Abstract
Background
The identification of effective dementia prevention strategies is a major public health priority, due to the enormous and growing societal cost of this condition. Consumption of a Mediterranean diet (MedDiet) has been proposed to reduce dementia risk. However, current evidence is inconclusive and is typically derived from small cohorts with limited dementia cases. Additionally, few studies have explored the interaction between diet and genetic risk of dementia.
Methods
We used Cox proportional hazard regression models to explore the associations between MedDiet adherence, defined using two different scores (Mediterranean Diet Adherence Screener [MEDAS] continuous and Mediterranean diet Pyramid [PYRAMID] scores), and incident all-cause dementia risk in 60,298 participants from UK Biobank, followed for an average 9.1 years. The interaction between diet and polygenic risk for dementia was also tested.
Results
Higher MedDiet adherence was associated with lower dementia risk (MEDAS continuous: HR = 0.77, 95% CI = 0.65–0.91; PYRAMID: HR = 0.86, 95% CI = 0.73–1.02 for highest versus lowest tertiles). There was no significant interaction between MedDiet adherence defined by the MEDAS continuous and PYRAMID scores and polygenic risk for dementia.
Conclusions
Higher adherence to a MedDiet was associated with lower dementia risk, independent of genetic risk, underlining the importance of diet in dementia prevention interventions.
Similar content being viewed by others
Background
Preventing dementia is a global public health priority due to the enormous and growing societal cost of this condition [1]. A key strategy to reduce dementia incidence is the identification of modifiable risk factors that can be targeted by personalized or public health interventions. These modifiable risk factors, in combination with genetic risk, play a key role in determining individual risk of Alzheimer’s disease and other forms of dementia [2,3,4]. Diet is an important modifiable risk factor for dementia that could be targeted for disease prevention and risk reduction [5, 6]. Healthier dietary patterns, especially the Mediterranean diet (MedDiet), have been proposed as a strategy to reduce dementia risk [7, 8]. Recent systematic [9] and umbrella [10] reviews have suggested higher adherence to the MedDiet may reduce cognitive decline, although evidence for a protective role of the MedDiet against dementia risk is inconsistent [11,12,13,14,15,16]. As most prior studies have been conducted in relatively small cohorts (n = 1000–6000) with limited numbers of dementia cases (n = 20–400), additional investigations which leverage large population-based cohorts are warranted. There is also currently no gold standard assessment of MedDiet adherence, and some variability in study findings may therefore be due to different dietary assessment methods and scoring systems [17]. Therefore, studies comparing different MedDiet scores directly and their associations with dementia risk are needed.
A healthy diet might also mitigate individual genetic risk for dementia. Previous studies exploring gene-diet interactions are limited, have reported inconsistent results, and, typically, focus on APOE genotype as the sole measure of genetic risk [13, 18,19,20]. Polygenic risk scores combining information from multiple weighted (i.e., according to the strength of their association with dementia) risk alleles predict incident all-cause dementia [21, 22] and are an important advance in facilitating in-depth exploration of potential gene-diet interactions.
The purpose of this study was to investigate associations between MedDiet adherence and dementia incidence in a large prospective cohort study, and to explore the interaction between diet and genetic risk for dementia.
Methods
Study population and design
The UK Biobank is an ongoing, multi-centre prospective cohort study of over half a million participants, that provides a resource for investigating the determinants of disease in middle and older age [23]. The design and methods of this study have been described elsewhere [24]. Briefly, between 2006 and 2010, men and women aged 40–69 years were recruited from across England, Scotland and Wales using National Health Service (NHS) patient registers. Participants attended one of 22 assessment centres where they completed a touchscreen questionnaire, verbal interview, and provided measures of physical function alongside biological samples. Subsequently, participants were invited to complete additional measures, including enhanced dietary assessments, imaging, and assessment of multiple health-related outcomes. UK Biobank also includes linkage to electronic healthcare records (death, cancer, inpatient and primary care records) for disease ascertainment. Ethical approval for the UK Biobank study was provided by the North West–Haydock Research Ethics Committee (REC reference: 16/NW/0274), and all participants provided electronic signed consent. The current study included participants who self-reported a racial/ ethnic background of white British, Irish or other white, were aged ≥ 60 years at recruitment with genetic data, appropriate dietary data (self-reported atypical dietary reports were excluded) and were not missing data for any of the included covariates (Additional file 1, Fig. S1).
Dietary assessment and calculation of Mediterranean diet scores
The Oxford WebQ is a web-based, self-administered 24-h dietary assessment tool, validated for use in large-scale observational studies [25, 26]. This tool collects information about the consumption of 206 types of foods and 32 types of drinks during the previous 24-h period, with participants selecting the number of standard portions for each item that they consumed. Participants recruited between April 2009 and September 2010 completed the Oxford WebQ as part of their baseline assessment centre visits. In addition, between February 2011 and June 2012, participants were invited to complete the Oxford WebQ assessment via their home computer every three to four months, up to a total of five assessments (including the baseline assessment). Consistent with previous investigations [17, 27], we energy-adjusted the dietary data (2000 kcal/d) for each time point via the residuals method to allow evaluation of diet quality independent of diet quantity [28]. Data were then averaged across all available time points (minimum 1, maximum 5) for each participant prior to calculation of MedDiet scores.
