Abstract
Background
Adverse childhood experiences (ACEs) have been implicated in the aetiology of a range of health outcomes, including multimorbidity. In this systematic review and meta-analysis, we aimed to identify, synthesise, and quantify the current evidence linking ACEs and multimorbidity.
Methods
We searched seven databases from inception to 20 July 2023: APA PsycNET, CINAHL Plus, Cochrane CENTRAL, Embase, MEDLINE, Scopus, and Web of Science. We selected studies investigating adverse events occurring during childhood (< 18 years) and an assessment of multimorbidity in adulthood (≥ 18 years). Studies that only assessed adverse events in adulthood or health outcomes in children were excluded. Risk of bias was assessed using the ROBINS-E tool. Meta-analysis of prevalence and dose–response meta-analysis methods were used for quantitative data synthesis. This review was pre-registered with PROSPERO (CRD42023389528).
Results
From 15,586 records, 25 studies were eligible for inclusion (total participants = 372,162). The prevalence of exposure to ≥ 1 ACEs was 48.1% (95% CI 33.4 to 63.1%). The prevalence of multimorbidity was 34.5% (95% CI 23.4 to 47.5%). Eight studies provided sufficient data for dose–response meta-analysis (total participants = 197,981). There was a significant dose-dependent relationship between ACE exposure and multimorbidity (p < 0.001), with every additional ACE exposure contributing to a 12.9% (95% CI 7.9 to 17.9%) increase in the odds for multimorbidity. However, there was heterogeneity among the included studies (I2 = 76.9%, Cochran Q = 102, p < 0.001).
Conclusions
This is the first systematic review and meta-analysis to synthesise the literature on ACEs and multimorbidity, showing a dose-dependent relationship across a large number of participants. It consolidates and enhances an extensive body of literature that shows an association between ACEs and individual long-term health conditions, risky health behaviours, and other poor health outcomes.
Similar content being viewed by others
Background
In recent years, adverse childhood experiences (ACEs) have been identified as factors of interest in the aetiology of many conditions [1]. ACEs are potentially stressful events or environments that occur before the age of 18. They have typically been considered in terms of abuse (e.g. physical, emotional, sexual), neglect (e.g. physical, emotional), and household dysfunction (e.g. parental separation, household member incarceration, household member mental illness) but could also include other forms of stress, such as bullying, famine, and war. ACEs are common: estimates suggest that 47% of the UK population have experienced at least one form, with 12% experiencing four or more [2]. ACEs are associated with poor outcomes in a range of physical health, mental health, and social parameters in adulthood, with greater ACE burden being associated with worse outcomes [1,2,3,4,5,6,7,8].
Over a similar timescale, multimorbidity has emerged as a significant heath challenge. It is commonly defined as the co-occurrence of two or more long-term conditions (LTCs), with a long-term condition defined as any physical or mental health condition lasting, or expected to last, longer than 1 year [9]. Multimorbidity is both common and age-dependent, with a global adult prevalence of 37% that rises to 51% in adults over 60 [10, 11]. Individuals living with multimorbidity face additional challenges in managing their health, such as multiple appointments, polypharmacy, and the lack of continuity of care [12,13,14]. Meanwhile, many healthcare systems struggle to manage the additional cost and complexity of people with multimorbidity as they have often evolved to address the single disease model [15, 16]. As global populations continue to age, with an estimated 2.1 billion adults over 60 by 2050, the pressures facing already strained healthcare systems will continue to grow [17]. Identifying factors early in the aetiology of multimorbidity may help to mitigate the consequences of this developing healthcare crisis.
Many mechanisms have been suggested for how ACEs might influence later life health outcomes, including the risk of developing individual LTCs. Collectively, they contribute to the idea of ‘toxic stress’; cumulative stress during key developmental phases may affect development [18]. ACEs are associated with measures of accelerated cellular ageing, including changes in DNA methylation and telomere length [19, 20]. ACEs may lead to alterations in stress-signalling pathways, including changes to the immune, endocrine, and cardiovascular systems [21,22,23]. ACEs are also associated with both structural and functional differences in the brain [24,25,26,27]. These diverse biological changes underpin psychological and behavioural changes, predisposing individuals to poorer self-esteem and risky health behaviours, which may in turn lead to increased risk of developing individual LTCs [1, 2, 28,29,30,31,32]. A growing body of evidence has therefore led to an increased focus on developing trauma-informed models of healthcare, in which the impact of negative life experiences is incorporated into the assessment and management of LTCs [33].
Given the contributory role of ACEs in the aetiology of individual LTCs, it is reasonable to suspect that ACEs may also be an important factor in the development of multimorbidity. Several studies have implicated ACEs in the aetiology of multimorbidity, across different cohorts and populations, but to date no meta-analyses have been performed to aggregate this evidence. In this review, we aim to summarise the state of the evidence linking ACEs and multimorbidity, to quantify the strength of any associations through meta-analysis, and to highlight the challenges of research in this area.
Methods
Search strategy and selection criteria
We conducted a systematic review and meta-analysis that was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO) on 25 January 2023 (ID: CRD42023389528) and reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
We developed a search strategy based on previously published literature reviews and refined it following input from subject experts, an academic librarian, and patient and public partners (Additional File 1: Table S1). We searched the following seven databases from inception to 20 July 2023: APA PsycNET, CINAHL Plus, Cochrane CENTRAL, Embase, MEDLINE, Scopus, and Web of Science. The search results were imported into Covidence (Veritas Health Innovation, Melbourne, Australia), which automatically identified and removed duplicate entries. Two reviewers (DS and BT) independently performed title and abstract screening and full text review. Discrepancies were resolved by a third reviewer (LC).
Reports were eligible for review if they included adults (≥ 18 years), adverse events occurring during childhood (< 18 years), and an assessment of multimorbidity or health status based on LTCs. Reports that only assessed adverse events in adulthood or health outcomes in children were excluded.
The following study designs were eligible for review: randomised controlled trials, cohort studies, case–control studies, cross-sectional studies, and review articles with meta-analysis. Editorials, case reports, and conference abstracts were excluded. Systematic reviews without a meta-analysis and narrative synthesis review articles were also excluded; however, their reference lists were screened for relevant citations.
