Abstract
Background
Observational studies have linked childhood obesity with elevated risk of colorectal cancer; however, it is unclear if this association is causal or independent from the effects of obesity in adulthood on colorectal cancer risk.
Methods
We conducted Mendelian randomization (MR) analyses to investigate potential causal relationships between self-perceived body size (thinner, plumper, or about average) in early life (age 10) and measured body mass index in adulthood (mean age 56.5) with risk of colorectal cancer. The total and independent effects of body size exposures were estimated using univariable and multivariable MR, respectively. Summary data were obtained from a genome-wide association study of 453,169 participants in UK Biobank for body size and from a genome-wide association study meta-analysis of three colorectal cancer consortia of 125,478 participants.
Results
Genetically predicted early life body size was estimated to increase odds of colorectal cancer (odds ratio [OR] per category change: 1.12, 95% confidence interval [CI]: 0.98–1.27), with stronger results for colon cancer (OR: 1.16, 95% CI: 1.00–1.35), and distal colon cancer (OR: 1.25, 95% CI: 1.04–1.51). After accounting for adult body size using multivariable MR, effect estimates for early life body size were attenuated towards the null for colorectal cancer (OR: 0.97, 95% CI: 0.77–1.22) and colon cancer (OR: 0.97, 95% CI: 0.76–1.25), while the estimate for distal colon cancer was of similar magnitude but more imprecise (OR: 1.27, 95% CI: 0.90–1.77). Genetically predicted adult life body size was estimated to increase odds of colorectal (OR: 1.27, 95% CI: 1.03, 1.57), colon (OR: 1.32, 95% CI: 1.05, 1.67), and proximal colon (OR: 1.57, 95% CI: 1.21, 2.05).
Conclusions
Our findings suggest that the positive association between early life body size and colorectal cancer risk is likely due to large body size retainment into adulthood.
Similar content being viewed by others
Background
Childhood obesity is a major global public health challenge with increasing prevalence observed in most geographic regions over the past three decades [1]. The rising prevalence of childhood obesity may have important consequences for population health, irrespective of adiposity later in life, given evidence from observational studies linking early life adiposity with elevated risks of chronic diseases, including cancer [1,2,3,4]. Colorectal cancer has a long latency period suggesting that important exposures might have occurred many years ago and therefore is plausible that childhood and adolescence constitute a critical period during which adiposity influences cancer risk during adulthood [5]. Additionally, obesity during childhood and adolescence has been linked with unfavorable metabolic profiles that may influence cancer risk [6]. Therefore, it is possible that the detrimental role of obesity on colorectal cancer risk during adulthood might have started earlier in life.
Two recent meta-analyses of observational studies have reported positive associations between early life body size measures (in adolescence and early adulthood) and later life colorectal cancer risk in both men and women [7, 8]. Despite these associations, causal inference of the health effects of early life adiposity on later life disease risk can be challenging, as individuals who are obese in childhood often remain so during adulthood [9]. Consequently, it is currently unknown if the prior positive associations between early life adiposity and colorectal cancer risk are a direct effect of early life obesity or to what extent they are mediated by later life adiposity.
Mendelian randomization (MR) uses germline genetic variants as proxies to allow causal inference between a given exposure and outcome [10]. Compared to traditional observational analyses, MR analyses should be less susceptible to conventional confounding and reverse causation, given the randomly allocated and fixed nature of genetic variants [11]. Additionally, multivariable MR allows the estimation of independent effects of multiple exposures (e.g., early life and adult body size) on disease outcomes [12,13,14]. Univariable MR can be used to estimate the total effect of early life body size on colorectal cancer (Fig. 1A), whereas multivariable MR can be used to estimate the effect of childhood obesity specifically on later life chronic disease risk, independently of adult body size [15, 16]. Under the multivariable framework, we hypothesize three scenarios in which early life body size affects colorectal cancer risk after considering adult body size (Fig. 1B–D): firstly, early life body size solely has direct effects on colorectal cancer, which are not influenced by adult body size (Fig. 1B); secondly, early life body size has only indirect effects on colorectal cancer through adult body size (Fig. 1C); thirdly, early life body size can have both direct and indirect effects on colorectal cancer risk (Fig. 1D). The same approach was applied recently to investigate whether early life body size influences risk of several diseases in later life, including breast and prostate cancer, and whether this effect is mediated by body size in adulthood [15].
