Abstract
Background
The study highlights the shedding pattern of Senecavirus A (SVA) during an outbreak of vesicular disease in a sow farm from the South-central Minnesota, USA. In this study, 34 individual, mixed parity sows with clinical signs of vesicular lesions and 30 individual piglets from 15 individual litters from sows with vesicular lesions were conveniently selected for individual, longitudinal sampling. Serum, tonsil, rectal, and vesicular swabs were collected on day1 post outbreak, and then again at 1, 2, 3, 4, 6, and 9 weeks post outbreak. Samples were tested at the University of Minnesota Veterinary Diagnostic Laboratory for SVA via Real Time Polymerase Chain Reaction (RT-PCR)
Results
In sows, vesicular lesions had the highest concentration of SVA, but had the shortest duration of detection lasting only 2 weeks. Viremia was detected for 1 week post outbreak, and quickly declined thereafter. SVA was detected at approximately the same frequency for both tonsil and rectal swabs with the highest percentage of SVA positive samples detected in the first 6 weeks post outbreak. In suckling piglets, viremia quickly declined 1 week post outbreak and was prevalent in low levels during the first week after weaning (4 weeks post outbreak) and was also detected in piglets that were co-mingled from a SVA negative sow farm. Similar to sows, SVA detection on rectal and tonsil swabs in piglets lasted approximately 6 weeks post outbreak.
Conclusion
The study illustrates the variation of SVA shedding patterns in different sample types over a 9 week period in sows and piglets, and suggests the potential for viral spread between piglets at weaning.
Similar content being viewed by others
Background
Senecavirus A (SVA) or commonly known as Seneca Valley Virus belongs to the family Pircornaviridae and has been associated with idiopathic vesicular lesions on the snout and coronary bands in swine [3, 8]. In 2014, SVA outbreaks were reported in Brazil and subsequent SVA outbreaks occurred in the United States (US) during the 2015 [2, 4, 5, 11, 12]. While not a new virus to the US or the world, the alarmingly high rate of severe clinical disease anecdotally reported in Brazil and the US, suggests a change in the epidemiology of SVA, such as the emergence of a new variant strain [7, 8, 10]. Some swine farms report reproductive issues, and weak piglets suffering from secondary infections in the nursery [9]. Vesicular disease caused by SVA infection cannot be differentiated from other US Foreign Animal Diseases (FADs), such as foot-and-mouth disease (FMD), swine vesicular disease (SVD), vesicular stomatitis, and vesicular exanthema. Therefore, SVA has important implications in the transport and slaughter of pigs, potentially leading to significant complications with international trade.
In early September of 2015, a sow farm in South-central Minnesota, US, exhibited the sudden and dramatic onset of severe vesicular lesions in nearly 80% of all sows on the site. Due to the relative paucity of information regarding this virus in the field, the objectives of this study were to assess the shedding patterns of SVA in a population of sows and suckling piglets, and assess the potential spread of SVA in weaned pigs. Not only will this information provide valuable insight into the infection dynamics of SVA within a population, but it may also aid in future FADs investigations, as well as assisting in the development of better control and elimination measures of SVA in sow herds.
Methods
A farm in South central Minnesota, US, containing two geographically different sow sites (sites 1 and 2, Fig. 1) consisting of 1300 sows per site were selected for this study based on the outbreak of vesicular lesions at one of the sow sites (site 1). This farm maintains ownership of pigs throughout all life stages, and at the time of weaning, piglets are transported from sites 1 and 2 to multiple different weaned pig sites (site 3, 4, 5, and 6) within their company (Fig. 1).
