured fish.
Similar content being viewed by others
Background
Vibrio spp. that have been associated with diseases in animals and human often possess virulence factors, which are not available or present in the environmental Vibrio [1]. However, since Vibrio possesses highly plastic genome, the probability of horizontal transfer of the virulence genes between pathogenic and environmental Vibrio is high. This contributes to the increased number of pathogenic Vibrio strains in aquatic environment [2]. Recently, more disease outbreaks following infections by Vibrio harveyi, V. alginolyticus, V. parahaemolyticus and V. campbellii in farmed fishes were reported in many tropical countries [3,4,5,6].
Several extracellular products that are known to contribute to the virulence of Vibrio include proteases, hemolysins, phospholipases, siderosphores, cytotoxins, biofilm formation, quorum sensing, and presence of phage [7,8,9]. Swarming motility of Vibrio has been consistently associated with their virulence [10], while hemolysin is a common virulence factor reported in Vibrio associated with both fish and human diseases [11]. In addition, virulence of several pathogenic Vibrio has also been attributed to quorum-sensing, the bacterial cell to cell communication [12].
Resistance to the bactericidal mechanisms is another important contributor to the virulence of fish pathogen. In the past few decades, antimicrobial resistance has emerged and evolved in Vibrio spp. due to the excessive use of antibiotics in human medicine, agriculture and aquaculture systems [13]. This issue gained great concern due to the increased resistance of pathogenic V. parahemolyticus, V. harveyi and V. vulnificus towards many clinically used antimicrobials [14,15,16,17,18]. Moreover, multiple antibiotic resistance (MAR) strains of V. harveyi and V. alginolyticus have caused severe economic setbacks to the aquaculture industry [19].
This study described the presence of virulence-associated genes and antibiotic resistance patterns of Vibrio spp. within the Harveyi clade, which were isolated from various aquaculture areas in Peninsular Malaysia. Three typical virulence genes that were possessed by V. harveyi (chiA, luxR and vhpA) and five atypical virulence genes that contributed to pathogenic Vibrio of both fish and human (flaC, hlyA, toxRvc, tdh and trh) were targeted. Furthermore, thermolabile hemolysin gene tlh, a species specific marker for V. parahaemolyticus was also included. In addition, resistance of the isolates towards 16 commercial antibiotics of various groups were determined to evaluate the potential responsiveness to the suite of antibiotic treatments that most frequently used in aquaculture.
Results
Generally, five out of the nine targeted virulence genes were present in the tested isolates (Fig. 1a). All (100%) 63 isolates of Harveyi clade possessed typical virulence genes of chiA and luxR. Forty-two isolates (67%) of studied Harveyi clade and all (100%) V. campbellii isolates were positive of vhpA gene. However, only two out of six virulence genes were detected in other Vibrio spp. tested in this study. The tlh was detected in all isolates except an isolate of V. campbellii.
The flaC gene was detected in 68% of the isolates (Fig. 1b). All V. harveyi and V. parahaemolyticus that were isolated from Pulau Ketam were positive for flaC. However, they lack the tdh, trh, hlyA and toxRvc genes. Moreover, 27 (43%) of the isolates carried all the virulence genes of chiA, luxR, vhpA, flaC and tlh, including all V. campbellii that were isolated from Pulau Ketam, and two V. parahaemolyticus from Banting. However, there was no correlation between the presence of virulence genes and the source of the isolates.
Amplification of chiA, luxR, vhpA, flaC and tlh from all species of Vibrio isolates, yielded products of approximately 232 bp, 618 bp, 201 bp, 580 bp, and 450 bp, respectively (Fig. 2). Figure 3 shows the phylogenetic tree of the virulence genes. Sequence analysis revealed that the chiA of V. campbellii GRO 230-L1 and V. harveyi SNA 143-L1 shared 99 and 89% similarity, respectively with chiA of V. harveyi, while V. alginolyticus SEA 124-S and V. parahaemolyticus GRO 286-E1 showed 95–99% similarity with chitinase A from V. parahaemolyticus [AF323471]. The luxR of V. harveyi SEA 131-K1, V. campbellii SEA 178-K1 and V. parahaemolyticus GRO 180-K1 were > 98% similar with luxR of V. harveyi. However, luxR of V. alginolyticus SNA 212-S1 was identical (99%) to luxR of V. alginolyticus [EF596781]. All vhpA in this study shared high similarity (> 98%) with vhpA of V. harveyi. Similarly, high similarity (> 89%) was also observed between tlh of Vibrio isolates in this study and tlh of V. parahaemolyticus JPW-8-11-1. In addition, flaC of V. harveyi SNA 143-L1 and V. alginolyticus GRO 144-E1 were highly identical (99%) with flaA of V. alginolyticus HY9901. On the other hand, flaC of V. parahaemolyticus shared 99% similarity with flaA of V. parahaemolyticus ATCC 17802, while flaC of V. harveyi SNA 143-L1 shared 96% with flaB of V. harveyi VIB645.