We quantified MedDiet adherence using two separate scores: the MedDiet Adherence Screener (MEDAS) score, and the MedDiet PYRAMID score. These scores define MedDiet adherence in different ways (e.g., using different dietary targets and food components) and therefore may differ in terms of their association with dementia.
MEDAS score
The MEDAS is a 14-point score developed as part of the Prevención con Dieta Mediterránea (PREDIMED) trial [29] that has been used widely in trials and observational studies [8, 30]. The MEDAS has been validated for use in the UK (the UK-validated version of the MEDAS was used to develop our MEDAS scores in this study) [31] and endorsed for use as a rapid diet assessment screening tool in clinical practice by the American Heart Association [32]. The MEDAS is conventionally calculated with a binary evaluation for each of the 14 food components, with one point awarded if the participant’s consumption meets a pre-defined cut-off (e.g., intake of a specific amount of vegetables), and zero points if they do not. The total possible score ranges from 0–14 points. We have shown previously that using the same dietary targets but implementing an alternative continuous scoring system using linear equation principles (y = ax + b, in which y is the number of points scored between 0 and 1, a is the slope and b is the intercept), in which points are awarded between zero and one depending upon proximity to the dietary targets, increases the sensitivity of this score in detecting differences in diet quality [17]. Therefore, this score, referred to here as the MEDAS continuous score, was used for the primary analyses in the present study. As a hypothetical example to illustrate the difference between the MEDAS and MEDAS continuous scores, an individual with a daily vegetable intake of 295 g or ~ 1.5 * 200 g servings of vegetables would be awarded 0 points for this specific MedDiet component for the MEDAS score, as they have not achieved the dietary target of 2 servings (i.e., 400 g) vegetable intake per day. By contrast, according to the MEDAS continuous score, this individual would be awarded ~ 0.74 points (y = 0.5 * 1.475 + 0 = 0.7375 points), based around how close they are to the specific dietary target (i.e., ~ 3/4 of the way towards achieving the dietary target). We repeated the analysis using the conventionally-scored MEDAS as a sensitivity analysis.
Both the MEDAS and MEDAS continuous scores award points for use of olive oil as the main culinary fat and, separately, for consumption of a target amount (4 or more tablespoons per day) of olive oil. Although we were able to determine use of olive oil as a culinary fat and to award points for consumption (1 point) or non-consumption (0 points) accordingly, it was not possible to determine the amount of olive oil consumed from the available dietary data, limiting the maximum possible scores for the MEDAS and MEDAS continuous to 13 points in this study.
PYRAMID score
The PYRAMID score is a 15-point MedDiet score used widely in epidemiological studies [9, 17, 27]. Each of the 15 individual components are coded on a continuous basis with scores ranging from zero to one (26). Further details of the calculation of each MedDiet score is provided in Additional file 1, Tables S1 and S2. For both MedDiet scores, higher values reflect greater adherence to the MedDiet.
Polygenic risk score
To estimate genetic risk of dementia, we used the polygenic risk score developed by Lourida and colleagues, who demonstrated that higher values of this score are associated with higher all-cause dementia risk in the UK Biobank cohort [22]. The score was based on a genome-wide association study of individuals of European ancestry [33]. Therefore, the current analysis was restricted to individuals who self-reported a racial/ ethnic background of white British, Irish or other white (who constitute > 90% of the UK Biobank cohort). For the primary analyses, the polygenic risk score was divided into quintiles, and participants were categorised into low (quintile 1), medium (quintiles 2–4) and high (quintile 5) risk groups. A total of 249,273 independent genetic variants were used to create the polygenic risk score. Further details of the polygenic risk score creation and this approach can be found elsewhere [22].
Dementia outcome ascertainment
All-cause incident dementia cases were ascertained using data linkage to hospital inpatient records and death registries. Diagnoses were recorded using the International Classification of Diseases (ICD) coding system [34]. Participants with a primary or secondary diagnosis of dementia were identified from hospital records or underlying/contributory cause of death from death registries using relevant ICD-9 and ICD-10 codes (Additional file 1, Table S3.). We used the censoring dates recommended by UK Biobank for death data and hospital inpatient data. These are the dates up to which the data is estimated to be over 90% complete in England, Scotland and Wales separately. At the time of analysis, the recommended censoring dates were 31st March, 2021 for England and Scotland, and 28th February, 2018 for Wales. The mean (SD) and median (interquartile range) follow up was 9.1 (1.7) and 9.3 (8.8–9.7) years, respectively. Follow up time was calculated from the most recent eligible dietary report used for MedDiet score creation and either the date of first dementia diagnosis, death, loss to follow-up, or censoring date, whichever was the earliest.
Statistical analysis
All analyses were conducted in SPSS version 27. Baseline characteristics of the analytic sample, stratified by dementia status, were summarised as mean ± SD for continuous variables and as percentages for categorical variables. Cox proportional hazard regression models were used to examine the association between MedDiet adherence and time to incident all-cause dementia, with the duration of follow-up in years used as the timescale. We also explored the association between the polygenic risk score and dementia incidence, to confirm the previously reported associations between these variables in this cohort [22]. The possible interaction between MedDiet adherence and polygenic risk for dementia was investigated by including an interaction term, with both variables expressed continuously.