Upon visual detection of vesicular lesions at site 1 (day 0 post outbreak (PO)), a FADs investigation was initiated and samples were collected by the state veterinary office and sent to the National Veterinary Services Laboratories (NVSL), the Foreign Animal Disease Diagnostic Laboratory at Plum Island Animal Disease Center and the University of Minnesota Veterinary Diagnostic Laboratory (UMNVDL). At NVSL, the samples were tested for Foot-and-Mouth Disease virus (FMDv), SVA, and Swine Vesicular Disease virus (SVDv) by real time RT-PCR. At the UMNVDL, the samples were tested for FMDv according the United States Department of Agriculture Standard Operation Procedure (SOP) titled, “Extraction of Total RNA Using a MagMax-96 Total RNA Isolation Kit for the Detection of Classical Swine Fever and Foot-and-Mouth Disease Viruses” (SOP-PVS-0004.1), which is publically available from the NVSL upon request. The detection of SVA occurred by real time Reverse Transcription Polymerase Chain Reaction (RT-PCR) using AgPath-ID One-Step RT-PCR Reagents (Thermo), forward (5′- TCTCTTGCCCTAACACTGGGG-3′) and reverse primers (5′- CTTGCCTCTAAGGACCACCACA-3′) and probe (5′- TGGCCCAAA/ZEN/GTCTCACCACTATGATCAATG-3′) with the following thermal cycling parameters: reverse transcription, 10 min at 45 °C; Taq activation, 10 min at 95 °C; followed by 40 cycles of 15 s at 95 °C, and 45 s at 60 °C.
On the second day post visualization of vesicular disease (day 1 PO), 34 individual, mixed parity sows in gestation from site 1 were conveniently selected based on the presence of vesicular lesions on their snouts and feet. In addition, 30 mixed gender, neonatal piglets from 15-mixed parity litters showing similar clinical signs were conveniently selected and tagged at site 1 between approximately 12 and 24 h of age. Tonsil, rectal, vesicular swabs (when vesicles were present), and serum samples were collected on day 1 and then 1, 2, 3, 4, 6, and 9 weeks PO from both sows and piglets. Last samples were collected at 9 weeks PO and did not occur at weeks 5, 7, and 8 due to concerns of increased stress of the pigs due to sampling. Vesicular swab samples were not collected from the piglets since they lacked clinical vesicular lesions. At week 3 PO (at weaning), the 30 piglets from site 1 were transported to site 6 and were comingled with 30 individually identified mixed gender pigs from site 2 and sampled at 4, 6, and 9 weeks PO (Fig. 1). In addition, at day 0 PO, 10 serum samples and a single oral fluid sample were collected (from three cotton ropes hung in pens within each site) at sites 3, 4, and 5 (Fig. 1) to determine if SVA was present in earlier groups of weaned pigs from before the detection of clinical signs in the system.
Samples were refrigerated overnight and sent next day to the University of Minnesota Veterinary Diagnostic Laboratory. The RNA was extracted using previously described methods [6] for SVA by real time RT-PCR.
Results
The swine farm initially reported concerns of increased lameness in a pen of group housed replacement gilts, as well as a slight decrease in feed intake (day −2 PO). Day −1 PO, lameness and decreased feed intake was more prevalent among the rest of the sows in the herd. Vesicular lesions were observed on day 0 PO, consisting of un-ruptured vesicles on the snouts and feet of approximately 5–10% of the animals on site 1, and the state veterinary office was notified. By day 2 PO, nearly 80% of the animals in the herd had detectable vesicular lesions (ruptured and un-ruptured) on the snouts and feet. Lameness, lethargy, and decreased feed intake were the main clinical concerns during this time; however, by 1 week PO, there was noticeable improvement in the clinical signs within the population. At no time was fever a major concern within the herd. In farrowing, there was an increase in pre-weaning mortality from an average of 12% to a high of 22% during the first 7 days PO, and returned to average levels by 2 weeks PO. The majority of the piglet mortality was associated with dehydration due to poor milk production by the sow, and piglets being laid on, potentially due to sow lameness. No other clinical signs were detected in piglets in farrowing, and there were no detectable signs at the nursery barns at any point during or after the outbreak.
The samples collected by the state veterinary office at site 1 on the day 0 PO were negative for SVDv at NVSL while the NVSL and UMNVDL had the same results for FMDv and SVA (negative and positive, respectively). Subsequent, SVA testing only occurred at the UMNVDL through the study.
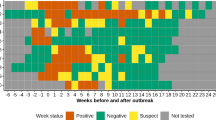
Sows displayed vesicular lesions on the snouts at day 0 PO, and SVA was present in 33 out of 34 (97%) of the vesicle swabs, with an average cycle threshold (Ct) value of 16 (range = 12–19). At 1 week PO, SVA was present in 34 out of 34 (100%) of the lesion swabs. At 2 weeks PO, most lesions had healed (only few skin tags and ruptured vesicles remained in the population), and SVA detection decreased to 12 out of 34 samples (35%). By 3 weeks PO, all of the vesicular lesions had healed, and no additional samples were collected.