The antibiotic resistance patterns are illustrated in Fig. 4. Highest resistance (79 to 99%) was observed against penicillin and the polypeptides group of antibiotics. All Vibrio spp. isolated from Pulau Ketam, with the exception of one isolate of V. alginolyticus and V. harveyi were resistance against AMP, P and VA. This was also exhibited by all V. campbellii isolates, regardless of their geographical origin. On the other hand, sensitivity towards AMP was mostly observed in V. parahaemolyticus isolated from Perak, Kedah, Kelantan and Johor. High resistance towards E was also observed in this study, where only 9% of the isolates showed sensitivity towards E.
Antibiotic resistance patterns showed by isolates of Harveyi clades isolated from sampled fishes. MAR index (MARi) indicated the multiple antibiotics resistance index for the isolates. AMP: Ampicillin (10 μg), P: Penicillin G (10 unit), CTX: Cefotaxime (30 μg), FEP: Cefepime (30 μg), KF: Cephalothin (30 μg), CN: Gentamycin (10 μg), K: Kanamycin (30 μg), S: Streptomycin (10 μg), TE: Tetracycline (30 μg), CIP: Ciprofloxacin (5 μg), NA: Nalidixic acid (30 μg), F: Nitrofurantoin (300 μg), SXT: Sulfamethoxazole/trimethoprim (1.25/23.75 μg), C: Chloramphenicol (30 μg), E: Erythromycin (15 μg), VA: Vancomycin (30 μg)
A total of 60 and 46% of isolates were resistance against at least one antibiotic from cephems and aminoglycosides groups. Lower resistance level was observed for CN, K and FEP, with less than 6% resistant isolates. However, more than 40% of the tested isolates were resistant against CTX, KF and S. The resistance against CTX was mostly showed by V. campbellii (78%), followed by V. harveyi (63%) and V. alginolyticus (62%). Low resistance (8%) towards quinolones group of antibiotic was observed in this study where none of V. alginolyticus and V. harveyi isolate was resistant towards CIP and NA. In addition, only one and seven isolates were resistant to C and SXT, respectively. None of the isolate in this study was resistant against F and TE.
The MAR index denotes the extent of environmental contamination by antimicrobial agents which potentially harmful to human health [20]. A MAR index higher than 0.2 indicated high-risk exposure to antibiotics. In this study, the MAR index ranged between 0.06 and 0.56. Approximately 75% of the isolates showed MAR index of higher than 0.20, including 80% from Pulau Ketam. However, no clear pattern was observed between the MAR index and the geographical origin. Nevertheless, an isolate of V. harveyi and two isolates of V. parahaemolyticus showed resistance against the nine antibiotics tested, with MAR index of 0.56. Among the three isolates, V. parahaemolyticus (GRO 286-E1) isolated from Pulau Ketam exhibited strongest resistance towards antibiotics tested, where only two antibiotics (TE and F) can inhibit the growth of this isolate.
Discussion
This study examines two factors that contribute to the pathogenicity of Vibrio spp., which previously isolated from cultured marine fishes in selected important farms and hatcheries in Peninsular Malaysia; the presence of virulence genes associated with pathogenic strains and the antibiotics resistance of Vibrio spp.
Virulence genes including chiA, luxR, toxRvh, vhpA, serine protease and vhh were widely distributed among pathogenic V. harveyi [21]. The distribution of these virulence genes in the closely related species of V. harveyi has also been documented [7]. In agreement to previous studies, chiA and luxR were found to presence in all isolates of Harveyi clade analysed in present study.