Data analysis
Two reviewers (DS and BT) independently performed data extraction into Microsoft Excel (Microsoft Corporation, Redmond, USA) using a pre-agreed template. Discrepancies were resolved by consensus discussion with a third reviewer (LC). Data extracted from each report included study details (author, year, study design, sample cohort, sample size, sample country of origin), patient characteristics (age, sex), ACE information (definition, childhood cut-off age, ACE assessment tool, number of ACEs, list of ACEs, prevalence), multimorbidity information (definition, multimorbidity assessment tool, number of LTCs, list of LTCs, prevalence), and analysis parameters (effect size, model adjustments). For meta-analysis, we extracted ACE groups, number of ACE cases, number of multimorbidity cases, number of participants, odds ratios or regression beta coefficients, and 95% confidence intervals (95% CI). Where data were partially reported or missing, we contacted the study authors directly for further information.
Two reviewers (DS and BT) independently performed risk of bias assessments of each included study using the Risk Of Bias In Non-randomized Studies of Exposures (ROBINS-E) tool [34]. The ROBINS-E tool assesses the risk of bias for the study outcome relevant to the systematic review question, which may not be the primary study outcome. It assesses risk of bias across seven domains; confounding, measurement of the exposure, participant selection, post-exposure interventions, missing data, measurement of the outcome, and selection of the reported result. The overall risk of bias for each study was determined using the ROBINS-E algorithm. Discrepancies were resolved by consensus discussion.
All statistical analyses were performed in R version 4.2.2 using the RStudio integrated development environment (RStudio Team, Boston, USA). To avoid repetition of participant data, where multiple studies analysed the same patient cohort, we selected the study with the best reporting of raw data for meta-analysis and the largest sample size. Meta-analysis of prevalence was performed with the meta package [35], using logit transformations within a generalised linear mixed model, and reporting the random-effects model [36]. Inter-study heterogeneity was assessed and reported using the I2 statistic, Cochran Q statistic, and Cochran Q p-value. Dose–response meta-analysis was performed using the dosresmeta package [37] following the method outlined by Greenland and Longnecker (1992) [38, 39]. Log-linear and non-linear (restricted cubic spline, with knots at 5%, 35%, 65%, and 95%) random effects models were generated, and goodness of fit was evaluated using a Wald-type test (denoted by X2) and the Akaike information criterion (AIC) [39].
Patient and public involvement
The Consortium Against Pain Inequality (CAPE) Chronic Pain Advisory Group (CPAG) consists of individuals with lived experiences of ACEs, chronic pain, and multimorbidity. CPAG was involved in developing the research question. The group has experience in systematic review co-production (in progress).
Results
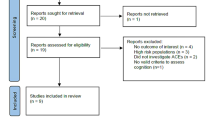
The search identified 15,586 records, of which 25 met inclusion criteria for the systematic review (Fig. 1) [40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. The summary characteristics can be found in Additional File 1: Table S2. Most studies examined European (n = 11) or North American (n = 9) populations, with a few looking at Asian (n = 3) or South American (n = 1) populations and one study examining a mixed cohort (European and North American populations). The total participant count (excluding studies performed on the same cohort) was 372,162. Most studies had a female predominance (median 53.8%, interquartile range (IQR) 50.9 to 57.4%).
All studies were observational in design, and so risk of bias assessments were performed using the ROBINS-E tool (Additional File 1: Table S3) [34]. There were some consistent risks observed across the studies, especially in domain 1 (risk of bias due to confounding) and domain 3 (risk of bias due to participant selection). In domain 1, most studies were ‘high risk’ (n = 24) as they controlled for variables that could have been affected by ACE exposure (e.g. smoking status) [40, 41, 43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. In domain 3, some studies were ‘high risk’ (n = 7) as participant selection was based on participant characteristics that could have been influenced by ACE exposure (e.g. through recruitment at an outpatient clinic) [45, 48, 49, 51, 53, 54, 58]. The remaining studies were deemed as having ‘some concerns’ (n = 18) as participant selection occurred at a time after ACE exposure, introducing a risk of survivorship bias [40,41,42,43,44, 46, 47, 50, 52, 55,56,57, 59,60,61,62,63,64].
Key differences in risk of bias were seen in domain 2 (risk of bias due to exposure measurement) and domain 5 (risk of bias due to missing data). In domain 2, some studies were ‘high risk’ as they used a narrow or atypical measure of ACEs (n = 8) [40, 42, 44, 46, 55, 56, 60, 64]; others were graded as having ‘some concerns’ as they used a broader but still incomplete measure of ACEs (n = 8) [43, 45, 48,49,50, 52, 54, 62]; the remainder were ‘low risk’ as they used an established or comprehensive list of ACE questions [41, 47, 51, 53, 57,58,59, 61, 63]. In domain 5, some studies were ‘high risk’ as they failed to acknowledge or appropriately address missing data (n = 7) [40, 42, 43, 45, 51, 53, 60]; others were graded as having ‘some concerns’ as they had a significant amount of missing data (> 10% for exposure, outcome, or confounders) but mitigated for this with appropriate strategies (n = 6) [41, 50, 56, 57, 62, 64]; the remainder were ‘low risk’ as they reported low levels of missing data (n = 12) [44, 46,47,48,49, 52, 54, 55, 58, 59, 61, 63].
Most studies assessed an exposure that was ‘adverse childhood experiences’ (n = 10) [41, 42, 50, 51, 53, 57, 58, 61, 63, 64], ‘childhood maltreatment’ (n = 6) [44,45,46, 48, 49, 59], or ‘childhood adversity’ (n = 3) [47, 54, 62]. The other exposures studied were ‘birth phase relative to World War Two’ [40], ‘childhood abuse’ [43], ‘childhood disadvantage’ [56], ‘childhood racial discrimination’ [55], ‘childhood trauma’ [52], and ‘quality of childhood’ (all n = 1) [60]. More than half of studies (n = 13) did not provide a formal definition of their exposure of choice [42,43,44,45, 49, 52,53,54, 57, 58, 60, 61, 64]. The upper age limit for childhood ranged from < 15 to < 18 years with the most common cut-off being < 18 years (n = 9). The median number of ACEs measured in each study was 7 (IQR 4–10). In total, 58 different ACEs were reported; 17 ACEs were reported by at least three studies, whilst 33 ACEs were reported by only one study. The most frequently reported ACEs were physical abuse (n = 19) and sexual abuse (n = 16) (Table 1). The exposure details for each study can be found in Additional File 1: Table S4.