Generally, viremia was detected up to 1 week PO in sows. A single positive sample was identified at 3 and 6 weeks PO (Fig. 2a). At the first sampling (day 1 PO), the Ct values from the sow serum averaged 33.1 (range = 17–36). Viremia was detected in only 7 out of 34 (20%) of the sows at 1 week PO. SVA viremia was not detected in 11 out of 34 (32%) of the sows at any point during the study. Viremia was detected in 18 out of 30 (60%) and 19 out of 30 (63%) in the suckling piglets from site 1. Additional SVA positive samples were detected at 3, 4, and 6 weeks PO. In suckling piglets, Ct values averaged 30.6 (range = 24–39.8). Similar to sows, viremia was not detected in 9 out of 30 (30%) of the site 1 piglets enrolled in the study. Viremia was detected in site 2 piglets at 4 (6 out of 30 (21%)), 6 (2 out of 30 (7%)), and 9 (1 out of 30 (3%)) weeks PO, with an average Ct value range of 35.7–38.0.
Tonsil swabs yielded the highest percentage of SVA detection in site 1 sows (32 out 34 (94%)) and piglets (25 out of 30 (83%))(Fig. 2b). In sows, the detection of SVA in the tonsils peaked at 1 week PO (33 out of 34 samples (97%)), with average Ct values of 24.8 (range = 20–27). In site 1 piglets, the peak shedding of SVA occurred at day 1 PO, with average Ct values of 27.1 (range = 20.3–34.6). The detection of SVA shedding decreased over time in sows and piglets, and a single sow and piglet tested positive at 9 weeks PO (Ct values of 35 and 36, respectively). SVA was only detected in 2 of the 30 piglets (7%) from site 2 at 5 weeks PO.
The peak of SVA detection from rectal swabs in sows (31 out of 34 samples (91%)) occurred at day 1 PO and continued to steadily decrease and was not detected at 9 weeks PO (Fig. 2c). In site 1 piglets, the detection of SVA peaked at 1 week PO when 27 of the 30 samples (90%) tested positive. While the detection of SVA decreased in site 1 piglets at 2 and 3 weeks PO, 19 out of 30 samples (64%) of the rectal swabs were positive at 4 weeks PO. At 6 weeks PO, the detection of SVA was same for both site 1 and 2 piglets (11%, n = 3); however, a single piglet from site 1 was still shedding SVA at 9 weeks PO.
The oral fluid and serum samples were positive for SVA from site 5, but negative from site 3 and 4. SVA was detected in 9 out of 10 (90%) of the serum samples (Ct values averaged 32.8, range = 31.8–35.9), and the single oral fluid sample had a (Ct value = 24.3), despite the fact that there were no obvious detectable clinical signs in site 5. Of importance is that site 5 appears to have received SVA positive weaned pigs from site 1 before the detection of clinical signs of decrease in feed intake and vesicular lesions.
Discussion
The study assessed the shedding pattern of SVA in sows and piglets during an outbreak on a farm in the US and investigated the spread of SVA between pigs during the post weaning period. In addition, the study suggests the spread of SVA to distant pig sites via piglets before the onset of vesicular lesions.
Fresh vesicular lesions on the sows had the greatest amounts of SVA; however, the lesions were only present for a very short period of time (2 weeks). This is important because state and federal veterinarians conducting the FAD investigations are trained to identify vesicles and collect their fluid. However, these data suggest a lower rate of detection of SVA in ruptured vesicles and dried skin tags (that are present after the first week of the outbreak) when compared to tonsil and rectal swabs, especially later in the outbreak when vesicles were no longer present. In sows, the detection of SVA in tonsil and rectal swabs was greater than 90% at 0 week PO and remained as high as 50% through 5 weeks PO, these sample types should be collected and submitted, in addition to vesicular lesion swabs and fluid (if present), as part of FAD investigations for the detection of SVA. In addition, the detection of SVA in oral fluid samples collected from site 5 and the high percentage of positive SVA tonsillar swabs suggests they may be valuable tools for the detection of SVA in pigs.
