Abstract
Background
The global dementia prevalence is surging, necessitating research into contributing factors. We aimed to investigate the association between metabolic syndrome (MetS), its components, serum uric acid (SUA) levels, and dementia risk.
Methods
Our prospective study comprised 466,788 participants without pre-existing MetS from the UK Biobank. We confirmed dementia diagnoses based on the ICD-10 criteria (F00-03). To evaluate the dementia risk concerning MetS, its components, and SUA levels, we applied Cox proportional hazards models, while adjusting for demographic factors.
Results
Over a median follow-up of 12.7 years, we identified 6845 dementia cases. Individuals with MetS had a 25% higher risk of all-cause dementia (hazard ratio [HR] = 1.25, 95% confidence interval [CI] = 1.19–1.31). The risk increased with the number of MetS components including central obesity, dyslipidemia for high-density lipoprotein (HDL) cholesterol, hypertension, hyperglycemia, and dyslipidemia for triglycerides. Particularly for those with all five components (HR = 1.76, 95% CI = 1.51–2.04). Dyslipidemia for HDL cholesterol, hypertension, hyperglycemia, and dyslipidemia for triglycerides were independently associated with elevated dementia risk (p < 0.01). MetS was further linked to an increased risk of all-cause dementia (11%) and vascular dementia (VD, 50%) among individuals with SUA levels exceeding 400 μmol/L (all-cause dementia: HR = 1.11, 95% CI = 1.02–1.21; VD: HR = 1.50, 95% CI = 1.28–1.77).
Conclusions
Our study provides robust evidence supporting the association between MetS, its components, and dementia risk. These findings emphasize the importance of considering MetS and SUA levels in assessing dementia risk, offering valuable insights for prevention and management strategies.
Similar content being viewed by others
Background
Dementia, marked by progressive cognitive decline and functional impairment, poses a substantial public health challenge with limited curative options. The increasing global prevalence of dementia, projected to exceed 78 million by 2030 and potentially reach 139 million by 2050, underscores the crucial need to precisely elucidate its risk factors. Obesity, hypertension, and diabetes are recognized as modifiable risk factors for the onset and progression of dementia, as well as being core conditions associated with metabolic syndrome (MetS) [1].
MetS, characterized by dysglycemia, elevated blood pressure and triglyceride levels; low high-density lipoprotein cholesterol, and central obesity, is established as a risk factor for cardiovascular disease, coronary heart disease, and type 2 diabetes mellitus [2, 3]. Despite this recognition, the association between MetS and dementia risk exhibits variability across studies. In a mid-life cohort study, an incremental dementia risk was identified with each additional MetS component, albeit constrained by data limitations [4]. Similarly, a systematic review linked high waist circumference to cognitive impairment but lacked subtype-specific data [5]. Population-based studies and additional research strengthened MetS’ connection to Alzheimer’s disease (AD) [6, 7]. A nationwide exploration revealed a gradual increase in dementia risk with cumulative MetS components, while a cohort study associated MetS with incident vascular dementia (VD) [8, 9]. Persistent or worsening MetS components in a decade-long cohort study were linked to heightened dementia risk, although subtype assessments were lacking [10]. A recent investigation [11] disclosed a 12% increased risk of all-cause dementia with MetS, grappling with subgroup classifications. Conversely, a recent meta-analysis with nine longitudinal studies and 18,313 participants found no statistically significant association between MetS and incident dementia or AD [12]. Notably, the meta-analysis highlighted an increased incidence of pure VD and progression from mild cognitive impairment to dementia. In addition, elevated serum uric acid (SUA) consistently predicts the development of conditions like MetS, diabetes, hypertension, renal disease, cardiovascular disease (CVD), and CVD-related mortality in numerous prospective studies [13,14,15,16,17,18,19]. Studies on the relationship between MetS and dementia have indicated the potential influence of SUA [20, 21]. However, the findings are inconclusive and conflicting. SUA is under scrutiny due to its dual role as an antioxidant and potential pro-oxidant, prompting discussions about its connection to dementia risk with studies even suggesting that elevated SUA levels might slow AD progression [22,23,24]. A cohort study [25] revealed a correlation between high mid-life SUA and fast cognitive decline rather than risk of dementia, while hyperuricemia reduced the risk of vascular-type dementia [26]. Interestingly, gout patients exhibited a lower dementia risk [27], and a meta-analysis suggested AD risk with low UA concentrations [28]. In a cohort of 4618 participants aged 55 and older, higher SUA levels was found to be associated with reduced dementia risk [29]. Previous findings underscore limitations in understanding dementia subtypes and generalizability from small populations. Bridging these limitations is critical for a comprehensive grasp of how metabolic factors, including SUA, intersect with dementia risk, underscoring the need to explore specific dementia subtypes associated with MetS. To address these limitations, our study evaluates the association between MetS and its components in relation to the risk of incident dementia and its subtypes (AD and VD) in a population-based cohort of 466,377 participants observed over a median follow-up of 12.7 years. Additionally, our research explores the interaction between MetS and SUA in relation to the risks stratified by dementia subtypes.
