Abstract
Background
To study the shared genetic structure between autoimmune diseases and B-cell acute lymphoblastic leukemia (B-ALL) and identify the shared risk loci and genes and genetic mechanisms involved.
Methods
Based on large-scale genome-wide association study (GWAS) summary-level data sets, we observed genetic overlaps between autoimmune diseases and B-ALL, and cross-trait pleiotropic analysis was performed to detect shared pleiotropic loci and genes. A series of functional annotation and tissue-specific analysis were performed to determine the influence of pleiotropic genes. The heritability enrichment analysis was used to detect crucial immune cells and tissues. Finally, bidirectional Mendelian randomization (MR) methods were utilized to investigate the casual associations.
Results
Our research highlighted shared genetic mechanisms between seven autoimmune disorders and B-ALL. A total of 73 pleiotropic loci were identified at the genome-wide significance level (P < 5 × 10–8), 16 of which had strong evidence of colocalization. We demonstrated that several loci have been previously reported (e.g., 17q21) and discovered some novel loci (e.g., 10p12, 5p13). Further gene-level identified 194 unique pleiotropic genes, for example IKZF1, GATA3, IKZF3, GSDMB, and ORMDL3. Pathway analysis determined the key role of cellular response to cytokine stimulus, B cell activation, and JAK-STAT signaling pathways. SNP-level and gene-level tissue enrichment suggested that crucial role pleiotropic mechanisms involved in the spleen, whole blood, and EBV-transformed lymphocytes. Also, hyprcoloc and stratified LD score regression analyses revealed that B cells at different developmental stages may be involved in mechanisms shared between two different diseases. Finally, two-sample MR analysis determined causal effects of asthma and rheumatoid arthritis on B-ALL.
Conclusions
Our research proved shared genetic architecture between autoimmune disorders and B-ALL and shed light on the potential mechanism that might involve in.
Similar content being viewed by others
Background
B-cell acute lymphoblastic leukemia (B-ALL) is a prevalent subtype of leukemia characterized by its highly malignant nature, primarily originating from the clonal expansion and abnormal proliferation of B lymphocytes within the hematopoietic system [1]. Autoimmune disorders are characterized by a disruption in self-tolerance, resulting in pathological alterations and clinical symptoms arising from immune responses targeting self-components [2]. Concurrently, the pathogenesis of several autoimmune disorders is intricately interwoven with the malfunctioning of B cells within the humoral immune system. The excessive activation of self-reactive B cells precipitates an overproduction of autoantibodies and immune complexes, which, in turn inflict damage upon a multitude of tissues and organs, culminating in the emergence of various autoimmune disorders [3]. To summarize, B cells assume a pivotal role in the orchestration of humoral immune responses, and their deregulation markedly contributes to the onset of autoimmune diseases and B-cell malignancies [4].
Epidemiological investigations have discovered associations between autoimmune disorders and B-cell malignancies. For example, rheumatoid arthritis (RA) patients exhibit a twofold increased risk of concomitant B-cell lymphomas when compared to their healthy counterparts [5]. In the case of systemic lupus erythematosus (SLE) and Sjögren’s syndrome patients, the risk amplifies significantly to 2.7–7.5 times [6] and 9–18 times [6], respectively. Previous studies observed that the standardized incidence ratio of ALL was estimated to be 2.77 after RA onset [7]. Studies also showed that at the time of diagnosis of malignancy, 15–30% of patients present with many of the typical features of rheumatic diseases [8]. However, current research focused primarily on the onset of autoimmune diseases on hematological malignancies risk, particularly diffuse large B-cell lymphoma and follicular lymphoma. This leaves a clear gap in understanding the pleiotropic mechanisms and bidirectional causations between B-ALL (a disease also derived from B lymphocytes) and autoimmune diseases. Only Li et al. have reported the shared mechanism between autoimmunity and B-ALL, specifically demonstrating the essential role of DYRK1a in mediating the noncanonical NF-κB activation induced by BAFF [9]. This underscores the existence of substantial knowledge gaps in this field, highlighting the urgent need to ascertain shared risk loci between these two disorders. It is worth noting that traditional clinical or epidemiological research may encounter challenges in ensuring the statistical effectiveness of such investigations.
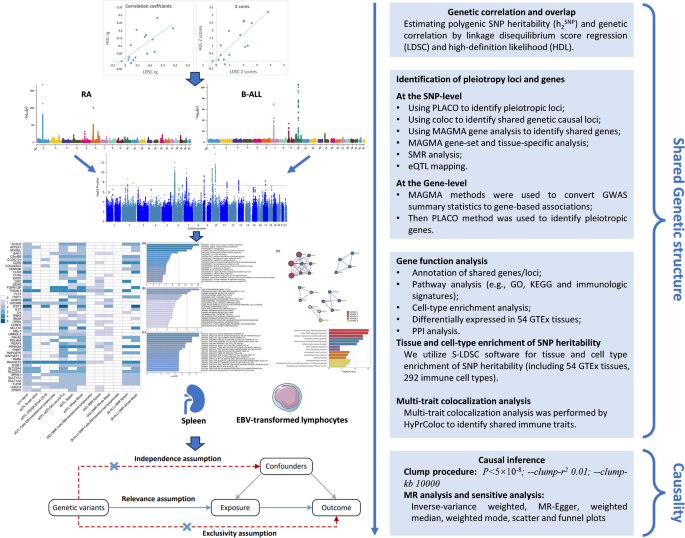
Recently, the linkage disequilibrium (LD) score regression (LDSC) approach has been developed to indicate whether there exists a genetic correlation between the two types of disease [10]. It is unclear whether the overall genetic correlation is attributable to a few loci or the entire genome. Few studies to date have systematically evaluated genetic overlap, shared susceptibility genes, and causality between autoimmune diseases and B-ALL. Cross-trait analyses that utilize the correlation of GWAS signals to study polyvalent genetic variants or loci between multiple traits have been shown to accurately identify shared loci between diseases or traits [11,12,13]. These pleiotropic loci can be targeted for intervention to potentially prevent or treat these diseases simultaneously. Recently, a novel method called “PLACO” was developed to identify pleiotropy at the SNP-level based on a level-α intersection–union test (IUT) [14]. Therefore, it is important to determine specific genetic variants or loci that lead to genome-wide genetic correlations or to delve into the shared genetic etiology of these two types of diseases. Our research flowchart is shown in Fig. 1.
Methods
GWAS summary data source
GWAS summary statistics for 16 autoimmune diseases were all publicly available from large-scale GWAS or GWAS meta-analyses: adult-onset asthma (AOA) [15, 16], childhood-onset asthma (COA) [15, 16], Graves’ disease (GD) [17, 18], Hashimoto’s disease (HD) [17, 18], hypothyroidism (HT) [17, 18], primary biliary cirrhosis (PBC) [19, 20], primary sclerosing cholangitis (PSC) [21, 22], inflammatory bowel disease (IBD) [23, 24], Crohn’s disease (CD) [23, 24], ulcerative colitis (UC) [23, 24], RA [25, 26], SLE [27, 28], multiple sclerosis (MS) [29, 30], systemic sclerosis (SS) [31, 32], type 1 diabetes (T1D) [17, 18], and vitiligo [33, 34]. GWAS summary statistics for B-ALL were generated in a meta-analysis of four GWAS including a total of 5321 cases and 16,666 controls of European ancestry [35, 36]. The same quality control procedure was followed for each study, the association between ALL status and SNP genotypes in each study was assessed using logistic regression, and genetic principal components were used as covariates in the association analysis. Risk estimates were finally combined by fixed-effects inverse variance weighted (IVW) meta-analysis. The sources and details of these datasets are summarized in Additional file 2: Table S1.
