Abstract
Background
Technology-enabled inpatient-level care at home services, such as virtual wards and hospital at home, are being rapidly implemented. This is the first systematic review to link the components of these service delivery innovations to evidence of effectiveness to explore implications for practice and research.
Methods
For this review (registered here https://osf.io/je39y), we searched Cochrane-recommended multiple databases up to 30 November 2022 and additional resources for randomised and non-randomised studies that compared technology-enabled inpatient-level care at home with hospital-based inpatient care. We classified interventions into care model groups using three key components: clinical activities, workforce, and technology. We synthesised evidence by these groups quantitatively or narratively for mortality, hospital readmissions, cost-effectiveness and length of stay.
Results
We include 69 studies: 38 randomised studies (6413 participants; largely judged as low or unclear risk of bias) and 31 non-randomised studies (31,950 participants; largely judged at serious or critical risk of bias). The 69 studies described 63 interventions which formed eight model groups. Most models, regardless of using low- or high-intensity technology, may have similar or reduced hospital readmission risk compared with hospital-based inpatient care (low-certainty evidence from randomised trials). For mortality, most models had uncertain or unavailable evidence. Two exceptions were low technology-enabled models that involve hospital- and community-based professionals, they may have similar mortality risk compared with hospital-based inpatient care (low- or moderate-certainty evidence from randomised trials). Cost-effectiveness evidence is unavailable for high technology-enabled models, but sparse evidence suggests the low technology-enabled multidisciplinary care delivered by hospital-based teams appears more cost-effective than hospital-based care for those with chronic obstructive pulmonary disease (COPD) exacerbations.
Conclusions
Low-certainty evidence suggests that none of technology-enabled care at home models we explored put people at higher risk of readmission compared with hospital-based care. Where limited evidence on mortality is available, there appears to be no additional risk of mortality due to use of technology-enabled at home models. It is unclear whether inpatient-level care at home using higher levels of technology confers additional benefits. Further research should focus on clearly defined interventions in high-priority populations and include comparative cost-effectiveness evaluation.
Trial registration
Similar content being viewed by others
Background
There are huge pressures on health systems globally; in large part due to an ageing population and a corresponding increase in demands on health care services. The COVID-19 pandemic placed additional pressure on a stretched system. In the UK National Health Service (NHS), between April 2021 and March 2022, adults aged 60 and above were in receipt of half (50.2%) of the 19.6 million NHS hospital consultant episodes recorded [1]. In the USA, those older than 65 years accounted for 36% of hospitalisations in 2017 [2]. Current inpatient provision cannot keep up with changing demographic and health care demands. Alternative service delivery models have been developed to provide inpatient-level care outside hospital settings. Such home-based inpatient care models are designed to prevent inpatient admission into hospital (step-up into hospital-based care) or to facilitate early discharge (step down from hospital-based care).
Service delivery models that offer inpatient care at home have been in use in various formats for several years. Recently, in countries including the UK, there has been increasing investment in the provision of inpatient care at home models [3]. A commonly described model is ‘virtual wards’. This term broadly refers to care services that offer a limited period of a hospital ward-level acute care at a patient’s place of residence and involve use of technology [4]. ‘Virtual wards’ entail variable face-to-face activity component and in some cases patients may be solely managed remotely. ‘Hospital at home’ is another common model and is broadly used to describe face-to-face provision of, often multidisciplinary, inpatient care at home that would otherwise be delivered in hospital [5]. As such ‘hospital at home’ could stand alone or be a component of a virtual ward.
The terms ‘virtual ward’ and ‘hospital at home’ have historically been used somewhat interchangeably, but this can complicate evidence synthesis and its interpretation. In this review, we use ‘inpatient-level care at home’ as an umbrella term for a set of complex interventions that allow people to receive inpatient-level acute care outside of hospital. Such complex interventions involve various components, including technology involvement, workforce structure, clinical activities, information and support provision, and use of information systems.
Our scoping search identified 11 published systematic reviews of interventions described as ‘hospital at home’ or ‘virtual wards’, compared with hospital-based inpatient care, in a range of populations [6]. These reviews included not only inpatient-level care at home including face-to-face delivered care models but also remote monitoring that may not involve inpatient-level care [6]. There is a lack of existing evidence on technology-enabled models that are the focus of this review. Whilst models with different components may differ in their effectiveness, [7] care at home models included in previous reviews have not been described in detail in terms of their constituent components. Clearly describing components of existing models and, where possible, linking the components to effectiveness evidence are important for informing implementation of future innovations.
Objectives
We aimed to (1) identify and describe the components of ‘inpatient-level care at home’ models reported in comparative effectiveness research; and (2) synthesise identified research to assess the clinical, cost-effectiveness and safety of ‘inpatient-level care at home’ models, compared with hospital-based inpatient care, in people with any health conditions.
Methods
We follow generic Cochrane Systematic Review Methodology to conduct this systematic review and meta-analysis and follow the 2020 PRISMA guideline to report it [8, 9]. The pre-registered protocol is available at the Open Science Framework (https://osf.io/je39y) [10].
Search strategy and selection criteria
We searched Ovid MEDLINE including In-Process & Other Non-Indexed Citations (1946), Ovid Embase (1974), Cochrane Central Register of Controlled Trials (CENTRAL), and EBSCO CINAHL Plus (1937) from the inception until 30 November 2022 for English language publications. See Additional file 1: Text 1 for full search methods. We also searched the ClinicalTrials.gov and World Health Organization International Clinical Trials Registry Platform for ongoing studies; the reference lists of the two up-to-date Cochrane Reviews; [11, 12] and MedRxiv.org between 2019 and 2022 for recently completed but unpublished studies.
Using Rayyan to support study selection processes, two authors (CS, FR) independently assessed titles and abstracts for potentially eligible studies. Two review authors (CS and FR, AU or SB) then independently inspected the full-text of these potentially eligible studies. At each screening stage, these two review authors resolved disagreements through discussion and by involving a third review author (JD) and a clinician expert (EV) if necessary.
We included randomised trials of any designs and non-randomised studies using the designs of non-randomised trials (i.e., experimental trials without random allocations), controlled before-after studies, controlled interrupted time series studies, cohort studies aiming for comparative effectiveness evaluations, and studies with regression discontinuity designs [13, 14]. Eligible non-randomised studies had to evaluate intervention groups comparatively over a defined follow-up time in clearly defined participants and adjust for confounding in the analysis or by study design [14].
We considered studies that compared ‘inpatient-level care at home’ with hospital-based inpatient care as the comparator. We excluded studies that used interventions for care that was not considered acute. Eligible interventions had to use technologies of some form, which could include telephone contact or digital technologies such as apps, wearables. For completeness, we included studies that did not report sufficient information on technology use for us to make a judgement on this criterion, but we did not consider these studies in data analysis. We however explored assumptions around these studies as part of sensitivity analyses (see later section).
Data extraction
Two authors (CS and FR, AU or SB) independently extracted data for 5% of the included studies to pilot the data extraction form. Then the remaining studies were split into two batches, where one review author extracted data and the other review author checked the data extracted. The pre-prepared data extraction form is publicly accessible at Qualtrics (https://www.qualtrics.manchester.ac.uk/jfe/form/SV_3qQedEEEuYMrhMG) [15]. Our primary outcomes in this review included (1) mortality, measured as proportions of participants who died during study follow-up and (2) number of hospital readmission events following discharge from the episode of care. We considered cost-effectiveness, length of inpatient-level care stay, and adverse events as secondary outcomes.
