Abstract
Background
Iruplinalkib (WX-0593) is an anaplastic lymphoma kinase (ALK)/c-ros oncogene 1 (ROS1) tyrosine kinase inhibitor. Here we reported the single-arm, phase II study (INTELLECT) results of the efficacy and safety of iruplinalkib for ALK-positive crizotinib-resistant advanced non-small cell lung cancer (NSCLC) patients.
Methods
ALK-positive crizotinib-resistant advanced NSCLC patients aged ≥18 years, with Eastern Cooperative Oncology Group performance status of 0–2 were eligible. Patients received iruplinalkib 180 mg orally once daily for a 21-day cycle with a 7-day lead-in phase at 60 mg orally once daily. The primary endpoint was the independent review committee (IRC)-assessed objective response rate (ORR).
Results
From August 7, 2019, to October 30, 2020, 146 patients were included. As of the data cut-off date on November 30, 2021, the median follow-up time was 18.2 months (95% confidence interval [CI] 16.8–18.8). IRC-assessed ORR and disease control rate (DCR) were 69.9% (95% CI 61.7–77.2%) and 96.6% (95% CI 92.2–98.9%), respectively. Investigator-assessed ORR and DCR were 63.0% (95% CI 54.6–70.8%) and 94.5% (95% CI 89.5–97.6%), respectively. Investigator-assessed median duration of response and progression-free survival (the same as median time to progression) were 13.2 months (95% CI 10.4–17.7) and 14.5 months (95% CI 11.7–20.0), respectively. Corresponding IRC-assessed results were 14.4 months (95% CI 13.1–not evaluable [NE]), 19.8 months (95% CI 14.5–NE), and NE (95% CI 14.5–NE), respectively. Investigator-assessed intracranial ORRs were 46% (41/90, 95% CI 35–56%) in patients with central nervous system metastases and 64% (27/42, 95% CI 48–78%) in patients with measurable intracranial lesions. Overall survival data were immature. Treatment-related adverse events (TRAEs) occurred in 136/146 (93.2%) patients. The most common TRAEs were aspartate aminotransferase increased (63 [43.2%]), alanine aminotransferase increased (54 [37.0%]), and blood creatine phosphokinase increased (51 [34.9%]). Dose interruption, reduction, and discontinuation due to TRAEs occurred in 21 (14.4%), 16 (11.0%), and four (2.7%) patients, respectively.
Conclusions
In this study, iruplinalkib (WX-0593) demonstrated favorable efficacy and manageable safety profiles in patients with ALK-positive crizotinib-resistant advanced NSCLC. Iruplinalkib could be a new treatment option for this patient population.
Trial registration
Center for Drug Evaluation of National Medical Products Administration of China: CTR20190789, registered on April 28, 2019; ClinicalTrials.gov: NCT04641754, registered on November 24, 2020.
Similar content being viewed by others
Background
According to GLOBOCAN 2020, lung cancer incidence and mortality rate are far ahead worldwide [1]. Non-small cell lung cancer (NSCLC) accounts for approximately 80–85% of lung cancer [2]. Anaplastic lymphoma kinase (ALK) rearrangement occurred in 3–7% of NSCLC patients and has been proved to be an important carcinogenic driver [3,4,5]. The first-generation ALK tyrosine kinase inhibitor (TKI) crizotinib significantly improved the prognosis of patients with ALK-positive NSCLC [6]. However, most patients progressed within one year after crizotinib treatment. The main reason for drug resistance is insufficient penetration into the central nervous system (CNS) [7]. Second-generation ALK TKIs such as ceritinib, alectinib, brigatinib, ensartinib, and conteltinib (CT-707) have effectively addressed the issues of crizotinib resistance. For these novel ALK TKIs, objective response rates (ORR) were 33.3–56% and median progression-free survival (PFS) approximately ranged from six to 17 months in crizotinib-resistant disease [8,9,10,11,12]. Overcoming crizotinib resistance and developing new ALK TKIs are urgent clinical needs.