Analyses were adjusted simultaneously for: age, sex, socioeconomic status (Townsend Index categorised as low [quintile 1], moderate [quintiles 2–4], high [quintile 5] deprivation), education (higher [college/university/other professional qualification], vocational [NVQ/HND/HNC], upper secondary [A-levels], lower secondary [O-levels/GCSEs /CSEs] or none), smoking status (never, past, current), typical sleep duration (< 7, 7–8, > 8 h), physical activity (international physical activity questionnaire [IPAQ] group, categorised as low, medium, high), energy intake (kcal/d), third-degree relatedness of individuals in the sample, and the first 20 principal components of ancestry. Models which included the polygenic risk score were additionally adjusted for the number of alleles included in the score, to account for SNP-level variation [22]
Sensitivity analyses
Sensitivity analyses were performed to test the robustness of associations between MedDiet adherence and dementia incidence. First, we used the conventional binary MEDAS score. Secondly, we included participants with a minimum of two, 24-h diet recalls to provide a more stringent measure of habitual dietary intake [26]. Thirdly, we excluded participants with 24-h recalls with extreme energy intakes (defined as < 800 or > 4200 kcal/d for males and < 600 or > 3500 kcal/d for females) [35]. Fourth, to assess whether any individual components of the MedDiet drove the observed associations, we repeated the analyses after sequentially removing each MedDiet component from the total score. Fifth, in consideration of the potential for reverse causality, we repeated the primary analyses after excluding participants with less than 2- and less than 5-years of follow-up, respectively. Sixth, we repeated the analyses including potential mediators individually; stroke history (yes/no for any type of stroke diagnosed prior to dementia diagnosis or end of follow-up for those who remained dementia-free), self-reported depressive symptoms (yes/no for reporting feeling down/depressed/hopeless on ‘several days’, ‘more than half the days’ or ‘nearly every day’), and body mass index (BMI) category (< 25, 25–29.9, > 30 kg/m2). Seventh, as an alternative method of exploring whether associations between MedDiet adherence and dementia risk were influenced by polygenic risk score, we conducted stratified analyses exploring associations between MedDiet adherence and dementia risk in low, medium and high genetic risk categories. Eighth, we investigated the interaction between MedDiet adherence and genetic risk, with genetic risk defined by Apolipoprotein E (APOE) genotype only (a more common but less comprehensive measure of genetic risk, which may be easier to apply in clinical practice). APOE ε4 carriers were defined as higher risk, whilst non-carriers were defined as lower risk. Nineth, to evaluate the influence of missing data, we repeated analyses following imputation of missing dietary and covariate data using multiple imputations by chained Eqs. (70 imputations, 20 iterations) [36]. We included all analytic variables (covariates and outcome data) as predictors in the model. In addition, we created abbreviated MedDiet scores using dietary data from the UK Biobank touchscreen questionnaire (data available for all participants) which were used as auxiliary variables in the imputation model. Tenth, we carried out separate analyses for fatal and non-fatal cases of dementia. Eleventh, we conducted stratified analyses in individuals with higher (college/university/other professional qualification) and lower (vocational, upper secondary, lower secondary, and none) education levels.
Results
Cohort characteristics
A total of 502,536 participants underwent baseline assessment as part of the UK Biobank study, of whom 60,298 participants were included in this analysis (See Additional file 1, Fig. S1 for the study inclusion flow diagram). Baseline characteristics of the participants, stratified by level of MedDiet adherence (low, medium, and high MEDAS continuous scores), are provided in Table 1. During a mean (SD) follow up of 9.1 (1.7) years and a total of 549,999 person years, there were 882 cases of incident all-cause dementia. Participants with a higher MedDiet adherence according to the MEDAS continuous score were more likely to be female, have a BMI within the healthy range (< 25 kg/m2), have a higher educational level, and be more physically active than those with lower MedDiet adherence. The mean MEDAS continuous and PYRAMID scores in this cohort were 6.1 ± 1.7 and 7.5 ± 1.8, respectively.
Mediterranean diet adherence and risk of incident dementia
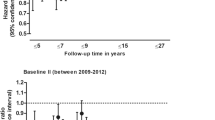
Higher adherence to the MedDiet was associated with 4.2–6.9% lower risk for dementia for the MEDAS continuous (HR per one point increase in MedDiet score: 0.931; 95% CI: 0.895–0.969; p < 0.001) and PYRAMID (HR per one point increase in MedDiet score: 0.958; 95% CI: 0.922–0.996; p = 0.031) scores. When divided into tertiles, relative to low MedDiet, high but not moderate adherence was associated with lower dementia risk (Fig. 1).