The form included items of the Template for Intervention Description and Replication (TIDieR) checklist to facilitate full description of models and their components [16]. There is no consensus guidance on the components required for an ‘inpatient-level care at home’ model. We developed our taxonomy using the relevant NHS guidance Virtual ward including Hospital at Home [17] and the chronic care model, [18] alongside iterative discussion with health professionals including a virtual ward service lead who contributed substantially to develop and implement virtual ward services. We identified the following five components required to adequately describe innovations in a clinically meaningful way (Additional file 1: Table S1):
-
technology involvement (to capture the type of technology being used in the model);
-
workforce (to capture who was delivering care);
-
clinical activities (to capture what care was being delivered);
-
information and support provision (to capture the wider infrastructure and support involved in care delivery); and
-
clinical management system used (to capture what types of health record systems were used to support care provision).
Following data extraction, the lead review author (CS) checked and coded components. Then 10% of the included studies were randomly selected, and an independent author (GN) checked the accuracy of the extraction and coding of the models they used.
To synthesise evidence linking the intervention components to effectiveness, we followed the clinically meaningful-element approach to addressing the heterogeneity and complexity of inpatient-level care at home interventions. This approach facilitated us to group those with similar components together, and we were able to further specify clinically important elements using component categories [19, 20]. Whilst we had extracted data on five components, after further discussion and iteration with stakeholders, the main components used to describe the innovations were reduced to three: workforce, clinical activities, and technology involvement only. We considered these three components as the substantive elements needed for inpatient-level care at home to be functional. The two remaining components (information and support provision, and clinical management systems) were considered as secondary features of less direct relevance, and models without these two components may still be functional.
Using a similar approach as with the components themselves, we developed the categories for the three components used across the review to group the models (Table 1). The categories used aimed to capture variation in care models that were relevant to and recognised by those designing and delivering care.
Quality assessment of included studies
We used the first version of the Cochrane Risk of Bias tool for randomised controlled trials (RCTs) and the Risk Of Bias In Non-randomised Studies—of Interventions (ROBINS-I) tool for non-randomised studies [21, 22]. In using the ROBINS-I tool for assessing non-randomised studies, we considered age, the severity of primary health conditions, health status, co-morbidities and socioeconomic status as key confounder domains and non-acute care elements as the co-interventions. We acknowledged the importance of considering health inequity issues in this area, and that there were a range of potentially relevant socioeconomic variables. We followed the PROGRESS Plus framework to ensure the thorough consideration of socioeconomic status-related factors reflecting health inequities in this review [23]. The risk of bias assessment involved one author (CS) undertaking the assessment and another author (GN) checking this. All discrepancies were resolved between review authors through discussion.
We referred to the above risk of bias results to appraise the credibility of cost-effectiveness evidence where relevant. We are aware of the reporting checklist Consolidated Health Economic Evaluation Reporting Standards (CHEERS), [24] but we considered it inappropriate to use a reporting checklist for assessing risk of bias of cost-effectiveness analysis. Indeed, developers of the CHEERS checklist clearly advise not to use it as a risk of bias tool [24].
Data synthesis
We summarised the characteristics of the included studies. Where appropriate, we used meta-analysis with the DerSimonian-Laird random-effects model to combine data across included studies. We assessed heterogeneity from clinical, methodological and statistical perspectives, in which we used the Chi2 test and I2 statistic to quantify statistical heterogeneity but not to guide effects model choice. We analysed RCTs and non-randomised studies separately.
Rather than performing component-specific analysis, [25, 26] we pooled data by the inpatient-level care at home model groups i.e. those with the same component categories [19]. In this analysis, we took the view that the components of each model have to act in combination to impact on clinical outcomes. We did not then perform a pre-planned sensitivity analysis using component network meta-analysis. We present data using forest plots and present risk ratios (RRs) and mean differences (MDs) with 95% confidence intervals (CIs) for binary and continuous outcomes, respectively.
Whilst our main analyses focus on inpatient-level care at home models grouped by collective component types, we also performed single meta-analyses (one for RCTs and one for non-RCTs) that compared all inpatient-level care at home models with the comparator of hospital-based inpatient care. These post-hoc analyses were undertaken for two purposes: (1) to allow us to broadly assess the consistency between non-randomised and RCT estimates, thus informing appropriateness of using non-randomised evidence as a complement where RCT evidence is unavailable; [27] (2) to reflect the ‘lumping’ approach of previous reviews which has guided decision making to date, in comparison with our more nuanced approach based on component grouping for analyses.
When not undertaking meta-analysis, we considered synthesis of relevant data following the synthesis without meta-analysis in systematic reviews reporting guideline [3, 28]. We used tabular approaches to present available data and report results of narrative synthesis.
We present the main, pooled results of the review in ‘Summary of findings’ tables and assessed the evidence certainty using the Grading Recommendations Assessment and Development Evidence (GRADE) approach [27]. We assessed publication bias using funnel plots and by assessing the comprehensiveness of literature searches [29].
We also performed additional analyses as described in Additional file 1: Text 2 including sensitivity analyses for testing the sensitivity of main analyses to the changes of analysis assumptions.
Results
Study selection and characteristics
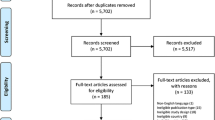
We included a total of 69 studies (Fig. 1) [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139]: eight (11.6%) were trial registries or protocols for ongoing studies that are still not completed upon writing this report [30, 44,45,46, 79, 80, 96, 97, 102, 139].
The 69 studies included 38 (55.1%) randomised trials [30,31,32,33,34,35,36,37,38,39,40,41,42, 44, 45, 48,49,50, 54,55,56, 62,63,64,65,66,67, 72, 73, 77,78,79,80,81,82,83, 92,93,94, 97, 99, 102,103,104,105,106,107,108,109,110, 115,116,117,118,119,120,121,122, 125,126,127, 130,131,132,133,134,135,136,137,138,139] and 31 (44.9%) non-randomised studies [43, 46, 47, 51,52,53, 57,58,59,60,61, 68,69,70,71, 74,75,76, 84,85,86,87,88,89,90,91, 95, 96, 98, 100, 101, 111,112,113,114, 123, 124, 128, 129] (Table 2 and Additional file 1: Table S2). The included RCTs were largely judged as overall low (1/38; 2.6%) and unclear risk of bias (25/38; 65.8%; Table 2 and Additional file 1: Table S3). The non-randomised studies were largely at serious (5/31; 16.1%) and critical risk of bias (14/31; 45.2%; Table 2 and Additional file 1: Table S4).
The 69 studies described 63 unique inpatient-level care at home interventions (Additional file 1: Table S57), of which 25 (39.7%) were reported as aiming to avoid hospital admissions, 22 (34.9%) were for early discharge, 4 (6.3%) were for both purposes, and 12 (19.0%) had no relevant detail. Based on the three components of focus, the 63 interventions formed eight model groups for analyses (Table 2).
