Abstract
Background
Overall survival (OS) is the gold standard endpoint to assess treatment efficacy in cancer clinical trials. In metastatic breast cancer (mBC), progression-free survival (PFS) is commonly used as an intermediate endpoint. Evidence remains scarce regarding the degree of association between PFS and OS. Our study aimed to describe the individual-level association between real-world PFS (rwPFS) and OS according to first-line treatment in female patients with mBC managed in real-world setting for each BC subtype (defined by status for both hormone-receptor [HR] expression and HER2 protein expression/gene amplification).
Methods
We extracted data from the ESME mBC database (NCT03275311) which gathers deidentified data from consecutive patients managed in 18 French Comprehensive Cancer Centers. Adult women diagnosed with mBC between 2008 and 2017 were included. Endpoints (PFS, OS) were described using the Kaplan–Meier method. Individual-level associations between rwPFS and OS were estimated using the Spearman’s correlation coefficient. Analyses were conducted by tumor subtype.
Results
20,033 women were eligible. Median age was 60.0 years. Median follow-up duration was 62.3 months. Median rwPFS ranged from 6.0 months (95% CI 5.8–6.2) for HR-/HER2 − subtype to 13.3 months (36% CI 12.7–14.3) for HR + /HER2 + subtype. Correlation coefficients were highly variable across subtypes and first-line (L1) treatments. Among patients with HR − /HER2 − mBC, correlation coefficients ranged from 0.73 to 0.81, suggesting a strong rwPFS/OS association. For HR + /HER2 + mBC patients, the individual-level associations were weak to strong with coefficients ranging from 0.33 to 0.43 for monotherapy and from 0.67 to 0.78 for combined therapies.
Conclusions
Our study provides comprehensive information on individual-level association between rwPFS and OS for L1 treatments in mBC women managed in real-life practice. Our results could be used as a basis for future research dedicated to surrogate endpoint candidates.
Similar content being viewed by others
Background
Despite a decrease of the mortality rate, breast cancer (BC) is still the major cause of cancer death among women worldwide [1]. BC is a heterogeneous disease and can be classified according to the tumor immunohistochemical profile, characterized by the presence or absence of hormone-receptor expression (HR-positive [HR +]/HR-negative [HR −] status) and/or human epidermal growth factor receptor 2 (HER2) protein expression and/or HER2gene amplification (HER2 − positive [HER2 +]/HER2 − negative status [HER2 −]) in the tumor cells. Up to 30% of BC patients will experience distant metastases over their lifetime [2]. Metastatic breast cancer (mBC) is a disease with poor prognosis, with median overall survival (OS) ranging from 14.8 months for triple-negative mBC (TN mBC, defined as the lack of HR expression and of HER2 over-expression/amplification) to around 5 years for HER2 + disease respectively [3]. The selection of the most appropriate treatment depends on the characteristics of the patient (age, performance status), the adjuvant treatment (type and duration of therapies for early BC), and the metastatic disease (number of metastatic sites, type of involved organs, time to metastatic occurrence, molecular profile). In the metastatic setting, chemotherapy, endocrine therapy, or targeted therapy are the recommended treatments, as induction or maintenance treatment, according to the tumor molecular profile [4].
In cancer randomized controlled trials (RCT), drug efficacy is assessed using OS, considered as the gold standard efficacy endpoint [5,6,7]. However, in the advanced setting, the multiple lines of treatments may affect OS and thus bias the assessment of the true treatment effect. In addition, observing a benefit on OS may require a large number of patients and extensive follow-up, limiting the feasibility of clinical trials based on OS as the primary outcome. In this context, alternative endpoints that could capture treatment benefit accurately and be measurable earlier is central for the evolution of clinical research in oncology. Progression-free survival (PFS), although recognized as presenting some limitations [8], has been used increasingly over the past decades [9] and is now the most common primary efficacy endpoint in mBC clinical trials [10]. The use of PFS relies on the hypothesis that it can adequately replace OS, i.e., be a valid surrogate of OS; otherwise, this might lead to the marketing of drugs that do not ultimately improve OS [11, 12]. PFS however has not been validated as a surrogate endpoint in the context of mBC [13].
Real-world data (RWD) are defined as observational data from other sources than clinical trials, such as electronic medical records, registries, insurance claims, pharmacy records, death certificates, and other patient-generated data [14]. RWD bring information on patient’s profiles not included in RCT and supplement with real-life knowledge on patient management, treatment strategies, and long-term survival. As such, they complement results of RCT by allowing one (i) to assess the generalizability of survival outcomes reported in RCT to the real-life setting, (ii) to expand generalizability of trials’ results to underrepresented or specific populations, and (iii) to generate scientific hypotheses. In mBC, the availability of large datasets for researchers such as the longitudinal Epidemiology Strategy and Medical Economics (ESME) mBC Database are a unique opportunity to investigate real-life survival outcomes for distinct subgroups of mBC patients. These could subsequently be used to validate efficacy data observed in published RCT or generate study hypotheses when estimating sample size for a future RCT. ESME-mBC-derived data have been published, either to describe treatment patterns and patient outcomes for some specific subgroups [15,16,17,18,19,20] or to report specifically on OS and associated prognostic factors in mBC [3, 21, 22].
