Abstract
Background
Colorectal cancer (CRC) screening reduces all-cause and CRC-related mortality. New research demonstrates that the faecal haemoglobin concentration (f-Hb) may indicate the presence of other serious diseases not related to CRC. We investigated the association between f-Hb, measured by a faecal immunochemical test (FIT), and both all-cause mortality and cause of death in a population-wide cohort of screening participants.
Methods
Between 2014 and 2018, 1,262,165 participants submitted a FIT for the Danish CRC screening programme. We followed these participants, using the Danish CRC Screening Database and several other national registers on health and population, until December 31, 2018. We stratified participants by f-Hb and compared them using a Cox proportional hazards regression on all-cause mortality and cause of death reported as adjusted hazard ratios (aHRs). We adjusted for several covariates, including comorbidity, socioeconomic factors, demography and prescription medication.
Results
We observed 21,847 deaths in the study period. Our multivariate analyses indicated an association relationship between increasing f-Hb and the risk of dying in the study period. This risk increased steadily from aHR 1.38 (95% CI: 1.32, 1.44) in those with a f-Hb of 7.1–11.9 μg Hb/g faeces to 2.20 (95% CI: 2.10, 2.30) in those with a f-Hb ≥60.0 μg Hb/g faeces, when compared to those with a f-Hb ≤7.0 μg Hb/g faeces. The pattern remained when excluding CRC from the analysis. Similar patterns were observed between incrementally increasing f-Hb and the risk of dying from respiratory disease, cardiovascular disease and cancers other than CRC. Furthermore, we observed an increased risk of dying from CRC with increasing f-Hb.
Conclusions
Our findings support the hypothesis that f-Hb may indicate an elevated risk of having chronic conditions if causes for the bleeding have not been identified. The mechanisms still need to be established, but f-Hb may be a potential biomarker for several non-CRC diseases.
Similar content being viewed by others
Background
Colorectal cancer (CRC) screening with faecal testing significantly impacts CRC mortality sufficiently to affect all-cause mortality [1,2,3,4,5,6,7,8]. The quantitative faecal immunochemical test (FIT) is the most frequently used test [9]. In the Danish CRC screening programme, participants with a positive FIT (faecal haemoglobin concentration (f-Hb) ≥100 ng Hb/mL buffer or ≥20 μg Hb/g faeces) are referred for colonoscopy. 37.0% of these colonoscopies reveal no neoplastic findings that explain the bleeding [10]. This issue is not unique to the Danish screening programme, and the sensitivity of the FIT is often discussed [11]. The presence of haemoglobin in the faeces without an obvious source of bleeding could indicate early-stage non-communicable chronic disease [12]. Recent studies suggest that f-Hb is associated with several seemingly unrelated chronic conditions and causes of death, including cardiovascular disease (CVD), respiratory disease and/or neuropsychological disorders [13,14,15,16]. However, these studies are limited by either the use of guaiac-based faecal occult blood test (gFOBT) instead of FIT, the lack of prescription medication associated with gastrointestinal (GI) bleeding as a covariate and/or the lack of individual-level adjustment for confounders. Therefore, we conducted a register-based study investigating the association between f-Hb and both all-cause mortality and cause-specific mortality in a population-based, FIT-tested cohort of participants in the Danish CRC screening programme.