Effects of inpatient-level care at home
We present evidence separately for RCTs and non-randomised studies for mortality, hospital readmission and length of stay (Additional file 1: Table S6; Additional file 2: Fig. S1 to S6). Non-randomised data clearly overestimated the effectiveness in our post hoc exploratory analyses compared with randomised data (Additional file 1: Text 2), and we summarise analyses of RCT data below and only present non-randomised data where RCT evidence is unavailable (Table 3). As random-effects models were used, all findings are average effects.
Mortality
RCT evidence is uncertain or unavailable for six models (Table 3 and Additional file 1: Table S6; Additional file 2: Fig. S1 and Fig. S2). There is low- or moderate-certainty evidence of, on average, similar or lower mortality risk than hospital-based inpatient care for two models: general inpatient-level care (RR 0.29, 95% CI 0.09 to 0.95; low-certainty RCT evidence) and extended multidisciplinary inpatient-level care (RR 0.96, 95% CI 0.79 to 1.16; moderate-certainty RCT evidence), delivered by hospital- and community-based professionals, with low-intensity technology involvement.
Hospital readmission
Six at home models have low-certainty evidence available, all suggesting, on average, at least a similar or lower risk of hospital readmission in people allocated to the inpatient-level care at home arms. Two models have uncertain or unavailable evidence (Table 3 and Additional file 1: Table S6; Additional file 2: Fig. S3 and Fig. S4).
Cost-effectiveness
Only two UK studies reported cost‐effectiveness and/or cost-utility analyses (Additional file 1: Text 3), both based on well-conducted trials but reporting conflicting evidence [65, 66]. A small trial-based analysis (118 participants, with 90-day follow-up) suggested that the model evaluated (i.e. extended multidisciplinary inpatient-level care, delivered by hospital- or community-based professionals, with low technology involvement) may be less expensive but more effective than hospital-based inpatient care for people with COPD exacerbations. The reported probability of this model being cost-effective was 90% at the National Institute for Health and Care Excellence’s threshold of £30,000 per quality-adjusted life year (QALY) gained [65]. The other analysis, based on a larger trial (457 stroke participants with 12-month follow-up), did not specify the technology its model used but suggested that its probability of being cost-effective is only 42% at the above threshold [66].
Length of stay
People receiving an at home model composed of extended multidisciplinary inpatient-level care, delivered by hospital- and community-based professionals, with low-intensity technology involvement may have, on average, a length of stay 4.85 days (95% CI 1.8 to 7.9 days) longer than hospital-based inpatient care (low-certainty RCT evidence). Three of the other models had, on average, similar or shorter length of care stays compared with hospital-based inpatient care. Four models have unavailable or uncertain evidence (Table 3 and Additional file 1: Table S6; Additional file 2: Fig. S5 and Fig. S6).
Adverse events
Four RCTs and seven non-randomised studies reported this outcome [31,32,33, 36,37,38, 52, 53, 60, 68, 74, 81,82,83,84, 87,88,89,90,91, 101]. We performed no synthesis for this outcome as the outcomes used were defined inconsistently, and outcome data were incomplete in four studies. The data, where available, appear to suggest conflicting evidence on the adverse effects of using hospital-level care at home (Additional file 1: Table S7).
Additional analyses
Pre-specified and post hoc sensitivity analyses suggest that the main analyses were not sensitive to analysis assumptions used (Additional file 1: Text 2), including: assuming that low technologies were used for interventions that had no information on technology use in the related studies (n = 22).
Our post hoc explanatory analyses that grouped all interventions into a broader comparison between inpatient-level care at home and hospital-based inpatient care suggested no statistical difference in mortality and readmission risk between groups. There appears to be an increase in the length of stay in inpatient-level inpatient care (Additional file 1: Text 2). Contrasting these findings with the model group-specific analyses above highlights the value of linking components to effectiveness.
Discussion
Summary of findings
Use of technology-enabled inpatient-level care at home (including models with low-intensity technology such as phone contact) may result in a similar risk of readmission to hospital following discharge compared with those receiving their initial care in hospital. The evidence is largely unavailable or uncertain for mortality and is mixed for length of stay. Where there is evidence on mortality, there may be no additional risk of mortality due to use of technology-enabled at home models. Cost-effectiveness evidence is unavailable for high technology-enabled models, and there is only limited evidence suggesting that the low technology-enabled multidisciplinary care model delivered by hospital-based teams may be cost-effective for people with COPD exacerbations.
Evidence in context
The current pressures in urgent and emergency services are promoting the expansion of technology-enabled, innovative care at home models, including virtual wards. Over the medium to longer term, expanding such innovations represents a paradigm shift in how acute care is delivered so that hospital occupancy can be better managed [140]. Care at home models are expected to become more integrated parts of future healthcare system with the continuing development of relevant technologies such as telehealth platforms, wearables, predictive algorithms including artificial intelligence. NICE health technology evaluation guidance (HTE13) has summarised key features that future virtual ward platform technologies should have, including interoperability with electronic record systems and medical devices; risk-stratified alerts; trend-based alerts; and patient interface with a user-centred design [141].
Our novel review highlights the value of disaggregating inpatient-level care at home models into constituent components to allow a more nuanced presentation of existing research findings. Our detailed analyses by component permutations provide a framework for future evaluations. The rapid scale-up of virtual wards in the UK and internationally includes a strong emphasis on high-intensity technology involvement [17, 142]. Limited available evidence means we are unclear whether use of high-intensity technology in these models confers additional benefit compared with hospital-based inpatient care. Further implementation of these models will benefit from concomitant evaluation with a focus on the added value of more complex technologies. Where the availability of high-intensity technology is a barrier to the implementation and evaluation of inpatient-level care at home models, lower-intensity models can be considered, again with evaluation.
For the key component of workforce, this review suggests that inpatient-level care at home delivered by hospital- and community-based professionals could result in similar readmission and/or mortality incidence to hospital-based inpatient care. This suggests the importance of coordination between hospital- and community-based teams to ensure the continuity of inpatient care in clinical practice [143]. The impacts of the team coordination on primary care workforce and provision require evaluations but are seldom reported. Existing evaluations focus on inpatient care provision and outcomes as opposed to relevant issues in primary care settings.
When considering the relevance of the evidence base in the UK, respiratory conditions, heart failure, and frailty are high-priority populations for inpatient-level care at home [17]. Whilst almost a quarter of the studies we included focused on acute respiratory conditions, our review highlights the limited available evidence for populations with frailty (four RCTs, with 1735 participants), and heart failure (three RCTs with 224 participants). The development of inpatient-level care at home models for frail and other high-priority populations should be informed by relevant existing evidence whilst recognising the likely need for carefully planned, likely rapid, evaluations.
Implications for research
In future, rigorous evaluation research is required to support the on-going development and implementation of technology-enabled inpatient-level care at home models and guide future decision-making about the value gained for investment. RCTs would be the ideal study design, but there are challenges to this. Non-randomised studies are more feasible, and these designs should evaluate intervention groups comparatively over a clearly defined, sufficient follow-up time in well-defined participants and appropriately adjust for confounding [14]. There is a crucial role for routinely collected data to allow rapid evaluation of this service delivery model, and identifying flags for service use should be added to data systems as far as possible. Intervention design and the corresponding evaluation should map to the approach of this review or other work that has considered the key elements of technology-enabled models [143, 144]. Important outcomes are not limited to those used in this review but also include the experiences of patient and caregivers.
