Abstract
Background
The COVID-19 vaccine supply shortage in 2021 constrained roll-out efforts in Africa while populations experienced waves of epidemics. As supply improves, a key question is whether vaccination remains an impactful and cost-effective strategy given changes in the timing of implementation.
Methods
We assessed the impact of vaccination programme timing using an epidemiological and economic model. We fitted an age-specific dynamic transmission model to reported COVID-19 deaths in 27 African countries to approximate existing immunity resulting from infection before substantial vaccine roll-out. We then projected health outcomes (from symptomatic cases to overall disability-adjusted life years (DALYs) averted) for different programme start dates (01 January to 01 December 2021, n = 12) and roll-out rates (slow, medium, fast; 275, 826, and 2066 doses/million population-day, respectively) for viral vector and mRNA vaccines by the end of 2022. Roll-out rates used were derived from observed uptake trajectories in this region. Vaccination programmes were assumed to prioritise those above 60 years before other adults.
We collected data on vaccine delivery costs, calculated incremental cost-effectiveness ratios (ICERs) compared to no vaccine use, and compared these ICERs to GDP per capita. We additionally calculated a relative affordability measure of vaccination programmes to assess potential nonmarginal budget impacts.
Results
Vaccination programmes with early start dates yielded the most health benefits and lowest ICERs compared to those with late starts. While producing the most health benefits, fast vaccine roll-out did not always result in the lowest ICERs. The highest marginal effectiveness within vaccination programmes was found among older adults. High country income groups, high proportions of populations over 60 years or non-susceptible at the start of vaccination programmes are associated with low ICERs relative to GDP per capita. Most vaccination programmes with small ICERs relative to GDP per capita were also relatively affordable.
Conclusion
Although ICERs increased significantly as vaccination programmes were delayed, programmes starting late in 2021 may still generate low ICERs and manageable affordability measures. Looking forward, lower vaccine purchasing costs and vaccines with improved efficacies can help increase the economic value of COVID-19 vaccination programmes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Since COVID-19 vaccines were first authorised in late 2020, many countries achieved considerable coverage in a short period [1], reducing disease and economic burdens enormously. However, accruing COVID-19 vaccine coverage in many African countries has been slow before 2022, with one of the largest contributors being vaccine shortage [2]. Given roll-out efforts by the end of 2021, it would be a substantial challenge for the region to achieve the 70% coverage target by mid-2022 as set out by the World Health Organization (WHO) and the Africa Centres for Disease Control and Prevention (Africa CDC) [3–5].
The vaccine shortage has eased significantly since early 2022 [4]. By this time, however, many African Union member states had already experienced several epidemic waves involving multiple variants of concern [1, 6]. A key question that these countries face is whether rolling out COVID-19 vaccines remains an impactful strategy representing good value for money, having missed key windows of opportunities from early- to mid-2021. Continuing vaccination raises issues in respect of both cost-effectiveness and affordability in the region due to potentially low health impacts and high health opportunity costs to other health services due to nonmarginal budgetary impact [7, 8].
This study aims to inform future decisions about vaccine roll-out and investment by retrospectively examining the impacts of implementation timing and speed on COVID-19 transmission using a combined epidemiological and economic modelling approach. This approach allowed us to factor in the potentially high seroprevalence of the region, the emergence of multiple variants of concerns, and population characteristics key to the transmission of SARS-CoV-2 (e.g. population age structure, contacts, non-pharmaceutical interventions) and the economic evaluation of vaccination programmes (gross domestic product per capita (GDP per capita), general public health expenditures).
Given that the COVID-19 vaccine roll-out occurred relatively late and slowly among African countries, we focused on answering two key questions: (i) how much health impacts and value for money could have been achieved had the vaccine roll-out been earlier and faster? (ii) could late COVID-19 vaccine roll-out efforts (in late 2021) still provide considerable health impacts and value for money? To answer these questions, we evaluated different vaccine roll-out scenarios in terms of health impacts, cost-effectiveness, and relative affordability by vaccine types among Africa Union member states.
This is the first multi-country regional analysis of COVID-19 vaccine strategies for Africa that links epidemiological models and economic evaluation with findings that reflect on between-country heterogeneities. The lessons learned could inform regional decision-makers on future vaccine roll-out decisions in Africa, particularly if reformulated vaccines become available in response to future variants. To our knowledge, this is also the first study to empirically examine the impacts of implementation timing while appraising COVID-19 vaccine policies.
Methods
We estimated the prevalence of infection-induced protection against COVID-19 by fitting a dynamic transmission model to country-level daily reported COVID-19 deaths. This procedure allowed us to capture different epidemic histories experienced by African Union member states. Cumulative health outcomes and disability-adjusted life years (DALYs) averted associated with different vaccine roll-out scenarios were then simulated. Combining health outcomes with data on the costs of vaccine delivery and COVID-19-related health services, we estimated the overall cost-effectiveness and relative affordability from a health sector perspective by the end of 2022. This section provides essential information to understand the methods. Further details are presented in Additional File 1.
All analyses were done in R (4.1.0). All data used are publicly available. Code and intermediate results can be accessed via our GitHub repository archived at Zenodo [9].
Characterising vaccine roll-out scenarios
By the time most African Union member states achieved 1% COVID-19 vaccine coverage (August 2021), the United Kingdom (UK) had vaccinated 74.6% (as of 31 August 2021) of its population [1]. By February 2022, vaccine coverage in over 35% of African Union member states was still below 10% (Fig. 1a) [1]. We explored possible alternative vaccine roll-out scenarios that could have taken place in 2021 by varying two parameters: vaccination programme start date and vaccine roll-out rates. The programme start date was defined as the date at which the first person in a country was vaccinated and was varied between 01 January 2021 and 01 December 2021 in 1-month increments. We limited the testing window for start dates to before 2022, as all African Union member states had introduced COVID-19 vaccines by then [1].
Model parameterisation and vaccine roll-out scenarios setup. (a) Observed vaccine uptake among African Union member states. Each line represents a country, and its colour indicates the roll-out rate levels (orange - slow; red - medium; blue - fast). (b) Vaccine supply trajectories of 36 vaccine roll-out scenarios (12 programmes starting date × 3 roll-out rates) and the population level vaccine coverage levels they realise
Vaccine roll-out rate was defined as the number of doses administered per million population-day. In this study, we varied this parameter along three levels: 275, 826, and 2066 doses/million population-day (hereafter ‘slow’, ‘medium’, and ‘fast’, respectively). These levels were derived from observed vaccine roll-out trajectories using a univariable linear model (cumulative doses per million population-day ~ date) and represent the median vaccine roll-out rates by tertile observed among African Union members [1].