Methods
Participants
Our study drew upon the UK Biobank's extensive dataset (https://www.ukbiobank.ac.uk), encompassing biological and medical information from approximately 500,000 adults aged between 40 and 70 years. Information regarding the study design and survey methods employed in the UK Biobank cohort can be found in previously published materials [30]. Ethical approvals were granted by the UK Biobank review committees (application number 51671, approved in August 2019). At baseline, 502,410 participants were recruited in this analysis. Participants with baseline cancer diagnoses (excluding non-melanoma skin cancer ICD-10 C44, n = 34,825) and pre-existing dementia diagnoses (n = 202) were excluded. Additionally, individuals with incomplete measurements for the five components of MetS (n = 595) were also excluded, resulting in a final analysis cohort of 466,788 participants, with a median follow-up of 12.7 years (Fig. 1).
Assessment of outcomes
The diagnosis of dementia was determined through medical history and linkage to the hospital statistics from England, Scotland and Wales, utilizing the International Classification of Diseases (ICD-10), specifically F00-03. Participants eligible for the study contributed person-years from their date of enrolment until the first dementia diagnosis, date of death, or the last date of follow-up (December 31, 2021), whichever came first.
Assessment of covariates
Participants completed a touchscreen questionnaire at the assessment center collect information on socio-demographic characteristics (i.e., age, gender, ethics, index of multiple deprivation), lifestyle factors (such as smoking status, alcohol consumption, physical activity, diet habits), and medication. Physical activity was assessed using adapted questions from the validated International Physical Activity Questionnaire (IPAQ), while dietary intake was assessed using a food frequency questionnaire, both of which have been validated in previous studies. During the physical examination, components of MetS were measured, including height, weight, waist circumference, and blood pressure. Fasting blood samples from each participant were collected by trained phlebotomists, and serum concentrations of glucose, high-density lipoprotein cholesterol, and triglycerides were measured using the Beckman Coulter AU5800 analyzer (Beckman Coulter (UK) Ltd., High Wycombe, UK). SUA was also measured using the Uricase PAP enzymatic method on the Beckman Coulter AU5800.
Definition for metabolic syndrome (MetS)
The definition of MetS follows the International Diabetes Federation (IDF) criteria [31]. Participants with more than three components were classified as a MetS at baseline. According to the criteria, central obesity was defined as a waist circumference ≥ 94 cm for males or ≥ 80 cm for females. High blood pressure was defined as systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥85 mmHg, or a prior diagnosis of hypertension under antihypertensive treatment. Elevated triglycerides were defined as plasma triglyceride (TG) levels ≥ 1.70 mmol/L (150 mg/dL) or currently receiving medication for hypertriglyceridemia. Low high-density lipoprotein (HDL) cholesterol was defined as male HDL < 1.03 mmol/L, female HDL < 1.29 mmol/L, or undergoing medication for lowering HDL cholesterol levels. High blood sugar was defined as fasting blood glucose ≥ 5.56 mmol/L or a previous diagnosis of type 2 diabetes.
Statistical analysis
In descriptive analysis, numerical values were presented as the mean (standard deviation) for continuous variables or as numbers (percentages) for categorical variables. For covariates where respondents select “no response” or “don’t know,” or in cases of missing data, an “unknown/missing” response category was generated.
Cox proportional hazard regression analyses were performed to calculate the hazard ratio (HR) and 95% confidence interval (95% CI). The proportional hazards assumption was validated through Schoenfeld residuals. In basic model 1, we stratified jointly by age, gender, and UK Biobank assessment centers to estimate the crude associations of MetS and its components with dementia risk and its subtypes. We additionally adjusted for race (white or other), index of multiple deprivation (a measure of socioeconomic status), smoking status (never smoked, previous smoker, current smoker), alcohol consumption (never or special occasions only, one to three times a month, one to four times a week, daily or almost daily), physical activity (high, low, moderate or unknown/missing), portions of fruit and vegetable intake (< 5 portions per day, ≥ 5 portions per day, or unknown/missing), fish intake (< 3 times per week, ≥ 3 times per week) in model 2. In the fully adjusted model 3, we additionally adjusted for regular medications [multivitamin use (yes or no), mineral supplement use (yes or no), non-steroidal anti-inflammatory drugs use (yes or no), aspirin use (yes or no)], and history of Alzheimer’s disease/dementia (yes or no). Additionally, the restricted cubic method was employed to evaluate the potential non-linearity association of each MetS component with the risk of dementia. We used a restricted cubic spline regression model with three knots at the 10th, 50th, and 90th percentiles of each MetS component to achieve the best fit. Non-linearity associations were investigated by using a likelihood ratio test comparing the model with only a linear term against the model with linear and cubic spline terms. To assess the joint effect of SUA on the association between MetS and dementia subtypes, four risk levels based on MetS (presence or absence) or SUA (with an SUA cut-off at 400 μmol/L) [32] and HRs for dementia subtypes risk were calculated with reference to “No MetS and SUA < 400 μmol/L group”. To further investigate potential effect modifiers, we conducted subgroup analyses stratified by age, sex, smoking habits, alcohol consumption, physical activity, fruit and vegetable intake, regular use of aspirin, and non-steroidal anti-inflammatory. In sensitivity analysis, we lagged the exposure for 2 years to avoid potential reverse causation. All statistical tests were two-tailed, and significance was defined as p < 0.05. Statistical analyses were conducted using the R software (version 4.1.0, R Foundation for Statistical Computing, Vienna, Austria).