Methods
Study population
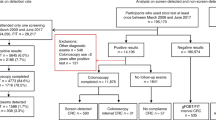
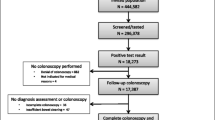
Our study population consisted of all individuals invited to the Danish Colorectal Cancer Screening Program (DCCSP) who had submitted an eligible FIT in the first round of screening. The programme started in 2014, and the last invitations for the first screening round were distributed in 2017. All Danish citizens in the eligible age group (50–74) were invited to participate by submitting a faeces sample. The participant collects one sample of approximately 10 mg of faeces using the OC-Auto Sampling bottle 3 (Eiken Chemical Co, Japan) with the supplied probe and places it in a vial containing 2.0 mL of buffer. The vial is then shipped by prioritized mail to one of five medical laboratories, where it is analysed on the day of receipt. Each medical laboratory covers all FIT assays from their respective healthcare regions and is accredited by the ISO 15189 standard for quality and competencies. If it arrives during the weekend, the test is refrigerated and analysed the coming Monday. The sample remains staple for 7 days at room temperature and 14 days at 2–10°C. The result is sent to the participants within 7 days. If a sample is ineligible for analysis (due to e.g. absence of identification label or inadequate sample material), the patient is sent a new collection kit and a letter explaining the issue. All analyses have been completed using the OC-Sensor Diana instruments (Eiken Chemical Co, Japan). The manufacturer-reported lower limit of detection of the OC-Sensor analyser is 10 μg Hb/g, but a lower limit of 3.8 μg Hb/g faeces has been reported [17, 18]. Mean concentration in unspiked collection tubes has been reported at 2.1 μg Hb/g faeces for the analyser [18]. A quantification limit of 4 μg Hb/g faeces with a variation coefficient of 20% was reported in a recent study [19]. In the DCCSP, the quantitative FIT is considered positive when f-Hb concentration above the positivity threshold of 20 μg Hb/g faeces is detected. All FIT concentrations between 0 and 7.0 μg Hb/g faeces are reported as 7.0 μg Hb/g faeces by the DCCSD. Therefore, all participants with ≤7.0 μg Hb/g faeces were grouped together. In the period 2014–2016, the FIT participation rate was 62.6%, with a positivity rate of 6.9%. 89.1% of those testing FIT positive subsequently underwent colonoscopy [20]. The study baseline was set as the FIT analysis date for each individual. Participants from the first round of screening were followed from baseline to death, migration or end of follow-up (31/12/2018). The flow of participants is presented in Fig. 1.
Data sources
We conducted an individual-level follow-up for all participants linking national registers on health and population. The study population was identified through the Danish Colorectal Cancer Screening Database (DCCSD), which contains data from the integrated Invitation and Administration Module, the Danish National Patient Register (DNPR) and the National Pathology Register [21]. The DCCSD provides data on screening participation, basic demographics, screening course and screening outcomes. We supplemented this with data from the DNPR on hospital admissions, diagnoses and treatments [22]. Information on prescription medication was obtained from the Danish National Prescription Register [23]. We obtained data on time and cause of death from the Danish Register of Cause of Death (DRCD). Both the underlying and contributing causes registered in the DRCD were used to identify the cause of death to reduce the impact of registration errors [24]. Furthermore, data on socioeconomic factors were obtained by the Danish Education Register [25] and the Income Statistics Register [26].
Outcome and exposure
We considered all-cause mortality and disease-specific causes of death as individual outcomes. We also included all-cause mortality excluding CRC as an outcome to allow a more nuanced interpretation of the results. The cause of death designation was based on ICD-10 codes. We used the following categories for the cause of death: respiratory disease (excluding lung cancer), diabetes, CVD, other (non-colorectal) cancers and CRC. The definitions are presented in Additional file 1: Table S1. Our main exposure was the f-Hb for all participants presented as an ordinal variable. The concentrations were categorized as ≤7.0 μg Hb/g faeces, 7.1–11.9 μg Hb/g faeces, 12.0–19.9 μg Hb/g faeces, 20.0–59.9 μg Hb/g faeces and ≥60.0 μg Hb/g faeces.
Covariates
We included a range of baseline covariates using data from the different registers. These were sex, age, highest level of completed education, annual income, conditions suspected of causing GI bleeding (such as inflammatory bowel disease and diverticular disease), prescription medication (e.g. aspirin) and comorbidity. Age was divided into the following categories “<55 years”, “55-59 years”, “60-64 years”, “65-69 years” and “>69 years”. The highest level of completed education was defined as “Elementary school”, “Vocational/high school”, “Short-medium length education” and “Long education”. We used person-equated inflation-adjusted income that considered the number of household adults and children of each participant. To allow for income fluctuations, we included the measurement as a 5-year average from baseline and back and categorized participants into quartiles based on income levels. Category 1 is the lowest, and category 4 the highest. We identified participants diagnosed with conditions suspected of causing overt GI bleeding and, therefore a positive FIT, up to 5 years before baseline. The definitions are presented in Additional file 1: Table S2. We included prescription medications known to cause GI bleeding as a covariate for the same reasons. We included all prescriptions collected up to 16 weeks before the FIT analysis date. The medication is detailed in Additional file 1: Table S2.