Directed acyclic graphs displaying four possible scenarios that could explain a causal effect between body size at age 10 years and colorectal cancer within the univariable and multivariable Mendelian randomization analysis. (Top left) The total effect of early life body size on colorectal cancer risk, (top right) early life body size has a direct effect on colorectal cancer risk independent of adult body size, (bottom left) early life body size has an indirect effect on colorectal cancer risk only through adult body size, and (bottom right) early life body size has both direct and indirect effects on colorectal cancer risk. The dashed arrows allude to the assumption that genetic instruments should not be associated with confounding factors
We used a two-sample multivariable MR framework to examine potential causal associations between early life body size and colorectal cancer risk, independent of adult body size. We combined genetic variants associated with recalled early life and measured adult body size from a recent genome-wide association study (GWAS) of 453,169 adults [15] and then examined the association of these variants with colorectal cancer risk in a large consortium of up to 125,478 adults (58,131 cases and 67,347 controls) [17, 18].
Methods
Data on early life and adult body size
Genetic variants associated with early life and adult body size were identified from a recent GWAS of 453,169 participants of European descent from the UK Biobank [15]. For early life body size, participants were asked at baseline: “When you were 10 years old, compared to average would you describe yourself as thinner, plumper, or about average?”. For adult body size, body mass index (BMI) was derived using height (centimeters) and weight (kilograms) measured at baseline. To improve comparability between the different body size traits, BMI in adults was converted into a categorical variable with three groups like early life body size. A linear regression model was applied, assuming similar effects of a given single nucleotide polymorphism (SNP) on moving from the lowest to the middle and from the middle to the highest category of the body size variables. The genome-wide significant (P < 5 × 10−8) variants identified in this GWAS were pruned based on a linkage disequilibrium (LD) level of R2 < 0.001 using genotype data from European individuals from phase 3 (version 5) enrolled in the 1000 genomes project as a reference panel [15]. The resulting instruments for early life body size (305 SNPs) and adult body size (557 SNPs) explained 4.5% and 6.4% of variability in these traits, respectively. Early life and adult body size genetic instruments were comprised of 138 and 215 SNPs for women and 68 and 159 SNPs for men, respectively (Additional file 1: Table S1). These genetic instruments have been used in prior MR studies and validated in external cohorts [15, 16, 19, 20]. The genetic correlation between early life and adult body size in UK Biobank was found to be 0.61 [15].
Data on colorectal cancer
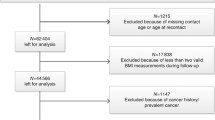
Summary data for the associations of the early life and adult body size related genetic variants with colorectal cancer (overall and by site: colon, proximal colon, distal colon, rectum) were obtained from a GWAS of 125,478 adults (58,131 cancer cases and 67,347 controls) within the ColoRectal Transdisciplinary Study (CORECT), the Colon Cancer Family Registry (CCFR), and the Genetics and Epidemiology of Colorectal Cancer (GECCO) consortium [17]. Imputation was performed using the Haplotype Reference Consortium (HRC) r1.0 reference panel and regression models were further adjusted for age, sex, genotyping platform, and genomic principal components as detailed here [17]. Subsite specific estimates by sex were obtained from a GWAS of 112,373 adults (48,214 cancer cases and 64,159 controls) within the same consortia [18]. Colorectal cancer estimates for each SNP are presented in Additional file 1: Table S2. The summary statistics that were provided in the current analysis did not include UK Biobank study to avoid potential overlap between the two datasets. The final sample included 98,715 participants (52,775 cancer cases and 45,940 controls). Additional file 1: Table S3 presents the final number of cancer cases by sex and subsite.
Statistical analysis
Power calculations
The a priori statistical power was calculated using an online tool at http://cnsgenomics.com/shiny/mRnd/ [21]. For early life body size, given a type 1 error of 5%, there was sufficient power (> 80%) to detect an odds ratio (OR) ≥ 1.10 for overall colorectal cancer per increase in odds conferred for each category change for both sexes combined, while an OR ≥ 1.15 was needed for the cancer sub-site analyses. In sex specific analyses, there was 80% power to detect an OR ≥ 1.21 and 1.16 or men and women, respectively. For adult body size, given a type 1 error of 5%, there was > 80% power to detect an OR ≥ 1.09 for overall colorectal cancer increase in odds conferred for each category change and an OR ≥ 1.17 in sex specific analyses. Additional file 1: Table S4 presents in more detail the statistical power for the two exposures and all outcomes in sex combined and specific analyses.