The vaccine roll-out scenarios combining these two parameters (n = 12 start dates × 3 roll-out rates) are visualised in Fig. 1b (age-specific example presented in Additional File 1: Fig. S1). We capped the maximum population-level vaccine coverage at 70%, consistent with the target set by the WHO and the Africa CDC [3, 5]. Assuming a two-dose schedule for COVID-19 vaccines, a country with a population size of n will receive a maximum of 1.4n doses of vaccines, likely via early and fast roll-out scenarios.
Older adults (60 + years of age) were prioritised in the vaccine roll-out process based on international guidelines and common practices observed in the region [10, 11]. In the transmission model, older adults were also characterised by higher susceptibility, higher percentages of infection showing symptoms, and high percentages of infections progressing to severe, critical, and fatal outcomes. We assume the maximum vaccine uptake rates among older adults to be 80% and other adults 60% [12].
Fitting and simulation using a dynamic transmission model
The epidemic model used in this study was an adaptation of CovidM, an age-specific dynamic transmission model of COVID-19, which has been previously used to explore the impact of different vaccination strategies in the UK, European countries, and Pakistan [13,14,15,16]. Between-country heterogeneity has been captured by a wide range of health (e.g. age structure, life expectancy, reported COVID-19-related deaths), behaviour (e.g. community mobility, social contacts), economic (e.g. GDP per capita, government public health expenditure, costs), and policy (e.g. COVID-19 response stringency index) factors using local data. The conceptual diagram of the transmission model is presented in Additional File 1: Fig. S2 [14]; the model is further described in Additional file 1: Methods S1 [14, 17–22] and S3 [13, 14, 23–25]; complete parameter tables and their data sources can be found in Additional File 1: Table S1-6 [1, 3, 10–20, 22–44] by category.
We fitted this model to country-level daily reported COVID-19 deaths during 2020–2022, before the wide use of COVID-19 vaccines, using maximum likelihood estimation and differential evolutionary algorithm (full optimisation procedure described in Additional File 1: Methods S2) [14, 45]. The following parameters were estimated: (1) the basic reproduction numbers (R0); (2) infection introduction dates; (3) COVID-19 death reporting rate; and (4) variants of concern introduction dates. The fitting process aims at approximating the immune profiles (i.e. the levels of infection-induced immunity by age and the distribution of non-susceptible individuals within the population by compartments) against SARS-CoV-2 before vaccine roll-out.
Among the 55 African Union member states, we identified 27 with sufficient data for model fitting.Footnote 1 The remaining 28 member states were excluded from further analysis due to either data sparsity (i.e. < = 10 deaths/day throughout the fitting period, n = 26) or potential reporting artefacts (i.e. with single day accounting for > 5% of the cumulative COVID-19 deaths since early 2020, n = 2). These countries, despite of interest among regional decision makers, are spatially clustered (Additional File 1: Fig. S3), which makes reasonable extrapolation unfeasible. The epidemic curves describing COVID-19-related deaths among 27 members included in this analysis are presented in Additional File 1: Fig. S4-6 [1].
We explored vaccine roll-out scenarios with either mRNA vaccines (with characteristics similar to those of the Pfizer-BioNTech COVID-19 vaccines) or viral vector vaccines (with characteristics similar to those of Oxford/AstraZeneca vaccines). Six types of vaccine effect mechanisms were incorporated into the model: infection-, disease-, severe case-, critical case-, mortality-reducing, and onward transmission-blocking.
The vaccine efficacy estimates we used are presented in Table 1. An alternative efficacy estimate set based on the lower bounds of their uncertainty ranges in existing literature was tested as a sensitivity analysis. We assumed infection-induced immunity among naïve individuals to last 3 years on average [22]. Given the time horizon of this study, we did not consider the protection waning among fully vaccinated individuals. Decreased vaccine efficacy due to immune evasiveness (e.g. against Omicron) was implemented through changes in susceptibility among vaccinated individuals. However, we assumed immune evasiveness only affects infection- and disease-reducing vaccine efficacies—other types of vaccine efficacies remained constant.
We projected four health outcomes under each vaccine roll-out scenario: (a) symptomatic infections; (b) severe cases that require hospitalisation; (c) critical cases that require intensive care unit (ICU) admission; and (d) deaths (see Additional File 1: Table S2 for parameter sources and Additional File 1: Methods S4 [14, 15, 23] for technical details). All outcomes were aggregated from 01 January 2021 to 31 December 2022. We kept the time horizon relatively short due to the uncertainty around future emerging variants. However, vaccination programmes that start late may require time to present impacts. We extended the time horizon to 30 June 2023 as a sensitivity analysis.
Calculating DALYs
We used projected health outcomes to calculate DALYs incurred, which capture both mortality and morbidity burden. Mortality outcomes were converted to years of life lost (YLLs) using country-specific life expectancy [26]. Morbidity outcomes (symptomatic infections, severe cases, critical cases and those who experience long-term health effects (“Long-COVID”)) were converted to years lived with disability (YLDs). We discounted YLLs over remaining life expectancy and additionally discounted DALYs according to the year in which COVID-19 health outcomes occurred. In line with the WHO guidelines on the economic evaluation of immunisation programmes, we used an annual discount rate of 3% [47]. More information on calculating DALYs can be found in Additional File 1: Methods S5-6 [26, 48–51].
Estimating the vaccine delivery and health service costs
We collected data on country-specific unit costs per vaccine delivered by vaccine type from a health sector perspective in three countries (Ethiopia (a low-income country), Nigeria (a lower-middle-income country), and South Africa (an upper-middle-income country)). For South Africa, we included observed vaccine delivery costs. However, at the time of this study, realistic vaccine delivery costs were not available for any other country in the region. Thus, in this study, we used a normative (i.e. per-protocol) ingredient-based (i.e. itemised) approach (see Additional File 1: Methods S7) [52–54]. The unit cost includes vaccine purchasing costs and related components and activities involved in the planning and delivery of the vaccine (e.g. planning and coordination, cold chain, transportation, and waste disposal) [52]. Costs were then validated by experts knowledgeable of country-level immunisation efforts. In the case of Ethiopia and Nigeria, this validation step was part of the health technology assessment process used to support decision-making around COVID-19 vaccinations.