Comorbidity
We expected existing comorbidity at baseline to affect both survival and cause of death. We, therefore, employed a standard Charlson Comorbidity Index (CCI) score categorized as “0”, “1” and “>1” for the survival analysis [27]. For the causes of death, we expected that having e.g. CVD at baseline would strongly influence the risk of dying from it. Hence, using only CCI to adjust for comorbidity would lead to an underestimation of the actual effect since there would be no distinction between the condition of interest and the other diseases included in the CCI. Therefore, we created five additional versions of the CCI, one for each of the causes of death. We removed any condition suspected of contributing extensively to the outcome from the CCI and added it as a separate covariate instead. Using diabetes as an example, this allowed us to investigate the association between f-Hb and diabetes mortality while accounting for both the effect of having diabetes and the effect of having other serious diseases at baseline. As a result, we have one covariate for the baseline presence of disease for each of the causes of death, reported as disease present at baseline “Yes” or “No”. The modified CCI scores are reported just like the unmodified version: “0”, “1” and “>1”. The excluded parts are presented in Additional file 1: Table S3.
Statistics
Descriptive statistics were employed to compare the groups of study participants. Kaplan-Meier curves were used to present mortality and cause of death developments. Cox proportional hazards regression models were used to investigate differences between the exposure groups. The control group was those with a f-Hb of <7.0 μg Hb/g faeces. Results are reported as adjusted hazard ratios (aHRs) or crude hazard ratios (HRs) both with 95% confidence intervals. Both univariate and multivariate analyses were conducted for all outcomes. Log-log plots were used to assess the proportional hazards assumptions. Participants with missing data on one or more covariates were excluded from the analysis. We investigated the presence of effect modification on all outcomes and significant covariates. A sensitivity analysis excluding the participants with a prescription for any anticoagulant medication (ATC-code: B01A) was conducted. All analyses were performed in Stata 17 [28].
Results
Characteristics
During the first round of screening in Denmark, 1,262,165 invited residents submitted an eligible FIT sample. We excluded 33,800 (2.68%) due to either missing data or registry errors. The remaining study population comprised 1,228,365 (97.3%) participants, and it was divided into five categories based on the f-Hb. Table 1 demonstrates the observed differences between our exposure groups’ several covariates. Increasing the f-Hb was associated with an increase in the proportion of males, participants who were older and of lower education level and income, had GI bleeding at baseline, were issued prescription medications suspected of causing GI bleeding, had lower education, had lower income and had a higher frequency of comorbidity.
21,857 (1.78%) participants died in the study period, 630 from CRC and 21,227 of causes other than CRC (Table 2). The overall tendency was an increase in the proportion of deaths with increasing f-Hb, which rose steadily from 1.41% of those with a f-Hb ≤7.0 μg Hb/g faeces to 5.18% of those with f-Hb ≥60.0 μg Hb/g faeces. A similar pattern was observed when comparing the f-Hb groups on the probability of dying from the different causes of death, as presented in Figs. 2 and 3. The median follow-up time for the entire population was 2.68 (interquartile range, 1.77–3.67) years with an average follow-up of 2.70 (±1.12) years. Among those who died, the median follow-up was 1.88 (interquartile range, 0.97–2.71) years, with an average follow-up of 1.88 (±1.11) years.
Faecal haemoglobin concentration, all-cause mortality and cause of death
The results of our multivariate analyses are presented in Fig. 4. All-cause mortality aHR increased with f-Hb from 1.38 (95% CI: 1.32, 1.44) in those with a f-Hb of 7.1–11.9 μg Hb/g faeces to 2.20 (95% CI: 2.10, 2.30) for those with f-Hb ≥60.0 μg Hb/g faeces when compared to those ≤7.0 μg Hb/g faeces. Considering all-cause mortality after excluding CRC deaths showed the same increasing trend in aHRs from 1.37 (95% CI: 1.31, 1.43) in those with f-Hb 7.1–11.9 μg Hb/g faeces to 1.98 (95% CI: 1.89, 2.08) in those with f-Hb ≥60.0 μg Hb/g faeces.