Univariable MR analysis to estimate the total effect of early and adult body size on colorectal cancer
A two-sample MR approach using summary data and the fixed-effect inverse-variance weighted method was implemented. Where Cochran’s Q statistics identified heterogeneity across the individual SNPs in the early life and adult body size instruments, random-effect inverse-variance weighted analyses were conducted [22,23,24]. Univariable MR analyses for men and women combined were conducted to estimate the effect of both early life and adult body size independently on colorectal cancer risk. Analyses according to sex and tumor anatomical subsite were also conducted. Heterogeneity of associations according to sex and colorectal anatomical subsites was assessed by calculating the χ2statistic [25]. The strength of each instrument was measured by calculating the F-statistic using the following formula: F = R2 ∗ (N − 2)/(1 − R2), where R2 is the proportion of the variability of the phenotype explained by each instrument and N the sample size of the GWAS for the exposures, with values below 10 denoting the presence of weak instrument bias [26, 27].
Multivariable MR analysis to estimate the independent (direct) effects of early and adult body size on colorectal cancer
Due to the correlation between genetic determinants of early life and adult body size, any difference between the total and independent (direct) effects of later life body size is likely driven by pleiotropy if there is also a direct effect of early body size on colorectal cancer [15]. Consequently, the focus of our analyses was on the total effect of early body size and the direct effects of early and adult body size. We therefore conducted multivariable MR analyses to estimate the direct effects of early and later life body size on colorectal cancer risk. For multivariable MR, we calculated three quantities: the conditional Fearly life body size, Fadult body size, and Qa. Fearly life body size and Fadult body size to examine the variance explained by the genetic variants on the main (i.e., early life body size) and secondary exposures (i.e., adult body size); again, values over 10 were interpreted to suggest little evidence of weak instrument bias [13]. Qa is a generalization of the Q statistic for the multivariable scenario, where high values based again on a \({\chi}_{L-2}^2\) distribution denote heterogeneity and potential pleiotropy even when corrected for adult body size [13].
Sensitivity analyses
Sensitivity analyses were used to check and correct for the presence of pleiotropy in the estimates. To evaluate the extent to which directional pleiotropy may have affected the causal estimates for the early and adult body size and colorectal cancer association, we used MR-Egger regression [28, 29]. We also computed OR estimates using the complementary weighted-median method which can give valid MR estimates under the presence of horizontal pleiotropy when up to 50% of the included instruments are invalid [30].
Odds ratio estimates from MR analyses reflect the increase in odds conferred for each category change (i.e., thinner to average and average to plumper) in the early and adult life body size phenotypes. All analyses were undertaken using R (version 3.6.3) using the MendelianRandomisation package [31, 32]. LD clumping between early life and adult body size SNPs in the multivariable MR analyses was done using the ieugwasr R package (based on linkage disequilibrium R2 = 0.001) and the plots were created using the forestplot R package [33, 34]. Summary statistics were harmonized using the harmonise_data function within the TwoSampleMR R package. All GWAS were assumed to be coded on the forward strand. The list of SNPs included in the multivariable MR analyses is given in Additional file 1: Table S5.
Reporting guidelines for MR studies were followed (MR STROBE checklist outlined in Additional file 1: Table S6) [35, 36].
Results
Early life body size
Genetically predicted early life body size was estimated to increase risk of colorectal cancer (OR per category change: 1.12, 95% confidence interval [CI]: 0.98–1.27), colon cancer (OR: 1.16, 95% CI: 1.00–1.35), and distal colon cancer (OR: 1.25, 95% CI: 1.04–1.51), although for overall colorectal cancer, the confidence interval crosses the null, while proximal colon and rectal cancer were minimally influenced by early life body size (OR: 1.11, 95% CI: 0.93, 1.32 and OR: 1.14 95% CI: 0.93, 1.38, respectively) (Fig. 2, Additional file 1: Table S7). In the multivariable models, the direct effect estimates of early life body size were attenuated to null for colorectal (OR: 0.97, 95% CI: 0.77–1.22) and colon cancer (OR: 0.97, 95% CI: 0.76–1.25), while an estimate of similar magnitude with more imprecision was observed for distal colon cancer (OR: 1.27, 95% CI: 0.90–1.77) (Fig. 2). In multivariable models for proximal colon and rectal cancer, there was little evidence for a direct effect of genetically predicted early life body size (OR: 0.82, 95% CI: 0.61, 1.09 and OR: 1.05 95% CI: 0.76, 1.45, respectively).
Forest plot showing the estimated direct and indirect effects for genetically predicted early (age 10 years) and adult body size (thinner, plumper, or about average) on colorectal cancer both overall and by cancer sub-site. The error bars correspond to the odds ratios with 95% confidence intervals and those within the grey frameworks represent the results of the univariable Mendelian randomization (MR) analysis
For women, early life body size was estimated to increase colorectal cancer (OR: 1.20, 95% CI: 0.97–1.48) and colon cancer (OR 1.20, 95% CI: 0.95–1.51) (Fig. 3, Additional file 1: Table S7). These genetically predicted effects attenuated towards the null in the multivariable model (colorectal cancer, OR: 1.08, 95% CI: 0.81–1.45; colon cancer, OR: 0.99, 95% CI: 0.71–1.36) with similar patterns for the other subsite models. For men, little evidence for an effect of early life body size on colorectal cancer risk was observed in the univariable model (OR: 0.96, 95% CI: 0.73–1.26) with an inverse point estimate observed in the multivariable model (OR: 0.74, 95% CI: 0.49–1.12) (Fig. 3).