Results
In our analysis encompassing 466,788 participants (46.4% male, 53.6% female, Table 1), we observed a higher prevalence of former smokers and lower physical activity and fruit/vegetable intake among the MetS cohort. Participants with MetS tended to be older and have a higher multiple deprivation index, higher BMI, blood pressure and serum concentrations of triglyceride, fasting glucose, and lower serum concentrations of HDL-cholesterol than the non-MetS group.
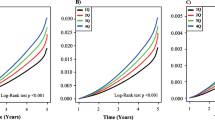
During a median follow-up period of 12.7 years, we observed 6,845 cases of dementia. Table 2 presents the significant association between MetS and dementia, including its subtypes. In general, individuals with MetS exerted a 25% higher risk of all-cause dementia (HR = 1.25, 95% CI = 1.19–1.31), a trend consistent across AD and VD. However, after comprehensive adjustments (sociodemographic characteristics, lifestyle factors, medications, and dementia history), the association with AD weakened (HR = 0.98, 95% CI = 0.90–1.07), while all-cause dementia (HR = 1.06, 95% CI = 1.01–1.12) and VD (HR=1.28, 95% CI = 1.15–1.43) associations remained significant. Subgroup analyses yielded consistent risk estimates, demonstrating the robustness of the association between MetS and all-cause dementia across various risk factors, including age, sex, smoking and drinking status, physical activity, fruit and vegetable intake, NSAIDS use, and aspirin use (Fig. 2). In a sensitivity analysis incorporating a 2-year exposure lag, the positive association persisted between MetS and all-cause dementia as well as VD (Additional file 1: Table S1).
The risk of dementia increased with an increasing number of MetS components (p trend < 0.001). Participants manifesting all five abnormal components experienced significantly elevated risks of all-cause dementia (HR = 1.76, 95% CI = 1.51–2.04) and VD (HR = 3.72, 95% CI = 2.64–5.24). Among the individual MetS components, dyslipidemia for HDL cholesterol (HR = 1.07, 95% CI = 1.01–1.14), hypertension (HR = 1.09, 95% CI = 1.02–1.17), hyperglycemia (HR = 1.43, 95% CI = 1.35–1.51), and dyslipidemia for triglycerides (HR = 1.05, 95% CI = 1.00–1.11) exhibited significant positive associations with dementia risk (p < 0.01). Moreover, all MetS components were associated with a higher risk of VD (Table 3).
Exploring the interaction between MetS and SUA levels, we found that MetS was linked to an elevated risk of all-cause dementia and VD in both groups stratified by SUA levels. Notably, the risk substantially escalated in participants with both MetS and SUA levels exceeding 400 μmol/L (all-cause dementia: HR = 1.11, 95% CI = 1.02–1.21; VD: HR = 1.50, 95% CI = 1.28–1.77) when compared to participants without MetS and SUA levels at or below 400 μmol/L in the fully adjusted model 3 (Table 4).
Furthermore, our analysis of the non-linear associations of continuous individual MetS components with dementia risk unveiled that higher fasting glucose was linked to significantly heightened risks of all-cause dementia, without evidence of non-linearity. Conversely, U-shaped patterns characterized the associations between other MetS components and dementia risk. Similar results were observed for the associations of MetS components with VD risk (Fig. 3 and Additional file 1 Figure S1).
Non-linear association between metabolic syndrome components and all-cause dementia risk. Restricted cubic spline models, with knots at the 10th, 50th, and 90th percentiles, were employed. Reference levels (HR fixed at 1.0) for each plot: A BMI: 27.42 kg/m2; B cholesterol: 5.69 mmol/L; C HDL: 1.45 mmol/L; D LDL: 3.56 mmol/L; E glucose: 5.12 mmol/L; F HbA1c: 36.08 mmol/L; G SBP: 139.6 mm Hg; H DBP: 82.25 mm Hg; I WC: 90.27 cm; J TG: 1.74 mmol/L Adjustments were made for age, gender, and UK Biobank assessment centers. Additional adjustments included race, index of multiple deprivation, smoking status, alcohol consumption, physical activity, portions of fruit and vegetable intake, regular medications (multivitamin use, mineral supplement, non-steroidal anti-inflammatory drugs, and aspirin), and history of dementia
Discussion
In this comprehensive study utilizing UK Biobank data, we explored the association between MetS and dementia, focusing on VD and AD. Our analysis encompassed data from 466,788 adults aged 40 to 70 years with genders balanced. The study unveiled a substantial 25% increased risk of all-cause dementia associated with MetS. Notably, this association exhibited a more pronounced effect for VD than AD. The risks for AD became apparent only after a 2-year exposure lag. This lag was strategically incorporated into our study design to account for potential delays in the manifestation of dementia symptoms following exposure to MetS. Additionally, as individuals accumulated abnormal metabolic components, their risk of both VD and all-cause dementia increased. Elevated SUA levels above 400 μmol/L were associated with a higher risk of all-cause dementia and VD, but not AD. These findings underscore the significant relationship between MetS and SUA in dementia risk and highlight the importance of considering these factors across different dementia subtypes.