Iruplinalkib (WX-0593) is a novel and highly selective ALK/c-ros oncogene 1 (ROS1) TKI, which was developed by Qilu Pharmaceutical Co., Ltd., Jinan, China. The results of preclinical studies showed that it could effectively inhibit proliferation of crizotinib-sensitive or -resistant tumor cell lines, and ALK-positive crizotinib-sensitive or -resistant tumor growth in mouse models (data unpublished). The results of its phase I study showed that iruplinalkib had potent antitumor activity and manageable safety profile in crizotinib- or second-generation ALK TKI-resistant, or ALK TKI-naïve patients with ALK- or ROS1-positive advanced NSCLC [13]. For ALK-positive patients in the phase I study, ORRs were 63.0% (95% confidence interval [CI] 47.5–76.8%) in the dose-escalation phase and 58.2% (95% CI 47.4–68.5%) in the dose-expansion phase. Treatment-related adverse events (TRAEs) occurred in 92% of the patients at a dose of 180 mg orally once daily. It is noteworthy that lead-in phase had been implemented in the phase I study for safety concerns, and 180 mg orally once daily with a 7-day lead-in phase at 60 mg orally once daily was determined as the recommended phase 2 dose (RP2D) of iruplinalkib.
Here we reported the single-arm, phase II study (INTELLECT) results of the efficacy and safety of iruplinalkib for ALK-positive crizotinib-resistant advanced NSCLC patients.
Methods
Study design and participants
This study was conducted at 41 hospitals in China, in accordance with the Declaration of Helsinki and the International Council for Harmonisation guidelines for Good Clinical Practice. The study protocol was approved by the ethics committees of each participating hospital and registered on the Center for Drug Evaluation of National Medical Products Administration of China (CTR20190789) and ClinicalTrials.gov (NCT04641754). All patients provided written informed consent before participating in the study.
Eligible patients were ≥ 18 years old; with ALK-positive advanced NSCLC confirmed by histopathology or cytology; expected survival time ≥ 12 weeks; an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2; progression after continuous treatment with crizotinib ≥ 12 weeks; ≥ one measurable extracranial lesion; and adequate organ function: absolute neutrophil count ≥ 1.5×109/L, platelet count ≥ 90×109/L, hemoglobin ≥ 90 g/L, total bilirubin ≤ 1.5×upper limit of normal (ULN) (for Gilbert’s syndrome: total bilirubin ≤ 3.0×ULN and direct bilirubin ≤ 1.5×ULN), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ≤ 2.5×ULN (for liver metastatic disease: ALT ≤ 5.0×ULN and AST ≤ 5.0×ULN), creatinine ≤ 1.5×ULN, and left ventricular ejection fraction ≥ 50%. Patients with asymptomatic brain metastases or those with symptomatic brain metastases who have been treated and stable for ≥ four weeks before iruplinalkib treatment initiation were allowed. Any surgery and radiotherapy (except palliative) must be completed ≥ four weeks before iruplinalkib treatment initiation. Palliative radiotherapy must be completed ≥ 48 h before iruplinalkib treatment initiation. ALK-alterations were tested before prior crizotinib treatment using immunohistochemical test (Ventana ALK [D5F3] CDx assay; Roche Tissue Diagnostics, Oro Valley, AZ, USA), amplification-refractory mutation system polymerase chain reaction (PCR), real-time PCR, fluorescence in situ hybridization, or next-generation sequencing.
Key exclusion criteria included previous ALK inhibitors other than crizotinib; continuous steroids > 30 days; planned to receive drugs that prolong QTc interval such as azithromycin or previously treated with those drugs within 14 days before iruplinalkib treatment initiation; or planned to receive strong inducer or inhibitor of CYP3A4 such as phenobarbital or previously treated with those drugs within seven days before iruplinalkib treatment initiation; with meningeal metastases; any clinically relevant cardiovascular or cerebrovascular disease such as myocardial infarction, heart failure within six months before iruplinalkib treatment initiation; or previous history of diffuse or bilateral lung interstitial fibrosis.
Procedure
Patients received iruplinalkib 180 mg orally once daily for a 21-day cycle with a 7-day lead-in phase at 60 mg orally once daily until disease progression, intolerable toxicity, withdrawal of consent, loss of follow-up, start of other antitumor treatment, or death. If grade ≥ 2 cardiac or renal dysfunction or other grade ≥ 3 adverse events (AEs) occurred, iruplinalkib treatment would be suspended. At the discretion of investigator, iruplinalkib treatment would continue at the previous or a reduced dose after AEs reverted to grade ≤ 1 within 14 days. Two steps of iruplinalkib dose reduction to 120 mg and to 90 mg were allowed. If the same grade ≥ 3 AEs occurred again at a dose of 90 mg, treatment would discontinue. If dose suspension exceeded 14 cumulative days in a 21-day cycle or 14 continuous days, iruplinalkib dose discontinuation would also be required.