A similar trend was seen in the risk of dying from respiratory disease and CVD. For diabetes, the cause-specific mortality increased with increasing f-Hb for the groups ≥20.0 μg Hb/g faeces. However, confidence intervals were wide and overlapping. The risk of dying from other cancers than CRC also increased with increasing f-Hb, from an aHR of 1.33 (95% CI: 1.24, 1.42) at f-Hb 7.1–11.9 μg Hb/g faeces to 1.77 (95% CI: 1.64, 1.91) at f-Hb ≥60.0 μg Hb/g faeces. In the sensitivity analysis, we repeated the multivariate regression without the 17.7% of participants with a prescription for anticoagulant medication. The analysis did not have any significant impact on the results.
For each outcome, we also conducted univariate analyses yielding the same overall conclusion as the multivariate analyses, which, with the exception of diabetes, presented with a more pronounced association to f-Hb. These findings are presented in Additional file 1: Fig. S1. The predictive value of f-Hb for all-cause mortality was further investigated by exploring the margins of the multivariate Cox regression. The results are presented in Additional file 1: Fig. S2.
Colorectal cancer mortality
Results from the univariate and multivariate analysis are presented in Fig. 5 and show an increase in the risk of CRC death with increasing f-Hb when compared to those with f-Hb ≤7.0 μg Hb/g faeces. Multivariate analysis showed that participants with f-Hb of 7.1–11.9 μg Hb/g faeces had a 1.84 (95% CI: 1.35, 2.50) times higher risk of dying from CRC. This increased to 4.71 (95% CI: 3.41, 6.53) for those with f-Hb of 12.0–19.9 μg Hb/g faeces. For those with f-Hb of 20.0–59.9 μg Hb/g faeces, the risk of dying from CRC was 4.08 (95% CI: 3.05, 5.46) times higher. Participants with a f-Hb of ≥60 μg Hb/g were 16.22 (95% CI: 13.51, 19.49) times more likely to die from CRC than the control group.
Discussion
Our results indicate that f-Hb is associated with all-cause mortality and seemingly unrelated causes of death in a cohort of screening participants in the Danish national CRC screening program. The significance of our findings is underlined by a significant association relationship between f-Hb and all-cause mortality. For causes of death, this effect was especially notable with CVD and respiratory disease. However, the increased risk of dying from other cancers seems to plateau at f-Hb of ≥20.0 μg Hb/g faeces. In addition, an association between diabetes mortality and f-Hb was observed, although a low number of events and a wide CI reduce interpretability. Since diabetes is a common disease, it is possible that the cause of death in some diabetics may be registered to complications of the disease rather than diabetes itself [29]. Less surprising was the observed relationship between f-Hb and CRC, which was particularly pronounced in the group with the highest f-Hb. However, the increase in aHR appeared to plateau in participants with f-Hb ≥20.0 μg Hb/g faeces, i.e. those just above the Danish positivity threshold. This should be interpreted cautiously as we have a low number of CRC deaths in each category, but one suggested explanation is that this stagnation in mortality around the positivity threshold shows the impact of CRC screening on the mortality curve.
The role of f-Hb in diseases with a systemic inflammatory component represents a growing area of research. A recent review summarizes the hypotheses for the observed patterns, suggesting that elevated f-Hb is an indicator of systemic inflammation and possibly a marker of susceptibility to non-communicable diseases [12]. Recently, Scottish and Danish researchers presented findings that indicated a relationship between f-Hb (measured by gFOBT) and both all-cause mortality and seemingly unrelated causes of death in CRC screening populations with very long follow-up [15, 16]. Korean researchers categorized a FIT-based screening population as either FIT positive or negative, concluding that FIT-positive participants had a higher risk of dying from non-CRC causes, circulatory disease and respiratory disease [30]. Furthermore, in a study from Taiwan, there was a similar dose-response relationship between f-Hb concentration on all-cause mortality and CRC mortality in a cohort of CRC screening participants [31].