Surprisingly, SVA was detected in serum and oral fluids from site 5, which had received weaned pigs approximately −2 weeks PO, before the detection of clinical signs at site 1. Furthermore, obviously detectable clinical signs were lacking in piglets at site 5, indicating the potential to spread SVA without realization through weaned pig movements. This is a major concern for epidemiologists as well as state and federal veterinarians because the prevalence of SVA within the US swine herd could be greatly underestimated. Therefore, as part of any epidemiological investigation of SVA outbreaks (particularly on sow farms), a social network analysis, which details all pig movements to the level of the specific trailers and trucks used during the previous month should be conducted to identify the potential scope of virus spread.
Commingling of piglets at weaning is common in the US swine industry. When the piglets were commingled at week 4 PO (approximately 1 week after weaning), 12 of the 30 (40%) of the piglets sampled from sites 1 and 2 were viremic. Interestingly, site 1 and 2 piglets had the same level SVA shedding in the tonsil and rectal swabs (2 out of 30 (7%), and 3 out of 30 (10%), respectively) at 6 weeks PO. These results suggest the potential spread of SVA to site 2 piglets, and SVA may be transmitted during commingling of piglets. Therefore, caution should be exercised when considering the movement of weaned pigs during an outbreak, which includes avoiding the comingling of piglets from different sow farms.
While SVA was detected in site 2 piglets, the levels of virus were minimal in the tonsil and rectal swabs potentially implying some underlying immunity to SVA in site 2 piglets that limited viral infection and shedding. While site 2 tested negative by PCR, indicating that the herd was not infected at that time, a proper immunological assay, which may help elucidate if the herd had been infected in the past and has some level of immunity, is lacking at the time of writing this manuscript. With that in mind, immunological assays including several different ELISA targets are currently under development at several US veterinary diagnostic laboratories [1]. In addition, these immunological assays would help to understand the prevalence of this virus in the US swine herd (in particular sow herds), which would provide better planning and allocation of resources for future SVA outbreaks that would initiate FAD investigations needed by state and federal veterinarians.
Our results suggest that SVA can be detected in a population of sows in tonsil and rectal swabs for up to 9 weeks; however, the viability of SVA at 9 weeks is currently unknown. Future studies should be developed to discern the viability of the virus from these samples, especially in the later stages of the outbreak. This information would be beneficial to veterinarians working on eliminating SVA from affected sow farms since it would dictate the duration of sow herd closure (no entry of potentially immunologically naïve animals for the duration of the shedding period) in order to elimination the virus from that population. Additionally, as part of any plan to eliminate SVA from sow farms, achieving rapid and homogeneous exposure of all animals is crucial. Therefore, studies are needed to identify the most efficient route of SVA exposure to eliminate the virus from infected sow herds.
Conclusion
In conclusion, this study highlights the detection of SVA in vesicular lesions, serum, tonsil and rectal swabs and suggests the spread of SVA between piglets during the post weaning period. In addition, this study suggests that tonsil and rectal swabs, and oral fluids may be useful for monitoring populations SVA positive animals over time.
Abbreviations
- CT:
-
Cycle threshold
- FAD:
-
Foreign Animal Disease
- FMD:
-
Foot-and-mouth disease
- NVSL:
-
National veterinary service laboratories
- PO:
-
Post outbreak
- RT-PCR:
-
Reverse Transcription Polymerase Chain Reaction
- SOP:
-
Standard operating procedure
- SVA:
-
Senecavirus A
- SVD:
-
Swine vesicular disease
- US:
-
United States
References
Gimenez-Lirola LG, Rademacher C, Linhares D, Harmon K, Rotolo M, Sun Y, Baum DH, Zimmerman J, Pineyro P. Serological and molecular detection of Senecavirus a associated with an outbreak of swine idiopathic vesicular disease and neonatal mortality. J Clin Microbiol. 2016;54:2082–9.
Guo B, Pineyro PE, Rademacher CJ, Zheng Y, Li G, Yuan J, Hoang H, Gauger PC, Madson DM, Schwartz KJ, Canning PE, Arruda BL, Cooper VL, Baum DH, Linhares DC, Main RG, Yoon KJ. Novel Senecavirus a in swine with vesicular disease, United States, July 2015. Emerg Infect Dis. 2016;22:1325–7.