On the other hand, typical virulence gene of vhpA was observed in > 50% of the isolates in this study, with 100% prevalence in V. campbellii isolates. Even though vhpA was reported as a typical virulence gene harboured by V. harveyi, only 50% of our V. harveyi isolates was positive for this gene. Ruwandeepika et al. [21], reported a 100% prevalence of vhpA among V. harveyi but conversely, vhpA was reported to be absent in all V. harveyi isolated from diseased cultured fish in China, regardless of the virulence level [2, 19].
Studies demonstrated that the presence of chitinase and metalloprotease in ECP of Vibrio can cause disease in wide range of aquatic animals including fish, oyster and shrimp [7, 22]. In addition, quorum sensing was reported to regulate the production of these ECP and other virulence genes in Gram-negative fish pathogens [12]. For instance, Defoirdt [23] described on the virulence of V. harveyi controlled by quorum sensing. Another study by Croxatto et al. [24] demonstrated the involvement of quorum sensing in the secretion of metalloprotease EmpA and biofilm formation in V. anguillarum.
In this study, flaC was widely distributed in 60% of V. alginolyticus, 63% of V. parahaemolyticus, and 78% V. campbellii isolates. In addition, all V. harveyi harboured flaC gene. In a study conducted by Bai et al. [25], 37.5% of the V. harveyi isolates carried the flaC gene. They also reported that the flaC gene was widely distributed in other Vibrio spp. including V. anguillarum, V. alginolyticus, V. campbellii, V. fischeri, V. fluvialis, V. mimicus, V. natriegens and V. parahaemolyticus. Another study revealed that flaC was detected in 60% of the Vibrio in the Harveyi clade [21]. Similarly, the sequence diversity of flaC observed in this study coincided with a previous report [25], and flagella play an important role in infecting host because it increase the motility of bacteria for colonization, or act as adhesive component [26].
The thermolabile hemolysin gene tlh was previously used as species specific marker to identify V. parahaemolyticus [27]. However in this study, tlh recovered from non-parahaemolyticus strains showed highly similar sequence with those from V. parahaemolyticus. The results indicate that tlh cannot be used as species specific marker for detection of V. parahaemolyticus due to possible detection of false-positive results. Furthermore, previous study showed an increase in the environmental V. parahaemolyticus strains carrying the tdh and/or trh genes in Malaysia [28]. Interestingly, all isolates in this study lacked the virulence genes associated pathogenic Vibrio of human, which are the tdh, trh, hlyA and toxRvc genes, indicating low potential risk for human health. On the other hand, higher percentage of pathogenic Vibrio were positive to trh (40%) and tdh (12.3%) was detected in aquatic animals in other studies [29, 30].
In general, similar virulence genes were widely distributed in the Harveyi clade, indicating that the genes are readily transferred among the Vibrionaceae species. This horizontal transferability of virulence genes might be due to their survival benefits in a variety of environments and host organisms [31].
Antibiotics are commonly used in fish farms either as feed additives, prophylaxis or therapy. Oxytetracycline, TE, quinolones, nitrofurans, potentiated sulfonamides, trimethoprim, sarafloxacin, flumequine and oxolinic acid are among the permissible antibiotics that have been used to combat vibriosis [32]. This study revealed that the Harveyi clade were highly resistance to AMP and VA. Similarly, all Vibrio isolates collected from Malaysian coastal area were resistant to AMP at the rate between 42 and 82% [33], including 100% of isolates from farmed fish [34]. The prevalence of AMP resistance in Vibrio isolates from marine environment is generally high, which probably due to the wide usage of AMP. Moreover, resistance to AMP or other penicillin were also well documented from environmental isolated Vibrio, ranging from 56 to 100% in China, Italy and U.S. [19, 35, 36].
FEP is one of the newer fourth generation cephalosporins [33]. While none of the isolates in this study showed resistance towards FEP, 84% of the isolates showed immediate sensitive towards this antibiotic. On the other hand, TE and nitrofurans were effective to inhibit the growth of Vibrio isolates in this study, thus can be used to treat Vibrio infection in Malaysian farm. However, prolonged and misused of antibiotics possess the danger of developing antibiotic resistant genes, that cause the Vibrio to develop resistance [13].