Our primary objective was to describe the individual-level association between rwPFS and OS according to first-line (L1) treatment in women treated for mBC as a potential surrogate endpoint. Secondary objectives included description of treatment patterns, rwPFS and OS, overall and by mBC subtype.
Methods
Data source
The Epidemio-Strategy and Medical Economic (ESME) Research Program is a French academic initiative supporting the centralization of structured and non- structured data documented in the electronic health records (EHR) (clinical notes, pathology reports and radiology reports) of patients treated for malignant conditions in a unique secured web-based data platform available for researchers. The ESME mBC data platform is an EHR-derived database that gathers exhaustive data on consecutive patients who initiated a L1 treatment for mBC between 01 January 2008 and 31 December 2017 in one of 18 French Comprehensive Cancer Centers (clinicaltrials.gov; NCT 03,275,311). Patients who only received surgery of a breast-related metastatic lesion were not eligible for selection into the ESME mBC database. The full methodology is described elsewhere [23]. Data extraction took place on April 14, 2020, and the extracted dataset included deidentified individual data for about 23,000 patients, with up to a 12-year follow-up. Available data were demographics, tumor characteristics, clinical features, clinical events, and treatments.
Study population
We included all female patients older than 18 years diagnosed with mBC (de novo disease or first metastatic recurrence) between January 1, 2008, and December 31, 2017, and who received a L1 systemic treatment such as chemotherapy, endocrine therapy or targeted therapy, whatever the sequence (monotherapy or combination of therapies using distinct mechanisms of actions, i.e., polytherapy). A treatment line was defined as all anti-cancer treatments received in the absence of tumor progression. We excluded patients without informative data for tumor subtype (e.g., status for both HR expression and HER2 expression/gene amplification). Patients receiving radiation therapy or anti-resorptive drugs (e.g., bisphosphonates, denosumab) as unique treatment were not considered in the analysis. Patients were excluded if a second breast cancer was diagnosed before the onset of metastatic disease in order to limit potential inconsistencies between both breast cancer tumor subtypes and the metastases.
Variables and definitions
Age, tumor characteristics (histological grade, histologic type, HR status, HER2 status), the dates of first disease progression, start of metastatic lines of treatment, and last contact were derived from clinical patient records using standard definitions validated by the ESME Scientific Group.
HER2 status and HR status were derived from existing results about metastatic tissue sampling where available, or, if not available, from last sampling on early disease. Tumors were defined as HR positive (HR +) if estrogen receptor (ER) or progesterone receptor (PR) expression was > = 10% (immunohistochemistry), as per European guidelines. HER2 immunohistochemical (IHC) score 3 + or IHC score 2 + with a positive fluorescence in situ hybridization (FISH) or chromogenic in situ hybridization (CISH) classified the cancer as HER2 positive (HER2 +). On the other hand, all cancers with an IHC score 0–1 + or 2 + with a negative FISH/CISH test, as well as patients with a negative FISH/CISH test without IHC information, were considered as HER2 negative (HER2 −). Cancers with an IHC score 2 + without FISH/CISH test information were considered as HER2 indeterminate.
The four tumor subtypes are described as follows: triple-negative breast cancer was defined as ER expression < 10% and PR expression < 10% and HER2 non overexpressed and/or non amplified (TN mBC). Hormone receptor-positive and HER2-positive BC was defined as ER and/or PR > = 10% and HER2 protein overexpression (3 +) and/or gene amplification (HR + /HER2 + mBC). Hormone receptor-negative and HER2-positive BC was defined by ER and PR expression < 10% and HER2 protein overexpression (3 +) and/or gene amplification (HR − /HER2 + mBC). Finally, hormone receptor-positive (HR +) and HER2-negative (HER2 −) BC was defined by an expression of ER and/or PR > = 10% and no overexpression nor amplification of HER2 (HR + /HER2 − mBC).
De novo metastatic disease was considered when the first occurrence of metastatic disease was diagnosed within 6 months after the diagnosis of the primary BC. Metastasis-free interval (MFI) was defined as the time between initial diagnosis and metastatic relapse.
Histological grade and histologic type were derived using the first informative results on the primary tumor whatever breast surgical procedures (biopsy, tumorectomy, lumpectomy, and mastectomy).
The number of metastatic sites was calculated based on the number of organs involved with one or more metastases diagnosed within 1 month (30 days) from the diagnosis of the first metastatic occurrence.
The first-line (L1) therapy was defined as intravenous or per os therapeutic regimen (chemotherapy, targeted therapy, immunotherapy, and/or endocrine therapy) initiated at the metastatic diagnosis or within 12 weeks following it. Drug classifications are listed in Additional file 1 Table S1.