Forest plot showing the estimated direct and indirect effects for genetically predicted early (age 10 years) and adult body size (thinner, plumper, or about average) on colorectal cancer both overall and by cancer sub-site in men and women separately. The error bars correspond to the odds ratios with 95% confidence intervals and those within the grey frameworks represent the results of the univariable Mendelian randomization (MR) analysis
The effect estimates were similar across cancer subsite (P-heterogeneity ≥ 0.14) and by sex (P-heterogeneity ≥ 0.10) in both the univariable and multivariable analyses. For distal colon cancer opposing direct effect, estimates of early life body size with wide confidence intervals were observed for men (OR: 0.71, 95% CI: 0.41, 1.23) and women (OR: 1.32, 95% CI: 0.87, 2.03); P-heterogeneity = 0.08.
Adult life body size
In the sex-combined multivariable model, adult body size was estimated to directly increase the risk of colorectal cancer (OR per category change: 1.27, 95% CI: 1.03, 1.57), colon cancer (OR: 1.32, 95% CI: 1.05, 1.67), and proximal colon cancer (OR: 1.57, 95% CI: 1.21, 2.05), whereas estimates for distal colon (OR: 1.02, 95% CI: 0.76, 1.38) and rectal (OR: 1.13, 95% CI: 0.84, 1.52) cancers were of smaller magnitude (Fig. 2).
For women, adult body size was estimated to directly increase, albeit imprecisely, the risk of colon (OR: 1.20, 95% CI: 0.87, 1.65), proximal colon (OR: 1.63, 95% CI: 1.12, 2.39), and rectal cancer (OR: 1.22, 95% CI: 0.79, 1.92), whereas null estimates were observed for colorectal cancer (OR: 1.06, 95% CI: 0.79, 1.42) (Fig. 3). For men, adult body size was estimated to directly increase the risk of colorectal cancer (OR: 1.54, 95% CI: 1.06, 2.20), colon cancer (OR: 1.52, 95% CI: 1.01, 2.32), and distal colon cancer (OR: 1.75, 95% CI: 1.07, 2.89) with similar positive effect estimates found for proximal colon (OR: 1.34, 95% CI: 0.81, 2.23) and rectal cancer (OR: 1.34, 95% CI: 0.84, 2.14) (Fig. 3).
Similarly to the early life body size analysis, opposing multivariable analysis estimates were observed for the direct effect of adult body size on distal colon cancer in men and women (OR: 1.75, 95% CI: 1.07, 2.89 and OR: 0.83, 95% CI: 0.54, 1.25, respectively, P-heterogeneity = 0.02).
Sensitivity analyses
F-statistics for genetic instruments indicated sufficient strength to satisfy the “relevance” assumption—we found little evidence of weak instrument bias under the univariable framework (F statistics were > 10 for all SNPs included in the analysis). In multivariable MR Fearly life body size, Fadult body size statistics were all over 10 with the exception of the analysis in men where Fearly life body size was equal to 8, and Fadult body size was equal to 10 suggesting that weak instrument bias may influence the analyses for men (Additional file 1: Tables S1 and S8). We evaluated heterogeneity and pleiotropy to test the “exchangeability” and “exclusion restriction” assumptions (that the instrument does not share a common cause with the outcome and that any effect of the instrument on the outcome is exclusively through its potential effect on the exposure, respectively). There was evidence of heterogeneity in most of the analyses as denoted by the Q statistics (Additional file 1: Tables S7 and S8). Under the univariable framework, based on Egger’s intercept test, evidence of directional pleiotropy was found for the effect of early life body size on colorectal cancer (overall, women only) and proximal colon cancer (overall), with stronger positive effect estimates observed for the MR Egger regression models (overall colorectal cancer , OR 1.46, 95% CI: 1.09, 1.93; colorectal cancer women only, OR 1.84, 95% CI: 1.16, 2.92; and overall proximal colon cancer, OR 1.60, 95% CI: 1.09, 2.34) (Additional file 1: Table S7). Finally, the multivariable MR Egger’s intercept test identified some evidence of pleiotropy in the analyses of adult body size on colorectal, colon and proximal colon risk, but the related effect estimates replicated the positive direct effects of adult body size observed in the main analysis (Additional file 1: Table S9).