Comparisons with other studies
In a rapid evidence synthesis, our scoping search identified 11 published systematic reviews of interventions that were described as ‘hospital at home’ or ‘virtual wards’ in comparison with hospital-based inpatient care, in a range of populations [6]. We found that there is low- to moderate-certainty evidence, suggesting that the interventions described as ‘hospital at home’ are probably as good or better than hospital-based inpatient care in terms of clinical outcomes including mortality and readmission [6]. In the previous reviews, the evidence is inconsistent on virtual wards for readmission to hospital [6].
In comparison with other work, our review focused on technology-enabled inpatient-level care at home models for people with acute conditions who would otherwise require hospitalisation. Previous reviews identified in this area included not only inpatient-level care at home but also remote monitoring that may not involve inpatient-level care [6]. This is an important distinction and impacts on the generalisability of review findings to specific intervention types. Unlike previous reviews, we present evidence for eight permutations of three components (Table 3), allowing stakeholders to refer to relevant evidence based on characteristics of the models used in their practice.
In previous reviews, the evidence for ‘virtual wards’ has appeared inconsistent regarding the effectiveness in reducing hospital (re)admission, depending on care models and health conditions [6]. We found consistency in this outcome across inpatient-level care at home models, and the available evidence suggests the interventions may have at least an equivalent readmission incidence to hospital-based inpatient care.
Strengths and limitations of this review
This review has strengths partly due to use of standard Cochrane-based, prespecified review methods in minimising the risk of bias in the review process. Use of a comprehensive search identified far more studies than existing systematic reviews on this topic [6]. In defining eligibility criteria, we considered use of digital technologies and the substitution of hospital-based inpatient care in the home environment as two important elements for inpatient-level care at home [17]. This ensures that the evidence is in line with the current innovations [17]. Given that there is no consensus on ‘inpatient-level care at home’ components, we developed and defined a typology. This helps ensure that components chosen are clinically relevant and the process is trustworthy. We noted that the components chosen via our approach are reflected in the recent relevant reviews that identified key elements for building virtual wards [143, 144].
This review has limitations. These include the widely acknowledged challenge in identifying non-randomised studies for inclusion in a review, [13] and the lack of an agreed single approach to grouping interventions. For these issues, we followed the Cochrane Effective Practice and Organisation of Care (EPOC) guidance and Reeves et al.’s checklist to minimise study selection bias [13, 14]. Given inpatient-level care at home is a heterogeneous set of complex interventions whose theories of change are not well defined, we followed the clinically meaningful-element approach to grouping inpatient-level care at home interventions for informing subsequent synthesis [19]. That is, we grouped the interventions with similar components together whilst considering clinically important elements in specifying component categories [19]. This approach allows us to investigate which combinations of components are associated with intervention effectiveness [20]. We reached a consensus on the clinically sensible grouping in consultation with methodological and clinical experts including a clinical virtual ward lead. Further research is needed to build on the first attempt in defining intervention components presented in this review, and to develop and validate intervention taxonomies in this area. We were unable to determine which model components are important, as component-specific analysis was considered inappropriate in this review (as justified in the ‘Methods’ section). In coding components of the included interventions, the lead review author followed the agreed process, with a second review author checking 10% of the included studies for comparison. We noted that the decision of using 10% of the included studies for comparison was arbitrary but pragmatic. Whilst this approach may have increased the risk of coding errors, empirically, this risk was limited here because the agreement in the 10% of randomly sampled papers was very good. We suggest this was due to the careful development and piloting of the process and the experience of the reviewers involved. We did not assess the quality of cost-effectiveness evaluations included, [24] but we noted the relevant trials used in these evaluations were small with short follow-ups and had no substantial methodological limitations.
Conclusions
We found that a range of technology-enabled inpatient-level care at home models may result in similar or reduced readmission risk compared with hospital-based inpatient care. Impacts on mortality are more uncertain, except for two models showing no increased risk compared with hospital-based inpatient care. The certainty of current evidence means further research could change findings. Further implementation of inpatient-level care at home models should be alongside evaluation to explore the potential benefits of using specific technologies particularly to gain further insights into clinical and cost-effectiveness particularly in high-priority populations.
Availability of data and materials
Data extracted from included studies and data used for analyses are all reported in this paper or its appendices. No additional data are available.
Abbreviations
- CHEERS:
-
Consolidated Health Economic Evaluation Reporting Standards
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- EPOC:
-
Cochrane Effective Practice and Organisation of Care
- GRADE:
-
Grading Recommendations Assessment and Development Evidence
- MD:
-
Mean difference
- NHS:
-
National Health Service
- QALY:
-
Quality-adjusted life year
- RCT:
-
Randomised controlled trial
- ROBINS-I:
-
Risk Of Bias In Non-randomised Studies—of Interventions
- RR:
-
Risk ratio
- TIDieR:
-
Template for Intervention Description and Replication
References
NHS Digital. Hospital admitted patient care activity, 2021–22. 2022. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2021-22#. Accessed 9 Nov 2022.
Lo AX, Flood KL, Biese K, Platts-Mills TF, Donnelly JP, Carpenter CR. Factors associated with hospital admission for older adults receiving care in U.S. emergency departments. J Gerontol A Biol Sci Med Sci. 2017;72:1105–9.
NHS England. Delivery plan for recovering urgent and emergency care services. 2023. https://www.england.nhs.uk/long-read/delivery-plan-for-recovering-urgent-and-emergency-care-services-january-2023/. Accessed 7 June 2023.
Lewis G, Wright L, Vaithianathan R. Multidisciplinary case management for patients at high risk of hospitalization: comparison of virtual ward models in the United Kingdom, United States, and Canada. Popul Health Manag. 2012;15:315–21.
Morris DE. Santé Service Bayonne: a French approach to home care. Age Ageing. 1983;12:323–8.
Norman G, Bennett P, Vardy ERLC. Virtual wards: a rapid evidence synthesis and implications for the care of older people. Age Ageing. 2023;52:afac319.
Leff B. Defining and disseminating the hospital-at-home model. CMAJ. 2009;180:156–7.
Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). 2022. www.training.cochrane.org/handbook. Accessed 9 Nov 2022.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Shi C. The components and effectiveness of using inpatient-level care at home as an alternative to hospital-based inpatient care. A systematic review (protocol). Open Science Framework 2022. https://osf.io/je39y. Accessed 20 Feb 2024.
Goncalves-Bradley DC, Iliffe S, Doll HA, et al. Early discharge hospital at home. Cochrane Database Syst Rev. 2017;6:CD000356.
Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
Cochrane Effective Practice and Organisation of Care (EPOC). What study designs can be considered for inclusion in an EPOC review and what should they be called? 2017. http://epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources-for-authors2017/what_study_designs_should_be_included_in_an_epoc_review.pdf. Accessed 9 Nov 2022.
Reeves BC, Wells GA, Waddington H. Quasi-experimental study designs series—paper 5: a checklist for classifying studies evaluating the effects on health interventions—a taxonomy without labels. J Clin Epidemiol. 2017;89:30–42.