Other studies have focused on the relationship between f-Hb and the likelihood of having an underlying non-communicable disease. Japanese researchers have demonstrated an association between FIT positivity and concentrations of glycated Hb in an apparently healthy population [13]. Furthermore, a South Korean study showed increasing incidence rates of diabetes with an increasing number of positive FIT during a 6½-year follow-up period [32]. In a large community-based study, researchers from Taiwan suggested a significantly increased risk of developing and dying from CVD with increasing f-Hb [33]. Lastly, Moon et al. support these findings by showing an increased risk of myocardial infarction, ischemic stroke and all-cause mortality in the FIT-positive population [34]. Other studies have investigated the association between f-Hb and the development of inflammatory diseases. One study presents a 16.0% higher risk of developing rheumatoid arthritis among the FIT positive in a large population of Korean screening participants [35]. Moreover, a Taiwanese study on periodontal disease and f-Hb concentrations reported that participants with a concentration of ≥20.0 μg Hb/g faeces had a 33.0% higher risk of their periodontal disease deteriorating to severe when compared to those with f-Hb concentrations <20.0 μg Hb/g faeces [36]. In another study, the authors found that having a positive FIT result was associated with an increased risk of having psoriasis. The authors highlighted the association between psoriasis and a number of conditions documented to be associated with elevated f-Hb such as metabolic syndrome, dyslipidaemia, obesity, diabetes and hypertension [37]. Moreover, a Taiwanese study has shown an association between metabolic syndrome and elevated f-Hb concentrations [38]. In extension, a Taiwanese study on air pollution and CRC reports that high concentrations of ambient fine particulate matter (known to cause inflammation) increase the risk of having a positive FIT by 11.0% [39]. Scottish researchers found an association between the consumption of medication as a proxy marker for disease and f-Hb. Their findings suggest an increased risk of having heart diseases, diabetes, hypertension and depression if participants have a positive gFOBT result [14]. Our results suggest that several characteristics such as higher age, male sex, lower education and lower income are more common among those with elevated f-Hb concentrations. This is consistent with existing literature [40].
Considering the findings of these studies in the context of our results, there appears to be mounting evidence that f-Hb does indicate the presence of non-communicable diseases and since many of the reported associations have a systemic inflammatory component, the proposed relationship between inflammation and GI bleeding seems plausible. However, the mechanisms underlying these associations remain elusive. An explanation is that the use of medications known to cause GI bleeding, such as oral anticoagulants, aspirin, corticosteroids and non-steroidal anti-inflammatory drugs, could affect the associations presented in this study. However, the inclusion of these drug categories as a confounding factor in the multivariate analysis strengthens the interpretability of our results. This, combined with differing conclusions from recent meta-analyses, reduces our concern about the impact of these drugs on the overall interpretation [41, 42]. Another potentially contributing factor to the elevated f-Hb could be diseases that cause bleeding in the GI tract. The immunochemical response of the FIT should in theory, not detect bleedings originating from the upper GI tract due to the digestion of the haemoglobin proteins. However, FIT-positive participants with a negative colonoscopy have been reported to have a higher risk of some upper GI cancers [43]. While the scientific evidence on this topic is limited and findings incoherent, it cannot be ruled out that upper GI bleedings can affect the FIT measured f-Hb [43, 44]. It is also possible that lower GI bleedings from sources such as haemorrhoids, diverticular diseases and inflammatory bowel disease may impact the reported f-Hb to some degree. While a relationship between false-positive FIT result (positive FIT and negative colonoscopy) and both inflammatory bowel disease and other non-neoplastic findings (such as anal fissures) has been reported, no association was found with diverticulosis and haemorrhoids in the same studies [45, 46]. To mitigate this uncertainty, we accounted for the potential effect of both types of bleeding in our multivariate analyses.
The strengths of our study include a very large population of screening participants, extensive and individual-level adjustment for confounding effects and our ability to adjust for a numerical f-Hb. Limitations include the risk of misclassification in the registrations from the DRCD, which is affected by the input quality. We have addressed this by including contributing causes of death in the analyses and have no indications that misclassification could confound the results to any significant extent. Limited follow-up is another disadvantage of our study, but given the size of our population, the use of time-sensitive analyses and the coherence to other studies with longer follow-up, we do not believe that this affects the overall interpretation of our results. Data on over-the-counter medication is not available in Danish registers and represents another limitation. This may result in some underestimation of the effect medications such as aspirin have on the f-Hb. However, because we were able to adjust for the effect of prescription medication, the impact of this limitation is reduced.
Assuming f-Hb is an effective and practical biomarker for non-communicable diseases, several new pathways focusing on maximizing the diagnostic gain from CRC screening and diagnostic initiatives in other clinical areas could be established. For instance, a study from China suggests using f-Hb to predict complications and survival after R0 gastrectomy [47].