Genetic overlap at the genome-wide level
We used LDSC to evaluate the genetic structure shared between autoimmune disorders and B-ALL [10]. The LD scores used in LDSC were calculated based on genotypes of common SNPs from European ancestry samples in the 1000 genomes project [37]. Standard errors (SE) were estimated by the jackknife method in LDSC which was further used to correct for attenuation bias. Intercept of LDSC was used to evaluate potential population overlaps between studies from different consortiums [10]. It is worth noting that no actual population overlap between autoimmune disorders and B-ALL studies existed in our analysis. A likelihood-based method, called high-definition likelihood (HDL), can utilize GWAS summary statistics to estimate genetic associations, which could reduce the variance of genetic association estimates by about 60% compared with the LDSC method [38].
We further investigated whether SNP heritability of autoimmune diseases and B-ALL was enriched in specific cells and tissues using hierarchical LDSC regression. Stratified-LDSC (S-LDSC) was applied to different immune cell data to assess whether specific cell types had significant genetic enrichment in these tissues. We downloaded 54 human tissues datasets from GTEx [39] and 292 immune cell types from the ImmGen consortium [40] (including B cells, γ δ T cells, α β T cells, innate lymphocytes, myeloid cells, stromal cells, and stem cells). After adjusting for the baseline model and all gene sets, we assessed the significance of the SNP heritability enrichment estimated in each tissue and cell by using the regression coefficient Z-scores and corresponding P values.
Identification of pleiotropic loci and genes by using PLACO
A pleiotropic analysis under composite null hypothesis (PLACO) was used to identify pleiotropy among multiple autoimmune diseases and B-ALL at the SNP-level. SNPs reach genome-wide significant level (P < 5 × 10–8) and were viewed as pleiotropic variants. The functional mapping and annotation (FUMA) of GWAS was used to determine the genomic regions of these risk variants (i.e., pleiotropic loci) [41]. Also, a Bayesian colocalization analysis was conducted to determine the pleiotropic loci shared by autoimmune diseases and B-ALL [42]. To explore the shared mechanisms of the identified loci, nearby genes were mapped based on lead SNPs within each locus. Also, a generalized gene-set analysis of GWAS data (multi-marker analysis of genomic annotation, MAGMA) approach was used to determine the biological function of these pleiotropic loci. Specifically, we performed MAGMA gene analysis to identify pleiotropic genes by properly incorporating LD between markers and to detect multi-marker effects (P < 0.05/18,345 = 2.73 × 10–6) [43]. MAGMA gene-set analysis was performed to investigate the biofunction of lead SNPs [43], and a total of 10,678 gene sets including curated gene sets (c2.all) and go terms (c5.bp, c5.cc, and c5.mf) from Molecular Signatures Database (MSigDB) were finally tested [44]. Bonferroni correction was performed for all tested gene sets to avoid false positives (P < 0.05/10,678 = 4.68 × 10–6). Metascape webtools (metascape.org) performed a pathway enrichment analysis to determine the function of mapped genes based on MSigDB [44]. Genome-wide tissue-specific enrichment analysis was conducted based on 54 GTEx tissues [45] for the genome-wide pleiotropic results generated by PLACO. We also calculated the average expression (log2 transformed) of all identified pleiotropic genes in all 54 GTEx tissues [45] and tested tissue specificity by differentially expressed genes (DEGs) in each tissue (up- and down-regulated DEGs were precomputed by the signs of the t-statistics).
Summary-based Mendelian randomization
Summary-based Mendelian randomization (SMR) [46] method combined summary-level data from GWAS with data from expression quantitative trait loci (eQTL) studies to identify genes whose expression levels are associated with complex traits due to pleiotropy. It employs SMR and HEIDI methods to test pleiotropic associations between gene expression levels and complex traits of interest using summary-level data from GWAS and eQTL studies. This approach could be interpreted as an analysis to test whether the magnitude of SNP effects on phenotype is mediated by gene expression.
Multi-trait colocalization analysis
We utilized hypothesis prioritization for multi-trait colocalization (HyPrColoc) [47] method to perform multi-trait colocalization analysis to pinpoint the crucial roles that immune traits played in the onset of autoimmune disorders and B-ALL. Immune-wide GWAS data contains a total of 731 immune cells [48], which could be publicly available from the GWAS catalog (GCST0001391 ~ GCST0002121). Detailed information on the GWAS summary datasets for immune cells was added to Additional file 1: Supplementary Methods.
Causal association analysis
We performed a one-directional two-sample Mendelian randomization (MR) analysis to assess possible causal effects of autoimmune disorders on B-ALL risk. The “clumping” procedure in PLINK 1.9 software was used to extract independent significance SNPs for all autoimmune diseases (P < 5 × 10–8), where r2 was set to 0.001 and window size was set to a physical distance of 10,000 KB [49]. Notably, r2 was calculated based on the 1000 genomes project phase 3 as a reference panel. Proportion of variance explained (PVE) and F statistic (F > 10) was used to measure the strength of instrumental variables (IVs) (see Additional File 1: Supplementary Methods) [50]. To verify causality among these trait pairs, six MR approaches were performed with each set of IVs, i.e., IVW, Debiased-IVW (DIVW) [51], weighted median approach [52], MR pleiotropy residual sum and outlier (MR-PRESSO) [53], MR-Egger [54], MR robust adjusted profile score (MR-RAPS) [55], and mode-based estimate [56] method. Cochran’s Q statistics was used to examine the effect size heterogeneity across the IVs (see Additional File 1: Supplementary Methods) [57, 58]. Additionally, the intercept of MR-Egger regression and global test of MR-PRSSO were utilized to detect horizontal pleiotropy [53, 54]. Detailed information on used MR methods was described in Additional file 1: Supplementary Method.
Software and packages
The main statistical analysis was performed in R (version 3.5.3). LDSC and S-LDSC analysis were implemented with “LDSC” software (v1.0.1) [10]. PLACO was performed with “PLACO” package [14]. Bayesian colocalization analysis was performed with the “coloc” package (version 5.2.1) [42] and HyPrColoc was performed with the “hyprcoloc” package (version 1.0) [47]. Function analysis was performed by FUMA web tool [41]. MAGMA gene and gene-set analysis were performed by MAGMA software [43]. Two-sample MR analysis was conducted with “MendelianRandomization” (version 0.9.0) [59], mr.raps (version 0.4.1) [55], and MRPRESSO (version 1.0) [53] packages. A copy of the main code used in this research is available at: https://github.com/biostatYu/MRcode-/tree/main/AD_BALL.
Results
Shared genetic architecture between autoimmune disorders and B-ALL
We first evaluated the genetic correlation between autoimmune diseases and B-ALL and results from the LDSC and HDL methods were highly consistent (Table 1 and Additional file 2: Table S2). Specifically, by using the LDSC method, six traits were identified to be genetically correlated with B-ALL, including AOA, HT, IBD, CD, RA, and MS. While implementing the HDL method, significant genetic correlations were observed among AOA, HT, PBC, RA, MS, and B-ALL, leading to a final union set of seven pairwise traits for further analysis. However, we did not find significant genetic correlation between IBD and CD and HDL results. It was noting that only RA remained significantly genetical correlated with B-ALL risk after applying the Bonferroni correction (P = 0.003 < 0.05/16).