Our findings contribute to the body of evidence on the relationship between MetS components and dementia risk. Confirming prior research on the association between MetS and dementia risk [4, 33]. We affirm that individuals with MetS face a heightened risk of dementia [4, 10, 11], particularly in the case of VD [9, 12, 34, 35] and AD [6, 7]. This risk escalated progressively with each additional MetS component. Discrepancies arise in previous studies regarding the association between MetS and AD [7, 9]. In a comprehensive four-year study conducted in France involving 7,078 subjects found no substantial association between MetS and AD [9]. Conversely, an alternative investigation yielded dissimilar results, disclosing a positive correlation between MetS and AD while failing to establish a significant link with VD [7]. This divergence in results can be ascribed, at least partially, to disparities in demographic composition, study design, methodology, and sample size. Such that, the study exclusively focused on a cohort of 84,144 participants with an average age of 67 years, a population segment renowned for a notable increase in AD incidence with advancing age [36, 37]. Our study encompasses a larger cohort with a balanced gender distribution and an average age of 56.8 years which is more representative of the age range at which dementia risk assessments are typically conducted. We also took into account the multifactorial nature of MetS, subject to influences from lifestyle and mental health status [38,39,40], our analysis strikes a methodological balance, capturing diverse participants while mitigating susceptibility to reverse causation bias over an extended follow-up period of 12.7 years.
Examining modifiable factors associated with MetS, we uncovered a higher prevalence of former smokers and lower physical activity and fruit/vegetable intake among the MetS cohort. These associations imply potential modifications in cognitive reserve with the addition of each MetS component. Furthermore, we accounted for medication use, adjusting for NSAIDs [41], a critical factor as it may have influenced the observed reduction in dementia cases. Our analysis implies individuals with MetS, particularly those exhibiting four or five components, may derive potential benefits from early intervention and prevention measures to mitigate the risk of dementia.
Moving beyond the broad link between MetS and dementia, our analysis unveils a nuanced, non-linear relationship between individual MetS components and dementia risk. While higher fasting glucose and systolic blood pressure show linear associations, others, exhibit U-shaped patterns. This dynamic, possibly due to a threshold effect, implies a significant increase in dementia risk beyond specific MetS levels. The interplay of each component contributes significantly to this non-linear nature. Moreover, individual variability, influenced by lifestyle factors such as diet, physical activity, and waist circumference, may play a substantial role. Understanding this relationship demands comprehensive consideration of multifaceted factors, emphasizing the necessity for sophisticated approaches in both research and clinical practice. This underscores the notion that the connections between MetS components and dementia risk surpass a simplistic linear correlation.
In our analysis with a particular focus on VD concerning dyslipidemia for HDL cholesterol, hyperglycemia, and dyslipidemia for triglycerides, along with hypertension, it is evident that each of these factors independently contributes to an elevated risk of VD. These findings underscore the substantial impact of metabolic factors on the risk of dementia, even when considering other components of MetS. Regarding AD, we initially observed an association in the unadjusted model (HR = 1.11 95% CI: 1.02–1.20). However, this association lost statistical significance after accounting for various factors, including anti-inflammatory drug usage. This implies a potential role for anti-inflammatory drugs in mitigating AD risk and its associated symptoms within the dementia spectrum, warranting further investigation.
Refining our analysis of dementia subtypes, atherogenic dyslipidemia emerges as a noteworthy factor in dementia risk. The substantial association with dementia, particularly VD (HR = 1.33 (95% CI: 1.17–1.50), underscores its impact on the overall risk profile. This vascular dysfunction contributes to atherosclerotic lesions, particularly in carotid and vertebrobasilar systems, causing chronic hemispheric hypoperfusion [42, 43]. The heightened dementia risk persists even in cases of minor strategic infarcts within vital cognitive regions, as identified through voxel-based brain MRI analysis [44, 45].
Furthermore, hypertension emerges as another critical factor, with HRs of 1.09 (95% CI 1.02–1.17) for all-cause dementia, 1.15 (95% CI 1.03–1.28) for AD, and 1.33 (95% CI 1.12–1.58) for VD. The pronounced dementia risk associated with hypertension, particularly for VD, underscores its role in inducing early cerebral blood flow dysfunction and vascular remodeling [42, 46,47,48,49,50]. Cerebral small vessel disease, often prevalent in individuals with hypertension, predominantly affects white matter integrity due to various factors, including endothelial dysfunction, hypoperfusion, and blood-brain barrier disruption, leading to cerebral atrophies, and eventually cognitive dysfunction [42]. Our results also indicated that each MetS component is independently associated with an increased risk of AD, with a significance level of p-value < 0.05 over the five accumulative effects of the MetS components. Our study reaffirms the potential pathogenic interrelationships between metabolic factors especially hypertension to cognitive health.