Thirteen studies provided sufficient data to allow for a meta-analysis of the prevalence of exposure to ≥ 1 ACE; the pooled prevalence was 48.1% (95% CI 33.4 to 63.1%, I2 = 99.9%, Cochran Q = 18,092, p < 0.001) (Fig. 2) [41, 43, 44, 46, 47, 49, 50, 52, 53, 57, 59, 61, 63]. Six studies provided sufficient data to allow for a meta-analysis of the prevalence of exposure to ≥ 4 ACEs; the pooled prevalence was 12.3% (95% CI 3.5 to 35.4%, I2 = 99.9%, Cochran Q = 9071, p < 0.001) (Additional File 1: Fig. S1) [46, 50, 51, 53, 59, 63].
Thirteen studies explicitly assessed multimorbidity as an outcome, and all of these defined the threshold for multimorbidity as the presence of two or more LTCs [40,41,42, 44, 46, 47, 50, 55, 57, 60,61,62, 64]. The remaining studies assessed comorbidities, morbidity, or disease counts [43, 45, 48, 49, 51,52,53,54, 56, 58, 59, 63]. The median number of LTCs measured in each study was 14 (IQR 12–21). In total, 115 different LTCs were reported; 36 LTCs were reported by at least three studies, whilst 63 LTCs were reported by only one study. Two studies did not report the specific LTCs that they measured [51, 53]. The most frequently reported LTCs were hypertension (n = 22) and diabetes (n = 19) (Table 2). Fourteen studies included at least one mental health LTC. The outcome details for each study can be found in Additional File 1: Table S5.
Fifteen studies provided sufficient data to allow for a meta-analysis of the prevalence of multimorbidity; the pooled prevalence was 34.5% (95% CI 23.4 to 47.5%, I2 = 99.9%, Cochran Q = 24,072, p < 0.001) (Fig. 3) [40, 41, 44, 46, 47, 49,50,51,52, 55, 57,58,59,60, 63].
All studies reported significant positive associations between measures of ACE and multimorbidity, though they varied in their means of analysis and reporting of the relationship. Nine studies reported an association between the number of ACEs (variably considered as a continuous or categorical parameter) and multimorbidity [41, 43, 46, 47, 50, 56, 57, 61, 64]. Eight studies reported an association between the number of ACEs and comorbidity counts in specific patient populations [45, 48, 49, 51, 53, 58, 59, 63]. Six studies reported an association between individual ACEs or ACE subgroups and multimorbidity [42,43,44, 47, 55, 62]. Two studies incorporated a measure of frequency within their ACE measurement tool and reported an association between this ACE score and multimorbidity [52, 54]. Two studies reported an association between proxy measures for ACEs and multimorbidity; one reported ‘birth phase relative to World War Two’, and the other reported a self-report on the overall quality of childhood [40, 60].
Eight studies, involving a total of 197,981 participants, provided sufficient data (either in the primary text, or following author correspondence) for quantitative synthesis [41, 46, 47, 49,50,51, 57, 58]. Log-linear (Fig. 4) and non-linear (Additional File 1: Fig. S2) random effects models were compared for goodness of fit: the Wald-type test for linearity was non-significant (χ2 = 3.7, p = 0.16) and the AIC was lower for the linear model (− 7.82 vs 15.86) indicating that the log-linear assumption was valid. There was a significant dose-dependent relationship between ACE exposure and multimorbidity (p < 0.001), with every additional ACE exposure contributing to a 12.9% (95% CI 7.9 to 17.9%) increase in the odds for multimorbidity (I2 = 76.9%, Cochran Q = 102, p < 0.001).
Dose–response meta-analysis of the relationship between adverse childhood experiences and multimorbidity. Dose–response meta-analysis of the relationship between adverse childhood experiences and multimorbidity. Solid black line represents the estimated relationship; dotted black lines represent the 95% confidence intervals for this estimate. ACE, adverse childhood experience
Discussion
This systematic review and meta-analysis synthesised the literature on ACEs and multimorbidity and showed a dose-dependent relationship across a large number of participants. Each additional ACE exposure contributed to a 12.9% (95% CI 7.9 to 17.9%) increase in the odds for multimorbidity. This adds to previous meta-analyses that have shown an association between ACEs and individual LTCs, health behaviours, and other health outcomes [1, 28, 31, 65, 66]. However, we also identified substantial inter-study heterogeneity that is likely to have arisen due to variation in the definitions, methodology, and analysis of the included studies, and so our results should be interpreted with these limitations in mind.
Although 25 years have passed since the landmark Adverse Childhood Experiences Study by Felitti et al. [3], there is still no consistent approach to determining what constitutes an ACE. This is reflected in this review, where fewer than half of the 58 different ACEs (n = 25, 43.1%) were reported by more than one study and no study reported more than 15 ACEs. Even ACE types that are commonly included are not always assessed in the same way [67], and furthermore, the same question can be interpreted differently in different contexts (e.g. physical punishment for bad behaviour was socially acceptable 50 years ago but is now considered physical abuse in the UK). Although a few validated questionnaires exist, they often focus on a narrow range of ACEs; for example, the childhood trauma questionnaire demonstrates good reliability and validity but focuses on interpersonal ACEs, missing out on household factors (e.g. parental separation), and community factors (e.g. bullying) [68]. Many studies were performed on pre-existing research cohorts or historic healthcare data, where the study authors had limited or no influence on the data collected. As a result, very few individual studies reported on the full breadth of potential ACEs.