L1 treatment patterns were defined according to the therapeutic classes of drugs: chemotherapy only, targeted therapy only, endocrine therapy only, chemotherapy and endocrine therapy, chemotherapy and targeted therapy, chemotherapy, targeted therapy and endocrine therapy, endocrine therapy and targeted therapy, immunotherapy-based regimen, and other therapy. Anthracyclines, purine analogs, pyrimidine analogs, alkylating agents, platinum derivatives, taxanes, and vinca alkaloids were classified as “chemotherapy.” Protein kinase inhibitors, vascular endothelial growth factor (VEGF)/vascular endothelial growth factor receptor inhibitors, and other agents were classified as “targeted therapy.” Endocrine therapy was assigned for aromatase inhibitors, anti-estrogens, and luteinizing hormone-releasing hormone agonists.
For non-de novo mBC patients, the type of adjuvant therapy received at early BC stage was also described.
The first disease progression was derived using diagnosed clinical events recorded in the database (local relapse, progression in the involved organs, metastases in a new organ) and progression-related reason(s) for termination of drugs included in the L1 therapy.
rwPFS was defined as the time from initial diagnosis of mBC to the date of disease progression (regional recurrence, progression, appearance/occurrence of metastases and distant recurrence) or death (any cause), whichever came first. OS was defined as the time from diagnosis of mBC to the date of death from any cause.
The present study was validated by the ESME mBC Scientific Group. No formal dedicated informed consent was required but all patients had been informed about the re-use of their electronically recorded data in compliance with the General Data Protection Regulation. The ESME mBC database was authorized by the French data protection authority (Registration ID 1,704,113; authorization N°DE-2013.-117; complementary authorization was obtained on 14 October 2019 regarding the ESME research Data Warehouse). The analysis was approved by an independent ethics committee (Comité De Protection Des Personnes Sud-Est II- 2015–79).
Statistical analyses
Baseline characteristics were summarized using frequency and percentage for qualitative variables. Median and inter-quartile range were reported for quantitative variables. We reported frequencies and proportions for variables with missing or not documented information. No statistical test was performed for the descriptive analyses.
Median follow-up was estimated using the reverse Kaplan–Meier Method [24]. Survival data were estimated using Kaplan–Meier method and we reported median survival times with their respective 95% confidence interval (95%CI). Data for patients without the events of interest were censored at the date of last contact recorded in the database.
We estimated the individual-level association between rwPFS and OS using a Spearman rank correlation coefficient expressed as a value between 0 (no association) and 1 (perfect association) with 95% CI. Copula models allow one to jointly model two time-to-event variables [25]. We used a reviewed copula-based approach that introduced an iterative multiple imputation method for the estimation of the correlation coefficient [26]. The strength of the rwPFS/OS association was ranged according to the estimated correlation coefficient as follows: 0–0.19 was considered as very weak, 0.2–0.39 as weak, 0.40–0.59 as moderate, 0.6–0.79 as strong, and 0.8–1 as very strong correlation [27]. We estimated and reported individual rwPFS/OS associations according to mBC subtype and first-line mBC treatment.
Data were analyzed using R software (v 3.6.1).
Results
Characteristics and treatments
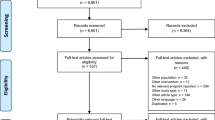
On the date of data extraction, the ESME mBC database included a total of 23,697 mBC subjects. Of those, 20,033 satisfied the eligibility criteria for our study population (Fig. 1). A total of 18 239 patients (91.0%) had at least one IHC score assessment for the primary tumor, and 1852 (9.2%) had at least one IHC score assessment for metastasis. The distribution of mBC subtypes was as follows: 66.3% for HR + /HER2 − (13 283 patients), 14.2% for TN (2 845 patients), 12.5% for HR + /HER2 + (2 502 patients), and 7.0% (1 403 patients) for HR − /HER2 + .
Study flow chart
ESME, epidemiological strategy and medical economics; mBC, metastatic breast cancer; HR + , presence of hormone receptor; HR − , absence of hormone receptor; HER2 + , human epidermal growth factor receptor 2 (HER2) protein overexpression; HER2 − , no HER2 protein overexpression. TN, triple negative
Clinical and tumor characteristics are presented overall and by tumor subtype (Table 1). De novo mBC was highly represented among HR − /HER2 + mBC patients (49.0%) and HR + /HER2 + mBC patients (40.6%). Most patients had two metastatic sites (79.0%). Overall, bone metastases were the most frequent (57.9%, ranging from 35.4% [TN mBC] to 65.3% [HR + /HER2 − mBC]), with HR − /HER2 + mBC patients presenting most often with liver metastases (38.9%) and TN mBC patients with metastatic lymph nodes (43.4%).
First-line treatments are described in Table 2. First-line treatments with a focus on anti-HER2 therapies are reported in Additional file 1 Table S2. Number of lines of treatment by tumor subtype are reported in Additional file 1 Table S3.
Outcomes
The median follow-up duration was 62.3 months (95% CI 58.4–63.6). Median time from initial diagnosis of mBC to the initiation of first-line treatment was 19 days, ranging from 18 days for HR + mBC to 25 days for TNBC. Survival outcomes are summarized in Fig. 2, by tumor subtype and first-line treatment.