Shi C. Data extraction form on Qualtrics. 2023. https://www.qualtrics.manchester.ac.uk/jfe/form/SV_3qQedEEEuYMrhMG. Accessed 20 Feb 2024.
Rhon DI, Fritz JM, Kerns RD, et al. TIDieR-telehealth: precision in reporting of telehealth interventions used in clinical trials-unique considerations for the Template for the Intervention Description and Replication (TIDieR) checklist. BMC Med Res Methodol. 2022;22:161.
NHS England. Virtual ward including hospital at home. 2022. https://www.england.nhs.uk/wp-content/uploads/2021/12/B1478-supporting-guidance-virtual-ward-including-hospital-at-home-march-2022-update.pdf. Accessed 9 Nov 2022.
Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775–9.
Shi C, Westby M, Norman G, Dumville JC, Cullum N. Node-making processes in network meta-analysis of nonpharmacological interventions should be well planned and reported. J Clin Epidemiol. 2018;101:124–5.
Melendez-Torres GJ, Bonell C, Thomas J. Emergent approaches to the meta-analysis of multiple heterogeneous complex interventions. BMC Med Res Methodol. 2015;15:1–7.
Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
O’Neill J, Tabish H, Welch V, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol. 2014;67(1):56–64.
Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated Reporting Guidance for Health Economic Evaluations. Value Health. 2022;25:3–9.
Bower P, Gilbody S, Richards D, Fletcher J, Sutton A. Collaborative care for depression in primary care: making sense of a complex intervention: systematic review and meta-regression. Br J Psychiatry. 2006;189:484–93.
Freeman SC, Scott NW, Powell R, Johnston M, Sutton AJ, Cooper NJ. Component network meta-analysis identifies the most effective components of psychological preparation for adults undergoing surgery under general anesthesia. J Clin Epidemiol. 2018;98:105–16.
Cuello-Garcia CA, Santesso N, Morgan RL, et al. GRADE guidance 24 optimizing the integration of randomized and non-randomized studies of interventions in evidence syntheses and health guidelines. J Clin Epidemiol. 2022;142:200–8.
Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890.
Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–5.
ACTRN12621000692831. Virtual care of acute diverticulitis. 2021. https://anzctr.org.au/ACTRN12621000692831.aspx. Accessed 4 Dec 2023.
Caplan GA, Ward JA, Brennan NJ, Coconis J, Board N, Brown A. Hospital in the home: a randomised controlled trial. Med J Aust. 1999;170(4):156–60.
Caplan GA, Coconis J, Woods J. Effect of hospital in the home treatment on physical and cognitive function: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2005;60(8):1035–8.
Board N, Brennan N, Caplan GA. A randomised controlled trial of the costs of hospital as compared with hospital in the home for acute medical patients. Aust N Z J Public Health. 2000;24(3):305–11.
Corwin P, Toop L, McGeoch G, Than M, Wynn-Thomas S, Wells JE, et al. Randomised controlled trial of intravenous antibiotic treatment for cellulitis at home compared with hospital. BMJ. 2005;330(7483):129.
Kalra L, Evans A, Perez I, Knapp M, Donaldson N, Swift CG. Alternative strategies for stroke care: a prospective randomised controlled trial. Lancet. 2000;356(9233):894–9.
Hendricks AM, Loggers ET, Talcott JA. Costs of home versus inpatient treatment for fever and neutropenia: analysis of a multicenter randomized trial. J Clin Oncol. 2011;29(30):3984.
Talcott JA, Yeap BY, Clark JA, et al. Safety of early discharge for low-risk patients with febrile neutropenia: a multicenter randomized controlled trial. J Clin Oncol. 2011;29(30):3977.
Adler MW, Waller JJ, Creese A, Thorne SC. Randomised controlled trial of early discharge for inguinal hernia and varicose veins. J Epidemiol Community Health. 1978;32(2):136–42.
Booth JE, Roberts JA, Flather M, et al. A trial of early discharge with homecare compared to conventional hospital care for patients undergoing coronary artery bypass grafting. Heart. 2004;90(11):1344–5.
Cotton MM, Bucknall CE, Dagg KD, et al. Early discharge for patients with exacerbations of chronic obstructive pulmonary disease: a randomised controlled trial. Thorax. 2000;55(11):902–6.
Lobato SD, Lorenzo FG, Mendieta MAG, Alises SM, Arechabala IM, Fernández-Montes CV. Evaluation of a home hospitalization program in patients with exacerbations of chronic obstructive pulmonary disease. Arch Bronconeumol (Engl Ed). 2005;41(1):5–10.
Skwarska E, Cohen G, Skwarski KM, et al. Randomised controlled trial of supported discharge in patients with exacerbations of chronic obstructive pulmonary disease. Thorax. 2000;55(11):907–12.
Gallier S, Atkin C, Reddy-Kolanu V, et al. Evaluating discharges and readmissions using a COVID Virtual Ward model: a retrospective data study assessing patient outcomes and the likely staffing commitment. medRxiv. 2021:2021–07.
NCT05920304. Early Discharge - Evaluating a Virtual Hospital at Home Model. https://classic.clinicaltrials.gov/show/NCT05920304. Accessed 4 Dec 2023.
NCT05256303. Rural Hospital-Level Care at Home for Acutely Ill Adults. https://classic.clinicaltrials.gov/show/NCT05256303. Accessed 4 Dec 2023.
NCT03490084. Impacts on Health Outcomes and Resources Utilization of Hospital-at-home for Elderly Patients With Multiple Myeloma. https://classic.clinicaltrials.gov/show/NCT03490084. Accessed 4 Dec 2023.
Aibar J, Fernandez-Martinez A, Seijas N, et al. Hospital at home in cancer patients. Support Care Cancer. 2013;21:S21.
Aimonino N, Amati D, Tibaldi V, Roglia D, Rocco M, Fabris F. Alternative approaches to traditional hospital care for elderly patients with advanced dementia: hospital at home. Proceedings of the 8th international conference on Alzheimer's disease and related disorders. 2002: AbstractNo206.
AimoninoRicauda N, Tibaldi V, Leff B, et al. Substitutive “hospital at home” versus inpatient care for elderly patients with exacerbations of chronic obstructive pulmonary disease: a prospective randomized, controlled trial. J Am Geriatr Soc. 2008;56(3):493–500.
NCT00369083. Hospitalization at home of elderly patients with exacerbated COPD. https://clinicaltrialsgov/show/NCT00369083. 2006. Accessed 4 Dec 2023.
Ansari K, Shamssain M, Farrow M, Keaney NP. Hospital-at-home care for exacerbations of chronic obstructive pulmonary disease: an observational cohort study of patients managed in hospital or by nurse practitioners in the community. Chron Respir Dis. 2009;6(2):69–74.
Federman AD, Soones T, DeCherrie LV, Leff B, Siu AL. Association of a bundled hospital-at-home and 30-day postacute transitional care program with clinical outcomes and patient experiences. JAMA Intern Med. 2018;178(8):1033–40.
Augustine MR, Siu AL, Boockvar KS, DeCherrie LV, Leff BA, Federman AD. Outcomes of hospital at home for older adults with and without high levels of social support. Home Healthc Now. 2021;39(5):261–70.
Bagust A, Haycox A, Sartain SA, et al. Economic evaluation of an acute paediatric hospital at home clinical trial. Arch Dis Child. 2002;87(6):489–92.