Discussion
In the current study, we investigated the effect of early life body size (at age 10 years) on risk of later life colorectal cancer and whether this effect remained robust after accounting for adult body size. Mendelian randomization estimates of the total effect of early life body size on colorectal cancer risk revealed that genetically predicted early life body size was estimated to increase odds of colorectal cancer risk which were strongest for colon and distal colon cancer. However, these associations were generally attenuated following adjustment for adult body size, suggesting no direct effects of early life body size on colorectal cancer risk. One exception was distal colon cancer, where the point estimate for the effect of early life body size on cancer risk remained in the multivariable scenario, though confidence intervals spanned the null. A similar pattern of results was reported in the sex-specific analyses, except for opposing effect estimates for both early life and adult body size on distal colon cancer under the multivariable framework, where greater early life body size appeared protective against distal colon cancer in male and detrimental in females and later life body size protective in females and detrimental in males, though both sets of estimates spanned the null.
Relatively few observational studies have examined the relationship between early life body size and risk of later life colorectal cancer. A joint Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS) found that higher body fatness in early life (childhood and adolescence) was associated with greater colorectal cancer risk for women but not men with similar results across cancer sub-sites [37], with these associations largely unchanged after multivariable adjustment for adult BMI [37]. A Swedish study of more than 200,000 men who had their height and weight measured in adolescence reported a more than twofold higher risk for later life colorectal cancer for the obese group (BMI ≥ 30 kg/m2) when compared with the normal weight group (BMI 18.5-< 25 kg/m2) [38]. A large Israeli study of almost 1.8 million men and women showed that being overweight and obese at adolescence was associated with higher colon cancer risk for both men (hazard ratio [HR] for overweight, 1.53; 95% CI, 1.28–1.84; HR for obesity, 1.54; 95% CI, 1.15–2.06; statistically significant from a BMI of 23.4 kg/m2) and women (HR for overweight, 1.54; 95% CI, 1.22–1.93; HR for obesity, 1.51; 95% CI, 0.89–2.57; significant from a BMI of 23.6 kg/m2) [39]. However, the Swedish and Israeli studies lacked data on information on important potential confounders including adult body size.
A recent MR study examined the associations between childhood obesity and cancer risk without taking into account adult body size, using a genetic instrument of 15 SNPs from a GWAS of 47,541 children from the Early Growth Genetics (EGG) consortium [40]. Effect estimates from this study did not support a positive relationship between childhood BMI and overall colorectal cancer (OR, 1.11; 95% CI, 0.93–1.32) [40]. Results from our analyses that crucially adjusted for adult body size also reflect little evidence of a positive relationship between early life body size and later life colorectal cancer risk. An additional MR study following a similar approach but using a different dataset for colorectal cancer also did not find any positive relationship between childhood BMI and overall colorectal cancer after adjusting for adult body size [20]. However, this study did not investigate further associations stratified by sex or cancer sub-site. Our findings suggest that positive effect estimates found between early life body size and colorectal cancer may be attributable to participants who were overweight or obese in childhood remaining this weight during adulthood, rather than any distinct harm from childhood adiposity itself. There was weak evidence for a direct effect of early life body size on distal colon cancer risk which warrants further investigation in new and larger studies with measured early life body size. Moreover, multivariable models estimated a greater effect of adult body size on CRC in men compared to women. This finding is consistent with a recent MR study, which found a stronger effect of adult BMI on CRC for men in sex-stratified analyses (OR per 1 standard deviation change: 1.23; 95% CI, 1.08–1.38 in men versus 1.09; 95% CI, 0.97–1.22 in women) [41].
We observed divergent effect estimates for early life body size and distal colon cancer risk for men and women in the multivariable models (P-heterogeneity = 0.08). However, caution is needed when interpreting the results for men as the estimates for early life body size might suffer from weak instrument bias based on the low conditional F statistic.
Strengths of the current study include our use of novel multivariable MR methods to disentangle the effects of early life body size from adult body size on colorectal cancer risk. Importantly, both early life and adult body size data were sourced from the identical participants within UK Biobank belonging to the same generation of individuals contributing data to the colorectal cancer GWAS used in this analysis; this should reduce bias that can be introduced to MR estimates when two samples do not represent the same underlying population [15]. We obtained summary stats for colorectal cancer from the Huyghe et al. GWAS excluding UK biobank participants; thus, independent datasets for the exposure and outcome phenotypes were used, avoiding potential bias due to sample overlap [42]. Finally, the large cancer sample size allowed us to conduct a more detailed analysis by sex and cancer sub-site.