In this study, the prevalence of MAR strains of Vibrio was at the alarming rate. The results indicate that the MAR strains of Vibrio existed widely in the aquaculture farm in this country. Ransangan et al. [34] and You et al. [33] reported high prevalence of multiple antibiotic Vibrio recovered from coastal seawater in Malaysia. While there is limited documentation on the information of the use of antibiotics in Malaysian fish farming, emergence of MAR Vibrio strains due to excessive utilization of antibiotics has been reported in other countries [2, 19, 37, 38]. For example, high MAR index (0.4) of Harveyi clade strains causing scale drop and muscle necrosis disease in groupers was reported in China [19]. Moreover, 77.3% of V. parahaemolyticus isolated from oyster in Korea demonstrated MAR to at least three antibiotics, with highest MAR index of 0.75 in one isolate [18].
In general, high prevalence of isolates that were resistant to multiple antibiotics was observed in Pulau Ketam, one of the extensive mariculture farms in Malaysia. While no antibiotic was recorded being used for treatment at our sampling site in Pulau Ketam, the Vibrio with multiple antibiotic resistance can be easily transmitted from nearby farms that used antibiotic via water column. In addition, Vibrio spp. may acquire and carry antibiotic resistance genes by horizontal genetic transference from and to neighbouring microorganisms. Out of seven sampling site, only the hatchery in Port Dickson reported on the utilization of antibiotic to treat bacterial infection. The rapid increase in antibiotic resistance rendered the treatment to be more difficult. The use of antibiotics in aquaculture also impacts the frequencies of resistance in human pathogens [15, 37]. Therefore, calls for the reduction of antibiotic use has been done worldwide [38, 39].
Other methods of vibriosis control are urgently needed. In recent year, the disruption of quorum sensing has recently been suggested as a cost-effective and environmental friendly method [12]. Several bacteria, micro-algae, macro-algae and aquatic sponges have been shown to inhibit quorum sensing properties in pathogenic Vibrio particularly V. harveyi [12, 23]. Given the wide distribution of quorum sensing regulated-gene in different Vibrio species found in this study, this method are promising to control the expression of virulence factors by different Vibrio species in aquaculture. In addition, immunostimulants, bacteriophage, vaccines and probiotics also have potentials to replace antibiotics in controlling and preventing vibriosis in fish farm [40].
Conclusion
In conclusion, low prevalence of virulence genes was detected in Vibrio spp. within the Harveyi clade in this study. However, majority of the isolates exhibited multiple resistance to tested antibiotics, highlighting the urgency for reducing the usage of antibiotic in fish farms. It is necessary to perform extensive studies on the spread of antibiotic resistance genes in Vibrio to understand the potential risk to public health. In the meantime, alternative non-antibiotic based methods such as quorum quenching and utilization of lytic bacteriophage for preventing and treating bacterial infections in fish farm are needed.
Methods
Bacterial strains
Large collection of Vibrio strains previously isolated from either healthy or diseased fish cultured in marine farm or hatchery were used in this study. The farm and hatchery included were intensive or semi-intensive farms culturing and producing finfish fry, including Asian seabass (Lates calcarifer), red snapper (Lutjanus sp.) and hybrid grouper (Epinephelus sp.). Seven sampling sites were selected as representative of important fish farming and fry producing area in Peninsular Malaysia; Pulau Ketam and Banting in Selangor, Port Dickson in Negeri Sembilan, Kuala Gula in Perak, Kukup in Johor, Kota Bharu in Kelantan, and Pulau Langkawi in Kedah (Table 1).
Identification of the Vibrio isolates were verified based on the partial sequencing of pyrH as described in previous reports [41, 42]. Based on the recovery rates, only four species of Vibrio were selected for this study. A total of 63 isolates representative of V. alginolyticus, V. harveyi, V. parahaemolyticus and V. campbellii were analysed. Forty of the isolates were isolated either from Asian seabass, red snapper and hybrid grouper cultured in Pulau Ketam, Selangor. Another 23 Vibrio isolates were recovered from hybrid groupers cultured in farm or hatchery located in different states in Peninsular Malaysia (Table 1). The code, species name, source of isolation, clinical sign/s of the host, month and year of isolation, and geographical origin of the isolates as listed in Appendix 1. All isolates were kept in 20% glycerol stock and stored at − 80 °C for further analysis.
Virulence genes detection
All isolates were sub-cultured from glycerol stock onto Tryptic Soy Agar (TSA) (HiMedia, Mumbai, India), supplemented with 1.5% NaCl and incubated at 30 °C for 18 h. Prior to PCR, genomic DNA of the isolates was extracted using Wizard Genomic DNA Purification Kit (Promega, WI, USA).