Pleiotropic loci and genes identified for multiple autoimmune disorders and B-ALL
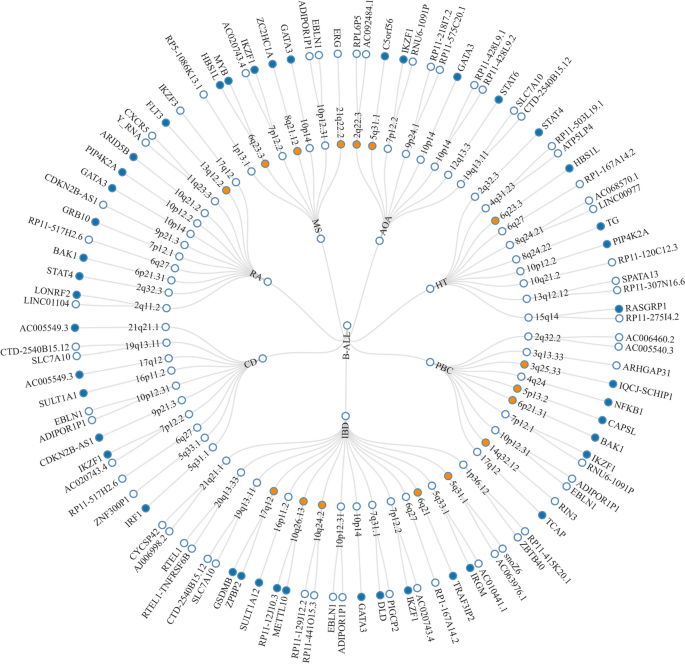
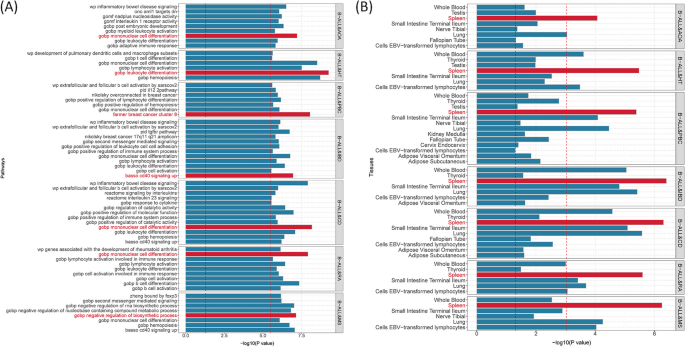
Given the shared genetic mechanisms between autoimmune diseases and B-ALL identified by LDSC and HDL, we used novel pleiotropy analyses (PLACO) to identify potential pleiotropic loci for both diseases (Additional file 1: Fig. S1). The QQ plots demonstrated no premature divergence between observed and expected values, ruling out the possibility of group stratification (Additional file 1: Fig. S2). Based on PLACO results, we identified a total of 73 pleiotropic genomic risk loci associated with both autoimmune disorders and B-ALL using FUMA (P < 5 × 10–8) (Fig. 2, Additional file 1: Fig. S1, and Additional file 2: Table S3). Colocalization analysis finally identified 16 of 73 (21.9%) potential pleiotropic loci with PP.H4 greater than 0.7 (e.g., 5p13) (Table 2). The regional plots for each trait pair are presented in Additional file 1: Fig. S3 ~ S8. Notably, some pleiotropic regions were shared between different pairs, for example, genome regions 7p12.2, 10p14, 6q27, and 10p12.31 were identified in four pairs (Additional file 2: Table S4). The MAGMA analysis of gene sets suggested that the identified pleiotropic loci may participate in the control of the immune system, hematopoiesis, and various other processes (Fig. 3A and Additional file 2: Table S5). Notably, significant monocyte differentiation pathway was found for all trait pairs, and significant leukocyte differentiation was found for all five trait pairs. Further tissue-specific analysis found these risk loci were enriched in several immune-related tissues (e.g., spleen, whole blood, Epstein–Barr virus (EBV)-transformed lymphocytes) (Fig. 3B and Additional file 2: Table S6). ANNOVAR category annotation showed that 28 of 73 lead SNPs (38.4%) were intronic variants and 30 of 73 (41.1%) were intergenic variants. Only 2 of 73 (3%) lead SNPs were exonic variants (Additional file 2: Table S3).
The circular diagram presents pleiotropic loci and genes identified by PLACO among seven trait pairs. Note: Shared loci identified by colocalization analysis are highlighted in orange; shared genes identified by MAGMA analysis are highlighted in blue. B-ALL B-cell acute lymphoblastic leukemia, AOA adult-onset asthma, HT hypothyroidism, PBC primary biliary cirrhosis, IBD inflammatory bowel disease, CD Crohn’s disease, RA rheumatoid arthritis, MS multiple sclerosis
Bar plot of MAGMA gene-set (A) and tissue-specific (B) analysis for genome-wide pleiotropic results. Note: The red dotted line represents the significance of 0.05 after multiple corrections, and the blue represents the significance of 0.05. B-ALL B-cell acute lymphoblastic leukemia, AOA adult-onset asthma, HT hypothyroidism, PBC primary biliary cirrhosis, IBD inflammatory bowel disease, CD Crohn’s disease, RA rheumatoid arthritis, MS multiple sclerosis
Pleiotropic genes identified and enrichment analysis
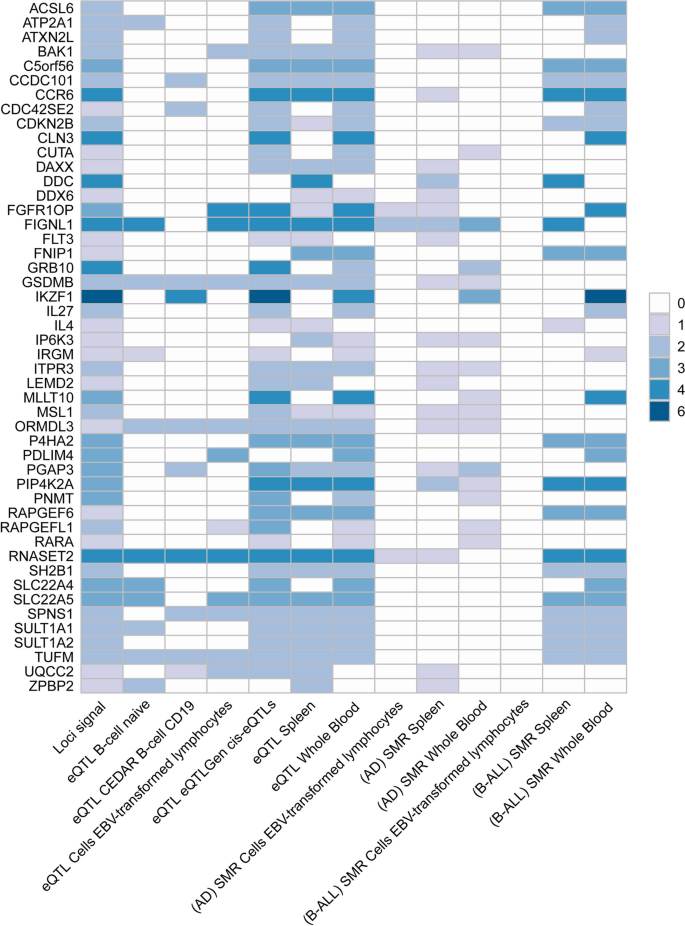
We used different methods to map the identified SNP-level signals into the gene-level signals. By using MAGMA gene analysis, a total of 341 significant pleiotropic genes were determined as pleiotropic genes between multiple autoimmune diseases and B-ALL (194 unique) (Additional file 2: Table S7 and Additional file 1: Fig. S9). Additional file 2: Table S8 lists the details of these genes. MAGMA gene analysis detected 92 repeated pleiotropic genes across different trait pairs, with IKZF1 identified as a pleiotropic gene for six pairs, followed by MLLT10, FIGNL1, RNASET2, CCR6, GATA3, CLN3, PIP4K2A, DDC, RP11-514O12.4, FGFR1OP, and GRB10 in four trait pairs. eQTL analysis identified multiple hits of IKZF1 in blood- and immune-related tissues (e.g., naïve B cell, CD19 B-cell, EBV-transformed lymphocytes cells, cis-eQTLs, trans-eQTLs, spleen, whole blood). Five genes (i.e., TUFM, ZC2HC1A, RNASET2, GSDMB, and ORMDL3) were observed to be significant in five different tissues. We summarized the landscape of pleiotropic genes identified in different methods and tissues in Fig. 4. We observed several genes (RNASET2 and FIGNL1) were significantly mapped in different tissues with different methods. The IKZF1 gene was also highlighted in whole blood tissues (Fig. 4).