Assessment
Efficacy examination was conducted within 28 days before iruplinalkib treatment initiation, every six weeks in the first 24 weeks after iruplinalkib treatment initiation, every nine weeks after 24 weeks, and every 12 weeks after one year until disease progression, withdrawal of consent, loss of follow-up, start of other antitumor treatment, or death, using computed tomography (CT) or magnetic resonance imaging according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. And the CNS efficacy was evaluated on the basis of the response assessment in neuro-oncology (RANO) brain metastases criteria [14]. The imaging method used for each patient was consistent throughout the study. After the termination of iruplinalkib treatment, survival follow-up was performed every 12 weeks. AEs were classified and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 from iruplinalkib treatment initiation throughout the treatment period and 28 days after the end of treatment. Plasma samples were obtained before administration of iruplinalkib on Cycle 1 Day 1, Cycle 1 Day 7, Cycle 1 Day 21, Cycle 2 Day 21, and Cycle 4 Day 21. Drug concentration of iruplinalkib was tested using liquid chromatography-mass spectrometry for pharmacokinetics (PK) evaluation.
Outcomes
The primary endpoint was the independent review committee (IRC)-assessed ORR. Secondary endpoints included investigator-assessed ORR, disease control rate (DCR), duration of response (DoR), PFS, time to progression (TTP), and intracranial ORR (iORR), overall survival (OS), safety, and PK parameter (minimum concentration at steady-state [Cmin,ss]). Additionally, IRC-assessed DCR, DoR, PFS, and TTP were post hoc exploratory endpoints.
ORR was defined as the proportion of patients with complete response (CR) or partial response (PR). DCR was defined as the proportion of patients with CR, PR, or stable disease (SD). DoR was defined as the time from the date of the first CR or PR to the first time of disease progression or death from any cause, whichever occurred first. PFS was defined as the time from enrollment to the first time of disease progression or death from any cause, whichever occurred first, and TTP was defined as the time from enrollment to disease progression. iORR was defined as the proportion of patients with investigator-assessed intracranial CR or PR according to RANO brain metastases criteria. OS was defined as the time from enrollment to death from any cause.
The efficacy endpoints were evaluated in the full analysis set (FAS). The per-protocol set (PPS) and response evaluable set (RES) were used for sensitivity analyses. The FAS included all patients who received at least one dose of iruplinalkib. The PPS included patients in the FAS who complied with the protocol. The RES included patients in the FAS with measurable lesions by IRC at baseline. The safety analysis was conducted in the safety set (SS) which included patients who received at least one dose of iruplinalkib and one post baseline safety evaluation. PK were analyzed in patients who received at least one dose of iruplinalkib and provided at least one blood sample for the measurement of iruplinalkib concentrations.
Statistical analysis
An ORR ≥ 40% was considered clinically acceptable and the expected ORR was 55%. One hundred and twenty-two patients were required to achieve a lower limit of the two-sided 95% CI of 40%, with a one-sided α of 0.025 and a power of 80%. Considering a dropout rate of 15%, a total sample size of 144 patients was required.
95% CIs for ORR and DCR were calculated using the Clopper-Pearson method. The median DoR, median PFS, median TTP, median OS, and corresponding 95% CIs were calculated using the Kaplan-Meier method. Cmin,ss were summarized using descriptive statistics. All statistical analyses were performed by SAS (version 9.4, SAS Institute Inc., Cary, NC, USA).
Results
Patients
From August 7, 2019, to October 30, 2020, a total of 183 patients with ALK-positive advanced NSCLC were screened. One hundred and forty-six ALK-positive crizotinib-resistant advanced NSCLC patients were enrolled in this study, all of whom were included in the FAS and the PPS. The flow chart of this study is presented in Fig. 1. Nine patients had no measurable lesion at baseline by IRC, thus, the RES included 137 eligible patients. In the FAS, the mean age (± standard deviation) of patients was 52.4 ± 10.4 years old. Seventy-seven (52.7%) patients were female. Forty-two (28.8%) patients had ECOG PS of 0, and 99 (67.8%) had ECOG PS of 1. Brain metastases were found in 90 (61.6%) patients, of which 20 (22%) had received brain radiotherapy and 42 (47%) had measurable intracranial lesions. There were 56 (38.4%) patients with prior chemotherapy. One (0.7%) and 89 (61.0%) patients had CR and PR to prior crizotinib treatment, respectively (Table 1). All patients progressed on crizotinib treatment before the iruplinalkib treatment initiation. Comorbidities in > 5% patients were summarized in Additional file 1: Table S1. As of the data cut-off date on November 30, 2021, 57 (39.0%) patients were still on iruplinalkib treatment, and the median follow-up time was 18.2 months (95% CI 16.8–18.8).