ACE research is often based on ACE counts, where the types of ACEs experienced are summed into a single score that is taken as a proxy measure of the burden of childhood stress. The original Adverse Childhood Experiences Study by Felitti et al. took this approach [3], as did 17 of the studies included in this review and our own quantitative synthesis. At the population level, there are benefits to this: ACE counts provide quantifiable and comparable metrics, they are easy to collect and analyse, and in many datasets, they are the only means by which an assessment of childhood stress can be derived. However, there are clear limitations to this method when considering experiences at the individual level, not least the inherent assumptions that different ACEs in the same person are of equal weight or that the same ACE in different people carries the same burden of childhood stress. This limitation was strongly reinforced by our patient and public involvement group (CPAG). Two studies in this review incorporated frequency within their ACE scoring system [52, 54], which adds another dimension to the assessment, but this is insufficient to understand and quantify the ‘impact’ of an ACE within an epidemiological framework.
The definitions of multimorbidity were consistent across the relevant studies but the contributory long-term conditions varied. Fewer than half of the 115 different LTCs (n = 52, 45.2%) were reported by more than one study. Part of the challenge is the classification of healthcare conditions. For example, myocardial infarction is commonly caused by coronary heart disease, and both are a form of heart disease. All three were reported as LTCs in the included studies, but which level of pathology should be reported? Mental health LTCs were under-represented within the condition list, with just over half of the included studies assessing at least one (n = 14, 56.0%). Given the strong links between ACEs and mental health, and the impact of mental health on quality of life, this is an area for improvement in future research [31, 32]. A recent Delphi consensus study by Ho et al. may help to address these issues: following input from professionals and members of the public they identified 24 LTCs to ‘always include’ and 35 LTCs to ‘usually include’ in multimorbidity research, including nine mental health conditions [9].
As outlined in the introduction, there is a strong evidence base supporting the link between ACEs and long-term health outcomes, including specific LTCs. It is not unreasonable to extrapolate this association to ACEs and multimorbidity, though to our knowledge, the pathophysiological processes that link the two have not been precisely identified. However, similar lines of research are being independently followed in both fields and these areas of overlap may suggest possible mechanisms for a relationship. For example, both ACEs and multimorbidity have been associated with markers of accelerated epigenetic ageing [69, 70], mitochondrial dysfunction [71, 72], and inflammation [22, 73]. More work is required to better understand how these concepts might be linked.
This review used data from a large participant base, with information from 372,162 people contributing to the systematic review and information from 197,981 people contributing to the dose–response meta-analysis. Data from the included studies originated from a range of sources, including healthcare settings and dedicated research cohorts. We believe this is of a sufficient scale and variety to demonstrate the nature and magnitude of the association between ACEs and multimorbidity in these populations.
However, there are some limitations. Firstly, although data came from 11 different countries, only two of those were from outside Europe and North America, and all were from either high- or middle-income countries. Data on ACEs from low-income countries have indicated a higher prevalence of any ACE exposure (consistently > 70%) [74, 75], though how well this predicts health outcomes in these populations is unknown.
Secondly, studies in this review utilised retrospective participant-reported ACE data and so are at risk of recall and reporting bias. Studies utilising prospective assessments are rare and much of the wider ACE literature is open to a similar risk of bias. To date, two studies have compared prospective and retrospective ACE measurements, demonstrating inconsistent results [76, 77]. However, these studies were performed in New Zealand and South Africa, two countries not represented by studies in our review, and had relatively small sample sizes (1037 and 1595 respectively). It is unclear whether these are generalisable to other population groups.
Thirdly, previous research has indicated a close relationship between ACEs and childhood socio-economic status (SES) [78] and between SES and multimorbidity [10, 79]. However, the limitations of the included studies meant we were unable to separate the effect of ACEs from the effect of childhood SES on multimorbidity in this review. Whilst two studies included childhood SES as covariates in their models, others used measures from adulthood (such as adulthood SES, income level, and education level) that are potentially influenced by ACEs and therefore increase the risk of bias due to confounding (Additional File 1: Table S3). Furthermore, as for ACEs and multimorbidity, there is no consistently applied definition of SES and different measures of SES may produce different apparent effects [80]. The complex relationships between ACEs, childhood SES, and multimorbidity remain a challenge for research in this field.
Fourthly, there was a high degree of heterogeneity within included studies, especially relating to the definition and measurement of ACEs and multimorbidity. Whilst this suggests that our results should be interpreted with caution, it is reassuring to see that our meta-analysis of prevalence estimates for exposure to any ACE (48.1%) and multimorbidity (34.5%) are in line with previous estimates in similar populations [2, 11]. Furthermore, we believe that the quantitative synthesis of these relatively heterogenous studies provides important benefit by demonstrating a strong dose–response relationship across a range of contexts.
Our results strengthen the evidence supporting the lasting influence of childhood conditions on adult health and wellbeing. How this understanding is best incorporated into routine practice is still not clear. Currently, the lack of consistency in assessing ACEs limits our ability to understand their impact at both the individual and population level and poses challenges for those looking to incorporate a formalised assessment. Whilst most risk factors for disease (e.g. blood pressure) are usually only relevant within healthcare settings, ACEs are relevant to many other sectors (e.g. social care, education, policing) [81,82,83,84], and so consistency of assessment across society is both more important and more challenging to achieve.
Some have suggested that the evidence for the impact of ACEs is strong enough to warrant screening, which would allow early identification of potential harms to children and interventions to prevent them. This approach has been implemented in California, USA [85,86,87]. However, this is controversial, and others argue that screening is premature with the current evidence base [88,89,90]. Firstly, not everyone who is exposed to ACEs develops poor health outcomes, and it is not clear how to identify those who are at highest risk. Many people appear to be vulnerable, with more adverse health outcomes following ACE exposure than those who are not exposed, whilst others appear to be more resilient, with good health in later life despite multiple ACE exposures [91] It may be that supportive environments can mitigate the long-term effects of ACE exposure and promote resilience [92, 93]. Secondly, there are no accepted interventions for managing the impact of an identified ACE. As identified above, different ACEs may require input from different sectors (e.g. healthcare, social care, education, police), and so collating this evidence may be challenging. At present, ACEs screening does not meet the Wilson-Jungner criteria for a screening programme [94].