A limitation of our study is that the early life body size phenotype was based on a self-reported questionnaire data than on direct measurements, so we cannot exclude potential recall bias in the measurements. However, the early life body size SNPs were successfully validated in three independent studies with direct measures of childhood BMI, demonstrating their validity as instruments for early life body size [15, 16, 19]. In the ALSPAC cohort, a genetic score based on the SNPs early life body size was found to be a stronger predictor of childhood BMI compared with the score based on the adult variants (area under the curve [AUC]: 0.64 vs 0.61; average age 9.9 year) with a similar finding found in the Young Finns Study (AUC 0.74 vs 0.62; range 3–18 years of age) [15, 19]. In the HUNT study, it was observed that the genetic score for early life body size explained a higher proportion of variance in the measured BMI than the genetic score for adult body size (6.7% vs 2.4%; age group 12–15.9 years) [16]. As exposures investigated were categorical variables with three broad categories, this may limit power to detect small effects; this did not appear to limit site and sex combined analyses, but power was lower in analyses according to sex and subsite, as evidenced by the imprecision of these effect estimates. Moreover, analyzing two correlated phenotypes together like early and adult body size may have introduced collinearity which leads to greater imprecision and possible bias in the multivariable MR effect estimates especially for early life body size; however, the same set of SNPs were strong enough to show independent effects of early life body size on breast cancer (OR: 0.59, 95% CI: 0.50, 0.71) [15]. Additionally, examining the direct of early life body size on colorectal cancer risk in the MVMR analysis may be biased due to mediator adjustment (i.e., induced collider bias due to mediator-outcome confounding). It would be interesting to investigate the role of key confounders of the adult body size relationship; however, an MVMR model including confounders would considerably weaken the power of exposure instruments (as illustrated by the weak conditional F statistic for early life body size and reduced power for stratified analyses in Supplementary Table 4). Furthermore, given the random allocation of genetic variants at conception, the genetic instrument for adult body size should not be correlated with confounding factors. Additionally, it is expected that body size-related variants could contribute to CRC risk via different metabolic pathways, e.g., lipids or glycemic traits. A recent MR study found effects of BMI or WHR on numerous lipids and glycemic traits—some consistent effects were observed for certain lipid metabolites, where levels were raised by BMI and positively associated with the risk of distal colon cancer [41]. A future approach to minimize heterogeneity in instrument selection could be to analyze the association between clusters of genetic determinants of BMI in relation to CRC, informed by phenotypic associations. The lack of ancestral diversity in UK Biobank limits the generalizability of results to diverse populations. Lastly, given the large number of comparisons, we cannot exclude that some of the observed effects might be due to chance.
Conclusions
In conclusion, we found that the suggested effects of early life body size on later life colorectal cancer risk are likely a consequence of individuals who are overweight or obese during childhood remaining this way into adulthood. Our results suggest that being overweight in childhood may not be detrimental in terms of colorectal carcinogenesis providing individuals reach and then maintain normal weight during adulthood. Further research is required to examine any possible role of early life body size on distal colon cancer development.
Availability of data and materials
The summary statistics used in this study are outlined in the Additional file 1 Tables S1, S2, S5. The summary-level GWAS data on outcomes used in this study are available following an application to the Genetics and Epidemiology of Colorectal Cancer Consortium (GECCO). https://www.fredhutch.org/en/research/divisions/public-health-sciences-division/research/cancer-prevention/genetics-epidemiology-colorectal-cancer-consortium-gecco.html.
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CCFR:
-
Colon Cancer Family Registry
- CORECT:
-
ColoRectal Transdisciplinary Study
- EGG:
-
Early Growth Genetics
- GECCO:
-
Genetics and Epidemiology of Colorectal Cancer
- GWAS:
-
Genome-wide association study
- HPFS:
-
Health Professionals Follow-up Study
- HR:
-
Hazard ratio
- LD:
-
Linkage disequilibrium
- MR:
-
Mendelian randomization
- NHS:
-
Nurses’ Health Study
- OR:
-
Odds ratio
- SNP:
-
Single nucleotide polymorphism
References
Collaborators GBDO, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27.
Bibbins-Domingo K, Coxson P, Pletcher MJ, Lightwood J, Goldman L. Adolescent overweight and future adult coronary heart disease. N Engl J Med. 2007;357(23):2371–9.
Hannon TS, Rao G, Arslanian SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics. 2005;116(2):473–80.
Xue F, Rosner B, Eliassen H, Michels KB. Body fatness throughout the life course and the incidence of premenopausal breast cancer. Int J Epidemiol. 2016;45(4):1103–12.