Study flow chart. ALK, anaplastic lymphoma kinase. NSCLC, non-small cell lung cancer. PD, progressive disease. ECOG, Eastern Cooperative Oncology Group. PS, performance status. FAS, full analysis set. PPS, per-protocol set. TRAEs, treatment-related adverse events. RES, response evaluable set. IRC, independent review committee. PK, pharmacokinetics
Efficacy
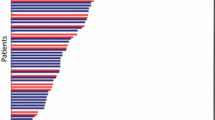
For all 146 patients in the FAS and PPS, 102 (69.9%) patients had PR, nine (6.2%) patients had non-CR/non-progressive disease (PD), and 30 (20.5%) patients had SD. IRC-assessed ORR was 69.9% (95% CI 61.7–77.2%; Table 2 and Fig. 2A).
Waterfall plot of systemic BOR (A) and forest plot of systemic objective response in subgroups (B). Response was assessed by IRC in the FAS (the same as the PPS). The dashed lines at 20% and -30% indicate the thresholds for PD and PR, respectively. There were nine and four patients with IRC-assessed BOR of non-CR/non-PD and NE, respectively. BOR, best objective response; CR, complete response; PR, partial response. SD, stable disease. PD, progressive disease. ORR, objective response rate. DCR, disease control rate. IRC, independent review committee. NE, not evaluable. FAS, full analysis set. PPS, per-protocol set
The corresponding lower limit of the 95% CI was superior to the pre-defined threshold 40%. Thus, the primary endpoint was met. The benefits were considerable, regardless of baseline brain metastases, previous brain radiotherapy, and previous chemotherapy or not. Systemic ORRs by IRC of patients without and with brain metastases were 80% (95% CI 68–90%) and 63% (95% CI 53–73%), respectively. The systemic ORR of patients not receiving prior brain radiotherapy and that of those receiving prior brain radiotherapy were 69% (95% CI 56–79%) and 45% (95% CI 23–68%), respectively. The forest plot and waterfall plots of the systemic best objective response by IRC in subgroups were shown in Fig. 2B and Additional file 1: Fig. S1, respectively. The DCR by IRC was 96.6% (95% CI 92.2–98.9%). Investigator-assessed ORR and DCR were 63.0% (95% CI 54.6–70.8%) and 94.5% (95% CI 89.5–97.6%), respectively (Additional file 1: Table S2 and Fig. S2). In the RES, IRC-assessed ORR was 74.5% (95% CI 66.3–81.5%) and DCR was 96.4% (95% CI 91.7–98.8%).
Investigator-assessed median DoR was 13.2 months (95% CI 10.4–17.7; Additional file 1: Table S2 and Fig. S3). Investigator-assessed median PFS was 14.5 months (95% CI 11.7–20.0; Fig. 3A, Additional file 1: Table S2 and Fig. S3). Investigator-assessed median TTP was the same as investigator-assessed median PFS (Fig. 3B, Additional file 1: Table S2 and Fig. S3). IRC-assessed median DoR, PFS, and TTP were 14.4 months (95% CI 13.1-not evaluable [NE]), 19.8 months (95% CI 14.5–NE), and NE (95% CI 14.5–NE), respectively (Table 2).
Among 90 patients with CNS metastasis, the iORR was 46% (41/90, 95% CI 35–56%). For 42 patients with measurable intracranial lesions, iORR was 64% (27/42, 95% CI 48–78%) (Table 2).
At the data cut-off date on November 30, 2021, OS data were immature, with 106 (72.6%) patients censored. The 12-month and 24-month survival rates were estimated to be 85.2% (95% CI 78.2–90.1%) and 57.9% (95% CI 44.2–69.4%), respectively (Table 2).