Conclusions
Our study is the first to investigate the association between f-Hb and mortality in a way that addresses many of the limitations of previous studies. We observed an association between f-Hb and both all-cause mortality and cause-specific mortality. Our results show that individuals with increased f-Hb are more prone to die from non-communicable diseases seemingly unrelated to CRC, including respiratory diseases, CVD and other cancers. In many of these associations, we observed an association between f-Hb and mortality. Overall, these findings emphasize the distinct potential of f-Hb as a biomarker for non-communicable conditions other than CRC and the need for future research in this area.
Availability of data and materials
The data used in this study have been collected from several different Danish registers and access using secure servers of Statistics Denmark. As we do not own this data, we are not able to make it available ourselves. Given the proper clearances however, others will be able to apply Statistics Denmark for access to the same raw data used in this study.
Abbreviations
- aHR:
-
Adjusted hazard ratio
- CCI:
-
Charlson Comorbidity Index
- CRC:
-
Colorectal cancer
- CVD:
-
Cardiovascular disease
- DCCSD:
-
The Danish Colorectal Cancer Screening Database
- DNPR:
-
The Danish National Patient Register
- DRCD:
-
The Danish Register of Cause of Death
- f-Hb:
-
Faecal haemoglobin concentration
- FIT:
-
Faecal immunochemical test
- gFOBT:
-
Guaiac faecal occult blood test
- GI:
-
Gastrointestinal
- HR:
-
Hazard ratio
References
Kronborg O, Fenger C, Worm J, Pedersen SA, Hem J, Bertelsen K, et al. Causes of death during the first 5 years of a randomized trial of mass screening for colorectal cancer with fecal occult blood test. Scand J Gastroenterol. 1992;27(1):47–52.
Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348(9040):1472–7.
Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365–71.
Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106–14.
Scholefield JH, Moss SM, Mangham CM, Whynes DK, Hardcastle JD. Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut. 2012;61(7):1036–40.
Shaukat A, Kaalby L, Baatrup G, Kronborg O, Duval S, Shyne M, et al. Effects of screening compliance on long-term reductions in all-cause and colorectal cancer mortality. Clin Gastroenterol Hepatol. 2021;19(5):967–75.e2.
Cardoso R, Guo F, Heisser T, Hackl M, Ihle P, De Schutter H, et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: an international population-based study. Lancet Oncol. 2021;22(7):1002–13.
Bretthauer M, Løberg M, Wieszczy P, Kalager M, Emilsson L, Garborg K, et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. 2022;387(17):1547–56.
Lee MW, Pourmorady JS, Laine L. Use of fecal occult blood testing as a diagnostic tool for clinical indications: a systematic review and meta-analysis. Am J Gastroenterol. 2020;115(5):662–70.
Danish Colorectal Cancer Screening Database. Dansk tarmkræftscreeningsdatabase årsrapport 2018 [Annual report of the Danish Colorectal Cancer Screening Database 2018]. 2020. Available from: https://www.rkkp.dk/siteassets/forside/databaser/arsrapporter/dts-arsrapport2018_anonymiseret_endelig_marts2020.pdf.
Kaminski MF, Robertson DJ, Senore C, Rex DK. Optimizing the quality of colorectal cancer screening worldwide. Gastroenterology. 2020;158(2):404–17.
Barnett KN, Clark GRC, Steele RJC, Fraser CG. Faecal haemoglobin estimated by faecal immunochemical tests-an indicator of systemic inflammation with real clinical potential. Diagnostics. 2021;11(11):2093.
Nakajima K, Suwa K. Association between positive fecal occult blood test and diabetes in a population undergoing health screening. Clin Biochem. 2017;50(1-2):97–100.
Libby G, Barnett KN, Fraser CG, Steele RJC. Association between faecal occult bleeding and medicines prescribed for chronic disease: a data linkage study. J Clin Pathol. 2021;74(10):664–7.
Kaalby L, Al-Najami I, Deding U, Berg-Beckhoff G, Steele RJC, Kobaek-Larsen M, et al. Cause of death, mortality and occult blood in colorectal cancer screening. Cancers. 2022;14(1):246.
Libby G, Fraser CG, Carey FA, Brewster DH, Steele RJC. Occult blood in faeces is associated with all-cause and non-colorectal cancer mortality. Gut. 2018;67(12):2116–23.