Wei EK, Wolin KY, Colditz GA. Time course of risk factors in cancer etiology and progression. J Clin Oncol. 2010;28(26):4052–7.
Biro FM, Wien M. Childhood obesity and adult morbidities. Am J Clin Nutr. 2010;91(5):1499S–505S.
Hidayat K, Yang CM, Shi BM. Body fatness at an early age and risk of colorectal cancer. Int J Cancer. 2018;142(4):729–40.
Garcia H, Song M. Early-life obesity and adulthood colorectal cancer risk: a meta-analysis. Rev Panam Salud Publica. 2019;43:e3.
Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;303(3):242–9.
Davey Smith G, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22.
Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey SG. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–63.
Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. 2015;181(4):251–60.
Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. 2019;48(3):713–27.
Sanderson E, Richardson TG, Morris TT, Tilling K, Davey Smith G. Estimation of causal effects of a time-varying exposure at multiple time points through multivariable mendelian randomization. PLOS Genet. 2022;18(7):e1010290.
Richardson TG, Sanderson E, Elsworth B, Tilling K, Davey SG. Use of genetic variation to separate the effects of early and later life adiposity on disease risk: mendelian randomisation study. BMJ. 2020;369:m1203.
Brandkvist M, Bjorngaard JH, Odegard RA, Asvold BO, Davey Smith G, Brumpton B, et al. Separating the genetics of childhood and adult obesity: a validation study of genetic scores for body mass index in adolescence and adulthood in the HUNT Study. Hum Mol Genet. 2021;29(24):3966–73.
Huyghe JR, Bien SA, Harrison TA, Kang HM, Chen S, Schmit SL, et al. Discovery of common and rare genetic risk variants for colorectal cancer. Nat Genet. 2019;51(1):76–87.
Huyghe JR, Harrison TA, Bien SA, Hampel H, Figueiredo JC, Schmit SL, et al. Genetic architectures of proximal and distal colorectal cancer are partly distinct. Gut. 2021;70:1325–34
Richardson TG, Mykkanen J, Pahkala K, Ala-Korpela M, Bell JA, Taylor K, et al. Evaluating the direct effects of childhood adiposity on adult systemic metabolism: a multivariable Mendelian randomization analysis. Int J Epidemiol. 2021;50(5):1580–92.
Mariosa D, Smith-Byrne K, Richardson TG, Ferrari P, Gunter MJ, Papadimitriou N, et al. Body size at different ages and risk of six cancers: a Mendelian randomization and prospective cohort study. J Natl Cancer Inst. 2022;114(9):1296–300.
Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42(5):1497–501.
Bowden J, Hemani G, Davey SG. Invited Commentary: Detecting individual and global horizontal pleiotropy in Mendelian randomization-a job for the humble heterogeneity statistic? Am J Epidemiol. 2018;187(12):2681–5.
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. 2017;36(11):1783–802.
Labrecque J, Swanson SA. Understanding the assumptions underlying instrumental variable analyses: a brief review of falsification strategies and related tools. Curr Epidemiol Rep. 2018;5(3):214–20.
Cochran WG. The combination of estimates from different experiments. Biometrics. 10(1):101–129.
Burgess S, Thompson SG, Collaboration CCG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40(3):755–64.
Shim H, Chasman DI, Smith JD, Mora S, Ridker PM, Nickerson DA, et al. A multivariate genome-wide association analysis of 10 LDL subfractions, and their response to statin treatment, in 1868 Caucasians. PLoS One. 2015;10(4):e0120758.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25.
Rees JMB, Wood AM, Burgess S. Extending the MR-Egger method for multivariable Mendelian randomization to correct for both measured and unmeasured pleiotropy. Stat Med. 2017;36(29):4705–18.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2020.
Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734–9.
Gibran Hemani. ieugwasr: R interface to the IEU GWAS database API. R package version 0.1.5. 2020.
Max Gordon, Thomas Lumley. forestplot: advanced forest plot using ‘grid’ graphics. R package version 1.10.1. 2020.
Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, VanderWeele TJ, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. 2021;375:n2233.
Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA. 2021;326(16):1614–21.
Zhang X, Wu K, Giovannucci EL, Ma J, Colditz GA, Fuchs CS, et al. Early life body fatness and risk of colorectal cancer in u.s. Women and men-results from two large cohort studies. Cancer Epidemiol Biomark Prev. 2015;24(4):690–7.
Kantor ED, Udumyan R, Signorello LB, Giovannucci EL, Montgomery S, Fall K. Adolescent body mass index and erythrocyte sedimentation rate in relation to colorectal cancer risk. Gut. 2016;65(8):1289–95.