Median survival outcomes (overall survival [OS], real-world progression-free-survival [rwPFS]) in metastatic breast cancer (mBC) patients after diagnosis of mBC, according to first-line treatment by tumor subtype (in months)
HR + , presence of hormone receptor; HR − , absence of hormone receptor; HER2 + , human epidermal growth factor receptor 2 (HER2) protein overexpression; HER2 − , no HER2 protein overexpression. CT, chemotherapy only; ET, endocrine therapy only; TT, targeted therapy only; CT& ET, chemotherapy and endocrine therapy; CT and TT, chemotherapy and targeted therapy; ET & TT, endocrine therapy and targeted therapy; CT, TT and ET, chemotherapy, targeted therapy and endocrine therapy. Median estimates are presented in months, with 95% confidence interval (95% CI)
Median rwPFS under first line treatment was 10.6 months (95% CI 10.4–10.8) for the whole population: 6.0 months (95% CI 5.8–6.2) for TN mBC patients, 11.4 months (95% CI 10.6–12.3) for HR − /HER2 + mBC patients, 11.9 months (95% CI 11.5–12.1) for HR + /HER2 − mBC patients, and 13.3 months (95% CI 12.7–14.3) for HR + /HER2 + mBC patients. rwPFS curves are reported in Fig. 3.
Estimated real-world progression-free survival curve, by tumor subtype, for study population (N = 20,033)
HR + , presence of hormone receptor; HR − , absence of hormone receptor; HER2 + , human epidermal growth factor receptor 2 (HER2) protein overexpression; HER2 − , no HER2 protein overexpression; TN, triple negative
Median OS was 39.5 months (95% CI 38.7–40.5) for the whole population: 14.7 months (95% CI 14.1–15.4) for TN mBC patients, 42.0 months (95% CI 38.8–45.4) for HR − /HER2 + mBC patients, 43.4 (95% CI 42.6–44.5) for HR + /HER2 − mBC patients, and 56.7 months (95% CI 54.9–60.2) for HR + /HER2 + mBC patients. OS curves are shown in Fig. 4.
Estimated overall survival curve, by tumor subtype for study population (N = 20,033)
OS, overall survival; HR + , presence of hormone receptor; HR − , absence of hormone receptor; HER2 + , human epidermal growth factor receptor 2 (HER2) protein overexpression; HER2 − , no HER2 protein overexpression; TN, triple negative
rwPFS-OS correlations
Individual-level associations between rwPFS and OS are presented in Table 3. Associations ranged from very weak to very strong, with correlation coefficients ranging from 0.33 (95% CI 0.12–0.52) for HR + /HER2 + mBC women treated with chemotherapy to 0.81 (95% CI 0.79–0.82) for TN mBC women treated with chemotherapy. High variability was observed for HR-positive subgroups with weak to strong associations. For HR + /HER2 + mBC patients, the correlation coefficient ranged from 0.33 to 0.43 when a single type of therapy was used (chemotherapy or endocrine), while it was 0.67 or more when multiple types of therapy were combined. For both TN and HR − /HER2 + subgroups, associations were at least strong. Among the 1804 patients with TN mBC treated with chemotherapy, the estimate was 0.81 (95% CI, 0.79–0.82). When targeted therapy was added to the initial chemotherapy regimen, the estimated correlation was 0.73.
Discussion
This retrospective analysis provided estimates on individual-level associations between rwPFS and OS in 20,033 women diagnosed in 2008–2017 with mBC who initiated first-line anti-cancer treatment. This large national cohort was a unique opportunity to report on individual-level associations between rwPFS and OS for each L1 treatment pattern, overall and by tumor subtype. Individual-level associations between rwPFS and OS were at least strong for both TN and HR − /HER2 + subtypes (rank correlation coefficient equal or higher than 0.67). For HR + subgroups with considerable variety in therapeutic options, associations were highly variable (weak to strong) depending on the treatment. Overall, within the 4 mBC subgroups, individual-level correlation between rwPFS and OS were at least strong for each dominant treatment class (i.e., chemotherapy for TN mBC, endocrine therapy for HR + /HER2 − , and anti-HER2 in either HER2 + subgroups).
Formal validation of a surrogate endpoint relies on the assessment of both individual- and trial-level associations, the latter being available through meta-analyses of RCTs. As of today however, few surrogate endpoints have been identified [13] and assessment of PFS as a surrogate for OS in mBC is still relevant.