Gies A, Cuk K, Schrotz-King P, Brenner H. Direct comparison of ten quantitative fecal immunochemical tests for hemoglobin stability in colorectal cancer screening. Clin Transl Gastroenterol. 2018;9(7):168.
Guildford Medical Device Evaluation Centre. Evaluation of quantitative faecal immunochemical tests for haemoglobin. Guildford: NHS Bowel Cancer Screening Southern Programme HUB; 2014. Available from: https://www.faecal-immunochemical-test.co.uk/wp-content/uploads/2018/06/FIT_007-Evaluation-of-Quantitative-Faecal-Immuncohemical-Tests-for-Haemoglobin.pdf
Plantener E, Deding U, Madsen JB, Kroijer R, Madsen JS, Baatrup G. Using fecal immunochemical test values below conventional cut-off to individualize colorectal cancer screening. Endosc Int Open. 2022;10(4):E413–e9.
Njor SH, Friis-Hansen L, Andersen B, Sondergaard B, Linnemann D, Jorgensen JCR, et al. Three years of colorectal cancer screening in Denmark. Cancer Epidemiol. 2018;57:39–44.
Thomsen MK, Njor SH, Rasmussen M, Linnemann D, Andersen B, Baatrup G, et al. Validity of data in the Danish Colorectal Cancer Screening Database. Clin Epidemiol. 2017;9:105–11.
Lynge E, Sandegaard J, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39:4.
Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 Suppl):38–41.
Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health. 2011;39(7 Suppl):26–9.
Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health. 2011;39(7 Suppl):91–4.
Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health. 2011;39(7 Suppl):103–5.
Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–82.
StataCorp. Stata Statistical Software: release 17. College Station, TX: StataCorp LLC.; 2021.
Pearson-Stuttard J, Bennett J, Cheng YJ, Vamos EP, Cross AJ, Ezzati M, et al. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 2021;9(3):165–73.
Jung YS, Lee J, Moon CM. Positive fecal immunochemical test results are associated with non-colorectal cancer mortality. Korean J Intern Med. 2022;37(2):313–21.
Chen L-S, Yen AM-F, Fraser CG, Chiu SY-H, Fann JC-Y, Wang P-E, et al. Impact of faecal haemoglobin concentration on colorectal cancer mortality and all-cause death. BMJ Open. 2013;3(11):e003740.
Kim KW, Lee HJ, Han K, Moon JM, Hong SW, Kang EA, et al. Risk of diabetes in subjects with positive fecal immunochemical test: a nationwide population-based study. Endocrinol Metab. 2021;36(5):1069–77.
Chien KL, Lin TY, Hsu CY, Chan CC, Chen TH, Chen LS. Association between faecal haemoglobin concentration and the risk of cardiovascular diseases among Taiwanese adults in a community-based screening cohort. BMJ Open. 2020;10(6):e032633.
Moon JM, Lee HJ, Han K, Kim DH, Hong SW, Soh H, et al. Occult blood in feces is associated with an increased risk of ischemic stroke and myocardial infarction: a nationwide population study. J Am Heart Assoc. 2021;10(1):e017783.
Noh C-K, Lee E, Park B, Ahn SS. A positive faecal immunochemical test result and its association with the incidence of rheumatoid arthritis, systemic lupus erythematosus, and psoriatic arthritis: an analysis of one-million national colorectal cancer screening programme results. BMC Med. 2022;20(1):226.
Yen AM, Lai H, Fann JC, Chiu SY, Chen SL. Relationship between community periodontal index and fecal hemoglobin concentration, an indicator for colorectal neoplasm. J Dent Res. 2014;93(8):760–6.
Lee HJ, Han K, Soh H, Koh SJ, Im JP, Kim JS, et al. Occult blood in feces is associated with increased risk of psoriasis. Dermatology. 2022;238(3):571–8.
Ku M-S, Fann JC-Y, Chiu SY-H, Chen H-H, Hsu C-Y. Elucidating bidirectional relationship between metabolic syndrome and elevated faecal haemoglobin concentration: a Taiwanese community-based cohort study. BMJ Open. 2019;9(3):e021153.