A total of nine virulence-associated genes (chiA, vhpA, luxR, flaC, hlyA, toxRvc, tlh, tdh and trh) of Vibrio were detected by PCR amplification. The sequence of primers used are as listed in Table 2. PCR amplifications were performed in a final volume of 30 μL, which contained 1× PCR buffer, 2 mM MgCl2, 200 uM dNTPs, 10 pmol of forward primer, 10 pmol of reverse primer, 5 U/μL Taq polymerase and 100 ng of template DNA (Promega). The amplification was performed under the following conditions: initial denaturation at 95 °C for 5 min, followed by 30 cycles of 95 °C for 1 min; 50 °C for 1 min (chiA, vhpA and luxR), 55 for 1 min (flaC), 60 for 1 min (hlyA and toxRvc) and 72 °C for 1 min, and a final extension of 72 °C for 10 min using Eppendorf Mastercycler Nexus Thermal Cycler (Eppendorf, Hamburg, Germany). The amplification of tlh, trh and tdh was performed under the following conditions: initial denaturation at 94 °C for 3 min, followed by 30 cycles of 94 °C for 1 min; 58 °C for 1 min and 72 °C for 1 min, and a final extension of 72 °C for 10 min.
Amplified PCR products were visualised on 1.2% agarose gel stained with ethidium bromide, run at 90 V for 40 min, and photographed using a gel documented system. The confirmation of the presence of genes were by partial sequencing (FirstBase, Kuala Lumpur, Malaysia) and BLAST comparison with GenBank (http://blast.ncbi.nlm.nih.gov/). Following multiple alignment of genes with their closed taxa by CLUSTAL W method, neighbour-joining trees were constructed using the Kimura 2-parameter model with MEGA version 7.0 with bootstraps of 1000 replicates [43].
Antibiotic sensitivity testings
The antibiotics sensitivity of the isolates were examined by the disc diffusion methods [44]. A total of 18 representative antimicrobial agents (Oxoid, London, UK), including penicillins (ampicillin (AMP): 10 μg; penicillin G (P): 10 units), cephems (cefotaxime (CTX): 30 μg; cefepime (FEP): 30 μg; cephalothin (KF): 30 μg), aminoglycosides (gentamycin (CN): 10 μg; kanamycin (K): 30 μg; streptomycin (S): 10 μg), and others such as nalidixic acid (NA): 30 μg; trimethoprim/sulfamethoxazole (SXT): 1.25/23.75 μg; chloramphenicol (C): 30 μg; nitrofurantoin (F): 300 μg; ciprofloxacin (CIP): 5 μg; tetracycline (TE): 30 μg; erythromycin (E): 15 μg; and vancomycin (VA): 30 μg were used.
Following incubation for 18–24 h, the isolates were then inoculated in sterile saline water to achieve turbidity equivalent to 0.5 MacFarland standard. The broth were evenly swabbed onto Mueller Hinton agar (HiMedia) supplemented with 1% of NaCl [45]. Antibiotic discs were aseptically placed on the swabbed plates. The plates were then incubated at 35 °C for 16–18 h, and the clearing zone was recorded. Testing was confirmed in duplicate. The resistance profiles (resistant, intermediate or susceptible) were assigned using criteria described by CLSI [44, 46] and Bauer et al. [47]. The multiple antibiotic resistance (MAR) index was determined for each isolate [20]. Table 3 summarized the list of antibiotics and the zone diameter interpretive criteria used in this study.
Abbreviations
- AMP:
-
Ampicillin
- C:
-
Chloramphenicol
- CIP:
-
Ciprofloxacin
- CN:
-
Gentamycin
- CTX:
-
Cefotaxime
- E:
-
Erythromycin
- F:
-
Nitrofurantoin
- FEP:
-
Cefepime
- K:
-
Kanamycin
- KF:
-
Cephalothin
- MAR:
-
Multiple antibiotic resistance
- NA:
-
Nalidixic acid
- P:
-
Penicillin G
- S:
-
Streptomycin
- SXT:
-
Trimethoprim/sulfamethoxazole
- TE:
-
Tetracycline
- TSA:
-
Tryptic soy agar
- VA:
-
Vancomycin
References
Bunpa S, Sermwittayawong N, Vuddhakul V. Extracellular enzymes produced by Vibrio alginolyticus isolated from environments and diseased aquatic animals. Procedia Chem. 2016;18:12–7.