To our knowledge, we report results of the first study assessing individual-patient level association between rwPFS and OS in the largest observational cohort including consecutive patients treated for mBC in the real-world setting. We assessed individual correlation based on the rank correlation approach, a rigorous tool used in the reference method for endpoint surrogacy assessment [28, 29]. Although RWD are not the primary source used for assessing surrogacy, our data source, ESME mBC database, offered a large dataset of individual-patient data [IPD] homogenously centralized to assess multiple endpoints. Secondly, exploring the value of RWD in the search of candidate endpoint for OS surrogacy, our IPD were sourced from a significant quantity of high-quality data. The ESME mBC cohort represented 23,000 + adult patients (without any upper limit regarding the age) consecutively treated in the French 18 participating centers over a 12-year time period. The BC subtypes distribution and de novo mBC disease proportion were consistent with the other observational studies published [30,31,32,33]. We relied on this highly reliable dataset updated on an annual basis (including update on patient status maintained update to date in each center supported by the local use of National registry of death certificates), and this leads to an objective estimation of OS and a strong external validity. Well-designed databases may lead to valid information even if conventional RCTs are still the reference for evidence [34]. Our retrospective analysis considered all 1L therapy and estimated rwPFS are consistent with published data [35, 36]. Finally, over the few past years, the ESME mBC database was extensively used to support post-marketing requirements for heath technology assessment body in France and to supplement clinical data package for marketing authorization file in Europe [37,38,39,40,41,42].
The comparison of our findings with published data on surrogate endpoints is complex due to differences in terms of statistical methods (meta-analytical approaches of RCTs using either IPD or aggregated data), recruitment period, distinct target populations (defined by HR expression or HER2 status), or distinct settings (first-line or subsequent lines of therapy). In the study published by Sherrill and al., individual-level association was weak (ρ = 0.38) based on 67 RCTs designed to assess different mBC therapies (mainly chemotherapy or endocrine therapy in monotherapy or combination) [43]. In a meta-analysis of RCTs assessing different types of therapy (including chemotherapy or endocrine therapy in monotherapy or combination) in multiple mBC setting and published between 1990 and 2020, moderate individual-level association was reported [44]. Based on the year of publication, most of RCTs (69.5% published earlier than 2005) were undertaken before our period of interest for mBC diagnosis (2008–2017) and 86.1% of RCTs included in the meta-analysis were not discriminant regarding the HR and HER2 status. Petrelli and al. reported a strong individual-level association (ρ = 0.81) in a meta-analysis of 20 RCTs assessing first-line targeted therapies in patients enrolled again before our period of interest [45]. All aforementioned meta-analyses were based on aggregated data and both PFS and time to progression merged as unique outcome when assessing correlation with OS at individual-patient level. Using IPD for meta-analyses, two studies reported individual-level association data between PFS and OS with a distinct definition for PFS [46, 47]. Burzykowski and al. reported a strong association (ρ = 0.81) at individual level in a meta-analysis of 11 RCTs comparing first-line anthracycline-based therapy with taxanes [46]. Again, no discrimination regarding the HR and HER2 status was retrieved among RCTs in the meta-analysis, which limits the comparison with our estimated associations for patients receiving chemotherapy alone with correlation coefficient ranging from 0.33 to 0.81 for HR + /HER2 + subtype and TN subtype respectively. The second meta-analysis investigated PFS surrogacy with OS in a set of 9 phase II/III RCTs evaluating anti-HER2 targeted therapies (trastuzumab or lapatinib), authors reported a strong individual-level association (ρ = 0.69) but 2 in 9 trials investigated drugs in second-line or more setting [47]. Our estimates of individual-level association between rwPFS and OS in HER2 + mBC patients exposed to L1 targeted therapy (range: 0.67–0.87) were consistent with those results focusing on drugs with similar mechanisms of action although all RCTs pooled in the meta-analysis included patients enrolled before 2008. As surrogacy assessment is specific to a disease and to the mechanisms of action of the drug, comparison of our results to published meta-analyses is complex as few of those are available. Investigations are supported using meta-analysis requiring large amount of individual patient data among terminated RCT assessing drug efficacy with the same mechanism of action.
Results of individual-patient level association using de-identified IPD collected in real-world setting are in line with published results seeking for PFS surrogacy with OS using RCTs data. This work shows the value of RWD to support the search of potential candidate surrogate endpoint for OS.
As RWD, the ESME mBC presents limitations [23, 48]. These include the lack of availability of electronic medical records data required to describe the global mBC management due to the low level of standardization of current electronic medical records as well as the retrospective patient selection-data collection. The interpretation of reported survival estimates may be limited by the presence of confounding factors inherent to the observational nature of RWD, as opposed to randomized controlled studies. As our primary objective was the association between rwPFS and OS, we only reported descriptive data for rwPFS and OS (crude estimates based on Kaplan–Meier) and refer the reader to earlier ESME mBC publications for further data on prognostic factors for rwPFS/OS (3, 21, 22). Concerning overall generalizability and external validity, the cohort centralizes data from patients treated in specialized cancer centers only, which thus may use different clinical practices compared with public hospitals and private institutions. Although we did assess real-world outcomes according to the first-line treatment by tumor subtypes, residual heterogeneity within treatment group remains. Indeed, we did not make any distinctions regarding the mechanism of action of the drugs among each treatment group, which affect individual-level association. As an example, we assigned to “targeted therapy” to both bevacizumab, VEGF-targeted therapy (component of mBC therapy affecting the tumor angiogenesis) and palbociclib, inhibitor of cyclin-dependent kinases 4 and 6 (CDK4/6) involved are the downstream of signaling pathways which lead to cellular proliferation). Antiangiogenics such as bevacizumab are unique in that PFS advantages appear to frequently be completely erased at OS, and this is the only therapy in breast cancer preclinically and clinically implicated to have a reversal effect in second and subsequent lines of therapy. This issue highlights that even within drug classes, residual variability in terms of mechanism of action may persist. Similarly, the impact of subsequent lines of treatment could be further investigated. Finally, in real-world setting, non-systematic RECIST-based progression evaluation may also introduce bias in our analyses, as compared to standardized RECIST-based assessment in RCTs.
