Abstract
There has been a renewed interest in the role of dietary therapies to manage irritable bowel syndrome (IBS), with diet high on the agenda for patients. Currently, interest has focussed on the use of traditional dietary advice (TDA), a gluten-free diet (GFD) and the low FODMAP diet (LFD). A consensus meeting was held to assess the role of these dietary therapies in IBS, in Sheffield, United Kingdom.
Evidence for TDA is from case control studies and clinical experience. Randomised controlled trials (RCT) have demonstrated the benefit of soluble fibre in IBS. No studies have assessed TDA in comparison to a habitual or sham diet. There have been a number of RCTs demonstrating the efficacy of a GFD at short-term follow-up, with a lack of long-term outcomes. Whilst gluten may lead to symptom generation in IBS, other components of wheat may also play an important role, with recent interest in the role of fructans, wheat germ agglutinins, as well as alpha amylase trypsin inhibitors. There is good evidence for the use of a LFD at short-term follow-up, with emerging evidence demonstrating its efficacy at long-term follow-up. There is overlap between the LFD and GFD with IBS patients self-initiating gluten or wheat reduction as part of their LFD. Currently, there is a lack of evidence to suggest superiority of one diet over another, although TDA is more acceptable to patients.
In view of this evidence, our consensus group recommends that dietary therapies for IBS should be offered by dietitians who first assess dietary triggers and then tailor the intervention according to patient choice. Given the lack of dietetic services, novel approaches such as employing group clinics and online webinars may maximise capacity and accessibility for patients. Further research is also required to assess the comparative efficacy of dietary therapies to other management strategies available to manage IBS.
Similar content being viewed by others
Background
Irritable bowel syndrome (IBS) is a common disorder of gut-brain interaction, with a reported global prevalence of around 4% using the Rome IV criteria [1]. The burden of IBS on patients is considerable and the costs to healthcare systems and society are substantial, driving the need for effective interventions [2]. Although there are many pharmacological options for the management of IBS, dietary management remains high on the agenda for the majority of patients [3]. A recent study highlighted that the most preferred treatment for individuals with IBS was dietary therapy (48%), followed by pharmacotherapy (29%) and psychotherapy (22%) [4]. In addition, 63 to 84% of people with IBS report symptoms related to eating specific food items, with a greater number of food triggers identified by individuals with more severe IBS [5, 6].
Research into dietary therapies in IBS has focussed on traditional dietary advice (TDA), a gluten-free diet (GFD) and the low FODMAP diet (LFD). Current guidelines vary globally in terms of recommendations for implementation of dietary therapies in IBS. British guidelines suggesting the use of TDA followed by the LFD for the dietary management of IBS, whereas American and Canadian guidelines suggest the use of the LFD, with no mention of TDA [7,8,9,10]. In addition, no guidelines recommend the use of a GFD due to insufficient evidence. However, further research has been published since the conception of these guidelines, and the aim of our UK consensus meeting was, therefore, to provide a meaningful framework for UK dietetic practice with respect to IBS management.
Format for consensus meeting
A consensus meeting between gastroenterologists and dietitians with a specialist interest in IBS was held in Sheffield, United Kingdom (UK) in June 2021. PubMed was searched to identify relevant studies pertaining to dietary therapies in IBS, using the MeSH terms diet, dietary therapies, irritable bowel syndrome, low FODMAP, gluten free and traditional diet to identify relevant articles. Existing literature was reviewed. Following the meeting, the literature was re-reviewed to assess for any updates until March 2022, with a consensus being made on the current evidence for dietary therapies in IBS.
Traditional dietary advice
TDA for patients with IBS consists of dietary and lifestyle management [7]. This encompasses practical measures such as reducing alcohol intake, reduction of caffeine intake, avoidance of spicy meals, reduction in fat intake and increase in fluid intake as well as alteration of fibre intake [7]. TDA reduces dietary triggers for people with IBS including some that are not FODMAP containing, which is an important consideration. With the exception of dietary fibre, the evidence base for recommending these dietary changes relies on case-control studies and clinical experience, rather than robust randomised controlled trials (RCTs). Although RCTs have demonstrated the benefit of fibre in IBS, this appears to be limited to soluble fibre, such as ispaghula, rather than insoluble fibre, like wheat bran [8]. To date, there have been no RCTs assessing the efficacy of TDA in comparison to a habitual diet or sham diet.