Overview of pleiotropic genes (highlighted in all three signals) for the autoimmune disorders and B-ALL. Note: The signals represent hits of genes across different trait pairs. eQTL expression quantitative trait loci, SMR summary-based Mendelian randomization, AD autoimmune disorders, B-ALL B-cell acute lymphoblastic leukemia, AOA adult-onset asthma, HT hypothyroidism, PBC primary biliary cirrhosis, IBD inflammatory bowel disease, CD Crohn’s disease, RA rheumatoid arthritis, MS multiple sclerosis
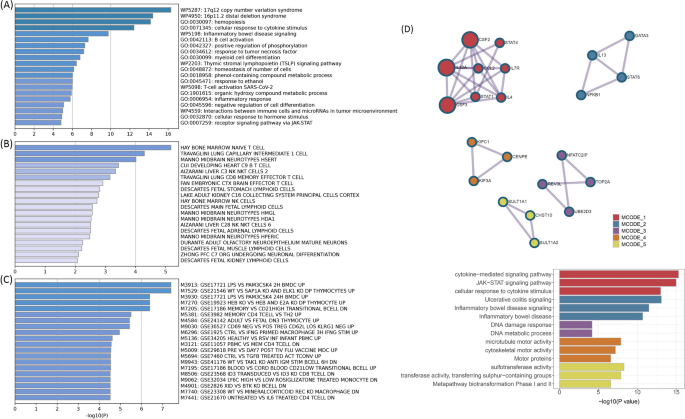
The shared mechanism between autoimmune diseases and B-ALL may involve specific organs or tissues involvement. Numerous genes (e.g., TOP2A, IKZF3, MYB, and CD80) showed significant differential expression in EBV-transformed lymphocytes, and APOBR, IKZF1, and IL7R showed significant differential expression in spleen and whole blood tissues (Additional file 1: Fig. S10 and Additional file 2: Table S9). Tissue enrichment analysis showed that these genes were also enriched into the spleen and EBV-transformed lymphocytes (Additional file 1: Fig. S11 and Additional file 2: Table S10). Additional S-LDSC based on multiple tissues identified significant SNP heritability enrichment for all autoimmune diseases (except AOA) in each of the monocytes, blood cells, and spleen, after adjusting for the baseline model (Additional file 1: Fig. S12 and Additional file 2: Table S11). Further enrichment analysis of the GO biological processes associated with these genes indicated higher enrichment in the cellular response to cytokine stimulation, B cell activation, response to tumor necrosis factor, inflammatory response, and receptor signaling pathway via JAK-STAT (Fig. 5A). These pathways play important roles in immune regulation and leukemogenesis. Cell type enrichment analysis showed the highest significance for bone marrow naïve T cells (Fig. 5B). Furthermore, we found that these genes were numerically enriched in several immunologic signatures (e.g., MEMORY VS CD21HIGH TRANSITIONAL BCELL DN) (Fig. 5C). The PPI analysis showed that five PPI networks were constructed, including the JAK-STAT signaling pathway and multiple pathways related to DNA damage were involved. And 22 proteins (e.g., STAT, NFKB1, and GATA3) could participate in these pathways (Fig. 5D). Also, the results suggest that heritability is enriched in the blood, EBV-transformed lymphocytes, whole blood, and palatine tonsil tissues among five or more autoimmune diseases and B-ALL.
Immune-related mechanisms shared between autoimmune disorders and B-ALL
The shared mechanism involving affected tissues such as the spleen, lymphocytes, and whole blood, suggested an important involvement of immune mechanisms in the inter-disease. We used the S-LDSC method to determine the heritability enrichment of pleiotropy in immune cells and the HyPrColoc method to identify immune cells with co-localization signals with pleiotropic motifs. S-LDSC observed heritability enrichment of B cells in both autoimmune diseases and B-ALL. When analyzing the enrichment of immune traits from ImmGen, we also observed that two cell traits in the B cell panel were enriched: B.FrE.BM (CD19+IgM+AA4.1+HSA+) and preB.FrD.BM (CD19+IgM−CD45R+CD43−). Additionally, numerous cell traits in the T cell panel were also identified, implying the potential immune mechanisms shared (Additional file 1: Fig. S12 and Additional file 2: Table S11). Then multi-trait colocalization analysis by using HyPrColoc was performed to pinpoint key immune cells (Additional file 2: Table S12). Results highlight 59 pleiotropic loci, of which 19 were unique, and these loci support the important role of 42 unique immune cells in autoimmune diseases and B-ALL by sharing causal variants. Our results support the critical influence of BAFF-R, CD4, CD45, and CD28 on different cells. Notably, a total of six BAFF-R-related immune traits were observed, including BAFF-R on B cell, BAFF-R on CD20−, BAFF-R on CD24+ CD27+, BAFF-R on IgD+ CD24−, BAFF-R on IgD+ CD24+, and BAFF-R on IgD+ CD38−. Interestingly, BAFF-R on B cell and BAFF-R on CD24+ CD27+ were both shared among three trait pairs (i.e., B-ALL&IBD, B-ALL&PBC, B-ALL&RA).
The causal relationship between autoimmune diseases and B-ALL estimated by MR
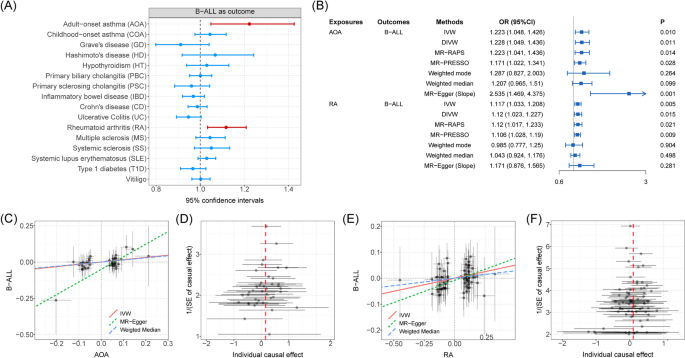
MR analyses using the IVW method showed significant positive associations between two autoimmune diseases (AOA and RA) and B-ALL risk (Fig. 6A and Additional file 2: Table S13). The risk of B-ALL was found to be able to be increased as the risk of AOA increases, the effect size was estimated by using the IVW method (OR = 1.223, 95%CI = 1.048 ~ 1.426, P = 0.010). Another four methods (DIVW, MR-RAPS, MR-PRESSO, and slope of MR-Egger) are consistent with the results of the IVW method. Although a significant intercept of MR-Egger might indicate the existence of potential horizontal pleiotropy, the global test of MR-PRESSO ruled out this possibility (P = 0.632). We also observed significant causal effects of RA onset on B-ALL risk by using the IVW method (OR = 1.117, 95%CI = 1.033 ~ 1.208, P = 0.005). DIVW, MR-RAPS, and MR-PRESSO support this association (Fig. 6B), where the intercept of MR-Egger and the global test of MR-PRESSO ruled out the possibility of horizontal pleiotropy (Additional file 2: Table S14). Additional scatter and funnel plots eliminate the possibility of potential outliers (Fig. 6C–D). However, after the Bonferroni adjustment, no causal associations between autoimmune disorders and B-ALL remained statistically significant (P = 0.003 < 0.05/16). Finally, reverse MR analysis ruled out the possibility of reverse-directional causality.
A The forest plot shows causal associations between autoimmune disorders and B-ALL by using one-directional MR analysis. Note: Causal effects were estimated by using IVW method. B Forest plot shows causal effects of AOA and RA on B-ALL risk estimated by using different methods. C Scatter plot shows significant causal association between AOA and B-ALL risk. D Funnel plot shows significant causal association between AOA and B-ALL risk. E Scatter plot shows significant causal association between RA and B-ALL risk. F Funnel plot shows significant causal association between RA and B-ALL risk. Associations highlighted with red represent that associations were significant in more than three main MR methods
Discussion
Given the critical contribution of B cells to autoimmune disorders and B-ALL, there may be a complex relationship between them [60]. The study employed comprehensive genetic methodologies to investigate the genetic correlation between autoimmune disorders and B-ALL. The study determined pleiotropic loci using cross-trait PLACO analysis and identified pleiotropic genes through the MAGMA method. Then the key pathways and immunological mechanisms involved were identified. Finally, comprehensive MR analysis and sensitive analysis established the causal relationships between autoimmune diseases and B-ALL.