Existing healthcare systems are poorly designed to deal with the complexities of addressing ACEs and multimorbidity. Possibly, ways to improve this might be allocating more time per patient, prioritising continuity of care to foster long-term relationships, and greater integration between different healthcare providers (most notably primary vs secondary care teams, or physical vs mental health teams). However, such changes often demand additional resources (e.g. staff, infrastructure, processes), which are challenging to source when existing healthcare systems are already stretched [95, 96]. Nevertheless, increasing the spotlight on ACEs and multimorbidity may help to focus attention and ultimately bring improvements to patient care and experience.
Conclusions
ACEs are associated with a range of poor long-term health outcomes, including harmful health behaviours and individual long-term conditions. Multimorbidity is becoming more common as global populations age, and it increases the complexity and cost of healthcare provision. This is the first systematic review and meta-analysis to synthesise the literature on ACEs and multimorbidity, showing a statistically significant dose-dependent relationship across a large number of participants, albeit with a high degree of inter-study heterogeneity. This consolidates and enhances an increasing body of data supporting the role of ACEs in determining long-term health outcomes. Whilst these observational studies do not confirm causality, the weight and consistency of evidence is such that we can be confident in the link. The challenge for healthcare practitioners, managers, policymakers, and governments is incorporating this body of evidence into routine practice to improve the health and wellbeing of our societies.
Availability of data and materials
No additional data was generated for this review. The data used were found in the referenced papers or provided through correspondence with the study authors.
Abbreviations
- ACE:
-
Adverse childhood experience
- AIC:
-
Akaike information criterion
- CAPE:
-
CONSORTIUM Against pain inequality
- CI:
-
Confidence interval
- CPAG:
-
Chronic pain advisory group
- IQR:
-
Interquartile range
- LTC:
-
Long-term condition
- PROSPERO:
-
International prospective register of systematic reviews
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- ROBINS-E:
-
Risk of bias in non-randomised studies of exposures
- SES:
-
Socio-economic status
References
Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. 2017;2:e356–66.
Bellis MA, Lowey H, Leckenby N, Hughes K, Harrison D. Adverse childhood experiences: retrospective study to determine their impact on adult health behaviours and health outcomes in a UK population. J Public Health Oxf Engl. 2014;36:81–91.
Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–58.
Maniglio R. The impact of child sexual abuse on health: a systematic review of reviews. Clin Psychol Rev. 2009;29:647–57.
Yu J, Patel RA, Haynie DL, Vidal-Ribas P, Govender T, Sundaram R, et al. Adverse childhood experiences and premature mortality through mid-adulthood: a five-decade prospective study. Lancet Reg Health - Am. 2022;15:100349.
Wang Y-X, Sun Y, Missmer SA, Rexrode KM, Roberts AL, Chavarro JE, et al. Association of early life physical and sexual abuse with premature mortality among female nurses: prospective cohort study. BMJ. 2023;381: e073613.
Rogers NT, Power C, Pereira SMP. Child maltreatment, early life socioeconomic disadvantage and all-cause mortality in mid-adulthood: findings from a prospective British birth cohort. BMJ Open. 2021;11: e050914.
Hardcastle K, Bellis MA, Sharp CA, Hughes K. Exploring the health and service utilisation of general practice patients with a history of adverse childhood experiences (ACEs): an observational study using electronic health records. BMJ Open. 2020;10: e036239.
Ho ISS, Azcoaga-Lorenzo A, Akbari A, Davies J, Khunti K, Kadam UT, et al. Measuring multimorbidity in research: Delphi consensus study. BMJ Med. 2022;1:e000247.
Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet Lond Engl. 2012;380:37–43.
Chowdhury SR, Das DC, Sunna TC, Beyene J, Hossain A. Global and regional prevalence of multimorbidity in the adult population in community settings: a systematic review and meta-analysis. eClinicalMedicine. 2023;57:101860.
Noël PH, Chris Frueh B, Larme AC, Pugh JA. Collaborative care needs and preferences of primary care patients with multimorbidity. Health Expect. 2005;8:54–63.
Chau E, Rosella LC, Mondor L, Wodchis WP. Association between continuity of care and subsequent diagnosis of multimorbidity in Ontario, Canada from 2001–2015: a retrospective cohort study. PLoS ONE. 2021;16: e0245193.
Nicholson K, Liu W, Fitzpatrick D, Hardacre KA, Roberts S, Salerno J, et al. Prevalence of multimorbidity and polypharmacy among adults and older adults: a systematic review. Lancet Healthy Longev. 2024;5:e287–96.
Albreht T, Dyakova M, Schellevis FG, Van den Broucke S. Many diseases, one model of care? J Comorbidity. 2016;6:12–20.
Soley-Bori M, Ashworth M, Bisquera A, Dodhia H, Lynch R, Wang Y, et al. Impact of multimorbidity on healthcare costs and utilisation: a systematic review of the UK literature. Br J Gen Pract. 2020;71:e39-46.
World Health Organization (WHO). Ageing and health. 2022. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health. Accessed 23 Apr 2024.
Franke HA. Toxic stress: effects, prevention and treatment. Children. 2014;1:390–402.
Parade SH, Huffhines L, Daniels TE, Stroud LR, Nugent NR, Tyrka AR. A systematic review of childhood maltreatment and DNA methylation: candidate gene and epigenome-wide approaches. Transl Psychiatry. 2021;11:1–33.
Ridout KK, Levandowski M, Ridout SJ, Gantz L, Goonan K, Palermo D, et al. Early life adversity and telomere length: a meta-analysis. Mol Psychiatry. 2018;23:858–71.
Elwenspoek MMC, Kuehn A, Muller CP, Turner JD. The effects of early life adversity on the immune system. Psychoneuroendocrinology. 2017;82:140–54.
Danese A, Baldwin JR. Hidden wounds? Inflammatory links between childhood trauma and psychopathology. Annu Rev Psychol. 2017;68:517–44.