Sartain SA, Maxwell MJ, Todd PJ, et al. Randomised controlled trial comparing an acute paediatric hospital at home scheme with conventional hospital care. Arch Dis Child. 2002;87(5):371–5.
ISRCTN11421664. Randomised controlled trial (RCT) of a children's hospital at home service. 2004. http://isrctn.com/ISRCTN11421664. Accessed 4 Dec 2023.
Cai S, Laurel PA, Makineni R, et al. Evaluation of a Hospital-in-Home program implemented among veterans. Am J Manag Care. 2017;23(8):482–7.
Cai S, Grubbs A, Makineni R, Kinosian B, Phibbs CS, Intrator O. Evaluation of the Cincinnati Veterans Affairs Medical Center hospital in home program. J Am Geriatr Soc. 2018;66(7):1392–8.
Cai S, Intrator O, Chan C, et al. Association of costs and days at home with transfer hospital in home. JAMA Netw Open. 2021:e2114920.
Campbell H, Karnon J, Dowie R. Cost analysis of a hospital-at-home initiative using discrete event simulation. J Health Serv Res Policy. 2001;6(1):14–22.
Cryer L, Shannon SB, Van Amsterdam M, Leff B. Costs for “hospital at home” patients were 19 percent lower, with equal or better outcomes compared to similar inpatients. Health Aff. 2012;31(6):1237–43.
Davies L, Wilkinson M, Bonnar S, Claverley PMA, Angus RM. A prospective randomised controlled trial of hospital at home versus hospital care in patients accepted for admission with exacerbations of COPD. Eur Respir J. 2000;16:412s.
Davies L, Wilkinson M, Bonner S, Calverley PMA, Angus RM. “Hospital at home” versus hospital care in patients with exacerbations of chronic obstructive pulmonary disease: prospective randomised controlled trial. BMJ. 2000;321(7271):1265–8.
Echevarria C, Hartley T, Gray J, et al. Hot decaf: A RCT comparing home treatment and inpatient care in COPD exacerbations selected by low risk decaf score. Thorax. 2016;71:A69.
Echevarria C, Gray J, Hartley T, et al. Home treatment of COPD exacerbation selected by DECAF score: A non-inferiority, randomised controlled trial and economic evaluation. Thorax. 2018;73:713–22.
Patel A, Knapp M, Perez I, Evans A, Kalra L. Alternative strategies for stroke care: cost-effectiveness and cost-utility analyses from a prospective randomized controlled trial. Stroke. 2004;35:196–203.
ISRCTN29082260. A study comparing home treatment of COPD exacerbations to usual hospital care. 2014. http://isrctn.com/ISRCTN29082260. Accessed 4 Dec 2023.
Escartin A, Mias MC, Gonzalez M, et al. Home hospitalization for the surgical and conservative treatment of acute calculous cholecystitis. Surg Pract. 2018;22(2):52–9.
Esmond G, Butler M, McCormack AM. Comparison of hospital and home intravenous antibiotic therapy in adults with cystic fibrosis. J Clin Nurs. 2006;15(1):52–60.
Gonzalez Barcala FJ, Pose Reino A, Paz Esquete JJ, et al. Hospital at home for acute respiratory patients. Eur J Intern Med. 2006;17(6):402–7.
Gruss AI, Reyes N, Mendez A, et al. Hospital vs. hospital-at-home programs for treatment of CAP in Uruguay. Am J Respir Crit Care Med. 2013;A1683.
Harris R. Randomised controlled trial of hospital at home programme for older people. Intern Med J. 2004;34(6):A60.
Harris R, Ashton T, Broad J, Connolly G, Richmond D. The effectiveness, acceptability and costs of a hospital-at-home service compared with acute hospital care: a randomized controlled trial. J Health Serv Res Policy. 2005;10(3):158–66.
Hatziagorou E, Chrysochoou E, Kirvassilis F, Tsanakas J. Home antibiotic treatment in cystic fibrosis: An effective, cost saving and preferred choice among patients with CF. J Cyst Fibros. 2015;14(SUPPL. 1):S127.
Hensher M, Fulop N, Hood S, Ujah S. Does hospital-at-home make economic sense? Early discharge versus standard care for orthopaedic patients. J R Soc Med. 1996;89(10):548–51.
Herranz C, González-Colom R, Baltaxe E, et al. Prospective cohort study for assessment of integrated care with a triple aim approach: hospital at home as use case. BMC Health Serv Res. 2022;22(1):1–12.
Ince AT, Senturk H, Singh VK, et al. A randomized controlled trial of home monitoring versus hospitalization for mild non-alcoholic acute interstitial pancreatitis: A pilot study. Pancreatology. 2014;14(3):174–8.
Ince AT, Yildiz K, Cinar A, et al. A prospective randomized controlled trial of home monitoring versus hospitalization for mild non-alcoholic acute interstitial pancreatitis. Gastroenterology. 2013;144(5):S139–40.
ISRCTN36101176. Hospital at home care in chronic obstructive pulmonary disease (COPD): a study on the associated health economy and disease-related quality of life. 2008. http://isrctn.com/ISRCTN36101176. Accessed 4 Dec 2023.
ISRCTN36662318. Efficacy, costs and user satisfaction of hospital at home as a model of early discharge from hospital in patients with low and moderate risk. 2009. http://isrctn.com/ISRCTN36662318. Accessed 4 Dec 2023.
Jakobsen AS, Laursen LC, Østergaard B, Rydahl-Hansen S, Phanareth KV. Hospital-admitted COPD patients treated at home using telemedicine technology in The Virtual Hospital Trial: methods of a randomized effectiveness trial. Trials. 2013;14(1):1–8.
Jakobsen AS, Laursen LC, Rydahl-Hansen S, et al. Home-based telehealth hospitalization for exacerbation of chronic obstructive pulmonary disease: findings from “the virtual hospital” trial. Telemed J E Health. 2015;21(5):364–73.
Schou L, Østergaard B, Rasmussen LS, et al. Telemedicine-based treatment versus hospitalization in patients with severe chronic obstructive pulmonary disease and exacerbation: effect on cognitive function. A randomized clinical trial. Telemed J E Health. 2014;20(7):640–6.
Jester R. Early discharge to hospital at home: should it be a matter of choice? Orthop Nurs. 2003;7(2):64–9.
Jester R, Hicks C. Using cost-effectiveness analysis to compare Hospital at Home and in-patient interventions. Part 1. J Clin Nurs. 2003;12(1):13–9.
Jester R, Hicks C. Using cost-effectiveness analysis to compare Hospital at Home and in-patient interventions. Part 2. J Clin Nurs. 2003;12(1):20–7.
Leff B, Burton L, Mader S, et al. Satisfaction with hospital at home care. J Am Geriatr Soc. 2006;54(9):1355–63.
Leff B, Burton L, Mader SL, et al. Comparison of functional outcomes associated with hospital at home care and traditional acute hospital care. J Am Geriatr Soc. 2009;57(2):273–8.
Leff B, Burton L, Mader SL, et al. Hospital at home: Feasibility and outcomes of a program to provide hospital-level care at home for acutely III older patients. Ann Intern Med. 2005;143(11):798–856.