Xu Y, Wang C, Zhang G, Tian J, Liu Y, Shen X, Feng J. ISCR2 is associated with the dissemination of multiple resistance genes among Vibrio spp. and Pseudoalteromonas spp. isolated from farmed fish. Arch Microbiol. 2017;199(6):891–6.
Khouadja S, Lamari F, Bakhrouf A. Characterization of Vibrio parahaemolyticus isolated from farmed sea bass (Dicentrarchus labrax) during disease outbreaks. Int Aquatic Res. 2013;5(1):13.
Abdullah A, Ramli R, Ridzuan MSM, Murni M, Hashim S, Sudirwan F, Abdullah SZ, Mansor NN, Amira S, Zamri-Saad M, Amal MNA. The presence of Vibrionaceae, Betanodavirus and Iridovirus in marine cage-cultured fish: role of fish size, water physicochemical parameters and relationships among the pathogens. Aquaculture Rep. 2017;7:57–65.
Dong HT, Taengphu S, Sangsuriya P, Charoensapsri W, Phiwsaiya K, Sornwatana T, Khunrae P, Rattanarojpong T, Senapin S. Recovery of Vibrio harveyi from scale drop and muscle necrosis disease in farmed barramundi, Lates calcarifer in Vietnam. Aquaculture. 2017;473:89–96.
Nurliyana M, Fauzul-Aidil MR, Amal MNA, Zamri-Saad M, Ina-Salwany MY, Nor-Amalina Z, Shaqinah NN. Natural concurrent infection of Vibrio harveyi and V. alginolyticus in cultured hybrid groupers in Malaysia. J Aquat Anim Health. 2019;31:88-96.
Aguirre-Guzmán G, Mejia Ruíz H, Ascencio F. A review of extracellular virulence product of Vibrio species important in diseases of cultivated shrimp. Aquac Res. 2004;35(15):1395–404.
Nakhamchik A, Wilde C, Rowe-Magnus DA. Cyclic-di-GMP regulates extracellular polysaccharide production, biofilm formation, and rugose colony development by Vibrio vulnificus. Appl Environ Microbiol. 2008;74(13):4199–209.
Rønneseth A, Castillo D, D'Alvise P, Tønnesen Ø, Haugland G, Grotkjær T, Engell-Sørensen K, Nørremark L, Bergh Ø, Wergeland HI, Gram L. Comparative assessment of Vibrio virulence in marine fish larvae. J Fish Dis. 2017;40:1373–85.
Frans I, Michiels CW, Bossier P, Willems KA, Lievens B, Rediers H. Vibrio anguillarum as a fish pathogen: virulence factors, diagnosis and prevention. J Fish Dis. 2011;34(9):643–61.
Austin B, Zhang XH. Vibrio harveyi: a significant pathogen of marine vertebrates and invertebrates. Lett Appl Microbiol. 2006;43(2):119–24.
Natrah FMI, Defoirdt T, Sorgeloos P, Bossier P. Disruption of bacterial cell-to-cell communication by marine organisms and its relevance to aquaculture. Mar Biotechnol. 2011;13(2):109–26.
Cabello FC, Godfrey HP, Tomova A, Ivanova L, Dolz H, Millanao A, Buschmann AH. Antimicrobial use in aquaculture re-examined: its relevance to antimicrobial resistance and to animal and human health. Environ Microbiol. 2013;15:1917–42.
Letchumanan V, Pusparajah P, Tan LTH, Yin WF, Lee LH, Chan K. Occurrence and antibiotic resistance of Vibrio parahaemolyticus from shellfish in Selangor, Malaysia. Front Microbiol. 2015;6:1417.
Elmahdi S, DaSilva LV, Parveen S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: a review. Food Microbiol. 2016;57:128–34.
Heng SP, Letchumanan V, Deng CY, Ab Mutalib NS, Khan TM, Chuah LH, Chan KG, Goh BH, Pusparajah P, Lee LH. Vibrio vulnificus: an environmental and clinical burden. Front Microbiol. 2017;8:997.
Lee LH, Ab Mutalib NS, Law JWF, Wong SH, Letchumanan V. Discovery on antibiotic resistance patterns of Vibrio parahaemolyticus in Selangor reveals carbapenemase producing Vibrio parahaemolyticus in marine and freshwater fish. Front Microbiol. 2018;9:2513.