The absolute risk of dementia in the low, medium and high MedDiet adherence groups defined by the MEDAS continuous score was 1.73%, 1.50% and 1.18%, respectively. Moderate and high MedDiet adherence groups had 0.23% (95% CI: -0.01–0.49%) and 0.55% (95% CI: 0.32–0.79%) lower absolute risk of dementia than those in the low MedDiet adherence group. Meanwhile, the absolute risk of dementia in the low, medium and high MedDiet adherence groups defined by the PYRAMID score was 1.67%, 1.53%, and 1.21%, respectively. Moderate and high MedDiet adherence groups had 0.14% (95% CI: -0.1–0.39%) and 0.46% (95% CI: 0.23–0.69%) lower absolute risk of dementia than those in the low MedDiet adherence group.
Mediterranean diet adherence, genetic risk and dementia incidence
A higher polygenic risk score was associated with greater risk for dementia (HR: 1.224, 95% CI: 1.102–1.360; p < 0.001). There was no significant interaction between polygenic risk for dementia and MedDiet adherence defined by the MEDAS continuous (HR: 1.036, 95% CI: 0.977–1.076; p = 0.070) or PYRAMID (HR: 1.011; 95% CI: 0.974–1.049; p = 0.572) scores.
Sensitivity analyses
The associations between high MedDiet adherence and lower dementia risk were robust to a range of sensitivity analyses. When we used the conventional (i.e., in which points for each MedDiet component are awarded on a binary basis) rather than continuous (i.e., in which points for each MedDiet component are awarded between 0 and 1 based on proximity to the dietary targets) MEDAS score, there was a similar, albeit slightly attenuated, association between MedDiet adherence and dementia risk. Specifically, each one-point increase in MEDAS score was associated with 4.5% lower risk of dementia (HR: 0.955; 95% CI: 0.918–0.993; p = 0.021) and, when split into tertiles, high (HR: 0.783, 95% CI: 0.651–0.943, p = 0.001) but not moderate (HR: 1.023, 95% CI: 0.873–1.199, p = 0.775) MedDiet adherence was associated with lower dementia risk versus low MedDiet adherence (Fig. 2). Results were similar when we repeated analyses for participants with a minimum of 2 dietary reports (Fig. 2 and Additional file 1, Table S4) and after excluding participants with extreme energy intakes (Fig. 2 and Additional file 1, Table S5). In analyses where MedDiet scores were derived after sequential removal of individual dietary components, the associations remained reasonably stable (Fig. 3 and Additional file 1, Table S6 and S7). Higher MedDiet adherence was associated with lower dementia risk when we repeated analyses after removing participants with less than two and less than five years of follow up to minimise risk of reverse causality (Fig. 2 and Additional file 1, Table S8), and when adjusting for potential mediators (BMI, history of depression, or stroke; Fig. 2 and Additional file 1, Table S9).
Association between MedDiet adherence and risk of dementia in sub-group and sensitivity analyses. MedDiet adherence level was split into tertiles, with the dashed line reflecting the low MedDiet adherence reference group for each MedDiet score. Analyses include: A) Primary analyses for the MEDAS, MEDAS continuous and PYRAMID scores (n = 60,298, including 882 dementia cases); B) Including participants with a minimum of 2 dietary reports (n = 38,794, including 479 dementia cases); C) After excluding participants with extreme energy intakes (n = 59,627, including 867 dementia cases); D) Excluding participants with less than 2 years (n = 59,594, including 843 dementia cases) and 5 years (n = 58,196, including 698 dementia cases) follow up; E) Adjusting for potential mediators, including BMI (n = 60,163, including 876 dementia cases), history of depression (n = 58,837, including 851 dementia cases), or stroke (n = 60,298, including 882 dementia cases); F) Stratified into low (n = 21,009, including 261 dementia cases), medium (n = 20,000, including 313 dementia cases) and high (n = 19,273, including 308 dementia cases) genetic risk categories; G) Stratified into APOE4 carriers (n = 16,644, including 467 dementia cases) and non-carriers (n = 43,651, including 415 dementia cases); H) With imputed missing data (n = 196,335, including 5001 dementia cases); I) Restricted to fatal (n = 59,627, including 260 dementia cases) and non-fatal (n = 60,038, including 622 dementia cases) dementia cases; and J) Stratified into higher (n = 33,281, including 430 dementia cases) and lower (n = 27,007, including 452 dementia cases) education status groups
Association between MedDiet adherence defined by the MEDAS (A), MEDAS continuous (B) and PYRAMID (C) scores and risk of dementia (n = 60,298, including 882 dementia cases) after sequentially removing each MedDiet component from the total score. Hazard ratios and 95% CIs were estimated per point increased in MedDiet score
When we repeated the analyses exploring the interaction between the MedDiet adherence and polygenic risk for dementia using the conventional MEDAS score we found a significant interaction (HR: 1.042, 95% CI: 1.003–1.082; p = 0.035). When analyses were stratified by polygenic risk category, higher MedDiet adherence according to the MEDAS continuous scores was associated with lower dementia incidence in individuals in the lower genetic risk category only (Fig. 2 and Additional file 1, Table S10). When we repeated the analysis using the conventional MEDAS score coded on a binary basis, similar results were observed. In addition, in individuals in the higher genetic risk category, moderate MedDiet adherence according to the conventional MEDAS score was associated with higher risk for dementia (Fig. 2 and Additional file 1, Table S10). When we explored the interaction between MedDiet adherence and genetic risk defined by APOE genotype, no diet-gene interactions were observed (MEDAS continuous HR: 1.035; 95% CI: 0.958–1.118; p = 0.386; MEDAS (binary coding) HR: 0.985; 95% CI: 0.913–1.064; p = 0.706; PYRAMID HR: 1.054; 95% CI: 0.978–1.136; p = 0.167). Likewise, when analyses were stratified by APOE genotype, there was a similar pattern of response (i.e., higher MedDiet adherence was associated with lower HRs) in APOE ε4 carriers/non-carriers (Fig. 2 and Additional file 1, Table S11). Similar associations were observed when we imputed missing data (Fig. 2 and Additional file 1, Table S12), for fatal and non-fatal dementia cases (Fig. 2 and Additional file 1, Table S13), and when analyses were stratified by education status (Fig. 2 and Additional file 1, Table S14).