Brindle RC, Pearson A, Ginty AT. Adverse childhood experiences (ACEs) relate to blunted cardiovascular and cortisol reactivity to acute laboratory stress: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2022;134: 104530.
Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016;17:652–66.
McLaughlin KA, Weissman D, Bitrán D. Childhood adversity and neural development: a systematic review. Annu Rev Dev Psychol. 2019;1:277–312.
Koyama Y, Fujiwara T, Murayama H, Machida M, Inoue S, Shobugawa Y. Association between adverse childhood experiences and brain volumes among Japanese community-dwelling older people: findings from the NEIGE study. Child Abuse Negl. 2022;124: 105456.
Antoniou G, Lambourg E, Steele JD, Colvin LA. The effect of adverse childhood experiences on chronic pain and major depression in adulthood: a systematic review and meta-analysis. Br J Anaesth. 2023;130:729–46.
Huang H, Yan P, Shan Z, Chen S, Li M, Luo C, et al. Adverse childhood experiences and risk of type 2 diabetes: a systematic review and meta-analysis. Metabolism. 2015;64:1408–18.
Lopes S, Hallak JEC, de Machado Sousa JP, de Osório F L. Adverse childhood experiences and chronic lung diseases in adulthood: a systematic review and meta-analysis. Eur J Psychotraumatology. 2020;11:1720336.
Hu Z, Kaminga AC, Yang J, Liu J, Xu H. Adverse childhood experiences and risk of cancer during adulthood: a systematic review and meta-analysis. Child Abuse Negl. 2021;117: 105088.
Tan M, Mao P. Type and dose-response effect of adverse childhood experiences in predicting depression: a systematic review and meta-analysis. Child Abuse Negl. 2023;139: 106091.
Zhang L, Zhao N, Zhu M, Tang M, Liu W, Hong W. Adverse childhood experiences in patients with schizophrenia: related factors and clinical implications. Front Psychiatry. 2023;14:1247063.
Emsley E, Smith J, Martin D, Lewis NV. Trauma-informed care in the UK: where are we? A qualitative study of health policies and professional perspectives. BMC Health Serv Res. 2022;22:1164.
ROBINS-E Development Group (Higgins J, Morgan R, Rooney A, Taylor K, Thayer K, Silva R, Lemeris C, Akl A, Arroyave W, Bateson T, Berkman N, Demers P, Forastiere F, Glenn B, Hróbjartsson A, Kirrane E, LaKind J, Luben T, Lunn R, McAleenan A, McGuinness L, Meerpohl J, Mehta S, Nachman R, Obbagy J, O’Connor A, Radke E, Savović J, Schubauer-Berigan M, Schwingl P, Schunemann H, Shea B, Steenland K, Stewart T, Straif K, Tilling K, Verbeek V, Vermeulen R, Viswanathan M, Zahm S, Sterne J). Risk Of Bias In Non-randomized Studies - of Exposure (ROBINS-E). Launch version, 20 June 2023. https://www.riskofbias.info/welcome/robins-e-tool. Accessed 20 Jul 2023.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60.
Schwarzer G, Chemaitelly H, Abu-Raddad LJ, Rücker G. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods. 2019;10:476–83.
Crippa A, Orsini N. Multivariate dose-response meta-analysis: the dosresmeta R Package. J Stat Softw. 2016;72:1–15.
Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–9.
Shim SR, Lee J. Dose-response meta-analysis: application and practice using the R software. Epidemiol Health. 2019;41: e2019006.
Arshadipour A, Thorand B, Linkohr B, Rospleszcz S, Ladwig K-H, Heier M, et al. Impact of prenatal and childhood adversity effects around World War II on multimorbidity: results from the KORA-Age study. BMC Geriatr. 2022;22:115.
Atkinson L, Joshi D, Raina P, Griffith LE, MacMillan H, Gonzalez A. Social engagement and allostatic load mediate between adverse childhood experiences and multimorbidity in mid to late adulthood: the Canadian Longitudinal Study on Aging. Psychol Med. 2021;53(4):1–11.
Chandrasekar R, Lacey RE, Chaturvedi N, Hughes AD, Patalay P, Khanolkar AR. Adverse childhood experiences and the development of multimorbidity across adulthood—a national 70-year cohort study. Age Ageing. 2023;52:afad062.
Cromer KR, Sachs-Ericsson N. The association between childhood abuse, PTSD, and the occurrence of adult health problems: moderation via current life stress. J Trauma Stress. 2006;19:967–71.
England-Mason G, Casey R, Ferro M, MacMillan HL, Tonmyr L, Gonzalez A. Child maltreatment and adult multimorbidity: results from the Canadian Community Health Survey. Can J Public Health. 2018;109:561–72.
Godin O, Leboyer M, Laroche DG, Aubin V, Belzeaux R, Courtet P, et al. Childhood maltreatment contributes to the medical morbidity of individuals with bipolar disorders. Psychol Med. 2023;53(15):1–9.
Hanlon P, McCallum M, Jani BD, McQueenie R, Lee D, Mair FS. Association between childhood maltreatment and the prevalence and complexity of multimorbidity: a cross-sectional analysis of 157,357 UK Biobank participants. J Comorbidity. 2020;10:2235042X1094434.
Henchoz Y, Seematter-Bagnoud L, Nanchen D, Büla C, von Gunten A, Démonet J-F, et al. Childhood adversity: a gateway to multimorbidity in older age? Arch Gerontol Geriatr. 2019;80:31–7.
Hosang GM, Fisher HL, Uher R, Cohen-Woods S, Maughan B, McGuffin P, et al. Childhood maltreatment and the medical morbidity in bipolar disorder: a case–control study. Int J Bipolar Disord. 2017;5:30.
Hosang GM, Fisher HL, Hodgson K, Maughan B, Farmer AE. Childhood maltreatment and adult medical morbidity in mood disorders: comparison of unipolar depression with bipolar disorder. Br J Psychiatry. 2018;213:645–53.
Lin L, Wang HH, Lu C, Chen W, Guo VY. Adverse childhood experiences and subsequent chronic diseases among middle-aged or older adults in China and associations with demographic and socioeconomic characteristics. JAMA Netw Open. 2021;4: e2130143.