Kang CH, Shin Y, Jang S, Yu H, Kim S, An S, So JS. Characterization of Vibrio parahaemolyticus isolated from oysters in Korea: resistance to various antibiotics and prevalence of virulence genes. Marine Poll Bull. 2017;118(1–2):261–6.
Zhu ZM, Dong CF, Weng SP, He JG. The high prevalence of pathogenic Vibrio harveyi with multiple antibiotic resistance in scale drop and muscle necrosis disease of the hybrid grouper, Epinephelus fuscoguttatus (♀) × E. lanceolatus (♂), in China. J Fish Dis. 2017;19(4):191–8.
Krumperman PH. Multiple antibiotic indexing of E. coli to identify high-risk sources of fecal contamination of foods. Appl Environ Microbiol. 1985;46:165–70.
Ruwandeepika HAD, Defoirdt T, Bhowmick PP, Shekar M, Bossier P, Karunasagar I. Presence of typical and atypical virulence genes in vibrio isolates belonging to the Harveyi clade. J Appl Microbiol. 2010;109(3):888–99.
Hasegawa H, Lind EJ, Boin MA, Häse CC. The extracellular metalloprotease of Vibrio tubiashii is a major virulence factor for pacific oyster (Crassostrea gigas) larvae. Appl Environ Microbiol. 2008;74(13):4101–10.
Defoirdt T, Boon N, Sorgeloos P, Verstraete W, Bossier P. Quorum sensing and quorum quenching in Vibrio harveyi: lessons learned from in vivo work. ISME J. 2008;2:19–26.
Croxatto A, Chalker VJ, Lauritz J, Jass J, Hardman A, Williams P, Camara M, Milton DL. VanT, a homologue of Vibrio harveyi LuxR, regulates serine, metalloprotease, pigment, and biofilm production in Vibrio anguillarum. J Bacteriol. 2002;184(6):1617–29.
Bai F, Pang L, Qi Z, Chen J, Austin B, Zhang XH. Distribution of five vibrio virulence-related genes among Vibrio harveyi isolates. J Gen Appl Microbiol. 2008;54(1):71–8.
Chen Q, Yan Q, Wang K, Zhuang Z, Wang X. Portal of entry for pathogenic Vibrio alginolyticus into large yellow croaker Pseudosciaena crocea, and characteristics of bacterial adhesion to mucus. Dis Aquat Org. 2008;80(3):181–8.
Bej AK, Patterson DP, Brasher CW, Vickery MCL, Jones DD, Kaysner CA. Detection of total and hemolysin-producing Vibrio parahaemolyticus in shellfish using multiplex PCR amplification of tl, tdh and trh. J Microbiol Methods. 1999;36:215–25.
Sujeewa AK, Norrakiah AS, Laina M. Prevalence of toxic genes of Vibrio parahaemolyticus in shrimps (Penaeus monodon) and culture environment. Int Food Res J. 2009;16:89–95.
Al-Othrubi SM, Kqueen CY, Mirhosseini H, Hadi YA, Radu S. Antibiotic resistance of Vibrio parahaemolyticus isolated from cockles and shrimp sea food marketed in Selangor, Malaysia. Clin Microbiol. 2014;3:148–54.
Letchumanan V, Yin WF, Lee LH, Chan KG. Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from retail shrimps in Malaysia. Front Microbiol. 2015;6:33.
Nakamura Y, Itoh T, Matsuda H, Gojobori T. Biased biological functions of horizontally transferred genes in prokaryotic genomes. Nat Genet. 2004;36:760–6.
Rico A, Satapornvanit K, Haque MM, Min J, Nguyen PT, Telfer TC, van den Brink PJ. Use of chemicals and biological products in Asian aquaculture and their potential environmental risks: a critical review. Rev Aquacult. 2012;4:75–93.
You KG, Bong CW, Lee CW. Antibiotic resistance and plasmid profiling of Vibrio spp. in tropical waters of peninsular Malaysia. Environ Monit Assess. 2016;188(3):171.
Ransangan J, Imm LKL, Lal TM, Sade A. Phenotypic characterization and antibiotic susceptibility of Vibrio spp. isolated from aquaculture waters on the west coast of Sabah, Malaysia. Int J Res Pure Appl Microbiol. 2013;3(3):58–66.