Discussion
Using data from over 60,000 participants, we demonstrated that higher adherence to the MedDiet is associated with lower risk of incident all-cause dementia. Specifically, participants with the highest MedDiet adherence had 23% lower risk of developing dementia in comparison with those with the lowest level of adherence (highest vs. lowest MEDAS continuous tertiles), which was equivalent to an absolute risk difference (reduction) of 0.55%. We found no significant interaction between MedDiet adherence, defined by both the MEDAS continuous and PYRAMID scores, and polygenic risk for dementia. In addition, we found that a continuous MEDAS score was a more sensitive predictor of dementia risk when compared with a binary MEDAS or PYRAMID scores.
Previous studies exploring associations between MedDiet adherence and dementia risk have produced inconsistent findings. Indeed, a systematic review by Limongi and colleagues [9] reported lower risk of Alzheimer’s disease and all-cause dementia in four out of seven and zero out of five studies (with the other studies reporting null findings), respectively. A more recent cohort study analysis found lower risk of all-cause and non-Alzheimer’s, but not Alzheimer’s, dementia among those with higher MedDiet adherence [16]. Previous investigations have used different approaches for collecting dietary intake data (e.g., food frequency questionnaires and 24-h recall methods), and have employed various MedDiet scoring systems, each of which define adherence to this dietary pattern in distinctly different ways. Such heterogeneity could hinder efforts to interpret and compare results from different studies [9]. Indeed, although we observed broadly consistent findings across the different MedDiet scores in this study, the strength of association with dementia risk differed. Whilst diet may be an important tractable risk factor for dementia, it is not emphasised in all dementia prevention guidelines (e.g., [2]), which may reflect the lack of consistent evidence about the dietary patterns that are associated with lower dementia risk. A better understanding of the best ways to operationalize a healthy dietary pattern (including the MedDiet) will be valuable for future research studies and for the formulation of dietary guidelines to minimise dementia risk.
There is limited and inconclusive evidence about the interaction between diet (defined by MedDiet adherence or another dietary index) and genetic risk on dementia incidence [13, 18,19,20]. For example, higher MedDiet adherence was associated with lower dementia risk in APOE ε4 carriers but not non-carriers in one study [13]. In contrast, other studies have reported that higher adherence to both the MIND diet (a hybrid between the MedDiet and Dietary Approach to Stop Hypertension) [18] and a ‘healthy’ diet [19] are more protective against dementia in APOE ε4 non-carriers. In the present study, we found no significant interaction between polygenic risk for dementia and MedDiet adherence defined by the MEDAS continuous or PYRAMID scores in our primary analyses. Likewise, when we explored the interaction between MedDiet adherence and genetic risk defined by APOE genotype, there was a similar pattern of response for both APOE ε4 carriers/non-carriers. Thus, our findings suggest similar associations between MedDiet adherence and dementia risk irrespective of genetic risk for this condition. Nevertheless, we acknowledge a degree of uncertainty in this conclusion, given that findings were not consistent across all sensitivity analyses. Further research into the interaction between diet and genetics on dementia risk is therefore warranted.