Conclusions
This study reports comprehensive information related to individual-level association between rwPFS and OS according to BC subtype and L1 mBC treatment. Overall, within the 4 mBC subgroups, individual-level correlation between rwPFS and OS were at least strong for each dominant treatment class (i.e., chemotherapy for TN mBC, endocrine therapy for HR + /HER2 − , and anti-HER2 in either HER2 + subgroups). Those results support the value of RWD when searching for candidate surrogate endpoints for OS. Data could be used subsequently to investigate or generate research hypotheses for future surrogate endpoint candidates. We also provided researchers with treatment patterns and rwPFS according to the 1L treatment received by tumor subtype. These estimates could be used when designing future research, in particular to provide information not readily available from the published trial literature for underrepresented populations.
Availability of data and materials
The data that support the findings of this study are available from UNICANCER but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of UNICANCER.
Abbreviations
- BC:
-
Breast cancer
- CISH:
-
Chromogenic in situ hybridization
- HER:
-
Electronic health records
- ESME:
-
Epidemiology Strategy and Medical Economics
- FISH:
-
Fluorescence in situ hybridization
- HER2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hormone-receptor
- IHC:
-
Immunohistochemistry
- IPD:
-
Individual-patient data
- L1:
-
First-line
- mBC:
-
Metastatic breast cancer
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- RW:
-
Real-world
- RCT:
-
Randomized controlled trials
- RWD:
-
Real-world data
- TN:
-
Triple-negative
- VEGF:
-
Vascular endothelial growth factor
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Deluche E, Antoine A, Bachelot T, Lardy-Cleaud A, Dieras V, Brain E, et al. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008–2016. Eur J Cancer. 2020;129:60–70.
Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5)†. Ann Oncol. 2020;31(12):1623-49.
Hirschfeld S, Pazdur R. Oncology drug development: United States Food and Drug Administration perspective. Crit Rev Oncol Hematol. 2002;42(2):137–43.
European Medicines Agency - Conditional marketing authorisation. 2018. Available from: https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/conditional-marketing-authorisation
Driscoll JJ, Rixe O. Overall survival: still the gold standard: why overall survival remains the definitive end point in cancer clinical trials. Cancer J Sudbury Mass. 2009;15(5):401–5.
Tannock IF, Pond GR, Booth CM. Biased evaluation in cancer drug trials—how use of progression-free survival as the primary end point can mislead. JAMA Oncol. 2022;8(5):679-80.
Gourgou-Bourgade S, Cameron D, Poortmans P, Asselain B, Azria D, Cardoso F, et al. Guidelines for time-to-event end point definitions in breast cancer trials: results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials). Ann Oncol. 2015;26(5):873–9.
Del Paggio JC, Berry JS, Hopman WM, Eisenhauer EA, Prasad V, Gyawali B, et al. Evolution of the randomized clinical trial in the era of precision oncology. JAMA Oncol. 2021;7(5):728–34.
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–76.
Carpenter D, Kesselheim AS, Joffe S. Reputation and precedent in the bevacizumab decision. N Engl J Med. 2011;365(2): e3.
Savina M, Gourgou S, Italiano A, Dinart D, Rondeau V, Penel N, et al. Meta-analyses evaluating surrogate endpoints for overall survival in cancer randomized trials: A critical review. Crit Rev Oncol Hematol. 2018;123:21–41.
Khozin S, Blumenthal GM, Pazdur R. Real-world data for clinical evidence generation in oncology. J Natl Cancer Inst. 2017;109(11).
Annonay M, Gauquelin L, Geiss R, Ung M, Cristol-Dalstein L, Mouret-Reynier MA, et al. Treatment and outcomes of older versus younger women with HER2-positive metastatic breast cancer in the real-world national ESME database. The Breast. 2021;60:138–46.
Bringuier M, Carton M, Levy C, Patsouris A, Pasquier D, Debled M, et al. Enrollment of older metastatic breast cancer patients in first-line clinical trials: 9-year experience of the large-scale real-life multicenter French ESME cohort. Breast Cancer Res Treat. 2022;91(3):577–87.