We found that the unit cost of delivering mRNA vaccines to be substantially higher than that of viral vector vaccines (Table 2). These differences are driven by vaccine purchasing costs (see itemised costs by country, activity, and component in Additional File 1: Methods S7, Additional File 1: Tables S7-8)—for which we used the lowest purchasing price reported in the public domain for each vaccine [55, 56]. We extrapolated these vaccine unit costs for other countries (see Additional File 1: Methods S8 [57–60] for extrapolation methods; this process did not differentiate by country-specific purchasing agreements) and other vaccination programme setups (i.e. programme duration and daily vaccine roll-out rate, see Additional File 1: Methods S9 for the extrapolation methods, and Additional File 1: Table S9 for sample extrapolation results). The extrapolation results indicate that faster vaccine roll-out rates are associated with lower vaccine unit costs due to fixed costs being spread between more vaccine doses (Table 2). Our costing approach assumed no increases in the price of scarce resources (e.g. healthcare workforce).
For COVID-19-related health service costs, we used previously published country-specific estimates (Table 3) and lengths of hospital stay [31, 48]. All costs were converted to US$2020 using GDP deflators [61]. Similarly to DALYs, costs were discounted by 3% in line with WHO guidelines [47].
Measuring cost-effectiveness and relative affordability
We estimated incremental cost-effectiveness ratios (ICERs) from a healthcare payer perspective for different vaccination scenarios compared to a no-vaccination scenario by dividing the differences in total costs between alternatives (including vaccine delivery and COVID-19 health service costs) by the differences in DALYs incurred. Vaccination costs may be paid by country governments or external donors. To allow for between-country comparison, we calculated ICER as a proportion of GDP per capita and thus intrinsically assuming a proportion of GDP per capita may approximate the underlying health opportunity costs [62, 63].
We did not fix a single cost-effectiveness threshold and instead focused our discussion on ICER as a proportion of GDP per capita. This is because there are no generally accepted cost-effectiveness thresholds for any country in Africa or, indeed, most of the world [64]. Furthermore, COVID-19 vaccination is likely to have a large budget impact, and hence the thresholds calculated at the margin of budgets may not always apply [7, 8, 65]. In practice, a cost-effectiveness analysis using an overestimated cost-effectiveness threshold may incur high health opportunity costs in budget-constrained settings as the programme under evaluation could affect existing public health activities. In low- and middle-income countries, substantial uncertain remains around the appropriate cost-effectiveness threshold [63, 66]. In this study, for simplicity, we assumed conditions where ICERs account for more than 50% of GDP per capita are less likely to be considered cost-effective [63, 66].
We ran additional multivariable regression analyses to explore the country-specific metrics that can link to ICERs as proportions of GDP per capita. We tested population size [26], proportion of population over 60 years [26], proportion of population non-susceptible at the start of vaccine roll-out (model fitted in this study), vaccine unit cost (estimated in this study), government general health expenditure [33], and income group [34].
In this study, we defined the relative affordability of a vaccine programme in a given year as the annual incremental cost of the programme divided by annual government general health expenditure [7, 8]. The annual incremental cost of a programme is calculated by the sum of vaccination programme costs and the corresponding COVID-19-related healthcare costs minus the COVID-19-related healthcare costs without any vaccine during the entire time horizon and then standardised to the annual level. Since the external donor contribution may be uncertain (or redirected through different funding schemes) in the COVID-19 era, only domestic healthcare budgets were considered.
We estimated ICER as a proportion of GDP per capita and relative affordability to evaluate COVID-19 vaccination programmes. The two underlying streams of data used, GDP per capita and government general health expenditure, are not strictly correlated. As a result, cost-effectiveness and affordability outcomes may lead to different conclusions—vaccination programmes may be cost-effective but not affordable, and vice versa.
Results
The fitted model could broadly reproduce the observed epidemic history of COVID-19 before vaccine roll-out by country (see Additional File 1: Fig. S4-6 for model fits and S7 for fitted parameter distributions). The projected impacts of viral vector vaccines on cases, deaths, and DALYs by roll-out scenario are presented in Fig. 2 (see Additional File 1: Fig. S8-10 for intermediate results and results for using mRNA vaccines). We calculated relative reduction in disease burden instead of absolute differences to allow the comparison across countries with varying population sizes.
Outcomes associated with different vaccine roll-out scenarios for 27 African Union Members using viral vector vaccines. This figure presents the relative reduction in outcomes as a result of different vaccine roll-out scenarios (i.e. combinations of vaccination programme start dates and vaccine roll-out rates). Relative reduction is defined as (1 - outcome with vaccination/outcome without vaccination). Greater relative reductions indicate more effective vaccine roll-out scenarios. Results for intermediate health outcomes and for using mRNA vaccines can be found in Additional File 1: Fig. S8-10. DALYs: disability-adjusted life years
For a given vaccination programme start date, faster roll-out rates are associated with a substantially greater disease burden averted compared to the no-vaccination scenario. For example, compared to the no-vaccination scenario, vaccination programmes starting in August 2021 (when more than half of African Union member states with data (n = 53) reached 1% population-level vaccine coverage) could have reduced deaths by an average of 11.06% [median = 8.80%, interquartile range: 5.87–16.20%; n = 27 (countries)] under slow roll-out, 24.19% [24.43%, 21.78–27.18%] under medium roll-out, and 31.29% [32.57%, 25.72–36.68%] under fast roll-out.
For a given vaccine roll-out rate, earlier roll-out start dates are associated with greater disease burden averted compared to the no-vaccination scenario. For example, under medium roll-out, starting in January could have reduced deaths by a mean of 50.30% [51.29%, 48.97–54.95%]. However, starting the same programme in August 2021 could only have reduced deaths by a mean of 24.19% [24.32%, 21.78–27.18%]. These decreasing trends in relative impact reduction are steeper for more severe outcomes, which reflects different vaccine efficacies by outcome and the features of emerging variants of concern.
Given the same start date, faster programmes may be associated with lower ICERs in relation to GDP per capita (Fig. 3). At all income levels, medium and fast roll-out scenarios are associated with lower ICERs relative to GDP per capita compared to slow. However, medium roll-out scenarios may result in lower ICERs relative to GDP per capita compared to fast. This can be explained by differential marginal effectiveness by age group as the fast roll-out scenarios quickly move into younger adult populations (which are less cost-effective to vaccinate) after vaccinating older adults (Additional File 1: Methods S10). Compared to older adults, younger adults are less susceptible and less likely to progress to more severe outcomes.