This study has several strengths. The majority of previous studies exploring associations between MedDiet adherence and dementia risk have involved relatively small numbers of participants (n = 1000–6000) with limited dementia cases (n = 20–400) and may have lacked statistical power [9]. In contrast, our study involved a much larger cohort (n = ~ 60,000) with more dementia cases (n = 882) than most previous investigations. We defined genetic risk for dementia using a comprehensive polygenic risk score whereas most previous studies have explored gene-diet interactions for individual genetic variants (e.g., APOE genotype) [13, 18,19,20]. A further strength of this study is that we carried out a wide range of sensitivity analyses which demonstrate the robustness of our findings. Several limitations should also be considered. Firstly, the observational design of this study precludes drawing causal inferences. Nevertheless, our findings are supported by the results from randomised controlled trials. This includes data from the Navarra [37] and Barcelona [38] cohorts of the PREDIMED trial, which demonstrated clinically meaningful benefits of a MedDiet intervention on cognitive function. A further limitation is the potential risk of reverse causality, given lower MedDiet adherence could be a consequence rather than a cause of dementia [39]. Although we did not find any evidence of reverse causality in sensitivity analyses where we excluded participants who developed dementia in the first two or five years of follow up, this does not eliminate the possibility that diet quality declined earlier in individuals who developed dementia, given the long pre-clinical phase of this condition [40, 41]. The measurement of dietary intake is a major challenge in research, and there are specific limitations related to the assessment of dietary intake in this study which should be considered when interpreting our results. We used dietary data from the Oxford WebQ, a self-administered 24-h recall method which provides results broadly comparable to those achieved via interviewer-administered 24-h recalls [25], to derive our MedDiet scores. Since multiple 24-h recalls are required to provide a ‘true’ representation of habitual diet [42], and many participants in UK Biobank completed only one or two recalls, it is possible that calculated MedDiet scores are not fully representative of the participants usual dietary intake. However, 20,348 participants repeated the touchscreen questionnaire at median 4.4 years after the initial dietary assessment. Analyses of the resulting data showed that there was moderate to substantial agreement between the responses to the dietary touchscreen questions at baseline and at the repeat visit [43]. Based on this evidence, we conclude that the estimates of dietary intake available in UK Biobank represent habitual intake and that this limits the likelihood of participant misclassification. In addition, we were unable to determine the amount of olive oil consumed from the available dietary data, which increases the risk of misclassification, as individuals who consume large amounts of olive oil may have been awarded lower scores than if we had been able to accurately quantify their intake of this MedDiet component. Nevertheless, findings from our previous research suggest that few individuals in a UK setting consume the requisite amount of olive oil to be awarded a full point for this MedDiet component [17], suggesting that this is likely to have had a limited impact on our MedDiet scores overall. Similarly, it was difficult to quantify accurately intake of sofrito, a sauce containing typically tomatoes, onions, and garlic cooked with olive oil which is popular in Mediterranean cuisine and is one of the components of the MEDAS/MEDAS continuous scores. We used self-reported intake of tomato-based sauces as the closest proxy for sofrito intake, which may have resulted in some misclassification depending upon the ingredients and preparation method. A further limitation of our work is that dementia cases were ascertained via linkage to hospital inpatient records and death registry only, which may miss some cases [44, 45]. However, previous studies have suggested good agreement with dementia ascertainment through primary care records [45]. Finally, UK Biobank participants are generally healthier and of higher socioeconomic status than the wider UK population [46] but this is unlikely to jeopardise valid assessment of exposure-disease relationships that are widely generalizable [46]. Nevertheless, since we restricted our sample to individuals of European ancestry aged ≥ 60 years at recruitment, our findings require substantiation in other populations (e.g., different ethnicities).
Conclusions
In this large population-based prospective cohort study, higher adherence to a MedDiet was associated with reduced dementia risk. A continuous MEDAS score was the most sensitive predictor of dementia risk when compared with a binary MEDAS or PYRAMID score and could therefore be prioritised as a tool for defining MedDiet adherence in future observational studies. There was no clear evidence for an interaction with genetic risk. These results underline the importance of dietary interventions in future dementia prevention strategies regardless of genetic predisposition.
Availability of data and materials
Data are available from UK Biobank for all bona fide researchers for health-related research in the public interest.
Abbreviations
- BMI:
-
Body mass index
- ICD:
-
International classification of diseases
- IPAQ:
-
International physical activity questionnaire
- MEDAS:
-
Mediterranean diet adherence screener
- MedDiet:
-
Mediterranean diet
- NHS:
-
National Health Service
- PREDIMED:
-
Prevención con Dieta Mediterránea
- PYRAMID:
-
Mediterranean diet Pyramid score
- SNP:
-
Single nucleotide polymorphism
References
Nichols E, Steinmetz JD, Vollset SE, Fukutaki K, Chalek J, Abd-Allah F, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7:e105–25.
Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–734.
World Health Organization. Risk Reduction of Cognitive Decline and Dementia. Geneva: World Health Organization; 2019.
Licher S, Ahmad S, Karamujić-Čomić H, Voortman T, Leening MJG, Ikram MA, et al. Genetic predisposition, modifiable-risk-factor profile and long-term dementia risk in the general population. Nat Med. 2019;25:1364–9.
Morris MC. Nutrition and risk of dementia: overview and methodological issues. Ann N Y Acad Sci. 2016;1367:31–7.
Stevenson EJ, Shannon OM, Minihane AM, Adamson A, Burns A, Hill T, et al. NuBrain: UK consortium for optimal nutrition for healthy brain ageing. Nutr Bull. 2020;45:223–9.
Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17:1006–15.
Siervo M, Shannon OM, Llewellyn DJ, Stephan BC, Fontana L. Mediterranean diet and cognitive function: From methodology to mechanisms of action. Free Radic Biol Med. 2021;176:105–17.
Limongi F, Siviero P, Bozanic A, Noale M, Veronese N, Maggi S. The Effect of Adherence to the Mediterranean Diet on Late-Life Cognitive Disorders: A Systematic Review. J Am Med Dir Assoc. 2020;21:1402–9.