Gluten-free diet
There has been an exponential growth over the last decade in the use of a GFD outside the context of coeliac disease (CD) [11]. The prevalence of self-reported wheat sensitivity has been reported at approximately 10% globally [12]. Wheat appears to be a key trigger for symptoms in patients with IBS, reported at between 23 and 49% of individuals [5, 13,14,15].
In view of this, the role of a GFD has been explored in IBS. There have been a number of RCTs demonstrating its efficacy, with key studies outlined in Table 1. The majority of RCTs exploring the GFD in IBS have demonstrated its efficacy at short-term follow-up, between 4 and 6 weeks [16,17,18,19,20]. It is worth noting that a study by Biesiekierski et al. failed to show dose dependent effects of gluten after individuals were placed on a LFD [21]. However, this may have been due to the study design, with individuals having an anticipatory nocebo response to the intervention. There have also been non-randomised trials also demonstrating the efficacy of the GFD. A prospective study in 41 patients with IBS-D demonstrated that 71% had a clinical response, defined as drop in IBS Symptom Severity Score [IBS-SSS] of ≥ 50 points, 6 weeks after implementation of a GFD [22]. Interestingly, 72% of individuals with a clinical response planned to continue the diet in the long term, with symptom reduction, anthropometric and biochemical features (body mass index, haemoglobin, ferritin, folate, vitamin B12 and albumin) being maintained at 18 months [22]. Nevertheless, there remains a lack of data demonstrating the long-term efficacy of a GFD for IBS and further research is required.
In terms of predictors of responsiveness to a GFD, a prospective study of 50 patients with IBS demonstrated that individuals with antigliadin IgG and IgA reported less diarrhoea than those without antibodies (p = 0.03), using the Birmingham IBS symptom questionnaire [24]. Whilst this highlighted that antigliadin antibodies could potentially be used as a predictor for response to a GFD in IBS, the prevalence of antigliadin antibody positivity in the study was 50%, markedly higher than the 21% reported in the validation cohort [24]. Further studies are required to assess whether antigliadin antibodies are a potential biomarker for predicting response to a GFD in IBS. Interestingly, in this study, individuals with some gluten exposure on the GFD still had a clinical response [24]. Similar improvements in overall GI symptoms were seen amongst individuals with strict compliance and minimal transgressions on the GFD (Z difference − 3.5 minimal transgression (p < 0.001) vs − 3.2 (p = 0.001) strict compliance in IBS AGA positive) [24]. This suggests that individuals with IBS may not need to follow the strict GFD used for treating patients with coeliac disease. The threshold of gluten reduction for patients with IBS to derive symptom benefit is still unclear. This is supported by a recent RCT, which demonstrated that 58% of individuals on a GFD, where there may have been some level of cross contamination, still had a clinical response, defined as a reduction in IBS-SSS of ≥ 50 points [20].
Mean dietary intake of fructans in the UK has been reported at 4.0 g daily [25]. Although wheat contains relatively low quantities of fructans, the high frequency of bread consumption has led to wheat being a major contributor to fructan consumption in the UK, with 66% of daily fructan intake being from wheat [25, 26]. Therefore, many individuals who adopt a GFD are likely to reduce their fructan intake substantially [27]. Although the benefits of a GFD in IBS have been demonstrated, it has been postulated that this may be due to a reduction in fructans (FODMAPs) rather than gluten. This is supported by a double-blind crossover challenge in 59 individuals who self-administered a GFD, who were randomly assigned to diets containing gluten, fructans or placebo [28]. This study demonstrated significantly higher Gastrointestinal Symptom Rating Scale (GSRS) scores for individuals consuming fructans rather than gluten (p = 0.049) [28]. In addition, a recent double-blind placebo RCT in 110 patients with IBS demonstrated that consumption of FODMAPs led to modest increases in IBS-SSS compared to the consumption of gluten (240 vs 208, p = 0.013) [29]. However, a recent study in patients with IBS showed no correlation of GI symptoms with fructan intake [30].