Safety
All 146 patients were included in the SS, and 136 (93.2%) patients had TRAEs. The most common TRAEs were AST increased (63 [43.2%]), ALT increased (54 [37.0%]), blood creatine phosphokinase increased (51 [34.9%]), hypercholesterolemia (49 [33.6%]), hypertriglyceridemia (39 [26.7%]), and hypertension (26 [17.8%]). Grade 3-4 TRAEs occurred in 45 (30.8%) patients. Twenty-one (14.4%) and 16 (11.0%) patients suffered TRAEs leading to dose interruption and reduction of iruplinalkib, respectively. The most common reasons for dose reduction of iruplinalkib were AST increased (four [2.7%]), blood creatine phosphokinase increased, rash (three [2.1%], each), gamma-glutamyltransferase increased, and eczema (two [1.4%], each). Dose discontinuations of iruplinalkib due to TRAEs were reported in four (2.7%) patients (blood creatinine increased, interstitial lung disease, rash, and hemorrhage intracranial [0.7%, each]; Table 3). Three patients are recovering from their respective TRAE after discontinuing iruplinalkib, while one patient died of TRAE (hemorrhage intracranial). Additionally, two (1.4%) patients had pneumonitis, both of which were grade 2 and occurred on the 7th day after iruplinalkib treatment initiation, leading to one patient of dose interruption and one patient of dose reduction, respectively. Pneumonitis did not recur after the iruplinalkib treatment continuation.
PK analysis
PK data were available in 141 patients. Plasma iruplinalkib concentration reached a steady state at Cycle 1 Day 21 with a mean Cmin,ss of 255.4 ± 160.8 ng/mL. Cmin,ss at each time point were detailed in Additional file 1: Fig. S4.
Discussion
This phase II study showed that iruplinalkib (WX-0593) had favorable clinical efficacy and manageable safety profiles in patients with ALK-positive crizotinib-resistant advanced NSCLC.
Iruplinalkib achieved the primary endpoint with an IRC-assessed ORR of 69.9% (95% CI 61.7–77.2%) whose lower limit of the 95% CI exceeded the pre-defined threshold of 40%. In this study, investigator-assessed ORR was 63.0% (95% CI 54.6–70.8%), consistent with data from the phase I study of iruplinalkib with an investigator-assessed ORR of 63.0% (95% CI 47.5–76.8%) in the dose-escalation phase and 58.2% (95% CI 47.4–68.5%) in the dose-expansion phase [13]. In crizotinib-resistant patients, the proportions of patients achieving IRC-assessed objective response for other second-generation ALK TKIs were 39.1% for ceritinib [8], 50% for alectinib [9], 56% for brigatinib [10], 52% for ensartinib [11], 33.3% for conteltinib [12], and 73% for lorlatinib [15]. Iruplinalkib also showed a DCR of 96.6% (95% CI 92.2–98.9%) assessed by IRC and 94.5% (95% CI 89.5–97.6%) by investigator.
Subgroup analysis of ORR in previous clinical studies was limited. Studies of alectinib and brigatinib reported the results in patients with or without previous chemotherapy. In patients with previous chemotherapy, ORRs were 44% for alectinib [9], 54% for brigatinib [16], and 77% for iruplinalkib in this study. In patients not receiving previous chemotherapy, ORRs were 69%, 52%, and 66% for alectinib, brigatinib, and iruplinalkib, respectively. Additionally, in this study, patients without baseline brain metastases had numerically better systemic ORR compared to those with baseline brain metastases in FAS (80% [95% CI 68–90%] vs 63% [95% CI 53–73%]), patients with prior brain radiotherapy had numerically better ORR compared to those without prior brain radiotherapy in patients with CNS metastatis (69% [95% CI 56–79%] vs 45% [95% CI 23–68%]).
In this study, the median DoR were 14.4 months (95% CI 13.1–NE) by IRC and 13.2 months (95% CI 10.4-17.7) by investigator, while the median DoR for ceritinib was 6.9 months by IRC [8], for alectinib was 11.2 months by IRC [9], for brigatinib 180 mg was 13.8 months by investigator [10], and for conteltinib was 6.6 months by investigator [12]. In respect of median PFS, iruplinalkib had 14.5 months (95% CI 11.7–20.0) by investigator and 19.8 months (95% CI 14.5–NE) by IRC in this study. The median PFS for ceritinib was 5.4 months by IRC and 6.7 months by investigator [8], for alectinib was 8.9 months by IRC [9], for brigatinib 180 mg was 16.7 months by IRC and 15.6 months by investigator [10], for ensartinib was 9.6 months by investigator [11], and for conteltinib was 6.7 months by investigator [12]. In the dose-expansion phase of the phase I study of iruplinalkib, because of the short median follow-up period of 203 days (95% CI 194–219), PFS data were immature [13].