Barbaresko J, Lellmann AW, Schmidt A, Lehmann A, Amini AM, Egert S, et al. Dietary Factors and Neurodegenerative Disorders: An Umbrella Review of Meta-Analyses of Prospective Studies. Adv Nutr. 2020;11:1161–73.
Feart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues J-F, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302:638–48.
Roberts RO, Geda YE, Cerhan JR, Knopman DS, Cha RH, Christianson TJH, et al. Vegetables, Unsaturated Fats, Moderate Alcohol Intake, and Mild Cognitive Impairment. Dement Geriatr Cogn Disord. 2010;29:413–23.
Olsson E, Karlström B, Kilander L, Byberg L, Cederholm T, Sjögren P. Dietary patterns and cognitive dysfunction in a 12-year follow-up study of 70 year old men. J Alzheimers Dis. 2015;43:109–19.
Haring B, Wu C, Mossavar-Rahmani Y, Snetselaar L, Brunner R, Wallace RB, et al. No Association between Dietary Patterns and Risk for Cognitive Decline in Older Women with 9-Year Follow-Up: Data from the Women’s Health Initiative Memory Study. J Acad Nutr Diet. 2016;116:921-930.e1.
Larsson SC, Wolk A. The Role of Lifestyle Factors and Sleep Duration for Late-Onset Dementia: A Cohort Study. J Alzheimers Dis. 2018;66:579–86.
Andreu-Reinón ME, Chirlaque MD, Gavrila D, Amiano P, Mar J, Tainta M, et al. Mediterranean Diet and Risk of Dementia and Alzheimer’s Disease in the EPIC-Spain Dementia Cohort Study. Nutrients. 2021;13:700.
Shannon OM, Stephan BCM, Granic A, Lentjes M, Hayat S, Mulligan A, et al. Mediterranean diet adherence and cognitive function in older UK adults: the European Prospective Investigation into Cancer and Nutrition-Norfolk (EPIC-Norfolk) Study. Am J Clin Nutr. 2019;110:938–48.
Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2015;11:1007–14.
Samuelsson J, Najar J, Wallengren O, Kern S, Wetterberg H, Mellqvist Fässberg M, et al. Interactions between dietary patterns and genetic factors in relation to incident dementia among 70-year-olds. Eur J Nutr. 2022;61:871–84.
Hosking DE, Eramudugolla R, Cherbuin N, Anstey KJ. MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimers Dement. 2019;15:581–9.
Marden JR, Walter S, Tchetgen Tchetgen EJ, Kawachi I, Glymour MM. Validation of a polygenic risk score for dementia in black and white individuals. Brain Behav. 2014;4:687–97.
Lourida I, Hannon E, Littlejohns TJ, Langa KM, Hyppönen E, Kuźma E, et al. Association of Lifestyle and Genetic Risk With Incidence of Dementia. JAMA. 2019;322:430–7.
Ollier W, Sprosen T, Peakman T. UK Biobank: from concept to reality. Pharmacogenomics. 2005;6:639–46.
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLOS Med. 2015;12:e1001779.
Liu B, Young H, Crowe FL, Benson VS, Spencer EA, Key TJ, et al. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr. 2011;14:1998–2005.
Greenwood DC, Hardie LJ, Frost GS, Alwan NA, Bradbury KE, Carter M, et al. Validation of the Oxford WebQ Online 24-Hour Dietary Questionnaire Using Biomarkers. Am J Epidemiol. 2019;188:1858–67.
Tong TYN, Wareham NJ, Khaw K-T, Imamura F, Forouhi NG. Prospective association of the Mediterranean diet with cardiovascular disease incidence and mortality and its population impact in a non-Mediterranean population: the EPIC-Norfolk study. BMC Med. 2016;14:135.
Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S-1228S (discussion 1229S-1231S).
Martínez-González MÁ, Corella D, Salas-Salvadó J, Ros E, Covas MI, Fiol M, et al. Cohort profile: design and methods of the PREDIMED study. Int J Epidemiol. 2012;41:377–85.
Gregory S, Ritchie CW, Ritchie K, Shannon O, Stevenson EJ, Muniz-Terrera G. Mediterranean diet score is associated with greater allocentric processing in the EPAD LCS cohort: A comparative analysis by biogeographical region. Front Aging. 2022;3:1012598.
Papadaki A, Johnson L, Toumpakari Z, England C, Rai M, Toms S, et al. Validation of the English Version of the 14-Item Mediterranean Diet Adherence Screener of the PREDIMED Study, in People at High Cardiovascular Risk in the UK. Nutrients. 2018;10:138.
Vadiveloo M, Lichtenstein AH, Anderson C, Aspry K, Foraker R, Griggs S, et al. Rapid Diet Assessment Screening Tools for Cardiovascular Disease Risk Reduction Across Healthcare Settings: A Scientific Statement From the American Heart Association. Circ Cardiovasc Qual Outcomes. 2020;13:e000094.
Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45:1452–8.
World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Geneva: World Health Organization; 1992.
Perez-Cornago A, Pollard Z, Young H, van Uden M, Andrews C, Piernas C, et al. Description of the updated nutrition calculation of the Oxford WebQ questionnaire and comparison with the previous version among 207,144 participants in UK Biobank. Eur J Nutr. 2021;60:4019–30.