Sirieix J, Fraisse J, Mathoulin-Pelissier S, Leheurteur M, Vanlemmens L, Jouannaud C, et al. Management and outcome of male metastatic breast cancer in the national multicenter observational research program Epidemiological Strategy and Medical Economics (ESME). Ther Adv Med Oncol. 2020;12:1758835920980548.
Bertho M, Fraisse J, Patsouris A, Cottu P, Arnedos M, Pérol D, et al. Real-life prognosis of 5041 bone-only metastatic breast cancer patients in the multicenter national observational ESME program. Ther Adv Med Oncol. 2021;13:175883592098765.
Darlix A, Louvel G, Fraisse J, Jacot W, Brain E, Debled M, et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br J Cancer. 2019;121(12):991-1000.
Pasquier D, Darlix A, Louvel G, Fraisse J, Jacot W, Brain E, et al. Treatment and outcomes in patients with central nervous system metastases from breast cancer in the real-life ESME MBC cohort. Eur J Cancer. 2020;125:22–30.
Gobbini E, Ezzalfani M, Dieras V, Bachelot T, Brain E, Debled M, et al. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer. 2018;96:17–24.
Grinda T, Joyon N, Lusque A, Lefèvre S, Arnould L, Penault-Llorca F, et al. Phenotypic discordance between primary and metastatic breast cancer in the large-scale real-life multicenter French ESME cohort. Npj Breast Cancer. 2021;7(1):41.
Pérol D, Robain M, Arveux P, Mathoulin-Pélissier S, Chamorey E, Asselain B, et al. The ongoing French metastatic breast cancer (MBC) cohort: the example-based methodology of the Epidemiological Strategy and Medical Economics (ESME). BMJ Open. 2019;9(2):e023568.
Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–6.
Shih JH, Louis TA. Inferences on the association parameter in copula models for bivariate survival data. Biometrics. 1995;51(4):1384–99.
Schemper M, Kaider A, Wakounig S, Heinze G. Estimating the correlation of bivariate failure times under censoring. Stat Med. 2013;32(27):4781–90.
Correlation and regression. The BMJ. 2020. Available from: https://www.bmj.com/about-bmj/resources-readers/publications/statistics-square-one/11-correlation-and-regression
Buyse M, Molenberghs G, Burzykowski T, Renard D, Geys H. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostat Oxf Engl. 2000;1(1):49–67.
Burzykowski T, Molenberghs G, Buyse M, Geys H, Renard D. Validation of surrogate end points in multiple randomized clinical trials with failure time end points. J R Stat Soc Ser C Appl Stat. 2001;50(4):405–22.
Vaz-Luis I, Cottu P, Mesleard C, Martin AL, Dumas A, Dauchy S, et al. UNICANCER: French prospective cohort study of treatment-related chronic toxicity in women with localised breast cancer (CANTO). ESMO Open. 2019;4(5):e000562.
Kotsakis A, Ardavanis A, Koumakis G, Samantas E, Psyrri A, Papadimitriou C. Epidemiological characteristics, clinical outcomes and management patterns of metastatic breast cancer patients in routine clinical care settings of Greece: results from the EMERGE multicenter retrospective chart review study. BMC Cancer. 2019;19(1):88.
Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7(1–2):4–13.
Gilbert A, Williams C, Azuero A, Burkard ME, Kenzik K, Garrett-Mayer E, et al. Utilizing data visualization to identify survival and treatment differences between women with de novo and recurrent metastatic breast cancer. Clin Breast Cancer. 2021;21(4):292–301.
Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342(25):1878–86.
Yardley DA, Kaufman PA, Brufsky A, Yood MU, Rugo H, Mayer M, et al. Treatment patterns and clinical outcomes for patients with de novo versus recurrent HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2014;145(3):725–34.
Tripathy D, Brufsky A, Cobleigh M, Jahanzeb M, Kaufman PA, Mason G, et al. De novo versus recurrent HER2-positive metastatic breast cancer: patient characteristics, treatment, and survival from the SystHERs registry. Oncologist. 2020;25(2):e214–22.
Delaloge S, Pérol D, Courtinard C, Brain E, Asselain B, Bachelot T, et al. Paclitaxel plus bevacizumab or paclitaxel as first-line treatment for HER2-negative metastatic breast cancer in a multicenter national observational study. Ann Oncol Off J Eur Soc Med Oncol. 2016;27(9):1725–32.
Heudel P, Delaloge S, Parent D, Madranges N, Levy C, Dalenc F, et al. Real-world evaluation of oral vinorelbine in the treatment of metastatic breast cancer: an ESME-MBC study. Anticancer Res. 2020;40(7):3905–13.
Jacot W, Heudel P, Fraisse J, Gourgou S, Guiu S, Dalenc F, et al. Real-life activity of eribulin mesylate among metastatic breast cancer patients in the multicenter national observational ESME program. Int J Cancer. 2019;145(12):3359–69.