Leff B, Burton L, Mader SL, et al. Comparison of stress experienced by family members of patients treated in hospital at home with that of those receiving traditional acute hospital care. J Am Geriatr Soc. 2008;56(1):117–23.
Frick KD, Burton LC, Clark R, et al. Substitutive Hospital at Home for older persons: effects on costs. Am J Manag Care. 2009;15(1):49–56.
Levine DM, Ouchi K, Blanchfield BB, Pu CT, Schnipper JL. Hospital-level care at home for acutely ill adults: a randomized controlled trial. J Gen Intern Med. 2018;33(2):221–2.
Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172(2):77–85.
Mendoza H, Martín MJ, García A, et al. “Hospital at home” care model as an effective alternative in the management of decompensated chronic heart failure. Eur J Heart Fail. 2009;11(12):1208–13.
Morgan C, Woodfield R, Noble D, Munang L, Rimer J. Hospital at home reduces early readmission rates for older people with exacerbation of copd. Age Ageing. 2019;48: i39.
NCT04330378. A Hospital-at-Home pilot in Singapore. 2020. https://clinicaltrials.gov/study/NCT04330378. Accessed 4 Dec 2023.
NCT05360914. Hospital at home for elderly acute ill patients. 2022. https://clinicaltrials.gov/study/NCT05360914. Accessed 4 Dec 2023.
O’Cathain A. Evaluation of a hospital at home scheme for the early discharge of patients with fractured neck of femur. J Public Health Med. 1994;16(2):205–10.
Ojoo JC, Moon T, McGlone S, et al. Patients’ and carers’ preferences in two models of care for acute exacerbations of COPD: results of a randomised controlled trial. Thorax. 2002;57(2):167–9.
Oterino de la Fuente D, Ridao M, Peiro S, Marchan C. Hospital at home and conventional hospitalization. An economic evaluation. Med Clin (Barc). 1997;109(6):207–11.
Oterino-de-la-Fuente D, Peiro S, Ridao M, Marchan C. Variations in diagnostic and therapeutic intensity between home and conventional hospitalization. Int J Qual Health Care. 1998;10(4):331–8.
Pouw MA, Calf AH, Van Munster BC, Ter Maaten JC, Smidt N, De Rooij SE. Hospital at home care for older patients with cognitive impairment: a protocol for a randomised controlled feasibility trial. BMJ Open. 2018;8(3):e020332.
NTR6581. Hospital at home care for older patients with cognitive impairment and an acute medical illness. https://www.onderzoekmetmensen.nl/en/trial/22701. 2017. Accessed 4 Dec 2023.
Ricauda NA, Bo M, Molaschi M, et al. Home hospitalization service for acute uncomplicated first ischemic stroke in elderly patients: a randomized trial. J Am Geriatr Soc. 2004;52(2):278–83.
Ahrens J. Italian study concludes “home hospitalization” benefits stroke patients. Caring. 2004;23(8):40–5.
Ricauda NA, Tibaldi V, Marinello R, et al. Acute ischemic stroke in elderly patients treated in Hospital at Home: a cost minimization analysis. J Am Geriatr Soc. 2005;53(8):1442–3.
Richards SH, Coast J, Gunnell DJ, Peters TJ, Pounsford J, Darlow M. Randomised controlled trial comparing effectiveness and acceptability of an early discharge, hospital at home scheme with acute hospital care. BMJ. 1998;316(7147):1796–801.
Coast J, Richards SH, Peters TJ, Gunnell DJ, Darlow MA, Pounsford J. Hospital at home or acute hospital care? A cost minimisation analysis. BMJ. 1998;316(7147):1802–6.
Gunnell D, Coast J, Richards SH, Peters TJ, Pounsford JC, Darlow M. How great a burden does early discharge to hospital-at-home impose on carers? A randomized controlled trial. Age Ageing. 2000;29(2):137–42.
ISRCTN76045614. A randomised controlled trial to compare the effectiveness and cost effectiveness of the hospital at home scheme with the acute hospital. 2004. http://isrctn.com/ISRCTN76045614. Accessed 4 Dec 2023.
Rodríguez-Cerrillo M, Poza-Montoro A, Fernandez-Diaz E, Matesanz-David M, Iñurrieta RA. Treatment of elderly patients with uncomplicated diverticulitis, even with comorbidity, at home. Eur J Intern Med. 2013;24(5):430–2.
Rousseau C, Motti D, Perez F, et al. Comparing disposition and bounce backs following treatment for pneumonia between hospital at home and traditional hospitalization. J Am Geriatr Soc. 2019;67:S101.
Saenger PM, Ornstein KA, Garrido MM, et al. Cost of home hospitalization versus inpatient hospitalization inclusive of a 30-day post-acute period. J Am Geriatr Soc. 2022;70(5):1374–83.
Sequerios IM, Jarad N. Outcome of care for supported home treated cystic fibrosis (CF) pulmonary exacerbations (P EXS) versus hospital treated exacerbations-a prospective trial. Am J Respir Crit Care Med. 2013;187.
Shepperd S. A randomised controlled trial comparing hospital at home with in-patient hospital care. University of Oxford. 1998.
Shepperd S, Harwood D, Gray A, Vessey M, Morgan P. Randomised controlled trial comparing hospital at home care with inpatient hospital care. II: cost minimisation analysis. BMJ. 1998;316(7147):1791–6.
ISRCTN47826365. Randomised controlled trial comparing hospital at home with hospital care. 2004. http://isrctn.com/ISRCTN47826365. Accessed 4 Dec 2023.
Shepperd S, Harwood D, Jenkinson C, Gray A, Vessey G, Morgan P. Randomised controlled trial comparing hospital at home care with inpatient hospital care. I: three month follow up of health outcomes. BMJ. 1998;316(7147):1786–91.
Shepperd S, Cradduck-Bamford A, Butler C, et al. A multi-centre randomised trial to compare the effectiveness of geriatrician-led admission avoidance hospital at home versus inpatient admission. Trials. 2017;18:1–9.
Shepperd S, Butler C, Cradduck-Bamford A, et al. Is comprehensive geriatric assessment admission avoidance hospital at home an alternative to hospital admission for older persons? : A randomized trial. Ann Intern Med. 2021;174(7):889–98.
Singh S, Gray A, Shepperd S, et al. Is comprehensive geriatric assessment hospital at home a cost-effective alternative to hospital admission for older people? Age Ageing. 2022;51(1):1–11.
ISRCTN60477865. Comprehensive geriatric assessment in a HAH setting. 2014. http://isrctn.com/ISRCTN60477865. Accessed 4 Dec 2023.
Soones TN, Federman A, DeCherrie L, Wajnberg A, Leff B, Siu A. The mobile acute care team: Preliminary outcomes of a bundled-payment hospital at home program. J Am Geriatr Soc. 2016;64:S122.
Summerfelt WT, Sulo S, Robinson A. Scalable hospital at home with virtual physician visits: pilot study. Am J Manag Care. 2015;21(10):675–84.
Tibaldi V, Isaia G, Scarafiotti C, et al. Hospital at home for elderly patients with acute decompensation of chronic heart failure: a prospective randomized controlled trial. Arch Intern Med. 2009;169(17):1569–75.