Several studies suggested that CNS progression occurred frequently within one year during crizotinib treatment [7]. Second-generation ALK TKIs showed good intracranial antitumor activity. In this study, iruplinalkib demonstrated promising intracranial efficacy, with an iORR of 64% in patients with intracranial measurable lesions at baseline. That for alectinib was 57% [9]. And that for brigatinib 180 mg was 67% [10]. For conteltinib, iORR was 33.3% in six patients with measurable intracranial lesions [12]. Intracranial CR rate was 7% for iruplinalkib, numerically similar to ceritinib (8.7%) [17], alectinib (8.3%) [18], and brigatinib 90 mg (8%) [10]. However, no intracranial CR was reported in the brigatinib 180 mg [10]. These results suggested that iruplinalkib penetrates the blood-brain barrier and is an option for ALK-positive advanced NSCLC patients with brain metastases, similar to the other second-generation ALK TKIs.
In this study, OS data were immature, with a median follow-up of 18.2 months (95% CI 16.8–18.8). Estimated 12-month and 24-month OS rates were 85.2% (95% CI 78.2–90.1%) and 57.9% (95% CI 44.2–69.4%), respectively. The 12-month OS rates for alectinib and brigatinib 180 mg, and 24-month OS rate for brigatinib 180 mg were 71% [19], 80%, and 66% [10], respectively.
In terms of RP2D, in the dose-expansion phase of the previous iruplinalkib phase I study, two patients who received iruplinalkib 120 mg orally once daily without the lead-in dose died due to infectious pneumonia and respiratory failure, which occurred within 48 h after iruplinalkib treatment initiation, respectively [13]. Brigatinib without a lead-in phase may increase the risk of early-onset pulmonary events (EOPEs). And lead-in phase was implemented to lower the risk [20]. Although whether iruplinalkib could result in EOPEs was unconfirmed, dosing cohorts of 120 mg and 180 mg with a 7-day lead-in phase at 60 mg orally once daily were added to the iruplinalkib phase I study for safety concerns. And finally, iruplinalkib 180 mg orally once daily with a 7-day lead-in phase at 60 mg orally once daily was determined as the RP2D [13]. Further studies are needed to determine the mechanisms behind these AEs.
For safety, TRAEs for iruplinalkib occurred in 93.2% patients, with a numerically lower incidence of anemia, constipation, and rash than alectinib [9], brigatinib [10], and ensartinib [11], a numerically lower incidence of visual disturbances, diarrhea, edema, and renal impairment compared to alectinib [19], and a numerically lower incidence of elevated blood creatinine and fatigue compared to ensartinib [11]. Hypercholesterolemia and hypertriglyceridemia were also common with iruplinalkib, and it is unclear how dysregulation of lipid metabolism occurs. The majority of TRAEs in this study were grade 1 or 2. Dose interruption, reduction, and discontinuation due to TRAEs occurred in 14.4%, 11.0%, and 2.7% of patients receiving iruplinalkib, respectively. Dose discontinuation rate for alectinib was 8% [9]. Dose interruption, reduction, and discontinuation rates were 62%, 29%, and 11% for brigatinib 180 mg [10], 15%, 12%, and 3% for ensartinib [11], and 6.3%, 4.7%, and 3.1% for conteltinib [12], respectively. The incidences of grade 3-4 and serious TRAEs for iruplinalkib (30.8% and 6.8%) were numerically comparable to those for ensartinib (23% and 8%) [11]. The safety profiles of iruplinalkib reported in this study were generally consistent with that in the 180 mg group of the previous iruplinalkib phase I study (TRAEs: 95%; Grade ≥ 3 TRAEs: 23%; Serious TRAEs: 8%; Dose interruption, reduction, and discontinuation due to TRAEs: 18%, 3%, and 5%, respectively) [13]. Further, pneumonitis occurred in 1.4% patients in the present study. In 120 mg or 180 mg with or without lead-in phase in the iruplinalkib phase I study, pneumonitis (2%) only occurred in the 180 mg without lead-in phase. One treatment-related death of hemorrhage intracranial was reported. Together, iruplinalkib has manageable safety profiles. However, when brain metastatic patients with high cardiovascular disease risks receive iruplinalkib, special attention should be paid to hemorrhage intracranial.