Mendizabal A, Nathan CL, Khankhanian P, Anto M, Clyburn C, Acaba-Berrocal A, et al. Adverse childhood experiences in patients with neurologic disease. Neurol Clin Pract. 2022. https://doi.org/10.1212/CPJ.0000000000001134.
Noteboom A, Have MT, De Graaf R, Beekman ATF, Penninx BWJH, Lamers F. The long-lasting impact of childhood trauma on adult chronic physical disorders. J Psychiatr Res. 2021;136:87–94.
Patterson ML, Moniruzzaman A, Somers JM. Setting the stage for chronic health problems: cumulative childhood adversity among homeless adults with mental illness in Vancouver. British Columbia BMC Public Health. 2014;14:350.
Post RM, Altshuler LL, Leverich GS, Frye MA, Suppes T, McElroy SL, et al. Role of childhood adversity in the development of medical co-morbidities associated with bipolar disorder. J Affect Disord. 2013;147:288–94.
Reyes-Ortiz CA. Racial discrimination and multimorbidity among older adults in Colombia: a national data analysis. Prev Chronic Dis. 2023;20:220360.
Sheikh MA. Coloring of the past via respondent’s current psychological state, mediation, and the association between childhood disadvantage and morbidity in adulthood. J Psychiatr Res. 2018;103:173–81.
Sinnott C, Mc Hugh S, Fitzgerald AP, Bradley CP, Kearney PM. Psychosocial complexity in multimorbidity: the legacy of adverse childhood experiences. Fam Pract. 2015;32:269–75.
Sosnowski DW, Feder KA, Astemborski J, Genberg BL, Letourneau EJ, Musci RJ, et al. Adverse childhood experiences and comorbidity in a cohort of people who have injected drugs. BMC Public Health. 2022;22:986.
Stapp EK, Williams SC, Kalb LG, Holingue CB, Van Eck K, Ballard ED, et al. Mood disorders, childhood maltreatment, and medical morbidity in US adults: an observational study. J Psychosom Res. 2020;137: 110207.
Tomasdottir MO, Sigurdsson JA, Petursson H, Kirkengen AL, Krokstad S, McEwen B, et al. Self reported childhood difficulties, adult multimorbidity and allostatic load. A cross-sectional analysis of the Norwegian HUNT study. PloS One. 2015;10:e0130591.
Vásquez E, Quiñones A, Ramirez S, Udo T. Association between adverse childhood events and multimorbidity in a racial and ethnic diverse sample of middle-aged and older adults. Innov Aging. 2019;3:igz016.
Yang L, Hu Y, Silventoinen K, Martikainen P. Childhood adversity and trajectories of multimorbidity in mid-late life: China health and longitudinal retirement study. J Epidemiol Community Health. 2021;75:593–600.
Zak-Hunter L, Carr CP, Tate A, Brustad A, Mulhern K, Berge JM. Associations between adverse childhood experiences and stressful life events and health outcomes in pregnant and breastfeeding women from diverse racial and ethnic groups. J Womens Health. 2023;32:702–14.
Zheng X, Cui Y, Xue Y, Shi L, Guo Y, Dong F, et al. Adverse childhood experiences in depression and the mediating role of multimorbidity in mid-late life: A nationwide longitudinal study. J Affect Disord. 2022;301:217–24.
Liu M, Luong L, Lachaud J, Edalati H, Reeves A, Hwang SW. Adverse childhood experiences and related outcomes among adults experiencing homelessness: a systematic review and meta-analysis. Lancet Public Health. 2021;6:e836–47.
Petruccelli K, Davis J, Berman T. Adverse childhood experiences and associated health outcomes: a systematic review and meta-analysis. Child Abuse Negl. 2019;97: 104127.
Bethell CD, Carle A, Hudziak J, Gombojav N, Powers K, Wade R, et al. Methods to assess adverse childhood experiences of children and families: toward approaches to promote child well-being in policy and practice. Acad Pediatr. 2017;17(7 Suppl):S51-69.
Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–90.
Kim K, Yaffe K, Rehkopf DH, Zheng Y, Nannini DR, Perak AM, et al. Association of adverse childhood experiences with accelerated epigenetic aging in midlife. JAMA Network Open. 2023;6:e2317987.
Jain P, Binder A, Chen B, Parada H, Gallo LC, Alcaraz J, et al. The association of epigenetic age acceleration and multimorbidity at age 90 in the Women’s Health Initiative. J Gerontol A Biol Sci Med Sci. 2023;78:2274–81.
Zang JCS, May C, Hellwig B, Moser D, Hengstler JG, Cole S, et al. Proteome analysis of monocytes implicates altered mitochondrial biology in adults reporting adverse childhood experiences. Transl Psychiatry. 2023;13:31.
Mau T, Blackwell TL, Cawthon PM, Molina AJA, Coen PM, Distefano G, et al. Muscle mitochondrial bioenergetic capacities are associated with multimorbidity burden in older adults: the Study of Muscle, Mobility and Aging (SOMMA). J Gerontol A Biol Sci Med Sci. 2024;79(7):glae101.
Friedman E, Shorey C. Inflammation in multimorbidity and disability: an integrative review. Health Psychol Off J Div Health Psychol Am Psychol Assoc. 2019;38:791–801.
Satinsky EN, Kakuhikire B, Baguma C, Rasmussen JD, Ashaba S, Cooper-Vince CE, et al. Adverse childhood experiences, adult depression, and suicidal ideation in rural Uganda: a cross-sectional, population-based study. PLoS Med. 2021;18: e1003642.
Amene EW, Annor FB, Gilbert LK, McOwen J, Augusto A, Manuel P, et al. Prevalence of adverse childhood experiences in sub-Saharan Africa: a multicounty analysis of the Violence Against Children and Youth Surveys (VACS). Child Abuse Negl. 2023;150:106353.
Reuben A, Moffitt TE, Caspi A, Belsky DW, Harrington H, Schroeder F, et al. Lest we forget: comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. J Child Psychol Psychiatry. 2016;57:1103–12.