Considering inflammation as a potential common risk factor in both MetS and dementia, recent studies proposed a pivotal role for SUA in atherosclerosis [51] and its involvement in triggering systemic inflammation in MetS [52]. Despite the controversial dual role of SUA, our findings align with previous research indicating that elevated SUA levels, not in the context of MetS, are associated with an increased risk of VD [53]. Our analysis underscores a correlation between elevated SUA levels (> 400 μmol/L) and a heightened risk of all-cause dementia with HRs of 1.11 (95% CI 1.02–1.21), and VD with an increased HR of 1.50 (95% CI 1.28–1.77). This emphasizes the significant impact of elevated SUA on VD risk. Indeed, SUA’s role in atherosclerosis and its link to hippocampal inflammation suggests a potential avenue for future research into neuroinflammation, given the established role of the hippocampus in dementia development. Unlike previous studies solely investigating the connection between dementia risk across scaled urate ranges [54], our research comprehensively considers all five MetS components to dementia subtypes. Recognizing the limitations of using a single index [54], such as BMI, for assessing the impact of MetS, especially beyond three components, highlights the novel approach our study undertakes. Additionally, our analysis’ equal gender distribution minimizes potential gender bias. Suggesting interventions for dementia, considering gender and serum uric acid variations, necessitates personalized strategies to accommodate diverse responses and specific factors influencing treatment efficacy [55,56,57,58,59,60,61]. Furthermore hyperuricemia, characterized by elevated serum uric acid, is associated with well-established risk factors for dementia including cardiovascular disease, diabetes, and hypertension—and links to dementia [62]. While our study did not find an increased risk of AD associated with elevated SUA levels, the potential antioxidant neuroprotective role of SUA [63] is implicated in our results, suggesting SUA could be a potential biomarker for monitoring dementia development among MetS individuals—a novel avenue for future investigation.
Our research unravels the intricate relationship between SUA and dementia risk, particularly in the realms of AD and VD. Elevated SUA levels (> 400 μmol/L) reveal a robust and significant association, indicating an 11% heightened risk of all-cause dementia and a substantial 50% increased risk of VD among individuals with MetS. Interestingly, no significant association is observed with AD, suggesting a potential antioxidative effect of higher SUA levels on cognitive health possibly contributing to an overall reduction in AD risk. These findings align with previous research on SUA’s association with its potential neuroprotective role [64, 65]. Nevertheless, it is important to note that the relationship between SUA and AD remains a subject of controversy [21]. Our study enriches the growing body of evidence concerning the interplay between MetS, its components, and dementia risk. The identification of non-linear associations observed for specific MetS components, along with the varied impacts on different dementia subtypes, underscores the importance of personalized approaches in dementia risk assessment and management. These insights are invaluable for developing comprehensive care strategies that address both metabolic health and dementia prevention, aligning with global health initiatives focused on dementia care and prevention. Our findings align with global health initiatives focused on enhancing dementia care and prevention, underscoring the importance of deciphering these complex relationships for the improvement of public health.
To the best of our knowledge, our study is the first to comprehensively explore the association between SUA, MetS, and the risk of AD, VD, and all-cause dementia subtypes. This research introduces novelty and significance to the field, and ongoing scientific exploration and dialog are crucial for refining our understanding of these intricate relationships and their implications for public health.
Strength and limitations
In our study using UK Biobank data to assess the relationship between MetS and dementia, we recognize several limitations. The participant cohort, mainly from the UK, may not represent global populations, impacting the generalizability of our findings. This is especially relevant given the varied influences of genetic, gender, lifestyle, nutrition status, and environmental factors on MetS and dementia risks across different demographics. Being observational, our study establishes associations but not causality, and there is a potential for unmeasured confounding factors. The use of baseline measurements for SUA and MetS components might not reflect changes over the 12.7-year follow-up period. This is significant for diseases like Alzheimer’s, which develop progressively. While reverse causality is less likely, it's not entirely ruled out. Data reliability could be influenced by self-reported information and changes in diagnostic criteria. Participant dropouts or loss to follow-up may also bias the results. Furthermore, our findings may not apply to diverse age groups, ethnicities, or healthcare systems. Some subgroup analyses might have limited statistical power, affecting the robustness of conclusions.
These limitations emphasize the need for cautious interpretation of our results and suggest the value of future research with more diverse populations and dynamic measurements for a deeper understanding of the MetS-dementia relationship.
Conclusions
In conclusion, our extensive analysis of 466,788 UK Biobank participants reveals a nuanced relationship between MetS and dementia. We observe a 25% increased risk of all-cause dementia, with a more pronounced association for VD over AD. The emergence of AD risk becomes apparent after a 2-year exposure lag providing valuable insights into temporal dynamics. As participants accumulate abnormal metabolic components, risks for VD and all-cause dementia rise, particularly with elevated SUA levels above 400 μmol/L, highlighting the significant association between MetS, SUA, and dementia risk across subtypes. Notably, elevated SUA levels are associated with an 11% increased risk of all-cause dementia and a 50% increased risk of VD. Our study contributes novel insights by examining the cumulative impact of all five MetS components and SUA levels on VD risk. The identification of non-linear associations for specific MetS components underscores the necessity for personalized approaches in dementia risk assessment. Comprehensively examining a large cohort, our study distinguishes itself by moving beyond singular components and addressing potential delays in symptom manifestation, enhancing the accuracy of assessing the temporal relationship between MetS and AD.
In summary, our study reaffirms the MetS-dementia association while offering novel insights. The diverse impacts on different dementia subtypes, the influence of SUA, and the strategic lag contribute to a comprehensive understanding. Advocating for a shift toward personalized, targeted approaches in dementia risk evaluation and intervention, our findings align with global health initiatives for dementia care and prevention.