For PK analysis, mean Cmin,ss of iruplinalkib were 255.4 ± 160.8 ng/mL and 164.10 ± 77.76 ng/mL in the present study and phase I study [13], respectively. Large standard deviations of drug concentration of iruplinalkib indicated differences in individuals. Future population PK studies could assess the factors that influence PK of iruplinalkib.
This study has some limitations. The OS data were immature because of insufficient follow-up duration. Updated OS data were awaited. There was also a lack of detection of efficacy predictors before and resistance mutations after iruplinalkib treatment. These could be investigated in the further studies. Besides, this study only enrolled Chinese patients, thus caution must be paid when these results were interpreted for other ethnic patients.
Conclusions
This study demonstrates that iruplinalkib (WX-0593) had favorable efficacy and manageable safety profiles in patients with ALK-positive crizotinib-resistant advanced NSCLC. Iruplinalkib could be a new treatment option for this patient population. A phase III study to compare iruplinalkib and crizotinib in the first-line setting (NCT04632758) in ALK-positive NSCLC is ongoing.
Availability of data and materials
The datasets generated and analyzed during the current study cannot be shared. Reason: We may further analyze the raw data for other research objectives.
Abbreviations
- AE:
-
Adverse event
- ALK:
-
Anaplastic lymphoma kinase
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- CI:
-
Confidence interval
- Cmin,ss :
-
Minimum concentration at steady-state
- CNS:
-
Central nervous system
- CR:
-
Complete response
- CT:
-
Computed tomography
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- DCR:
-
Disease control rate
- DoR:
-
Duration of response
- ECOG:
-
Eastern Cooperative Oncology Group
- EOPE:
-
Early-onset pulmonary event
- FAS:
-
Full analysis set
- iORR:
-
Intracranial objecctive response rate
- IRC:
-
Independent review committee
- NE:
-
Not evaluable
- NSCLC:
-
Non-small cell lung cancer
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PCR:
-
Polymerase chain reaction
- PD:
-
Progressive disease
- PFS:
-
Progression-free survival
- PK:
-
Pharmacokinetics
- PPS:
-
Per-protocol set
- PR:
-
Partial response
- PS:
-
Performance status
- RANO:
-
Response Assessment in Neuro-Oncology
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- RES:
-
Response evaluable set
- ROS1:
-
c-ros oncogene 1
- RP2D:
-
Recommended phase 2 dose
- SD:
-
Stable disease
- SS:
-
Safety set
- TKI:
-
Tyrosine kinase inhibitor
- TRAE:
-
Treatment-related adverse event
- TTP:
-
Time to progression
- ULN:
-
Upper limit of normal
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–237.
Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol. 2013;24(9):2371–6.
Koivunen JP, Mermel C, Zejnullahu K, Murphy C, Lifshits E, Holmes AJ, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14(13):4275–83.
Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–6.
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–77.
Zhang S, Anjum R, Squillace R, Nadworny S, Zhou T, Keats J, et al. The potent ALK inhibitor Brigatinib (AP26113) overcomes mechanisms of resistance to first- and second-generation ALK inhibitors in Preclinical models. Clin Cancer Res. 2016;22(22):5527–38.
Shaw AT, Kim TM, Crinò L, Gridelli C, Kiura K, Liu G, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017;18(7):874–86.
Ou SH, Ahn JS, De Petris L, Govindan R, Yang JC, Hughes B, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase ii global study. J Clin Oncol. 2016;34(7):661–8.
Huber RM, Hansen KH, Paz-Ares Rodríguez L, West HL, Reckamp KL, Leighl NB, et al. Brigatinib in Crizotinib-refractory ALK+ NSCLC: 2-year follow-up on systemic and intracranial outcomes in the phase 2 ALTA trial. J Thorac Oncol. 2020;15(3):404–15.
Yang Y, Zhou J, Zhou J, Feng J, Zhuang W, Chen J, et al. Efficacy, safety, and biomarker analysis of ensartinib in crizotinib-resistant, ALK-positive non-small-cell lung cancer: a multicentre, phase 2 trial. Lancet Respir Med. 2020;8(1):45–53.