Naicker SN, Norris SA, Mabaso M, Richter LM. An analysis of retrospective and repeat prospective reports of adverse childhood experiences from the South African Birth to Twenty Plus cohort. PLoS ONE. 2017;12: e0181522.
Walsh D, McCartney G, Smith M, Armour G. Relationship between childhood socioeconomic position and adverse childhood experiences (ACEs): a systematic review. J Epidemiol Community Health. 2019;73:1087–93.
Ingram E, Ledden S, Beardon S, Gomes M, Hogarth S, McDonald H, et al. Household and area-level social determinants of multimorbidity: a systematic review. J Epidemiol Community Health. 2021;75:232–41.
Darin-Mattsson A, Fors S, Kåreholt I. Different indicators of socioeconomic status and their relative importance as determinants of health in old age. Int J Equity Health. 2017;16:173.
Bateson K, McManus M, Johnson G. Understanding the use, and misuse, of Adverse Childhood Experiences (ACEs) in trauma-informed policing. Police J. 2020;93:131–45.
Webb NJ, Miller TL, Stockbridge EL. Potential effects of adverse childhood experiences on school engagement in youth: a dominance analysis. BMC Public Health. 2022;22:2096.
Stewart-Tufescu A, Struck S, Taillieu T, Salmon S, Fortier J, Brownell M, et al. Adverse childhood experiences and education outcomes among adolescents: linking survey and administrative data. Int J Environ Res Public Health. 2022;19:11564.
Frederick J, Spratt T, Devaney J. Adverse childhood experiences and social work: relationship-based practice responses. Br J Soc Work. 2021;51:3018–34.
University of California ACEs Aware Family Resilience Network (UCAAN). acesaware.org. ACEs Aware. https://www.acesaware.org/about/. Accessed 6 Oct 2023.
Watson CR, Young-Wolff KC, Negriff S, Dumke K, DiGangi M. Implementation and evaluation of adverse childhood experiences screening in pediatrics and obstetrics settings. Perm J. 2024;28:180–7.
Gordon JB, Felitti VJ. The importance of screening for adverse childhood experiences (ACE) in all medical encounters. AJPM Focus. 2023;2: 100131.
Finkelhor D. Screening for adverse childhood experiences (ACEs): Cautions and suggestions. Child Abuse Negl. 2018;85:174–9.
Cibralic S, Alam M, Mendoza Diaz A, Woolfenden S, Katz I, Tzioumi D, et al. Utility of screening for adverse childhood experiences (ACE) in children and young people attending clinical and healthcare settings: a systematic review. BMJ Open. 2022;12: e060395.
Gentry SV, Paterson BA. Does screening or routine enquiry for adverse childhood experiences (ACEs) meet criteria for a screening programme? A rapid evidence summary. J Public Health Oxf Engl. 2022;44:810–22.
Morgan CA, Chang Y-H, Choy O, Tsai M-C, Hsieh S. Adverse childhood experiences are associated with reduced psychological resilience in youth: a systematic review and meta-analysis. Child Basel Switz. 2021;9:27.
Narayan AJ, Lieberman AF, Masten AS. Intergenerational transmission and prevention of adverse childhood experiences (ACEs). Clin Psychol Rev. 2021;85: 101997.
VanBronkhorst SB, Abraham E, Dambreville R, Ramos-Olazagasti MA, Wall M, Saunders DC, et al. Sociocultural risk and resilience in the context of adverse childhood experiences. JAMA Psychiat. 2024;81:406–13.
Wilson JM, Jungner G. Principles and practice of screening for disease. World Health Organisation; 1968.
Huo Y, Couzner L, Windsor T, Laver K, Dissanayaka NN, Cations M. Barriers and enablers for the implementation of trauma-informed care in healthcare settings: a systematic review. Implement Sci Commun. 2023;4:49.
Foo KM, Sundram M, Legido-Quigley H. Facilitators and barriers of managing patients with multiple chronic conditions in the community: a qualitative study. BMC Public Health. 2020;20:273.
Acknowledgements
The authors thank the members of the CAPE CPAG patient and public involvement group for providing insights gained from relevant lived experiences.
Funding
The authors are members of the Advanced Pain Discovery Platform (APDP) supported by UK Research & Innovation (UKRI), Versus Arthritis, and Eli Lilly. DS is a fellow on the Multimorbidity Doctoral Training Programme for Health Professionals, which is supported by the Wellcome Trust [223499/Z/21/Z]. BT, BS, and LC are supported by an APDP grant as part of the Partnership for Assessment and Investigation of Neuropathic Pain: Studies Tracking Outcomes, Risks and Mechanisms (PAINSTORM) consortium [MR/W002388/1]. TH and LC are supported by an APDP grant as part of the Consortium Against Pain Inequality [MR/W002566/1]. The funding bodies had no role in study design, data collection/analysis/interpretation, report writing, or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
DS and LC contributed to review conception and design. DC, BT, BS, TH, LM, and LC contributed to search strategy design. DS and BT contributed to study selection and data extraction, with input from LC. DS and BT accessed and verified the underlying data. DS conducted the meta-analyses, with input from BT, BS, TH, LM, and LC. DS drafted the manuscript, with input from DC, BT, BS, TH, LM, and LC. DC, BT, BS, TH, LM, and LC read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12916_2024_3505_MOESM1_ESM.docx
Additional File 1: Tables S1-S5 and Figures S1-S2. Table S1: Search strategy, Table S2: Characteristics of studies included in the systematic review, Table S3: Risk of bias assessment (ROBINS-E), Table S4: Exposure details (adverse childhood experiences), Table S5: Outcome details (multimorbidity), Figure S1: Meta-analysis of prevalence of exposure to ≥4 adverse childhood experiences, Figure S2: Dose-response meta-analysis of the relationship between adverse childhood experiences and multimorbidity (using a non-linear/restricted cubic spline model).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Senaratne, D.N.S., Thakkar, B., Smith, B.H. et al. The impact of adverse childhood experiences on multimorbidity: a systematic review and meta-analysis. BMC Med 22, 315 (2024). https://doi.org/10.1186/s12916-024-03505-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-024-03505-w