Through genetic correlation analysis, we observed significant genetic overlap between B-ALL and seven autoimmune disorders, including AOA, HT, IBD, CD, PBC, RA, and MS. We provide strong evidence for a shared genetic mechanism between RA and B-ALL, as well as MR evidence suggesting that patients with RA symptoms should be alerted to the risk of progression to ALL, which is consistent with previous studies [7, 8]. Additionally, study have shown that 34 of 699 ALL patients diagnosed and followed had previously received varying doses of steroids for aplastic events or arthritis-based rheumatic diseases [61]. By using genetic variables, MR methods could well avoid the influence of possible confounding factors. Therefore, we believe that in addition to the effect of immunosuppressants, RA itself will also play an important role in the risk of B-ALL. We also observed significantly causal effects of AOA on B-ALL risk, which was ambiguous in previous studies: a systematic review supported the protective effect of asthma on ALL [62], two types of research included showed significant high risks of ALL in patients with a history of asthma [63, 64].
We identified a series of genetically risk loci associated with both autoimmune diseases and B-ALL, and some of which were observed in multiple phenotype pairs (e.g., 7p12.2, 10p14, 6q27, 10p12.31). Previous studies gave the evidence of key role these loci played in the development of autoimmune disorders and B-ALL. For example, loci on 7p12.2 (IKZF1) had been proven to be associated with risk of childhood B-ALL [65], which was also identified as susceptibility genes for SLE [66]. After searching for the GWAS catalog, 7p12.2 had been reported to be associated with multiple autoimmune disorders, including CD [23], IBD [23], RA [25], and MS [67]. GATA3 (10p14) is a key regulator in the immune system, especially in the differentiation and function of type 2 helper (Th2) cells [68]. Th2 cells have been demonstrated to play a role in various autoimmune diseases, including SLE and IBD [69, 70]. Recent research also highlighted the role of noncoding genetic variation (rs3824462) in GATA3, linking it to an increased risk of Ph-like ALL, a common subtype of B-ALL. The study revealed that rs3824462 induced local and global changes in chromatin conformation, activating JAK-STAT pathway and promoting disease development [71].
We searched for the identified risk loci in the GWAS catalog (last update in 2023 December 20) [72] and found that some of the risk loci have been reported to be associated with both B-ALL and autoimmune disorders (Additional file 1: Fig. S13 and Additional file 2: Table S15). For instance, the 17q21 locus is implicated in various autoimmune diseases, including asthma [73, 74], IBD [75, 76], T1D [77], and SLE [78]. This locus, housing IKZF3, GSDMB, and ORMDL3, involved in lymphocyte development [79], pyroptosis [80], and inflammatory response [81], has been challenging to dissect. GSDMB and ORMDL3 represent the target genes of rs2290400, and its minor allele is associated with a protective effect against ALL [82]. IKZF3 polymorphism contributes to B-ALL with a 1.5-fold to twofold increase in relative risk [83]. Genes previously reported to be associated with leukemia have also been observed in our results to be correlated with autoimmune diseases: MLLT10 (10p12) participates in various chromosomal rearrangements associated with ALL and acute myeloid leukemia (AML) [84]. It is implicated in chromatin structure regulation and DNA damage response, deemed crucial for early development, maintenance, and differentiation of hematopoietic stem cells. While direct evidence for the impact of MLLT10 on autoimmune diseases has not been established, studies indicated a close association with C-reactive protein levels [85], widely recognized as a valuable indicator of disease activity in various autoimmune rheumatic diseases [86]. Simultaneously, certain genes previously reported to be associated with autoimmune disorders have also been found in our results to be associated with B-ALL. IRGM (5q33) encodes a member of the immunity-related GTPase family, crucial in innate immunity and inflammatory responses [87]. Previous studies have linked IRGM to CD [88,89,90], UC [91], and IBD [23]. CAPSL (5p13) has been reported to be associated with PBC [92], T1D [93], asthma [94], and SLE [95]. Although direct evidence of its association with ALL is lacking, increased mRNA levels have been observed in AML patients [96]. Additionally, the long non-coding RNA C5orf56 (5q31) has been identified for its protective role in IBD [97]. SCHIP1 (3q25) has been associated with SLE [98], while RNASET2(6q27) has been identified as a risk gene for both vitiligo [99] and GD [100].
Shared genetic structures observed in our research revealed common mechanisms between autoimmune disease and B-ALL. Identified genes were observed to participate in several pathways, like B cell activation, cellular response to cytokine stimulus, and inflammatory response. For each disease pair, we observed a significant enrichment of pleiotropy to the spleen, a critical site for B cell development. Notably, a substantial presence of BAFF-R-associated immune signature, a key regulator of B cell function and survival, was discerned in a multi-trait colocalization analysis. These findings collectively underscored the pivotal role played by B cells in both autoimmune disorders and B-ALL. In autoimmune conditions, B cells are exposed to antigens, undergo activation, and subsequently proliferate and expand clonally, thereby increasing the risk of accumulating genetic mutations, and finally leads to the emergence and progression of B-ALL [60]. We can think that ORMDL3 and IKZF3, mentioned earlier, play crucial roles in this context, as evidenced by prior literature reporting ORMDL3’s vital role in B cell survival [101], and IKZF3’s predominant regulation of B cell differentiation, activation response, and proliferation [102]. Furthermore, malignancies arising from B cells consistently exhibit concurrent autoimmune disorders at any stage, whereas those derived from T cells are less commonly linked to autoimmune phenomena [103]. Nevertheless, our findings also identified numerous cell traits in the T cell panel, and we speculate that this may be attributed to interactions between B and T cells. The JAK-STAT pathway may represent a crucial mechanism in this context, as it has been targeted in autoimmune diseases [104] and its role in B-ALL involves the disruption of preleukemic cells differentiation [105]. Our results highlighted the critical role of EBV infection as a trigger for both autoimmune disorders and B-ALL: tissue-specific analysis revealed enriched risk loci in EBV-transformed lymphocytes, and the central role of IKZF1 in this cell was also identified by gene-level analyses. EBV remains latent in memory B cells after infection, and reactivation can induce B cell clonal immortalization, promoting lymphomagenesis [106]. Additionally, EBV-induced autoimmunity has been reported to increase the risk of autoimmune diseases [107].
Limitations
Our study is not without limitations. Firstly, as with other similar studies, the data used in this study was summary-level, and individual-level datasets were not available. Further stratification of the population (e.g., gender, age, etc.) was therefore not possible. Secondly, the sample size of immune cell GWAS used in this study was limited. Therefore, caution should be exercised in interpreting the role of immune cells and drawing conclusions in our studies. Thirdly, it should be noted that our study was limited to European ancestry and may not be generalizable to other ancestries. It is important to be equally cautious in concluding our findings since the relatively small sample size of B-ALL may result in limited statistical power.
Conclusions
Our research has uncovered the intricate connections between autoimmune disorders, especially AOA, HT, IBD, CD, RA, and MS and B-ALL. Identification of pleiotropic risk loci (7p12, 10p14, 6q27, and 10p12) and genes (IKZF1, GATA3, IKZF3, GSDMB, and ORMDL3) shared between diseases suggested shared mechanisms, such as B cell activation and JAK-STAT pathway, common triggers like EBV infection. Additionally, our findings have shed light on and the causal links between autoimmune disorders (AOA and RA) and B-ALL.
Availability of data and materials
Data are available in public, open access repositories corresponding to the original studies (e.g., GWAS catalog). Main codes used in our research are available at https://github.com/biostatYu/MRcode/tree/main/AD_BALL.