Levi Z, Kark JD, Katz LH, Twig G, Derazne E, Tzur D, et al. Adolescent body mass index and risk of colon and rectal cancer in a cohort of 1.79 million Israeli men and women: A population-based study. Cancer. 2017;123(20):4022–30.
Fang X, Wang X, Song Z, Han D, Yin X, Liu B, et al. Causal association of childhood obesity with cancer risk in adulthood: a Mendelian randomization study. Int J Cancer. 2021;149(7):1421–5.
Bull CJ, Bell JA, Murphy N, Sanderson E, Davey Smith G, Timpson NJ, et al. Adiposity, metabolites, and colorectal cancer risk: Mendelian randomization study. BMC Med. 2020;18(1):396.
Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016;40(7):597–608.
Acknowledgments
Information on consortia acknowledgements is included in the Additional file 2: Acknowledgements.
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article, and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Funding
This work was supported by Cancer Research UK (C18281/A29019)
MJ and DJH received funding from the International Hundred K+ Cohorts Consortium/Global Genomic Medicine Collaborative (IHCC/G2MC) No.31312020.
C.J.B. is supported by the World Cancer Research Fund (WCRF UK), as part of the World Cancer Research Fund International grant program (IIG_2019_2009).
J.A.B. works in a unit funded by the UK MRC (MC_UU_00011/1) and the University of Bristol.
E.E.V. is supported by Diabetes UK (17/0005587) and the World Cancer Research Fund (WCRF UK), as part of the World Cancer Research Fund International grant program (IIG_2019_2009) and works within the CRUK Integrative Cancer Epidemiology Programme (C18281/A29019).
NJT is a Wellcome Trust Investigator (202802/Z/16/Z), is the PI of the Avon Longitudinal Study of Parents and Children (MRC & WT 217065/Z/19/Z), is supported by the University of Bristol NIHR Biomedical Research Centre (BRC-1215-20011) and the MRC Integrative Epidemiology Unit (MC_UU_12013/3), and works within the CRUK Integrative Cancer Epidemiology Programme (C18281/A19169).
GDS works in a unit funded by the UK Medical Research Council (MC_UU_00011/1) and the University of Bristol.
TGR and ES work within the Medical Research Council Integrative Epidemiology Unit at the University of Bristol (MC_UU_00011/1). TGR is a UKRI Innovation Research Fellow (MR/S003886/1).
KKT is supported by Cancer Research UK (PPRCPJT\100005)
Information on consortia funding is included in the Additional file 2: Funding
Author information
Authors and Affiliations
Contributions
NP and CJB contributed equally to this work. NP and CJB contributed to the analysis and interpretation of data and draft of the manuscript. TGR and NM contributed equally to this work. NM contributed to the conceptualization and interpretation of data, and TGR contributed to the conceptualization, data acquisition, and methodology. GDS and ES contributed to the methodology. JAB and EEV contributed to the interpretation of data. UP contributed to the data acquisition. NP, CJB, MJ, DJH, JAB, ES, NJT, GDS, DA, PTC, SK, LLM, CMU, KV, JCF, PAN, RKP, UP, KKT, JMAB, EEV, DM, MJG, TGR, and NM reviewed, contributed to, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics were approved by respective institutional review boards.
Consent for publication
Not applicable.
Competing interests
Tom G Richardson is an employee of GlaxoSmithKline outside of the research presented in this manuscript. The remaining authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Beta estimates for genome-wide significant SNPs for early/adult life body size in UK Biobank (overall, men, women). Table S2. Summary information on colorectal cancer risk for the SNPs used in the analysis. Table S3. Number of cancer cases by sex and subsite. Table S4. Sample size and power calculations for each phenotype and group in the Mendelian randomization study of early and adult life body size and risk of colorectal cancer. Table S5. Beta estimates for early/adult life body size in UK Biobank for the SNPs included in the MVMR analysis (overall, men, women). Table S6. STROBE-MR checklist of recommended items to address in reports of Mendelian randomization studies. Table S7. Univariable Mendelian randomization estimates between early and adult body size and colorectal cancer risk. Table S8. Fearly life body size, Fadult body size and Qa in the multivariable MR between early, adult body size and colorectal cancer. Table S9. Multivariable MR Egger analysis to assess the effect of both predicted early life and adult body size on colorectal cancer.
Additional file 2.
Funding, acknowledgements. Funding – Information on consortia funding. Acknowledgements – Information on consortia acknowledgements.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Papadimitriou, N., Bull, C.J., Jenab, M. et al. Separating the effects of early and later life adiposity on colorectal cancer risk: a Mendelian randomization study. BMC Med 21, 5 (2023). https://doi.org/10.1186/s12916-022-02702-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-022-02702-9