ICERs in relation to GDP per capita by vaccine roll-out scenarios. A. Low-income countries. B. Lower-middle-income countries. C. Upper-middle-income countries. Each y-axis tick represents a country; the y-axis is arranged based on ICERs as proportions of GDP per capita. The baseline for comparison is the no-vaccination scenario (01 January 2021–31 December 2022)
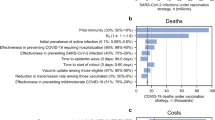
The results of multivariable regression analysis linking country characteristics and ICER as a proportion of GDP per capita revealed that a country’s income group was statistically significant (using type I error rate of 0.05) and had the largest explanatory power (i.e. effect size, Additional File 1: Fig. S11). Higher income groups were associated with low ICERs as proportions of GDP per capita. In upper-middle-income countries, ICERs account for, on average, − 2.52% and 20% of GDP per capita using viral vector and mRNA vaccines, respectively (Fig. 3C). Vaccination programmes may be cost-saving. In low-income countries, ICERs account for, on average, 82.59% and 313.52% of GDP per capita using viral vector and mRNA vaccines, respectively (Fig. 3A). These vaccination programmes are unlikely to be considered cost-effective. In low-income countries, only viral vector vectors and only when vaccination programmes start in the first half of 2021 could the average ICERs as proportions of GDP per capita have fallen below 50% (Fig. 3A).
The proportion of population above 60 years of age and the proportion of non-susceptible individuals at the start of vaccination programmes were also statistically significant and had the next largest effect sizes. Higher proportions of population above 60 years of age were associated with lower ICERs as proportions of GDP per capita. Higher proportions of non-susceptible population at the start of the vaccination programme were associated with higher ICERs as proportions of GDP per capita. All other variables, except for government general health expenditure, were statistically significantly associated with ICERs as proportions of GDP per capita but had smaller effect sizes relative to the aforementioned three variables.
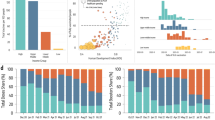
Our sensitivity analyses around the time horizon show that drawing the line on 31 December 2022 affects how ICERs compare to GDP per capita (Fig. 4, top panels). Of 972 country and roll-out scenario combinations (27 countries × 36 scenarios), 34.88% and 19.65% returned different results when we extended the time horizon by 6 months while using viral vector and mRNA vaccines, respectively. These differences are expressed as deviations from the diagonal line in Fig. 4. Deviations above the diagonal line indicate by extending the time horizon ICERs got larger. Stratified analyses revealed that most deviations above the diagonal lines had late start dates; most deviations below had early start dates. An extended time horizon makes the observed contrast in ICER over start date (Fig. 3) more extreme, indicating the robustness of our results to the analytical window.
Results from sensitivity analyses. Univariable sensitivity analysis dimensions: (top) time horizon; (bottom) vaccine efficacies. In each panel, a total of 972 data points (27 countries × 36 vaccine roll-out scenarios) have been allocated into each cell based on value, with colours indicating the overall count of data points in that cell. In the bottom right panel, for example, the dark cell on the top right indicates there are approximately 500 country-and-vaccine-roll-out-scenario combinations where the ICER relative to GDP per capita is greater than one in both the baseline analysis and when low vaccine efficacies were implemented. Deviations from the diagonal line indicate there are country-and-vaccine-roll-out-scenario combinations returning different results in terms of ICERs as proportions of GDP per capita resulting from the univariable sensitivity analysis
Using lower vaccine efficacies affects ICERs as proportions of GDP per capita to a much lesser extent. We expect low vaccine efficacy to be associated with high medical costs and low overall QALYs averted compared to the no vaccine used scenario and thus high ICERs as proportions to GDP per capita. This is the case with both viral vector and mRNA vaccines (Fig. 4, bottom panels; non-empty cells exist to the top left of the diagonal line). While using mRNA vaccines, lower vaccine efficacies may lead to lower ICERs as proportions to GDP per capita (Fig. 4, bottom right panel; non-empty cells exist on the bottom right of the diagonal line). In these cases, lower vaccine efficacy leads to larger outbreaks earlier on, resulting in more individuals being protected by hybrid immunity by the time that the more transmissible strain emerges.
The median relative affordability estimates for vaccination programmes involving mRNA vaccines implemented with slow, medium, and fast roll-out rates are 3.85% [interquartile range: 1.26–10.24%], 12.42% [3.43–29.84%], and 26.13% [7.82–61.61%], respectively, and for viral vector vaccines 1.09% [0.18–3.02%], 3.24% [0.61–6.61%], and 5.28% [0.70% -10.98%], respectively.
In the context of low- and middle-income countries, scenarios where ICERs account for more than 50% of GDP per capita are less likely to be cost-effective (Fig. 5, dotted cells) [63, 66]. Scenarios where ICERs account for less than 50% of GDP per capita and where programme cost less than 10% of government general health expenditure may be favourable policy options (Fig. 5, pattern-free cells). Some scenarios have negative net costs and ICERs, indicating they are cost-saving and health-improving. Other scenarios (5.22% and 1.11% of country-and-roll-out-scenario combinations for viral vector and mRNA vaccines, respectively) have low ICERs as proportions of GDP per capita but cost more than 10% of government health expenditure (Fig. 5, striped cells). In these cases, some COVID-19 vaccination programmes may still be cost-effective yet unaffordable. Decision makers may need to adjust their perceived health opportunity costs for a DALY, given COVID-19 is now a new and leading source of health burden [7, 8]. The need for this adjustment may be mitigated by shifting costs to external donors (e.g. via Gavi COVID-19 Vaccines Global Access (or COVAX), Advance Market Commitment) among health sector payers.
ICERs as proportions of GDP per capita and relative affordability of COVID-19 vaccine roll-out scenarios. In each panel, a total of 900 data points (25 countries × 36 vaccine roll-out scenarios) have been allocated in each cell, with colours indicating the overall count of data points in that cell. Only 25 of 27 countries with ICER results also had relative affordability results. Data on government general health expenditure for Somalia and Libya were not available. In the top panel, for example, there are over 150 country-and-vaccine-roll-out-scenario combinations where the ICERs account for 0–10% of the GDP per capita and where incremental costs of the vaccination programmes account for 0–10% of the country’s general healthcare expenditure. An unfavourable conclusion (dotted pattern cells) is extremely unaffordable (> 100% of general healthcare expenditure) or one where ICERs as proportions of GDP per capita are too high (> 0.5); a favourable conclusion (pattern-free cells) is one where ICERs as proportions of GDP per capita are low (< = 0.5) and programme costs are also low (< = 10% of general healthcare expenditure); a mixed conclusion (striped pattern cells) is one where ICERs as proportions of GDP per capita are low (< = 0.5), but programme costs are high (> = 10% of general healthcare expenditure)
Raw estimates behind the results presented above (including incremental costs, incremental DALYs, ICER, GDP per capita, and ICERs as proportions of GDP per capita) by each scenario and country can be downloaded from our GitHub repository.