Abbreviations
- AML:
-
Acute myeloid leukemia
- AOA:
-
Adult-onset asthma
- B-ALL:
-
B-cell acute lymphoblastic leukemia
- CD:
-
Crohn’s disease
- COA:
-
Childhood-onset asthma
- DEGs:
-
Differentially expressed genes
- DIVW:
-
Debiased-inverse variance weighted
- EBV:
-
Epstein–Barr virus
- eQTL:
-
Expression quantitative trait loci
- FUMA:
-
Functional mapping and annotation
- GD:
-
Graves’ disease
- GWAS:
-
Genome-wide association study
- HD:
-
Hashimoto's disease
- HDL:
-
High-definition likelihood
- HT:
-
Hypothyroidism
- HyPrColoc:
-
Hypothesis prioritization for multi-trait colocalization
- IBD:
-
Inflammatory bowel disease
- IUT:
-
Intersection–union test
- IVs:
-
Instrumental variables
- IVW:
-
Inverse variance weighted
- LD:
-
Linkage disequilibrium
- LDSC:
-
Linkage disequilibrium score regression
- MAGMA:
-
Multi-marker analysis of genomic annotation
- MR:
-
Mendelian randomization
- MR-PRESSO:
-
Mendelian randomization pleiotropy residual sum and outlier
- MR-RAPS:
-
Mendelian randomization robust adjusted profile score
- MS:
-
Multiple sclerosis
- MSigDB:
-
Molecular signatures database
- PBC:
-
Primary biliary cirrhosis
- PLACO:
-
Pleiotropic analysis under composite null hypothesis
- PSC:
-
Primary sclerosing cholangitis
- PVE:
-
Proportion of variance explained
- RA:
-
Rheumatoid arthritis
- SE:
-
Standard errors
- S-LDSC:
-
Stratified-linkage disequilibrium score regression
- SLE:
-
Systemic lupus erythematosus
- SMR:
-
Summary-based Mendelian randomization
- SS:
-
Systemic sclerosis
- T1D:
-
Type 1 diabetes
- UC:
-
Ulcerative colitis
References
Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541–52.
Chi X, Huang M, Tu H, Zhang B, Lin X, Xu H, et al. Innate and adaptive immune abnormalities underlying autoimmune diseases: the genetic connections. Sci China Life Sci. 2023;66(7):1482–517.
Lin X, Lu L. B Cell-mediated autoimmune diseases. Adv Exp Med Biol. 2020;1254:145–60.
Shaffer AL, Rosenwald A, Staudt LM. Lymphoid malignancies: the dark side of B-cell differentiation. Nat Rev Immunol. 2002;2(12):920–32.
Smedby KE, Hjalgrim H, Askling J, Chang ET, Gregersen H, Porwit-MacDonald A, et al. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst. 2006;98(1):51–60.
Ekström Smedby K, Vajdic CM, Falster M, Engels EA, Martínez-Maza O, Turner J, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111(8):4029–38.
Hemminki K, Huang W, Sundquist J, Sundquist K, Ji J. Autoimmune diseases and hematological malignancies: exploring the underlying mechanisms from epidemiological evidence. Semin Cancer Biol. 2020;64:114–21.
Cabral DA, Tucker LB. Malignancies in children who initially present with rheumatic complaints. J Pediatr. 1999;134(1):53–7.
Li Y, Xie X, Jie Z, Zhu L, Yang JY, Ko CJ, et al. DYRK1a mediates BAFF-induced noncanonical NF-κB activation to promote autoimmunity and B-cell leukemogenesis. Blood. 2021;138(23):2360–71.
Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh P-R, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47(11):1236.
Gong W, Guo P, Li Y, Liu L, Yan R, Liu S, et al. Role of the gut-brain axis in the shared genetic etiology between gastrointestinal tract diseases and psychiatric disorders: a genome-wide pleiotropic analysis. JAMA Psychiat. 2023;80(4):360–70.
Yu XH, Yang YQ, Cao RR, Cai MK, Zhang L, Deng FY, et al. Rheumatoid arthritis and osteoporosis: shared genetic effect, pleiotropy and causality. Hum Mol Genet. 2021;30(21):1932–40.
Lu H, Qiao J, Shao Z, Wang T, Huang S, Zeng P. A comprehensive gene-centric pleiotropic association analysis for 14 psychiatric disorders with GWAS summary statistics. BMC Med. 2021;19(1):314.
Ray D, Chatterjee N. A powerful method for pleiotropic analysis under composite null hypothesis identifies novel shared loci between type 2 diabetes and prostate cancer. Plos Genet. 2020;16(12):e1009218.
Ferreira MAR, Mathur R, Vonk JM, Szwajda A, Brumpton B, Granell R, et al. Genetic architectures of childhood- and adult-onset asthma are partly distinct. Am J Hum Genet. 2019;104(4):665–84.
Ferreira MAR, Mathur R, Vonk JM, Szwajda A, Brumpton B, Granell R, et al. Genetic architectures of childhood- and adult-onset asthma are partly distinct. https://www.ebi.ac.uk/gwas/studies/GCST007800. (2019).
Sakaue S, Kanai M, Tanigawa Y, Karjalainen J, Kurki M, Koshiba S, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet. 2021;53(10):1415–24.
Sakaue S, Kanai M, Tanigawa Y, Karjalainen J, Kurki M, Koshiba S, et al. A cross-population atlas of genetic associations for 220 human phenotypes. https://pheweb.jp/downloads. (2021).
Cordell HJ, Fryett JJ, Ueno K, Darlay R, Aiba Y, Hitomi Y, et al. An international genome-wide meta-analysis of primary biliary cholangitis: novel risk loci and candidate drugs. J Hepatol. 2021;75(3):572–81.
Cordell HJ, Fryett JJ, Ueno K, Darlay R, Aiba Y, Hitomi Y, et al. An international genome-wide meta-analysis of primary biliary cholangitis: novel risk loci and candidate drugs. https://www.ebi.ac.uk/gwas/studies/GCST90061440. (2021).
Ji SG, Juran BD, Mucha S, Folseraas T, Jostins L, Melum E, et al. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat Genet. 2017;49(2):269–73.
Ji SG, Juran BD, Mucha S, Folseraas T, Jostins L, Melum E, et al. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. https://www.ebi.ac.uk/gwas/studies/GCST004030. (2017).
de Lange KM, Moutsianas L, Lee JC, Lamb CA, Luo Y, Kennedy NA, et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet. 2017;49(2):256–61.
de Lange KM, Moutsianas L, Lee JC, Lamb CA, Luo Y, Kennedy NA, et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. https://www.ebi.ac.uk/gwas/studies/GCST004131. (2017).
Ishigaki K, Sakaue S, Terao C, Luo Y, Sonehara K, Yamaguchi K, et al. Multi-ancestry genome-wide association analyses identify novel genetic mechanisms in rheumatoid arthritis. Nat Genet. 2022;54(11):1640–51.
Ishigaki K, Sakaue S, Terao C, Luo Y, Sonehara K, Yamaguchi K, et al. Multi-ancestry genome-wide association analyses identify novel genetic mechanisms in rheumatoid arthritis. https://www.ebi.ac.uk/gwas/studies/GCST90132223. (2022).
Bentham J, Morris DL, Graham DSC, Pinder CL, Tombleson P, Behrens TW, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet. 2015;47(12):1457–64.
Bentham J, Morris DL, Graham DSC, Pinder CL, Tombleson P, Behrens TW, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. https://www.ebi.ac.uk/gwas/studies/GCST003156. (2015).
Consortium. IMSG. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science (New York, NY). 2019;365(6460):7188.
Consortium. IMSG. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. https://www.ebi.ac.uk/gwas/studies/GCST009597. (2019).
López-Isac E, Acosta-Herrera M, Kerick M, Assassi S, Satpathy AT, Granja J, et al. GWAS for systemic sclerosis identifies multiple risk loci and highlights fibrotic and vasculopathy pathways. Nat Commun. 2019;10(1):4955.
López-Isac E, Acosta-Herrera M, Kerick M, Assassi S, Satpathy AT, Granja J, et al. GWAS for systemic sclerosis identifies multiple risk loci and highlights fibrotic and vasculopathy pathways. https://www.ebi.ac.uk/gwas/studies/GCST009131. (2019).
Jin Y, Andersen G, Yorgov D, Ferrara TM, Ben S, Brownson KM, et al. Genome-wide association studies of autoimmune vitiligo identify 23 new risk loci and highlight key pathways and regulatory variants. Nat Genet. 2016;48(11):1418–24.