Ku MS, Liu CY, Hsu CY, Chiu HM, Chen HH, Chan CC. Association of ambient fine particulate matter (PM(2.5)) with elevated fecal hemoglobin concentration and colorectal carcinogenesis: a population-based retrospective cohort study. Cancer Control. 2021;28:10732748211041232.
Clark GRC, Strachan JA, McPherson A, Digby J, Mowat C, Steele RJC, et al. Faecal haemoglobin distributions by sex, age, deprivation and geographical region: consequences for colorectal cancer screening strategies. Clin Chem Lab Med. 2020;58(12):2073–80.
Nieuwenburg SAV, Vuik FER, Kruip M, Kuipers EJ, Spaander MCW. Effect of anticoagulants and NSAIDs on accuracy of faecal immunochemical tests (FITs) in colorectal cancer screening: a systematic review and meta-analysis. Gut. 2019;68(5):866–72.
Jung YS, Im E, Park CH. Impact of antiplatelet agents and anticoagulants on the performance of fecal immunochemical tests: a systematic review and meta-analysis. Surgical Endoscopy. 2022;36(6):4299–311.
Jung YS, Lee J, Moon CM. Positive fecal immunochemical test results are associated with increased risks of esophageal, stomach, and small intestine cancers. J Clin Med. 2020;9(7):2172.
Kim NH, Yang HJ, Park SK, Park JH, Park DI, Sohn CI, et al. Does low threshold value use improve proximal neoplasia detection by fecal immunochemical test? Dig Dis Sci. 2016;61(9):2685–93.
Stegeman I, de Wijkerslooth TR, Stoop EM, van Leerdam M, van Ballegooijen M, Kraaijenhagen RA, et al. Risk factors for false positive and for false negative test results in screening with fecal occult blood testing. Int J Cancer. 2013;133(10):2408–14.
Amitay EL, Cuk K, Niedermaier T, Weigl K, Brenner H. Factors associated with false-positive fecal immunochemical tests in a large German colorectal cancer screening study. Int J Cancer. 2019;144(10):2419–27.
Lu J, Xu B, Xu Y, Wu Y, Xie J, Wang J, et al. A novel insight into fecal occult blood test for the management of gastric cancer: complication, survival, and chemotherapy benefit after r0 resection. Front Oncol. 2020;10:526746.
Acknowledgements
We would like to acknowledge Open Patient data Explorative Network for their support in the early phases of the study.
Funding
This study was supported by The Danish Cancer Society (R100-A6747), Odense University Hospital (A3472/A2359) and the US Department of Veterans Affairs (36C24E18P0155) and the University of Southern Denmark. The funders did not influence or take part in the study in any way.
Author information
Authors and Affiliations
Contributions
LK, IA and GB conceptualized the study. Data curation was done by LK and UD. The formal analysis was designed by LK, UD, TBM, AK, GBB and GB. The formal analysis was completed by LK, UD and GB. Funding for the project was obtained by LK, UD and GB. LK, UD, IA, GBB, TBM, TL, AS, RS, AK, MR, MK and GB contributed to the development of the methodology. Project administration was handled by LK. Supervision was conducted by GB. The original draft was done by LK, supported by UD, IA, TL, AK and GB. LK, UD, IA, GBB, TBM, TL, AS, RS, AK, MR, MK and GB contributed to the review and editing process. UD has access to the underlying data. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable. In Denmark, register-based studies are exempted from obtaining ethical approvals and patient consent as per the Danish Scientific Ethical Committees Act § 2, part 2.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. The file contains a list of the ICD-10 classification codes used to define cause of death. Table S2. A list of diseases, indications and medications suspected of causing GI bleedings and the corresponding ICD-10 classification or ATC codes. These have all been included as covariates. Table S3. An overview of the adapted CCI’s used as measures of comorbidity. More precisely the table shows which ICD-10 classifications codes were “removed” from the index and added as a separate variable instead. Figure S1. Results from the univariate analysis on mortality outcomes and f-Hb. This provides readers some insight into the impact of adding covariates into the regression analyses. Figure S2. Predicted hazard ratios using the margins of the multivariate Cox regression on overall survival.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kaalby, L., Deding, U., Al-Najami, I. et al. Faecal haemoglobin concentrations are associated with all-cause mortality and cause of death in colorectal cancer screening. BMC Med 21, 29 (2023). https://doi.org/10.1186/s12916-022-02724-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-022-02724-3