Availability of data and materials
The data that support the findings of this study are available from the UK Biobank (application number 51671, approved August 2019) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the UK Biobank. UK Biobank is an open-access resource, and the study website https://www.ukbiobank.ac.uk/ has information on data availability and access procedures. Data sets used for the analysis will be made available upon reasonable request.
Abbreviations
- AD:
-
Alzheimer’s dementia
- ASP:
-
Aspirin
- BMI:
-
Body mass index
- DBP:
-
Diastolic blood pressure
- HbA1c:
-
Hemoglobin A1c
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- MetS:
-
Metabolic syndrome
- NASIDS:
-
Non-steroidal anti-inflammatory drugs
- SBP:
-
Systolic blood pressure
- SUA:
-
Serum uric acid
- TG:
-
Triglyceride
- VD:
-
Vascular dementia
References
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–46.
Wilson PWF, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and Type 2 Diabetes Mellitus. Circulation. 2005;112(20):3066–72.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640.
Machado-Fragua MD, Fayosse A, Yerramalla MS, van Sloten TT, Tabak AG, Kivimaki M, et al. Association of metabolic syndrome with incident dementia: role of number and age at measurement of components in a 28-year follow-up of the Whitehall II Cohort Study. Diabetes Care. 2022;45(9):2127–35.
Tang X, Zhao W, Lu M, Zhang X, Zhang P, Xin Z, et al. Relationship between central obesity and the incidence of cognitive impairment and dementia from cohort studies involving 5,060,687 participants. Neurosci Biobehav Rev. 2021;130:301–13.
Vanhanen M, Koivisto K, Moilanen L, Helkala EL, Hänninen T, Soininen H, et al. Association of metabolic syndrome with Alzheimer disease. a population-based study. Neurology. 2006;67(5):843–7.
Kim YJ, Kim SM, Jeong DH, Lee S-K, Ahn M-E, Ryu O-H. Associations between metabolic syndrome and type of dementia: analysis based on the National Health Insurance Service database of Gangwon province in South Korea. Diabetol Metab Syndr. 2021;13(1):4.
Cho Y, Han K, Kim DH, Park YM, Yoon KH, Kim MK, et al. cumulative exposure to metabolic syndrome components and the risk of dementia: a nationwide population-based study. Endocrinol Metab (Seoul). 2021;36(2):424–35.
Raffaitin C, Gin H, Empana JP, Helmer C, Berr C, Tzourio C, et al. Metabolic syndrome and risk for incident Alzheimer’s disease or vascular dementia: the Three-City Study. Diabetes Care. 2009;32(1):169–74.
Fan YC, Chou CC, You SL, Sun CA, Chen CJ, Bai CH. Impact of worsened metabolic syndrome on the risk of dementia: a nationwide cohort study. J Am Heart Assoc. 2017;6(9):e004749.
Qureshi D, Collister J, Allen NE, Kuźma E, Littlejohns T. Association between metabolic syndrome and risk of incident dementia in UK Biobank. Alzheimer’s & Dementia [Internet]. Dementia: the journal of Alzheimer's Association. 2023;20(1):447–58. Available from: https://doi.org/10.1002/alz.13439.
Atti AR, Valente S, Iodice A, Caramella I, Ferrari B, Albert U, et al. Metabolic syndrome, mild cognitive impairment, and dementia: a meta-analysis of longitudinal studies. Am J Geriatr Psychiatry. 2019;27(6):625–37.
Kawamoto R, Ninomiya D, Kasai Y, Senzaki K, Kusunoki T, Ohtsuka N, et al. Baseline and changes in serum uric acid independently predict 11-year incidence of metabolic syndrome among community-dwelling women. J Endocrinol Invest. 2018;41(8):959–68.
Sui X, Church TS, Meriwether RA, Lobelo F, Blair SN. Uric acid and the development of metabolic syndrome in women and men. Metabolism. 2008;57(6):845–52.
Yang T, Chu C-H, Bai C-H, You S-L, Chou Y-C, Chou W-Y, et al. Uric acid level as a risk marker for metabolic syndrome: a Chinese cohort study. Atherosclerosis. 2012;220(2):525–31.
Zurlo A, Veronese N, Giantin V, Maselli M, Zambon S, Maggi S, et al. High serum uric acid levels increase the risk of metabolic syndrome in elderly women: The PRO.V.A study. Nutr Metab Cardiovasc Dis. 2016;26(1):27–35.
Gonçalves JP, Oliveira A, Severo M, Santos AC, Lopes C. Cross-sectional and longitudinal associations between serum uric acid and metabolic syndrome. Endocrine. 2012;41(3):450–7.
Oda E. Serum uric acid is an independent predictor of metabolic syndrome in a Japanese health screening population. Heart Vessels. 2014;29(4):496–503.
Cicero AFG, Salvi P, D’Addato S, Rosticci M, Borghi C. for the Brisighella Heart Study g. Association between serum uric acid, hypertension, vascular stiffness and subclinical atherosclerosis: data from the Brisighella Heart Study. J Hypertens. 2014;32(1):57–64.
Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87(1):315–424.
Qiao M, Chen C, Liang Y, Luo Y, Wu W. the influence of serum uric acid level on Alzheimer’s Disease: a narrative review. Biomed Res Int. 2021;2021:5525710.