Jin Y, Andersen G, Yorgov D, Ferrara TM, Ben S, Brownson KM, et al. Genome-wide association studies of autoimmune vitiligo identify 23 new risk loci and highlight key pathways and regulatory variants. https://www.ebi.ac.uk/gwas/studies/GCST004785. (2016).
Vijayakrishnan J, Qian M, Studd JB, Yang W, Kinnersley B, Law PJ, et al. Identification of four novel associations for B-cell acute lymphoblastic leukaemia risk. Nat Commun. 2019;10(1):5348.
Vijayakrishnan J, Qian M, Studd JB, Yang W, Kinnersley B, Law PJ, et al. Identification of four novel associations for B-cell acute lymphoblastic leukaemia risk. https://www.ebi.ac.uk/gwas/studies/GCST009638. (2019).
Consortium GP. A global reference for human genetic variation. Nature. 2015;526(7571):68.
Ning Z, Pawitan Y, Shen X. High-definition likelihood inference of genetic correlations across human complex traits. Nat Genet. 2020;52(8):859–64.
GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45(6):580–5.
ImmGen O-S. mononuclear phagocytes. Nat Immunol. 2016;17(7):741.
Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017;8(1):1826.
Giambartolomei C, Vukcevic D, Schadt EE, Franke L, Hingorani AD, Wallace C, et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. Plos Genet. 2014;10(5):e1004383.
de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. Plos Comput Biol. 2015;11(4):e1004219.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci. 2005;102(43):15545–50.
Carithers LJ, Ardlie K, Barcus M, Branton PA, Britton A, Buia SA, et al. A novel approach to high-quality postmortem tissue procurement: the GTEx project. Biopreserv Biobanking. 2015;13(5):311–9.
Zhu Z, Zhang F, Hu H, Bakshi A, Robinson MR, Powell JE, et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet. 2016;48(5):481–7.
Foley CN, Staley JR, Breen PG, Sun BB, Kirk PDW, Burgess S, et al. A fast and efficient colocalization algorithm for identifying shared genetic risk factors across multiple traits. Nat Commun. 2021;12(1):764.
Orrù V, Steri M, Sidore C, Marongiu M, Serra V, Olla S, et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet. 2020;52(10):1036–45.
Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526(7571):68–74.
Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40(3):755–64.
Ye T, Shao J, Kang H. Debiased inverse-variance weighted estimator in two-sample summary-data Mendelian randomization. Ann Stat. 2021;49(4):2079–100.
Bowden J, Smith GD, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14.
Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693.
Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–89.
Zhao Q, Wang J, Hemani G, Bowden J, Small DS. Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. Ann Statist. 2020;48(3):1742–69. https://doi.org/10.1214/19-AOS1866.
Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46(6):1985–98.
Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18(20):2693–708.
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45(6):1961–74.
Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734–9.
Baecklund E, Smedby KE, Sutton LA, Askling J, Rosenquist R. Lymphoma development in patients with autoimmune and inflammatory disorders–what are the driving forces? Semin Cancer Biol. 2014;24:61–70.
Révész T, Kardos G, Kajtár P, Schuler D. The adverse effect of prolonged prednisolone pretreatment in children with acute lymphoblastic leukemia. Cancer. 1985;55(8):1637–40.
Zhou MH, Yang QM. Association of asthma with the risk of acute leukemia and non-Hodgkin lymphoma. Mol Clin Oncol. 2015;3(4):859–64.
Chang JS, Tsai YW, Tsai CR, Wiemels JL. Allergy and risk of childhood acute lymphoblastic leukemia: a population-based and record-based study. Am J Epidemiol. 2012;176(11):970–8.
Spector L, Groves F, DeStefano F, Liff J, Klein M, Mullooly J, et al. Medically recorded allergies and the risk of childhood acute lymphoblastic leukaemia. Eur J Cancer (Oxford, England : 1990). 2004;40(4):579–84.
Papaemmanuil E, Hosking FJ, Vijayakrishnan J, Price A, Olver B, Sheridan E, et al. Loci on 7p12.2, 10q21.2 and 14q11.2 are associated with risk of childhood acute lymphoblastic leukemia. Nat Genet. 2009;41(9):1006–10.
Han JW, Zheng HF, Cui Y, Sun LD, Ye DQ, Hu Z, et al. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet. 2009;41(11):1234–7.
Beecham AH, Patsopoulos NA, Xifara DK, Davis MF, Kemppinen A, Cotsapas C, et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet. 2013;45(11):1353–60.
Zhu J. GATA3 regulates the development and functions of innate lymphoid cell subsets at multiple stages. Front Immunol. 2017;8:1571.
Ramirez GA, Tassi E, Noviello M, Mazzi BA, Moroni L, Citterio L, et al. Histone‐specific CD4+ T cell plasticity in active and quiescent systemic lupus erythematosus. Arthritis Rheumatol. 2024.
Liu A, Liang X, Wang W, Wang C, Song J, Guo J, et al. Human umbilical cord mesenchymal stem cells ameliorate colon inflammation via modulation of gut microbiota-SCFAs-immune axis. Stem Cell Res Ther. 2023;14(1):271.
Yang H, Zhang H, Luan Y, Liu T, Yang W, Roberts KG, et al. Noncoding genetic variation in GATA3 increases acute lymphoblastic leukemia risk through local and global changes in chromatin conformation. Nat Genet. 2022;54(2):170–9.
Sollis E, Mosaku A, Abid A, Buniello A, Cerezo M, Gil L, et al. The NHGRI-EBI GWAS catalog: knowledgebase and deposition resource. Nucleic Acids Res. 2023;51(D1):D977–85.
Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448(7152):470–3.
Li X, Christenson SA, Modena B, Li H, Busse WW, Castro M, et al. Genetic analyses identify GSDMB associated with asthma severity, exacerbations, and antiviral pathways. J Allergy Clin Immunol. 2021;147(3):894–909.
Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24.
Chao KL, Kulakova L, Herzberg O. Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein. Proc Natl Acad Sci USA. 2017;114(7):E1128–37.
Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41(6):703–7.
Perez RK, Gordon MG, Subramaniam M, Kim MC, Hartoularos GC, Targ S, et al. Single-cell RNA-seq reveals cell type-specific molecular and genetic associations to lupus. Science (New York, NY). 2022;376(6589):eabf1970.
Morgan B, Sun L, Avitahl N, Andrikopoulos K, Ikeda T, Gonzales E, et al. Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation. EMBO J. 1997;16(8):2004–13.
Li X, Zhang T, Kang L, Xin R, Sun M, Chen Q, et al. Apoptotic caspase-7 activation inhibits non-canonical pyroptosis by GSDMB cleavage. Cell Death Differ. 2023;30(9):2120–34.
Zhang Y, Willis-Owen SAG, Spiegel S, Lloyd CM, Moffatt MF, Cookson W. The ORMDL3 asthma gene regulates ICAM1 and has multiple effects on cellular inflammation. Am J Respir Crit Care Med. 2019;199(4):478–88.
Wiemels JL, Walsh KM, de Smith AJ, Metayer C, Gonseth S, Hansen HM, et al. GWAS in childhood acute lymphoblastic leukemia reveals novel genetic associations at chromosomes 17q12 and 8q24.21. Nat Commun. 2018;9(1):286.
Cobaleda C, Vicente-Dueñas C, Sanchez-Garcia I. Infectious triggers and novel therapeutic opportunities in childhood B cell leukaemia. Nat Rev Immunol. 2021;21(9):570–81.
Forgione MO, McClure BJ, Yeung DT, Eadie LN, White DL. MLLT10 rearranged acute leukemia: incidence, prognosis, and possible therapeutic strategies. Genes Chromosomes Cancer. 2020;59(12):709–21.
Said S, Pazoki R, Karhunen V, Võsa U, Ligthart S, Bodinier B, et al. Genetic analysis of over half a million people characterises C-reactive protein loci. Nat Commun. 2022;13(1):2198.