Discussion
Using a combined epidemiological and economic modelling approach, we explored how the timing and speed of implementation (i.e. vaccination programme start date and vaccine roll-out rates) affect vaccination programmes’ health benefits, cost-effectiveness, and relative affordability among African countries. We found that vaccination programmes with earlier start dates lead to greater health benefits and are more likely to result in small ICERs in relation to GDP per capita. Fast vaccination roll-out leads to the greatest health benefits but does not always lead to the smallest ICERs in relation to GDP per capita. This is due to differential marginal effectiveness by age. Fast scenarios quickly move beyond the vulnerable and thus prioritised population (defined as 60 + years of age in this study, see Additional File 1: Methods S10). These results should not be extrapolated to other parts of the world without further validation; these results also should not be extrapolated to other vaccine products due to the fundamental differences in the underlying epidemiology.
Higher income groups and greater proportions of population over 60 years of age were associated with lower ICERs as proportions to GDP per capita. Greater proportions of populations non-susceptible at the start of vaccination programmes were associated with higher ICERs as proportions of GDP per capita. The proportions of populations non-susceptible at the start of vaccination programmes were estimated by fitting a dynamic transmission model in this study. In practice, decision-making based on this metric requires accurate serological surveys, which is particularly challenging to conduct in pandemic response settings.
There were missed opportunities in early- to mid-2021 for COVID-19 vaccination to save more lives in Africa. However, even with a late start, certain vaccine types and roll-out rates combinations may still generate relatively low ICERs. Two factors could further increase the economic value of vaccination programmes. First, our results are based on the known immune dynamics of SARS-CoV-2 and the performance of current COVID-19 vaccines. New vaccines with better effectiveness against emerging variants could decrease ICER by increasing health benefits. Emerging variants with the protection advantage of vaccine-induced immunity over infection-induced immunity may have the same effect.
Second, our results show that vaccine roll-out scenarios are much more likely to be cost-effective when using viral vector vaccines than mRNA vaccines. The relatively similar vaccine efficacy estimates against severe health outcomes (Table 1) and the drastically different vaccine unit costs (Table 2) may explain this. The main driver of vaccine unit costs is vaccine price. Some low-income countries may struggle to find COVID-19 vaccination programmes cost-effective after factoring in budget constraints and potential health opportunity costs. This highlights the importance of achieving even lower vaccine purchasing prices than those we assumed in this analysis. In this study, we estimated that the price of viral vector vaccines may be reduced by 10.32–28.69% and mRNA vaccines by 36.91-60.96% to reduce ICERs to 10–50% of GDP per capita (Additional File 1: Fig. S12).
We found a small number of countries where ICERs were small in relation to GDP per capita, yet the programmes were relatively unaffordable. The implication is that the investment in managing COVID-19, a new and leading cause of disease burden, may risk opportunity costs for existing public health and health service issues. Our affordability estimates are from a health sector perspective and have not explicitly accounted for the potential externalities on the local health systems and essential health services. Unless development partners provide subsidies that compensate for these effects, the perceived opportunity cost of a DALY, which is often approximated using a fraction of GDP per capita, may need to be further adjusted down from what we already know them for in low- and middle-income settings (which are low) [7, 8, 63, 66]. Affordability and ICERs in relation to GDP per capita are two of many metrics that stakeholders base their decisions on while planning for vaccine programmes.
This is the first study to examine the role of timing in the cost-effectiveness of COVID-19 vaccination programmes. We highlighted key issues to consider in the next pandemic (or next epidemic waves triggered by emerging variants of concern) to come. Timing has already been shown to influence the health impacts of vaccination programmes. Incorporating it into economic evaluation would lead to more accurate and realistic cost-effectiveness assessments [67, 68]. We fit a mathematical model with a range of plausible vaccine effect mechanisms to the COVID-19 epidemic histories in 27 African Union member states. Most existing modelling studies on the economic evaluation of COVID-19 vaccination strategies have either focused on a single country [69] or city [15] or used hypothetical epidemic histories that are not based on data from any country [70], which hinders the interpretability and generalisability of their results.
It is not the objective of this study to directly inform COVID-19 vaccine policies in individual countries. A regional analysis constrained us to use only data streams available in all countries under consideration. Thus, although we provided country-specific estimates in our GitHub repository, we caution national-level stakeholders against interpreting these results out of the regional context. Evidence for informing policies in individual countries may be generated by better-fitted transmission models (e.g. channelling more locally available data streams, such as more detailed non-pharmaceutical interventions and ICU occupancy) using the modelling framework we presented here (code available through our GitHub repository).
Our study has several limitations. First, our epidemic model has not captured the full complexity of the immune dynamics against SARS-CoV-2, which involves both vaccination and infections. For example, our model assumed that vaccinated individuals who had prior infection histories were completely protected against SARS-CoV-2. This design is intended to ensure that most individuals within the model receive a maximum of two doses of vaccine. However, we cannot capture a small number of breakthrough infections that may still happen. These tend to be mild cases and, thus, should not alter our results substantially [71]. Despite best efforts, there may be additional factors that may affect the epidemiology of COVID-19 that we have not implemented in this model (e.g. baseline prevalence of chronic health conditions). Second, we assumed the vaccine supply shortage was the only reason for slow uptake. In practice, uptake may also be slowed down by factors such as vaccine hesitancy [72]. The vaccine delivery unit costs may have been underestimated in this study as the vaccination programmes may require greater efforts in terms of social mobilisation. Third, we did not use observed costs of different vaccination programmes, as this data is not yet available. The normative approach (i.e. per-protocol) we took did not account for the resource wastes incurred in operations or the increased prices of scarce resources that may be associated with the pace of the roll-out, healthcare utilisation rate, or potential structural changes to local health systems as a result of COVID-19 pandemic response. As a result, ICERs may have been biased. Fourth, the cost-effectiveness analysis we presented is from a country’s perspective and does not consider COVID-19 transmission in nearby countries (e.g. transmission spillover). Finally, our analysis took on a health sector perspective and did not account for societal elements such as changes in productivity or the preservation of key functions (e.g. labour market, emergence response, healthcare, and education). These societal elements specific to the COVID-19 pandemic have been difficult to estimate and may vary substantially between countries—additional data and research on these elements could improve the comprehensiveness of vaccine policy evaluation in the future.
Conclusions
We assessed the impact of COVID-19 vaccination programmes’ timing and speed on the health benefits, cost-effectiveness, and relative affordability in 27 African countries. We found that earlier vaccination programmes led to greater health benefits and generated lower ICERs in relation to GDP per capita. Although fast vaccination programmes yielded the greatest health benefits, they did not always generate the lowest ICERs in relation to GDP per capita as they covered larger proportions of individuals who were less vulnerable. Lower vaccine purchasing costs and improved vaccine efficacies may improve the overall cost-effectiveness and affordability of COVID-19 vaccination programmes.