Xing P, Zhao Q, Zhang L, Wang H, Huang D, Hu P, et al. Conteltinib (CT-707) in patients with advanced ALK-positive non-small cell lung cancer: a multicenter, open-label, first-in-human phase 1 study. BMC Med. 2022;20(1):453.
Shi Y, Fang J, Hao X, Zhang S, Liu Y, Wang L, et al. Safety and activity of WX-0593 (Iruplinalkib) in patients with ALK- or ROS1-rearranged advanced non-small cell lung cancer: a phase 1 dose-escalation and dose-expansion trial. Signal Transduct Target Ther. 2022;7(1):25.
Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270-8.
Shaw AT, Solomon BJ, Besse B, Bauer TM, Lin C-C, Soo RA, et al. Resistance mutations and efficacy of Lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J Clin Oncol. 2019;37(16):1370–9.
Kim DW, Tiseo M, Ahn MJ, Reckamp KL, Hansen KH, Kim SW, et al. Brigatinib in patients with Crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35(22):2490–8.
Wu YL, Shi Y, Tan DSW, Xiaoqing L, Cheng Y, Zhou J, et al. Phase 1/2 study of ceritinib in Chinese patients with advanced anaplastic lymphoma kinase-rearranged non-small cell lung cancer previously treated with crizotinib: results from ASCEND-6. Lung Cancer. 2020;150:240–6.
Wolf J, Helland Å, Oh IJ, Migliorino MR, Dziadziuszko R, Wrona A, et al. Final efficacy and safety data, and exploratory molecular profiling from the phase III ALUR study of alectinib versus chemotherapy in crizotinib-pretreated ALK-positive non-small-cell lung cancer. ESMO Open. 2022;7(1):100333.
Shaw AT, Gandhi L, Gadgeel S, Riely GJ, Cetnar J, West H, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17(2):234–42.
Gettinger SN, Bazhenova LA, Langer CJ, Salgia R, Gold KA, Rosell R, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(12):1683–96.
Acknowledgements
We thank the patients, their families, and the teams who participated in this study. We also thank Dr. Liling Huang and Dr. Haizhu Chen (National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China) for providing editing assistance, and Yunjie Yu (an employee of Qilu Pharmaceutical Co., Ltd., Jinan, China) and Yan Sun (a former employee of Qilu Pharmaceutical Co., Ltd., Jinan, China) for providing medical writing support.
Funding
This work was funded by Qilu Pharmaceutical Co., Ltd., Jinan, China and partly supported by China National Major Project for New Drug Innovation (2017ZX09304015).
Qilu Pharmaceutical Co., Ltd., Jinan, China played a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication and as such is included in the author list and acknowledgments.
Author information
Authors and Affiliations
Contributions
Conception and design: YKS and MMS. Administrative support: YKS, MMS, and HZG. Provision of study material or patients: all authors. Collection and assembly of data: all authors. Data analysis and interpretation: YKS, MMS, and HL. Manuscript writing and revision: all authors. All authors read and approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonisation guidelines for Good Clinical Practice. The study protocol was approved by the ethics committees of each participating hospital. All patients provided written informed consent before participating in the study.
Consent for publication
Not applicable.
Competing interests
Meimei Si, Hui Li, and Xiaoyan Kang are employees and Huaize Geng was a former employee of Qilu Pharmaceutical Co., Ltd., Jinan, China. All the other authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Summary of comorbidities in ≥ 5% patients in FAS. Table S2. Investigator-assessed efficacy of iruplinalkib in FAS. Figure S1. Waterfall plots of the subgroup BOR of target lesions by IRC in FAS. Figure S2. Waterfall plot of BOR by investigator in FAS. Figure S3. Swimmer plot of iruplinalkib exposure and response by investigator in FAS. Figure S4. Minimum plasma iruplinalkib concentration at each time point in the pharmacokinetics analysis set.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shi, Y., Chen, J., Zhang, H. et al. Efficacy and safety of iruplinalkib (WX-0593) in ALK-positive crizotinib-resistant advanced non-small cell lung cancer patients: a single-arm, multicenter phase II study (INTELLECT). BMC Med 21, 72 (2023). https://doi.org/10.1186/s12916-023-02738-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-023-02738-5