Prasad M, Matteson EL, Herrmann J, Gulati R, Rihal CS, Lerman LO, et al. uric acid is associated with inflammation, coronary microvascular dysfunction, and adverse outcomes in postmenopausal women. Hypertension. 2017;69(2):236–42.
Scheepers LEJM, Boonen A, Dagnelie PC, Schram MT, van der Kallen CJH, Henry RMA, et al. Uric acid and blood pressure: exploring the role of uric acid production in The Maastricht Study. J Hypertens. 2017;35(10):1968–75.
Maarman GJ, Andrew BM, Blackhurst DM, Ojuka EO. Melatonin protects against uric acid-induced mitochondrial dysfunction, oxidative stress, and triglyceride accumulation in C2C12 myotubes. J Appl Physiol. 2016;122(4):1003–10.
Alam AB, Wu A, Power MC, West NA, Alonso A. Associations of serum uric acid with incident dementia and cognitive decline in the ARIC-NCS cohort. J Neurol Sci. 2020;414:116866.
Hong JY, Lan TY, Tang GJ, Tang CH, Chen TJ, Lin HY. Gout and the risk of dementia: a nationwide population-based cohort study. Arthritis Res Ther. 2015;17(1):139.
Min KH, Kang SO, Oh SJ, Han JM, Lee KE. Association between gout and dementia in the elderly: a nationwide population-based cohort study. Am J Geriatr Psychiatry. 2021;29(12):1177–85.
Zhou Z, Zhong S, Liang Y, Zhang X, Zhang R, Kang K, et al. Serum uric acid and the risk of dementia: a systematic review and meta-analysis. Front Aging Neurosci. 2021;13:625690.
Euser SM, Hofman A, Westendorp RGJ, Breteler MMB. Serum uric acid and cognitive function and dementia. Brain. 2008;132(2):377–82.
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779.
Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. a consensus statement from the international diabetes federation. Diabet Med. 2006;23(5):469–80.
Bardin T, Richette P. Definition of hyperuricemia and gouty conditions. Curr Opin Rheumatol. 2014;26(2):186–91.
Craft S. The role of metabolic disorders in alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66(3):300–5.
Solfrizzi V, Scafato E, Capurso C, D’Introno A, Colacicco AM, Frisardi V, et al. Metabolic syndrome and the risk of vascular dementia: the Italian Longitudinal Study on Ageing. J Neurol Neurosurg Psychiatry. 2010;81(4):433–40.
Kalmijn S, Foley D, White L, Burchfiel CM, Curb JD, Petrovitch H, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler Thromb Vasc Biol. 2000;20(10):2255–60.
Muller M, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. Metabolic syndrome and dementia risk in a multiethnic elderly cohort. Dement Geriatr Cogn Disord. 2007;24(3):185–92.
Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65(4):545–51.
Borges RL, Ribeiro AB, Zanella MT, Batista MC. Uric acid as a factor in the metabolic syndrome. Current Hypertension Reports. 2010;12(2):113–9.
Jeong J, Suh YJ. Association between Serum Uric Acid and Metabolic Syndrome in Koreans. J Korean Med Sci. 2019;34(48):e307.
Huang S, Liu X, Li H, Xu W, Jia H. Sex difference in the association of serum uric acid with metabolic syndrome and its components: a cross-sectional study in a Chinese Yi population. Postgraduate Med. 2017;129(8):828–33.
Marcum ZA, Gabriel N, Bress AP, Hernandez I. Association of New Use of Antihypertensives That Stimulate vs Inhibit Type 2 and 4 Angiotensin II Receptors With Dementia Among Medicare Beneficiaries. JAMA Network Open. 2023;6(1):e2249370-e.
Borshchev YY, Uspensky YP, Galagudza MM. Pathogenetic pathways of cognitive dysfunction and dementia in metabolic syndrome. Life Sci. 2019;237:116932.
Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurology. 2009;8(11):1006–18.
Biesbroek JM, Weaver NA, Biessels GJ. Lesion location and cognitive impact of cerebral small vessel disease. Clin Sci. 2017;131(8):715–28.
Duering M, Zieren N, Herve D, Jouvent E, Reyes S, Peters N, et al. Strategic role of frontal white matter tracts in vascular cognitive impairment: a voxel-based lesion-symptom mapping study in CADASIL. Brain. 2011;134:2366–75.
Lee JE, Shin DW, Han K, Kim D, Yoo JE, Lee J, et al. Changes in metabolic syndrome status and risk of dementia. J Clin Med. 2020;9(1):122.
Jennings JR, Muldoon MF, Ryan C, Price JC, Greer P, Sutton-Tyrrell K, et al. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology. 2005;64(8):1358–65.
Hammond CA, Blades NJ, Chaudhry SI, Dodson JA, Longstreth WT, Heckbert SR, et al. Long-Term Cognitive Decline After Newly Diagnosed Heart Failure Longitudinal Analysis in the CHS (Cardiovascular Health Study). Circ Heart Fail. 2018;11(3):e004476.
Alagiakrishnan K, Mah D, Ahmed A, Ezekowitz J. Cognitive decline in heart failure. Heart Failure Rev. 2016;21(6):661–73.