Availability of data and materials
We used publicly available aggregate data and provided appropriate citations. The CovidM modelling framework has been published previously and is available at https://github.com/nicholasdavies/covidm-alpha. All code used and country-specific intermediate results, original a GitHub repository, have been archived at Zenodo as Liu et al. (9). A CHEERS checklist (2022 version) is presented in Additional File 1: Table S10. CHEERS: Consolidated Health Economic Evaluation Reporting Standards.
Notes
In alphabetical order: People’s Democratic Republic of Algeria, Republic of Angola, Republic of Botswana, Republic of Cameroon, Democratic Republic of the Congo, Arab Republic of Egypt, Eswatini, Federal Democratic Republic of Ethiopia, Republic of Ghana, Republic of Kenya, Republic of Liberia, Libya, Republic of Madagascar, Republic of Malawi, Kingdom of Morocco, Republic of Mozambique, Republic of Namibia, Federal Republic of Nigeria, Republic of Rwanda, Republic of Senegal, Somali Republic, Republic of South Africa, Republic of The Sudan, Tunisian Republic, Republic of Uganda, Republic of Zambia, Republic of Zimbabwe.
Abbreviations
- Africa CDC:
-
Africa Centres for Disease Control and Prevention
- ARI:
-
Acute respiratory infection
- CHEERS:
-
Consolidated Health Economic Evaluation Reporting Standards
- DALY:
-
Disability-adjusted life year
- GDP:
-
Gross domestic product
- ICER:
-
Incremental cost-effectiveness ratio
- ICU:
-
Intensive care unit
- UK:
-
United Kingdom
- WHO:
-
World Health Organization
- YLDs:
-
Years lived with disability
- YLLs:
-
Years of life lost
References
Mathieu E, Ritchie H, Rodés-Guirao L, Appel C, Giattino C, Hasell J, et al. Coronavirus pandemic (COVID-19). Our World in Data. 2020;
WHO Regional Office for Africa. Less than 10% of African countries to hit key COVID-19 vaccination goal. WHO Regional Office for Africa. 2021;
World Health Organization. Achieving 70% COVID-19 immunization coverage by mid-2022. World Health Organization. 2021;
WHO Regional Office for Africa. Africa needs to ramp up COVID-19 vaccination six-fold. WHO Regional Office for Africa. 2022;
Africa CDC | African Union. Africa needs to vaccinate 70% of its population by the end of 2022 to have a chance of controlling the COVID19 pandemic. Africa CDC | African Union. 2021;
The African Academy of Sciences. Seroprevalence of SARS-CoV-2 in Africa - Policy Brief. The African Academy of Sciences. 2021;
Lomas J, Claxton K, Martin S, Soares M. Resolving the “cost-effective but unaffordable” paradox: estimating the health opportunity costs of nonmarginal budget impacts. Value Health. 2018;21(3):266–75.
Howdon DDH, Lomas JRS, Paulden M. Implications of nonmarginal budgetary impacts in health technology assessment: a conceptual model. Value Health. 2019;22(8):891–7.
Liu Y, Procter SR, Pearson CAB, Montero AM, Torres-Rueda S, Asfaw E, et al. Archived: yangclaraliu/covid_vac_africa. Zenodo: https://doi.org/10.5281/zenodo.7618749; 2023.
World Health Organization. WHO SAGE Roadmap for prioritizing uses of COVID-19 vaccines: an approach to optimize the global impact of COVID-19 vaccines, based on public health goals, global and national equity, and vaccine access and coverage scenarios. World Health Organization; 2022.
Gooding K, Webster J, Wiafe N, Kumar V. Real-time assessment of UNICEF’s ongoing response to COVID-19 in eastern and southern Africa. Oxford Policy Management; 2021.
Robinson E, Jones A, Lesser I, Daly M. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39(15):2024–34.
Hale T, Angrist N, Goldszmidt R, Kira B, Petherick A, Phillips T, et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat Hum Behav. 2021;5(4):529–38.
Liu Y, Pearson CAB, Sandmann FG, Barnard RC, Kim JH, CMMID COVID-19 Working Group, et al. Dosing interval strategies for two-dose COVID-19 vaccination in 13 middle-income countries of Europe: Health impact modelling and benefit-risk analysis. Lancet Reg Health Eur. 2022;17:100381.
Pearson CAB, Bozzani F, Procter SR, Davies NG, Huda M, Jensen HT, et al. COVID-19 vaccination in Sindh Province, Pakistan: a modelling study of health impact and cost-effectiveness. PLoS Med. 2021;18(10): e1003815.
Barnard RC, Davies NG, Centre for Mathematical Modelling of Infectious Diseases COVID-19 working group, Jit M, Edmunds WJ. Modelling the medium-term dynamics of SARS-CoV-2 transmission in England in the Omicron era. Nat Commun. 2022;13(1):4879.
Davies NG, Klepac P, Liu Y, Prem K, Jit M, CMMID COVID-19 working group, et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205–11.
Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol. 2020;35(12):1123–38.
Salje H, Tran Kiem C, Lefrancq N, Courtejoie N, Bosetti P, Paireau J, et al. Estimating the burden of SARS-CoV-2 in France. Science. 2020;369(6500):208–11.
Prem K, Zandvoort KV, Klepac P, Eggo RM, Davies NG, Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group, et al. Projecting contact matrices in 177 geographical regions: an update and comparison with empirical data for the COVID-19 era. PLoS Comput Biol. 2021;17(7):e1009098.
Krammer F, Srivastava K, Alshammary H, Amoako AA, Awawda MH, Beach KF, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384(14):1372–4.
Hall VJ, Foulkes S, Charlett A, Atti A, Monk EJM, Simmons R, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative healthcare workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet. 2021;397(10283):1459–69.
Liu Y, Sandmann FG, Barnard RC, Pearson CAB, Pastore R, Pebody R, et al. Optimising health and economic impacts of COVID-19 vaccine prioritisation strategies in the WHO European Region: a mathematical modelling study. Lancet Reg Health Eur. 2022;12: 100267.
Google Inc. COVID-19 Community Mobility Reports (2020–2022). Google Inc.: https://www.google.com/covid19/mobility/; 2022.
Davies NG, Barnard RC, Jarvis CI, Russell TW, Semple MG, Jit M, et al. Association of tiered restrictions and a second lockdown with COVID-19 deaths and hospital admissions in England: a modelling study. Lancet Infect Dis. 2021;21(4):482–92.