Commission de la Transparence. ENHERTU 100 mg: - AVIS CONDITIONNEL du 16 JUIN 2021/Première évaluation. Haute Autorité de Santé - FRANCE; 2021. Available from: https://www.has-sante.fr/upload/docs/evamed/CT-19098_ENHERTU_PIC_INS_AvisDef_CT19098.pdf
Commission d’évaluation économique et de santé publique. Tecentriq (atezolizumab) en association au nab-paclitaxel - Avis d’efficience. Haute autorité de Santé; 2020. Available from: https://www.has-sante.fr/plugins/ModuleXitiKLEE/types/FileDocument/doXiti.jsp?id=p_3186009
Francisco EM. Enhertu - Committee for Medicinal Products for Human Use (CHMP) - Assessment report. 2020. p. 149–52. Report No.: EMA/2446/2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/enhertu
Sherrill B, Amonkar M, Wu Y, Hirst C, Stein S, Walker M, et al. Relationship between effects on time-to-disease progression and overall survival in studies of metastatic breast cancer. Br J Cancer. 2008;99(10):1572–8.
Beauchemin C, Cooper D, Yelle L, Lachaine J, Lapierre ME. Progression-free survival as a potential surrogate for overall survival in metastatic breast cancer. Onco Targets Ther. 2014;7:1101-10.
Petrelli F, Barni S. Surrogate endpoints in metastatic breast cancer treated with targeted therapies: an analysis of the first-line phase III trials. Med Oncol Northwood Lond Engl. 2014;31(1):776.
Burzykowski T, Buyse M, Piccart-Gebhart MJ, Sledge G, Carmichael J, Lück HJ, et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(12):1987–92.
Michiels S, Pugliano L, Marguet S, Grun D, Barinoff J, Cameron D, et al. Progression-free survival as surrogate end point for overall survival in clinical trials of HER2-targeted agents in HER2-positive metastatic breast cancer. Ann Oncol Off J Eur Soc Med Oncol. 2016;27(6):1029–34.
Visvanathan K, Levit LA, Raghavan D, Hudis CA, Wong S, Dueck A, et al. Untapped potential of observational research to inform clinical decision making: american society of clinical oncology research statement. J Clin Oncol. 2017;35(16):1845–54.
Acknowledgements
We thank all patients for sharing their data for the outcome research. We thank the 18 French Comprehensive Cancer Centers (I. Curie, Paris/Saint-Cloud, G. Roussy, Villejuif, I. Cancérologie de l’Ouest, Angers/Nantes, C. F. Baclesse, Caen, ICM Montpellier, C. L. Bérard, Lyon, C. G-F Leclerc, Dijon, C. H. Becquerel, Rouen; I. C. Regaud, Toulouse; C. A. Lacassagne, Nice; Institut de Cancérologie de Lorraine, Nancy; C. E. Marquis, Rennes; I. Paoli-Calmettes, Marseille; C. J. Perrin, Clermont Ferrand; I. Bergonié, Bordeaux; C. P. Strauss, Strasbourg; I. J. Godinot, Reims; C. O. Lambret, Lille) for providing the data and each ESME coordinator for managing the project at the local level. Moreover, we thank the ESME Scientific Group and Strategic Committee for their ongoing support.
Funding
The ESME MBC database receives financial support from industrial partners (Roche, Pfizer, AstraZeneca, Merck Sharp and Dohme, Eisai and Daiichi Sankyo). Unicancer manages the ESME database (i.e., data collection, analysis and publication) independently.
CB received a grant from GIRCI-SOHO (Groupement Interrégional de Recherche Clinique et d’Innovation Sud-ouest Outre-Mer Hospitalier)—APITHEM call for project 2016 (Appel à Projets Interrégional Thématique).
Author information
Authors and Affiliations
Contributions
CC, SG and CB contributed to the conception, the design of the work, and to the analysis. CC, SG, CB, WJ and SD contributed to the interpretation of data. All authors (CC, SG, WJ, MC, OG, LV, AB, ML, DP, PM, CL, LU, GP, RS, FB, DP, MB, TP, TF, AL, SM, MR, SD, CB) contributed to the data acquisition. All authors drafted the work or substantively revised it. All authors have approved the submitted version (and any substantially modified version that involves the author’s contribution to the study); All authors have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was validated by the ESME mBC Scientific Group and approved by an independent ethics committee (Comité De Protection Des Personnes Sud-Est II- 2015–79). No formal dedicated informed consent was required, but all patients were informed about the re-use of their electronically recorded data in compliance with the General Data Protection Regulation. The ESME mBC database was authorized by the French data protection authority (Registration ID 1704113; authorization N°DE-2013.-117; complementary authorization was obtained on 14 October 2019 regarding the ESME research Data Warehouse).
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Drugclassification. TableS2. Therapeutic strategy during first-line therapyfor mBC disease for HER2+ mBC. Table S3. Number of metastatic lines of treatmentaccording to mBC subtype.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Courtinard, C., Gourgou, S., Jacot, W. et al. Association between progression-free survival and overall survival in women receiving first-line treatment for metastatic breast cancer: evidence from the ESME real-world database. BMC Med 21, 87 (2023). https://doi.org/10.1186/s12916-023-02754-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-023-02754-5