NCT00623571. Hospitalization at home of elderly patients with heart failure. 2008. https://clinicaltrials.gov/study/NCT00623571. Accessed 4 Dec 2023.
Tibaldi V, Isaia G, Bergerone S, et al. A randomized clinical trial on the efficacy of an early discharge to a hospital at home service of elderly patients with acute decompensation of severe chronic heart failure. G Gerontol. 2013;61(2):78–85.
Toral-Lopez I, Gonzalez-Carrion MP, Rivas-Campos A, et al. Evolution of care indicators after an early discharge intervention in preterm infants. Evolucion de indicadores de cuidados tras una intervencion de alta precoz en recien nacidos prematuros. 2017;27(4):235–40.
Tsiachristas A, Ellis G, Buchanan S, Langhorne P, Stott DJ, Shepperd S. Should I stay or should I go? A retrospective propensity score-matched analysis using administrative data of hospital-at-home for older people in Scotland. BMJ Open. 2019;9(5):e023350.
Utens CM, Goossens LM, Smeenk FW, et al. Effectiveness and cost-effectiveness of early assisted discharge for chronic obstructive pulmonary disease exacerbations: the design of a randomised controlled trial. BMC Public Health. 2010;10(1):618.
Utens CMA, Goossens LMA, van Schayck OCP, et al. Patient preference and satisfaction in hospital-at-home and usual hospital care for COPD exacerbations: results of a randomised controlled trial. Int J Nurs Stud. 2013;50(11):1537–49.
Utens CMA, van Schayck OCP, Goossens LMA, et al. Informal caregiver strain, preference and satisfaction in hospital-at-home and usual hospital care for COPD exacerbations: Results of a randomised controlled trial. Int J Nurs Stud. 2014;51(8):1093–192.
Vianello A, Savoia F, Pipitone E, et al. “Hospital at home” for neuromuscular disease patients with respiratory tract infection: A pilot study. Respir Care. 2013;58(12):2061–8.
Wilson A, Parker H, Wynn A. Management of Acute Conditions in Hospital or Hospital at Home: A Randomised Controlled Trial: University of Leicester, Department of General Practice and Primary Health Care; 1998.
Wilson A, Parker H, Wynn A, Jagger C, Spiers N, Jones J, et al. Randomised controlled trial of effectiveness of Leicester hospital at home scheme compared with hospital care. BMJ. 1999;319(7224):1542–6.
Wilson A, Parker H, Wynn A, Spiers N. Performance of hospital-at-home after a randomised controlled trial. J Health Serv Res Policy. 2003;8(3):160–4.
Wilson A, Wynn A, Parker H. Patient and carer satisfaction with “hospital at home”: quantitative and qualitative results from a randomised controlled trial. Br J Gen Pract. 2002;52(474):9–13.
Jones J, Wilson A, Parker H, et al. Economic evaluation of hospital at home versus hospital care: cost minimisation analysis of data from randomised controlled trial. BMJ. 1999;319(7224):1547–50.
Yao X, Paulson M, Maniaci MJ, Dunn AN, Nelson CR, Behnken EM, et al. Effect of hospital-at-home vs. traditional brick-and-mortar hospital care in acutely ill adults: study protocol for a pragmatic randomized controlled trial. Trials. 2022;23(1):1–12.
Royal College of Physicians of London (RCP London). The RCP view: Hospital at Home and virtual wards. 2023. https://www.rcplondon.ac.uk/guidelines-policy/rcp-view-hospital-home-and-virtual-wards. Accessed 18 Jan 2024.
National Institute for Health and Care Excellence (NICE). Virtual ward platform technologies for acute respiratory infections. 2023. https://www.nice.org.uk/guidance/hte13. Accessed 18 Jan 2024.
British Geriatrics Society. Bringing hospital care home: Virtual Wards and Hospital at Home for older people. 2022. https://www.bgs.org.uk/virtualwards. Accessed 5 June 2023.
[preprint] Westby MJ, Ijaz S, Savovic J, et al. Rapid realist review of virtual wards for people with frailty. medRxiv. 2023. https://doi.org/10.1101/2023.04.18.23288729.
Miranda R, Oliveira MD, Nicola P, Baptista FM, Albuquerque I. Towards a framework for implementing remote patient monitoring from an integrated care perspective: a scoping review. Int J Health Policy Manag. 2023;12:7299.
Acknowledgements
The authors of this research want to thank Dr Will Whittaker and Dr Fay Bradley for their insightful comments on the initial draft. We also thank Sophie Bishop, formerly the Information Specialist of the Cochrane Wounds Group, for advising on the design of search strategies.
Funding
This research was funded by the National Institute for Health and Care Research (NIHR) Applied Research Collaboration Greater Manchester (ARC-GM). The views expressed in this publication are those of the authors and not necessarily those of the National Institute for Health and Care Research or the Department of Health and Social Care.
EV has time funded by the NIHR Clinical Research Network Greater Manchester hosted through Manchester University hospitals NHS Foundation Trust. EV holds an Honorary Clinical Chair through the Manchester Academic Health Sciences Centre, School of Health Sciences, Faculty of Biology, Medicine and Health, University of Manchester. The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
CS, JD, PB and FR conceptualised the study. CS, JD, FR and PB designed the methodology. CS, FR, GN, AU, SB and EV did the data collection. CS and JD did the data analysis. JD, PB and EV supervised the data collection, analyses and/or interpretation. JD and GN verified the data collection and evidence interpretation. JD and PB acquired funding. CS prepared the original draft of the manuscript with the input of JD, PB and EV. All authors edited and reviewed the final manuscript. All authors have read and agreed to the final version of the manuscript and to the decision to submit. All authors had access to all the data. CS and GN have verified the data. All authors read and approved the final manuscript.
Authors’ Twitter handles
@chunhu_shi (Chunhu Shi).
@GN_ARC (Gill Norman).
@Bowercpcman (Peter Bower).
@emmvardy2 (Emma Vardy).
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
EV received speaker honoraria from GE healthcare and Vitaflo International. All other authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Text 1.
Literature search methods used. Table S1. Intervention components of inpatient level care at home and specifications. Text 2. Methods and results of subgroup and sensitivity analyses. Table S2. Characteristics of the included studies. Table S3. Risk of bias assessment results for RCTs. Table S4. Risk of bias assessment results for non-randomised studies. Table S5. Summarises of intervention components. Table S6. Summary of findings tables for individual outcomes. Text 3. Results of cost and cost-effectiveness analyses. Table S7. Results of adverse event outcomes.
Additional file 2: Fig. S1.
Meta-analyses of RCT data by care models for mortality. Fig. S2. Meta-analyses of non-randomised data by care models for mortality. Fig. S3. Meta-analyses of RCT data by care models for hospital readmission. Fig. S4. Meta-analyses of non-randomised data by care models for hospital readmission. Fig. S5. Meta-analyses of RCT data by care models for the length of care stay in days. Fig. S6. Meta-analyses of non-randomised data by care models for the length of care stay in days.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shi, C., Dumville, J., Rubinstein, F. et al. Inpatient-level care at home delivered by virtual wards and hospital at home: a systematic review and meta-analysis of complex interventions and their components. BMC Med 22, 145 (2024). https://doi.org/10.1186/s12916-024-03312-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-024-03312-3