United Nations Department of Economic and Social Affairs, Population Division. 2019 World Population Prospects. United Nations: https://population.un.org/wpp; 2019.
Davies NG, Kucharski AJ, Eggo RM, Gimma A, Edmunds WJ. Centre for the Mathematical Modelling of Infectious Diseases COVID-19 working group. effects of non-pharmaceutical interventions on COVID-19 cases, deaths, and demand for hospital services in the UK: a modelling study. Lancet Public Health. 2020;5(7):e375-85.
Bi Q, Wu Y, Mei S, Ye C, Zou X, Zhang Z, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(8):911–9.
Linton NM, Kobayashi T, Yang Y, Hayashi K, Akhmetzhanov AR, Jung SM, et al. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J Clin Med. 2020;9(2):538.
Nishiura H, Linton NM, Akhmetzhanov AR. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis. 2020;93:284–6.
Torres-Rueda S, Sweeney S, Bozzani F, Naylor NR, Baker T, Pearson C, et al. Stark choices: exploring health sector costs of policy responses to COVID-19 in low-income and middle-income countries. BMJ Glob Health. 2021;6(12):e005759.
World Bank. GDP per capita (current US$) | Data. World Bank: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD; 2022.
World Bank. Domestic general government health expenditure per capita (current US$) | Data. World Bank: https://data.worldbank.org/indicator/SH.XPD.GHED.PC.CD; 2022.
World Bank Data Help Desk. World Bank Country and Lending Groups. World Bank: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups; 2022.
World Health Organization. The Pfizer BioNTech (BNT162b2) COVID-19 vaccine: what you need to know. World Health Organization. 2022;
UK Health Security Agency. 14a. Guidance COVID-19: the Green Book. UK Health Security Agency; 2022.
Pearson CAB, Silal SP, Li MWZ, Dushoff J, Bolker BM, Abbott S, et al. Bounding the levels of transmissibility & immune evasion of the Omicron variant in South Africa. medRxiv. 2021;
Grint DJ, Wing K, Houlihan C, Gibbs HP, Evans SJW, Williamson E, et al. Severity of severe acute respiratory system coronavirus 2 (SARS-CoV-2) Alpha variant (B.1.1.7) in England. Clin Infect Dis. 2022;75(1):e1120-7.
Public Health England. SARS-CoV-2 variants of concern and variants under investigation in England - Technical Briefing 14. Public Health England; 2021.
UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England - Technical briefing: update on hospitalisation and vaccine effectiveness for Omicron VOC-21NOV-01 (B.1.1.529) . UK Health Security Agency; 2021.
Iuliano AD, Brunkard JM, Boehmer TK, Peterson E, Adjei S, Binder AM, et al. Trends in disease severity and health care utilization during the early omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146–52.
Pouwels KB, Pritchard E, Matthews PC, Stoesser N, Eyre DW, Vihta K-D, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021;27(12):2127–35.
UK Health Security Agency. COVID-19 vaccine surveillance report Week 2. UK Health Security Agency; 2022
Liu Y, CMMID COVID-19 working group, Funk S, Flasche S. The contribution of pre-symptomatic infection to the transmission dynamics of COVID-2019 [version; peer review: 3 approved]. Wellcome Open Res. 2020;
Mullen K, Ardia D, Gil D, Windover D, Cline J. deoptim: an r package for global optimization by differential evolution. J Stat Softw. 2011;40(6):1–26.
UK Health Security Agency. COVID-19 vaccine surveillance report Week 12. UK Health Security Agency; 2022.
World Health Organization. Guide for standardization of economic evaluations of immunization programmes. World Health Organization; 2019.
Leclerc QJ, Fuller NM, Keogh RH, Diaz-Ordaz K, Sekula R, Semple MG, et al. Importance of patient bed pathways and length of stay differences in predicting COVID-19 hospital bed occupancy in England. BMC Health Serv Res. 2021;21(1):566.
Wyper GMA, Assunção RMA, Colzani E, Grant I, Haagsma JA, Lagerweij G, et al. Burden of Disease methods: a guide to calculate COVID-19 disability-adjusted life years. Int J Public Health. 2021;66: 619011.
Salomon JA, Haagsma JA, Davis A, de Noordhout CM, Polinder S, Havelaar AH, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015;3(11):e712–23.
Haagsma JA, Maertens de Noordhout C, Polinder S, Vos T, Havelaar AH, Cassini A, et al. Assessing disability weights based on the responses of 30,660 people from four European countries. Popul Health Metr. 2015;13:10.
Griffiths U, Adjagba A, Attaran M, Hutubessy R, Van de Maele N, Yeung K, et al. Costs of delivering COVID-19 vaccine in 92 AMC countries Updated estimates from COVAX Working Group on delivery costs. World Health Organization; 2021.
Vassall A, Sweeney S, Kahn J, Gomez Guillen G, Bollinger L, Marseille E, et al. Reference case for estimating the costs of global health services and interventions. Global Health Cost Consortium; 2017.
Global Health Centre. COVID-19 vaccine access. Geneva Graduate Institute; 2022.
Nonvignon J, Owusu R, Asare B, Adjagba A, Aun YW, Yeung KHT, et al. Estimating the cost of COVID-19 vaccine deployment and introduction in Ghana using the CVIC tool. Vaccine. 2022;40(12):1879–87.
UNICEF Supply Division. COVID-19 Market Dashboard. UNICEF: https://www.unicef.org/supply/covid-19-market-dashboard; 2022.
Turner HC, Lauer JA, Tran BX, Teerawattananon Y, Jit M. Adjusting for inflation and currency changes within health economic studies. Value Health. 2019;22(9):1026–32.
World Bank. World Bank Open Data. World Bank: https://data.worldbank.org/; 2022.
Serje J, Bertram MY, Brindley C, Lauer JA. Global health worker salary estimates: an econometric analysis of global earnings data. Cost Eff Resour Alloc. 2018;16:10.
Maina J, Ouma PO, Macharia PM, Alegana VA, Mitto B, Fall IS, et al. A spatial database of health facilities managed by the public health sector in sub Saharan Africa. Sci Data. 2019;6(1):134.
Federal Reserve Economic Data. Gross domestic product: implicit price deflator. Federal Reserve : https://fred.stlouisfed.org/series/GDPDEF; 2022.
Ochalek J, Lomas J, Claxton K. Estimating health opportunity costs in low-income and middle-income countries: a novel approach and evidence from cross-country data. BMJ Glob Health. 2018;3(6): e000964.