Hales LM, Knowles NJ, Reddy PS, Xu L, Hay C, Hallenbeck PL. Complete genome sequence analysis of Seneca Valley virus-001, a novel oncolytic picornavirus. J Gen Virol. 2008;89:1265–75.
Leme RA, Oliveira TE, Alfieri AF, Headley SA, Alfieri AA. Pathological, Immunohistochemical and molecular findings associated with Senecavirus A-induced lesions in neonatal piglets. J Comp Pathol. 2016;155(2-3):145–55.
Leme RA, Zotti E, Alcantara BK, Oliveira MV, Freitas LA, Alfieri AF, Alfieri AA. Senecavirus a: an emerging vesicular infection in Brazilian pig herds. Transbound Emerg Dis. 2015;62:603–11.
Marthaler D, Rossow K, Gramer M, Collins J, Goyal S, Tsunemitsu H, Kuga K, Suzuki T, Ciarlet M, Matthijnssens J. Detection of substantial porcine group B rotavirus genetic diversity in the United States, resulting in a modified classification proposal for G genotypes. Virology. 2012;433:85–96.
Munday BL, Ryan FB. Vesicular lesions in swine – possible association with feeding of marine products. Aust Vet J. 1982;59:193.
Pasma T, Davidson S, Shaw SL. Idiopathic vesicular disease in swine in Manitoba. Can Vet J. 2008;49:84–5.
Segales J, Barcellos D, Alfieri A, Burrough E, Marthaler D. Senecavirus A. Vet Pathol. 2017;54(1):11–21.
Sensi M, Catalano A, Dinaro M, Mariotti C, Panieri C, Marchi S, Costelli S. Idiopathic vesicular disease (IVD): a case report in the centre of Italy. Proc Int Pig Vet Soc 005. 2010. p46
Vannucci FA, Linhares DC, Barcellos DE, Lam HC, Collins J, Marthaler D. Identification and complete genome of Seneca Valley virus in vesicular fluid and sera of pigs affected with idiopathic vesicular disease. Brazil Transbound Emerg Dis. 2015;62:589–93.
Zhang J, Pineyro P, Chen Q, Zheng Y, Li G, Rademacher C, Derscheid R, Guo B, Yoon KJ, Madson D, Gauger P, Schwartz K, Harmon K, Linhares D, Main R. Full-length genome sequences of Senecavirus a from recent idiopathic vesicular disease outbreaks in U.S. swine. Genome Announc. 2015;3 10.1128/genomeA.01270-15
Acknowledgments
We would like to thank the following people that have contributed time, resources, thoughts and permission for this study, specifically the owner and staff of the sow farm, the veterinarians caring for the animals including, Sam Holst of the Swine Vet Center, and all the staff at the University of Minnesota Veterinary Diagnostic Laboratory, especially the Molecular Diagnostic section.
Funding
The sample collection and veterinary services were supported by the Swine Health Information Center while the University of Minnesota Veterinary Diagnostic Laboratory supported the diagnostic testing.
Availability of data and materials
The datasets analyzed during the current study available from the corresponding author on reasonable request.
Contact information for the National Veterinary Services Laboratory and information regarding the SOP-PVS-0004.1 for the detection of Classical Swine Fever and Foot and Mouth Disease can be found at: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/lab-info-services/sa_about_nvsl/ct_about_nvsl.
Author information
Authors and Affiliations
Contributions
ST and DM contributed to study design, drafted and revised the manuscript. ST, LB and JS contributed to the study design, and oversaw data collection. FV and SR assisted with analysis and interpretation of data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
During the outbreak, the health of the pigs and sampling was managed under the supervision of veterinarians from Swine Vet Center. The client-owner of the pigs consented to sample collections.
Consent for publication
The authors received consent from the farmer to publish this manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Tousignant, S.J.P., Bruner, L., Schwartz, J. et al. Longitudinal study of Senecavirus a shedding in sows and piglets on a single United States farm during an outbreak of vesicular disease. BMC Vet Res 13, 277 (2017). https://doi.org/10.1186/s12917-017-1172-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-017-1172-7