Zanetti S, Spanu T, Deriu A, Romano L, Sechi LA, Fadda G. In vitro susceptibility of Vibrio spp. isolated from the environment. Int J Antimicrob Agents. 2001;17(5):407-9.
Fernández-Delgado M, Suárez P, Giner S, Sanz V, Peña J, Sánchez D, García-Amado MA. Occurrence and virulence properties of Vibrio and Salinivibrio isolates from tropical lagoons of the southern Caribbean Sea. Antonie Van Leeuwenhoek. 2017;110(6):833–41.
Igbinosa EO. Detection and antimicrobial resistance of Vibrio isolates in aquaculture environments: implications for public health. Microb Drug Resist. 2016;22(3):238–45.
Nguyen HNK, Van TTH, Coloe PJ. Antibiotic resistance associated with aquaculture in Vietnam. Microbiol Australia. 2016;37(3):108–11.
WHO. Report of a joint FAO/OIE/WHO Expert Consultation on Antimicrobial Use in Aquaculture and Antimicrobial Resistance. Seoul: World Health Organization; 2006.
Ina-Salwany MY, Nurhidayu A, Aslah M, Fathin-Amirah M, Aslizah MA, Amal MNA, Hisae K, Sayaka M, Tomoo S, Zamri-Saad M. Vibriosis in fish: a review on disease development and prevention. J Aquat Anim Health. 2019;31:3-22.
Nurliyana M, Amal MNA, Zamri-Saad M, Ina-Salwany MY. Possible transmission routes of Vibrio spp. in tropical cage-cultured marine fishes. Lett Appl Microbiol. 2019;68(6):485-96.
Nurliyana M, Mustafa M, Amal MNA, Mohd-Zamri S, Ina-Salwany MY, Al-saari N. Environmental factors associated with the presence of Vibrionaceae in tropical cage-cultured marine fishes. J Aquat Anim Health. 2019. https://doi.org/10.1002/aah.10062.
Kumar S, Stecher G, Tamura K. MEGA 7: molecular evolutionary genetics analysis version 7.0 for bigger dataset. Mol Biol Evol. 2016;33(7):1870–4.
CLSI. Methods for dilution antimicrobial diltion and disk susceptibility testing of infrequently isolated or fastidious bacteria; approved guideline-2nd ed., CLSI document M45-A2. 2010. Clinical and laboratory standards institute, Wayne, Pennsylvania 19087, USA.
Ottaviani D, Bacchiocchi I, Masini L, Leoni F, Carraturo A, Giammarioli M, Sbaraglia G. Antimicrobial susceptibility of potentially pathogenic halophilic vibrios isolated from sea- food. Int J Antimicrob Agents. 2001;18:135–40.
CLSI. Performance standards for antimicrobial susceptibility testing; twenty-third informational supplement, CLSI document M100-S23. 2013. Clinical and laboratory standards institute, Wayne, Pennsylvania 19087, USA.
Bauer AW, Kirby WMM, Shenis JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493.
Saravanan V, Sanath KH, Karunasagar I, Karunasagar I. Putative virulence genes of Vibrio cholerae from seafoods and the coastal environment of Southwest India. Int J Food Microbiol. 2007;119:329–33.
Miller VL, Taylor RK, Mekalanos JJ. Cholera toxin transcriptional activator ToxR is a transmembrane DNA binding protein. Cell. 1987;48:271–9.
Acknowledgements
The authors are grateful to farmers that helping the sampling activities.
Funding
This work was partially supported by the Universiti Putra Malaysia (GP-IPB/2016/9484101 and GP-IPS/2018/9619200) and the Higher Institution Centre of Excellence, the Ministry of Higher Education (Vote no.: 6369100). The funding sources had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
NM, MNAA, ISMY and NAZ conducted the bacterial sampling activities. NM and NAZ conducted laboratory works. NM, MNAA and NSN conducted data analyses. NM drafted the manuscript. MNAA, ISMY, MZS, MM and NSN were involved in critical reading, editing and final approval of the submitted version.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Mohamad, N., Amal, M.N.A., Saad, M.Z. et al. Virulence-associated genes and antibiotic resistance patterns of Vibrio spp. isolated from cultured marine fishes in Malaysia. BMC Vet Res 15, 176 (2019). https://doi.org/10.1186/s12917-019-1907-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-019-1907-8