Ovsenik A, Podbregar M, Fabjan A. Cerebral blood flow impairment and cognitive decline in heart failure. Brain Behav. 2021;11(6):e02176.
Kimura Y, Tsukui D, Kono H. Uric Acid in Inflammation and the Pathogenesis of Atherosclerosis. Int J Mol Sci. 2021;22(22):12394.
Spiga R, Marini MA, Mancuso E, Fatta CD, Fuoco A, Perticone F, et al. Uric acid is associated with inflammatory biomarkers and induces inflammation via activating the NF-κB signaling pathway in HepG2 cells. Arterioscler Thromb Vasc Biol. 2017;37(6):1241–9.
Latourte A, Soumaré A, Bardin T, Perez-Ruiz F, Debette S, Richette P. Uric acid and incident dementia over 12 years of follow-up: a population-based cohort study. Ann Rheum Dis. 2018;77(3):328–35.
Cao Z, Xu C, Yang H, Li S, Xu F, Zhang Y, et al. Associations of BMI and serum urate with developing dementia: a prospective cohort study. J Clin Endocrinol Metab. 2020;105(12):e4688–98.
Geraets AFJ, Leist AK. Sex/gender and socioeconomic differences in modifiable risk factors for dementia. Sci Rep. 2023;13(1):80.
Podcasy JL, Epperson CN. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci. 2016;18(4):437–46.
Kim Y-H, Kim NH, Jung M-H, Kim H-J. Sex differences in metabolic risk indicator of dementia in an elderly urban Korean population: a community-based cross-sectional study. Geriatr Gerontol Int. 2017;17(11):2136–42.
Azad NA, Al Bugami M, Loy-English I. Gender differences in dementia risk factors. Gender Med. 2007;4(2):120–9.
Rahman A, Jackson H, Hristov H, Isaacson RS, Saif N, Shetty T, et al. Sex and gender driven modifiers of alzheimer’s: the role for estrogenic control across age, race, medical, and lifestyle risks. front Aging Neurosci. 2019;11:315.
Sundarakumar JS, Stezin A, Menesgere AL, Ravindranath V. Rural-urban and gender differences in metabolic syndrome in the aging population from southern India: Two parallel, prospective cohort studies. eClinicalMedicine. 2022;47:101395.
Laudisio A, Marzetti E, Antonica L, Pagano F, Vetrano DL, Bernabei R, et al. Metabolic syndrome and quality of life in the elderly: age and gender differences. Eur J Nutr. 2013;52(1):307–16.
Liu N, Xu H, Sun Q, Yu X, Chen W, Wei H, et al. The Role of oxidative stress in hyperuricemia and xanthine oxidoreductase (XOR) Inhibitors. Oxid Med Cell Longev. 2021;2021:1470380.
Scheepers L, Jacobsson LTH, Kern S, Johansson L, Dehlin M, Skoog I. Urate and risk of Alzheimer’s disease and vascular dementia: a population-based study. Alzheimers Dement. 2019;15(6):754–63.
Du N, Xu D, Hou X, Song X, Liu C, Chen Y, et al. Inverse association between serum uric acid levels and alzheimer’s disease risk. Molecular Neurobiology. 2016;53(4):2594–9.
Chen X, Guo X, Huang R, Chen Y, Zheng Z, Shang H. Serum uric acid levels in patients with Alzheimer’s disease: A meta-analysis. PLoS ONE. 2014;9(4):e94084.
Acknowledgements
We extend our heartfelt gratitude to the participants and staff of the UK Biobank cohort for their invaluable contributions. We also appreciate all who have supported and inspired us as we align our efforts with the UN Sustainable Development Goals, especially in addressing global aging and dementia health challenges. Special thanks to WHO NCD Rehabilitation team, Dr. Alarcos Cieza, ICCC Professor Dianne Davis, Professor Jian-Guo Zhang, Professor Jun-Jun Ding, and ISPRM Professor Bryan O'Young for their unwavering support, inspiration, and mentorship.
Transparency
All authors affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted and that any discrepancies from the study as planned have been explained.
Funding
This work was supported by the 5010 Planning Project of Sun Yat-Sen University, China (Grant Number: 20140009).
Author information
Authors and Affiliations
Contributions
JY and DH, with full data access, ensure data integrity and accurate analysis. TC is the first author, and NM is the second co-first author. All authors and TC, CW, WL, YM, YT, DH, JY, and ZP contributed to conceptualization, design, acquisition, analysis, and interpretation. TC drafted the manuscript, revised by HL. Statistical analysis: JY, NM, TC. Supervision: DH, JY, ZP. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The data for this study were derived from the UK Biobank (application number 51671, granted approval in August 2019). Ethical approval was granted by authorities in England, Wales, and Scotland, with participants providing written informed consent in accordance with the Helsinki Declaration principles.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. The association between metabolic syndrome and risk of dementia after lagging for 2 years. Figure S1. Non-linear Association Between Metabolic Syndrome Components and Vascular Dementia Risk.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, T.S., Mi, NN., Lao, H.Y. et al. Investigating the nexus of metabolic syndrome, serum uric acid, and dementia risk: a prospective cohort study. BMC Med 22, 115 (2024). https://doi.org/10.1186/s12916-024-03302-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-024-03302-5