Zhang SL, Lin H, Huang F. Special diagnostic value of C-reactive protein in systemic autoimmune rheumatic diseases complicated with infections. Zhonghua Nei Ke Za Zhi. 2020;59(7):489–92.
Singh SB, Davis AS, Taylor GA, Deretic V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science (New York, NY). 2006;313(5792):1438–41.
Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42(12):1118–25.
Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–78.
Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohnʼs disease. Nat Genet. 2008;40(8):955–62.
Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47(9):979–86.
Juran BD, Hirschfield GM, Invernizzi P, Atkinson EJ, Li Y, Xie G, et al. Immunochip analyses identify a novel risk locus for primary biliary cirrhosis at 13q14, multiple independent associations at four established risk loci and epistasis between 1p31 and 7q32 risk variants. Hum Mol Genet. 2012;21(23):5209–21.
Baxter AG, Jordan MA. From markers to molecular mechanisms: type 1 diabetes in the post-GWAS era. The review of diabetic studies : RDS. 2012;9(4):201–23.
Johansson Å, Rask-Andersen M, Karlsson T, Ek WE. Genome-wide association analysis of 350 000 Caucasians from the UK Biobank identifies novel loci for asthma, hay fever and eczema. Hum Mol Genet. 2019;28(23):4022–41.
Wang YF, Zhang Y, Lin Z, Zhang H, Wang TY, Cao Y, et al. Identification of 38 novel loci for systemic lupus erythematosus and genetic heterogeneity between ancestral groups. Nat Commun. 2021;12(1):772.
Uhlen M, Zhang C, Lee S, Sjöstedt E, Fagerberg L, Bidkhori G, et al. A pathology atlas of the human cancer transcriptome. Science (New York, NY). 2017;357(6352):eaan2507.
Ma H, Hu T, Tao W, Tong J, Han Z, Herndler-Brandstetter D, et al. A lncRNA from an inflammatory bowel disease risk locus maintains intestinal host-commensal homeostasis. Cell Res. 2023;33(5):372–88.
Khunsriraksakul C, Li Q, Markus H, Patrick MT, Sauteraud R, McGuire D, et al. Multi-ancestry and multi-trait genome-wide association meta-analyses inform clinical risk prediction for systemic lupus erythematosus. Nat Commun. 2023;14(1):668.
Quan C, Ren YQ, Xiang LH, Sun LD, Xu AE, Gao XH, et al. Genome-wide association study for vitiligo identifies susceptibility loci at 6q27 and the MHC. Nat Genet. 2010;42(7):614–8.
Chu X, Pan CM, Zhao SX, Liang J, Gao GQ, Zhang XM, et al. A genome-wide association study identifies two new risk loci for Graves’ disease. Nat Genet. 2011;43(9):897–901.
Dang J, Bian X, Ma X, Li J, Long F, Shan S, et al. ORMDL3 facilitates the survival of splenic B cells via an ATF6α-endoplasmic reticulum stress-Beclin1 autophagy regulatory pathway. J Immunol (Baltimore, Md : 1950). 2017;199(5):1647–59.
John LB, Ward AC. The Ikaros gene family: transcriptional regulators of hematopoiesis and immunity. Mol Immunol. 2011;48(9–10):1272–8.
Porpaczy E, Jäger U. How I manage autoimmune cytopenias in patients with lymphoid cancer. Blood. 2022;139(10):1479–88.
Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77(5):521–46.
Fregona V, Bayet M, Bouttier M, Largeaud L, Hamelle C, Jamrog LA, et al. Stem cell-like reprogramming is required for leukemia-initiating activity in B-ALL. J Exp Med. 2024;221(1):20230279.
Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350(13):1328–37.
Vietzen H, Berger SM, Kühner LM, Furlano PL, Bsteh G, Berger T, et al. Ineffective control of Epstein-Barr-virus-induced autoimmunity increases the risk for multiple sclerosis. Cell. 2023;186(26):5705–18.e13.
Acknowledgements
We thank all the studies for making the summary association statistics data publicly available. We are also very grateful to the editor and two referees for their insightful and constructive comments, which substantially improved our original manuscript.
Funding
This work was supported by National Key Research and Development Program (2022YFC2502700) to Y.X. and National Natural Science Foundation of China (82020108003 to D.W., 82070187 to Y.X.). D.W. was supported by Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and Jiangsu Provincial Medical Innovation Center (CXZX202201). Y.C. is also supported by Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX23_3270). D.W. was supported by Suzhou Science and Technology Program Project (SLT201911). X.Y. was supported by Boxi Cultivation Program of the First Affiliated Hospital of Suzhou University (BXQN2023032).
Author information
Authors and Affiliations
Contributions
DW and YX designed the study. XY obtained the data. XY and YC cleared up the datasets. XY, HL, and YC mainly performed the data analyses. YX, XY, YC, JC, YF, and HL drafted the manuscript. YX, XY, YC, and DW revised the manuscript, and all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declared no potential conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Methods and Fig. S1-S3. Supplementary Methods - A supplementary document on GWAS quality control, PLACO method, colocalization analysis, MAGMA analysis, HyPrColoc method, immune cell data description, and Mendelian randomization analysis. Fig. S1. Manhattan plot of the PLACO results. Fig. S2. -QQ plots for pleiotropic results performed by PLACO. Fig. S3. Regional plots of each colocalized locus (PP.H4 > 0.7) identified for corresponding trait pair (B-ALL&AOA) by using the PLACO. Fig. S4. Regional plots of each colocalized locus (PP.H4 > 0.7) identified for corresponding trait pair (B- B-ALL&HT) by using the PLACO. Fig. S5. Regional plots of each colocalized locus (PP.H4 > 0.7) identified for corresponding trait pair (B-ALL&PBC) by using the PLACO. Fig. S6. Regional plots of each colocalized locus (PP.H4 > 0.7) identified for corresponding trait pair (B-ALL&IBD) by using the PLACO. Fig. S7. Regional plots of each colocalized locus (PP.H4 > 0.7) identified for corresponding trait pair (B-ALL&MS) by using the PLACO. Fig. S8. Regional plot of each colocalized locus (PP.H4 > 0.7) identified for corresponding trait pair (B- B-ALL&RA) by using the PLACO. Fig. S9. Manhattan plot of MAGMA gene analysis. Fig. S10. Heatmap for expression values of pleiotropic genes in different tissues identified by MAGMA analysis. Fig. S11. Gene-set enrichment for identified pleiotropic genes. Red panels represent significant tissues after Bonferroni adjustment. Fig. S12. Heatmap of tissues and immune traits shared between autoimmune disorders and B-ALL identified by S-LDSC. Fig. S13. Heatmap shows whether the identified risk loci have been reported to be associated with B-ALL and AD in the previous studies after searching the GWAS catalog.
Additional file 2: Table S1.
Data sources. Table S2. Genetic correlation analysis conducted by LDSC and HDL. Table S3. Shared pleiotropic loci identified by PLACO. Table S4. Shared pleiotropic loci among different trait pairs. Table S5. MAGMA Gene-set analysis. Table S6. MAGMA tissue-specific analysis. Table S7. MAGMA gene analysis. Table S8. Information of pleiotropy genes identified by MAGMA. Table S9. Expression value of pleiotropy genes identified by MAGMA in different tissues from GTEx. Table S10. Tissue-specific enrichment of pleiotropy genes identified by MAGMA in different tissues from GTEx. Table S11. S-LDSC cell-type heritability enrichment analysis. Table S12. Multi-trait colocalization analysis highlighted key role of immune cells (PP>0.7). Table S13. Bi-direction MR analysis and sensitive analysis. Table S14. Bi-direction MR analysis and sensitive analysis. Table S15. Identified loci reported in previous GWAS analysis for ALL and AD.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yu, X., Chen, Y., Chen, J. et al. Shared genetic architecture between autoimmune disorders and B-cell acute lymphoblastic leukemia: insights from large-scale genome-wide cross-trait analysis. BMC Med 22, 161 (2024). https://doi.org/10.1186/s12916-024-03385-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-024-03385-0