Ochalek J, Claxton K, Lomas J, Thompson KM. Valuing health outcomes: developing better defaults based on health opportunity costs. Expert Rev Pharmacoecon Outcomes Res. 2021;21(4):729–36.
Cameron D, Ubels J, Norström F. On what basis are medical cost-effectiveness thresholds set? Clashing opinions and an absence of data: a systematic review. Glob Health Action. 2018;11(1):1447828.
Leech AA, Kim DD, Cohen JT, Neumann PJ. Use and misuse of cost-effectiveness analysis thresholds in low- and middle-income countries: trends in cost-per-DALY studies. Value Health. 2018;21(7):759–61.
Woods B, Revill P, Sculpher M, Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19(8):929–35.
van de Wetering G, Woertman WH, Adang EM. Time to incorporate time in cost-effectiveness analysis. Eur J Health Econ. 2012;13(3):223–6.
Haghpanah F, Lin G, Levin SA, Klein E. Analysis of the potential impact of durability, timing, and transmission blocking of COVID-19 vaccine on morbidity and mortality. EClinicalMedicine. 2021;35: 100863.
Debrabant K, Grønbæk L, Kronborg C. The cost-effectiveness of a COVID-19 vaccine in a Danish context. Clin Drug Investig. 2021;41(11):975–88.
Siedner MJ, Alba C, Fitzmaurice KP, Gilbert RF, Scott JA, Shebl FM, et al. Cost-effectiveness of coronavirus disease 2019 vaccination in low- and middle-income countries. J Infect Dis. 2022;226(11):1887–96.
Hall V, Foulkes S, Insalata F, Kirwan P, Saei A, Atti A, et al. Protection against SARS-CoV-2 after COVID-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207–20.
Orangi S, Pinchoff J, Mwanga D, Abuya T, Hamaluba M, Warimwe G, et al. Assessing the level and determinants of COVID-19 vaccine confidence in Kenya. Vaccines (Basel). 2021;9(8):936.
Acknowledgements
The authors acknowledge the contribution of Dr Nicholas G. Davies and Dr Rosanna C. Barnard to developing the general CovidM framework. The authors thank the reviewers for helping us improve this work.
Funding
The contributions of YL, SRP, CABP, AMM, ST-R, EA, BSC (Uzochukwu), TD, EB, RME, FR, JN, MJ, and AN are supported by the International Decision Support Initiative, which is funded by the Bill and Melinda Gates Foundation (OPP1202541). The contributions of CABP is supported by the World Health Organization. MJ has received funding from the European Union’s Horizon 2020 research and innovation programme—project EpiPose (Grant agreement number 101003688); MJ and RME have received funding from the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Modelling and Health Economics at Imperial College and LSHTM in partnership with UKHSA. The European Commission is not responsible for any use that may be made of the information it contains. The views expressed are those of the author(s) and not necessarily those of the National Health Service (UK) (NHS), the NIHR, the Department of Health, or UKHSA. The contribution of RME is also supported by the Health Data Research UK (HDR UK) (grant: MR/S003975/1). YL has also received funding from the Bill and Melinda Gates Foundation via grant INV-003174.
Author information
Authors and Affiliations
Contributions
YL, NN, JN, MJ, AV, FR, RME, and TD conceived the idea. YL, JN, MJ, and AV designed the study. YL, SRP, AMM, ST-R, BSC, EB, EA, and FR collected the data. YL, SRP, CABP, AMM, and ST-R analysed the data, built the models, and conducted the analysis. YL conducted the literature search and visualised the data and results. YL and SRP wrote the manuscript. All co-authors have contributed to interpreting the results, reviewing, and providing feedback to the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Our cost estimation and epidemiological modelling were based on publicly available data sources as well as validation from the authors and therefore did not require ethics approval. The use and collection of data on Nigeria was based on a wider health technology assessment exercise of COVID-19 vaccination which was approved by the University of Nigeria Teaching Hospital Research Ethics Committee (Ref: NHREC/05/01/2008B-FWA00002458-1RB00002323).
Consent for publication
Not applicable. This manuscript does not contain any individual person’s data in any form.
Competing interests
One of the senior authors of this manuscript, Prof. Mark Jit (who contributed equally with Dr Justice Nonvignon and Prof. Anna Vassall), is an editorial board member of the BMC Medicine for his expertise in Health Economics, Quality and Reporting.
We declare no additional competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Figures: Figure S1.
Vaccine roll-out trajectories by age group. Figure S2. Diagram of Transmission Model Structure. Figure S3. Countries included and excluded from this analysis. Figure S4-6. Performance of model fitting process, Part 1-3 (in alphabetical order). Figure S7. Fitted parameters. Figure S8-10. Health Outcomes Associated with Vaccine Roll-out Scenarios (by vaccine type and by health outcome). Figure S11. The association between the proportion of DALYs averted attributable to older adults and the performance of medium and fast scenarios. Figure S11. Effect sizes estimated in the multivariable regression model linking country characteristics to ICERs as proportions of GDP per capita. Figure S12. Target vaccine price under different perceived cost-effectiveness thresholds. Supplemental Tables: Table S1. Model Equations. Table S2. Epidemic and Healthcare Process Parameters. Table S3. Additional Data Sources. Table S4. Other vaccine and vaccination programme characteristics. Table S5. Variants of Concern Introduction. Table S6. List of countries with fitted models. Table S7. Itemised cost per dose per activity for base countries - viral vector / AstraZeneca-like vaccine. Table S8. Itemised Cost per dose per activity for base countries - mRNA / Pfizer-like vaccine. Table S9. Cost per dose for countries with fitted models. Table S10. CHEERS 2022 Checklist. Supplemental Methods: Methods S1. Further Model Descriptions. Methods S2. Fitting Process. Methods S3. Characterising behavioural change using data on non-pharmaceutical intervention and mobility. Methods S4. Calculating COVID-19 severe, critical, and death cases. Methods S5. Lengths of Stay (LoSs). Methods S6. Calculating Disability-adjusted Life Years (DALYs). Methods S7. Estimating Vaccine Delivery Costs. Methods S8. Extrapolating unit costs from base countries to other countries in Africa. Methods S9. Extrapolating vaccine unit costs for different roll-out scenarios. Methods S10. ICERs and Proportions of DALYs averted by those above 60 years.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Y., Procter, S.R., Pearson, C.A.B. et al. Assessing the impacts of COVID-19 vaccination programme’s timing and speed on health benefits, cost-effectiveness, and relative affordability in 27 African countries. BMC Med 21, 85 (2023). https://doi.org/10.1186/s12916-023-02784-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-023-02784-z