White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–99.
Martínez-Lapiscina EH, Clavero P, Toledo E, Estruch R, Salas-Salvadó J, Julián BS, et al. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry. 2013;jnnp-:2012–304792.
Valls-Pedret C, Sala-Vila A, Serra-Mir M, Corella D, de la Torre R, Martínez-González MÁ, et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern Med. 2015;175:1094–103.
Akbaraly TN, Singh-Manoux A, Dugravot A, Brunner EJ, Kivimäki M, Sabia S. Association of Midlife Diet With Subsequent Risk for Dementia. JAMA. 2019;321:957–68.
Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–16.
Wagner M, Dartigues J-F, Samieri C, Proust-Lima C. Modeling Risk-Factor Trajectories When Measurement Tools Change Sequentially During Follow-up in Cohort Studies: Application to Dietary Habits in Prodromal Dementia. Am J Epidemiol. 2018;187:845–54.
Jackson KA, Byrne NM, Magarey AM, Hills AP. Minimizing random error in dietary intakes assessed by 24-h recall, in overweight and obese adults. Eur J Clin Nutr. 2008;62:537–43.
Bradbury KE, Young HJ, Guo W, Key TJ. Dietary assessment in UK Biobank: an evaluation of the performance of the touchscreen dietary questionnaire. J Nutr Sci. 2018;7:e6.
Sommerlad A, Perera G, Singh-Manoux A, Lewis G, Stewart R, Livingston G. Accuracy of general hospital dementia diagnoses in England: Sensitivity, specificity, and predictors of diagnostic accuracy 2008–2016. Alzheimers Dement J Alzheimers Assoc. 2018;14:933–43.
Wilkinson T, Schnier C, Bush K, Rannikmäe K, Henshall DE, Lerpiniere C, et al. Identifying dementia outcomes in UK Biobank: a validation study of primary care, hospital admissions and mortality data. Eur J Epidemiol. 2019;34:557–65.
Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, et al. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am J Epidemiol. 2017;186:1026–34.
Acknowledgements
None.
Funding
This research was conducted as part of the NuBrain consortium, which is funded by the UK Nutrition Research Partnership (UK NRP), an initiative supported by the Medical Research Council (MRC), Biotechnology and Biological Sciences Research Council (BBSRC) and the National Institute for Health Research (NIHR) (MR/T001852/1).
Author information
Authors and Affiliations
Contributions
OMS, JMR, TRH, AA, MS, AMM, G M-T, CR, JCM, DJL and ES conceived and designed the study. OMS, HM, CM, ML, AM, CM, AG, JM, MS, JCM and ES derived the MedDiet scores. OMS conducted the statistical analysis, with support from JMR, SG, ML, MS, GM-T, JCM, DJL, and ES. JMR and DJL facilitated data access, carried out data processing, and derived key variables used in the analysis. JMR updated the dementia data. OMS, JMR, JCM, DJL and ES wrote the initial draft of the manuscript, with OMS taking a lead role. TRH, AA, AMM, GMT, CR, and ES obtained funding to support the analysis. All authors participated in the interpretation of the results and critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for the UK Biobank study was provided by the North West–Haydock Research Ethics Committee (REC reference: 16/NW/0274), and all participants provided electronic signed consent. The study was performed in accordance with the tenets of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Text S1.
Dietary assessment and creation of the MedDiet scores. Table S1. Components and scoring of the MEDAS and MEDAS Continuous Mediterranean diet scores. Table S2. Components and scoring of the PYRAMID Mediterranean diet adherence score. Table S3. ICD-9 and ICD-10 codes for dementia diagnosis. Figure S1. Participant flowchart. Table S4. Risk of incident dementia according to Mediterranean diet adherence, with analyses restricted to individuals with a minimum of 2 dietary reports. Table S5. Risk of incident dementia according to Mediterranean diet adherence excluding participants with extreme energy intakes. Table S6. Influence of each component of the MEDAS and MEDAS Continuous scores on dementia risk. Table S7. Influence of each component of the PYRAMID score on dementia risk. Table S8. Risk of incident dementia according to Mediterranean diet adherence, excluding participants with less than 2 years and 5 years of follow up. Table S9. Sensitivity analyses adjusting for potential effect mediators (BMI, Depression and stroke). Table S10. Association between MedDiet adherence and risk of dementia in analyses stratified by polygenic risk score tertiles. Table S11. Associations between MedDiet adherence and dementia incidence in APOE ε4 non-carriers and carriers. Table S12. Associations between MedDiet adherence and dementia incidence in analyses where missing data were imputed. Table S13. Risk of incident dementia according to Mediterranean diet adherence restricted to fatal and non-fatal dementia cases. Table S14. Risk of incident dementia according to Mediterranean diet adherence for individuals with higher and lower education levels.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shannon, O.M., Ranson, J.M., Gregory, S. et al. Mediterranean diet adherence is associated with lower dementia risk, independent of genetic predisposition: findings from the UK Biobank prospective cohort study. BMC Med 21, 81 (2023). https://doi.org/10.1186/s12916-023-02772-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-023-02772-3