Whilst fructan intake has been explored as a key component of wheat in symptom generation, several other components may also trigger symptoms in IBS. Gluten itself may trigger symptoms, with a gluten containing diet showing an association with higher small bowel permeability, as well as a decrease in the expression of tight junction proteins [17, 31]. In addition, several other components of wheat may also play a role in the pathophysiology, with alpha-1 amylase trypsin inhibitors (ATIs) and wheat germ agglutinins (WGAs) also been postulated as triggers [32, 33].
Potential nutritional concerns remain with implementation of a GFD. This data is extrapolated from individuals with CD. In terms of macronutrient intake, fat intake appears to be increased on a GFD, with some studies showing a high consumption of saturated fatty acids potentially increasing the risk of cardiovascular disease [34]. In addition, individuals may have a reduced carbohydrate and fibre intake [34]. Micronutrient deficiencies of iron, calcium and magnesium have also been noted [34]. There is little data on the nutritional adequacy of a GFD in IBS. A recent RCT in IBS, where patients received a GFD, demonstrated no changes in macronutrient intake following a GFD, but a reduction in micronutrient intake of magnesium and thiamine was noted [20]. However, it is worth noting that this study did not employ the strict GFD employed in CD [20].
The impact of a GFD on the gut microbiome in patients with IBS is currently unclear. In healthy individuals, a GFD appears to affect bacterial populations, such as a decreasing pro-inflammatory bacteria such as Veillonellaceae [35, 36]. Reduction in beneficial gut populations such as Bifidobacterium have also been noted in healthy individuals on a GFD [37], as well as in CD [36]. There is little data on the effects of a GFD in patients with IBS. A recent study demonstrated a reduction in abundance of Actinobacteria, Parabacteroides johnsonii, and Eubacteriumrectale, as well as Ruminococcusalbus and R. bromii following a GFD in IBS [20]. Further research is required to elucidate the effect of a GFD on the gut microbiome in patients with IBS.
Low FODMAP diet
Fermentable oligo-, di-, mono- saccharides and polyols (FODMAPs) are short chain carbohydrates, which are poorly absorbed, increasing small bowel water content by osmosis, and releasing gases, predominantly carbon dioxide and hydrogen, from bacterial fermentation [38]. Both healthy individuals and patients with IBS have similar physiological responses following FODMAP ingestion, as demonstrated by MRI imaging [39]. However, it is likely that individuals with IBS have increased symptoms following FODMAP consumption as a result of visceral hypersensitivity [40].
There has been significant interest in the role of the LFD to manage patients with IBS, particularly over the last decade. The LFD is implemented through a multiphasic approach. The initial phase, which is generally implemented over 4-8 weeks, involves the strict reduction of all FODMAP groups. If individuals have an improvement in symptoms, then the next phase is implemented, over a 6- to 10-week period. FODMAPs are re-introduced, enabling patients to identify specific FODMAP triggers that induce symptoms. Finally, patients enter the long-term personalisation phase, where a less restrictive diet is consumed, and only those FODMAPs which induce symptoms are excluded [41].
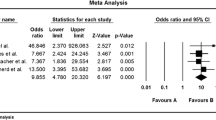
The majority of studies assessing the LFD have focussed on the initial short-term elimination phase, with a large number of RCTs demonstrating its efficacy (Table 2). Recent meta-analyses, which pooled outcome data amongst studies that used IBS-SSS to assess clinical response, demonstrated a mean reduction of around 50 points following the LFD [42, 43]. It is worth noting that studies assessing the LFD have had variable comparator diets, such as a sham diet, habitual diet, TDA, Australian diet and high FODMAP diet, highlighting the heterogeneity of studies to date [44].
Currently, there are limited long-term studies demonstrating the efficacy of a low FODMAP diet. A study in 90 patients with IBS, with a mean follow-up of 16 months, demonstrated that symptom improvement was maintained during longer term follow-up, with adherence reported at 76% [53]. Likewise, a RCT in 74 patients with IBS demonstrated sustained symptom response at 6 months in 82% of patients on a LFD [54]. More recently, two studies from the UK have demonstrated ongoing symptom relief with the LFD during long-term follow-up, with adequate relief of symptoms reported in 57% (6–18 months follow-up) and 60% (44 months mean follow-up) of individuals, respectively [55, 58]. In addition, between 65 to 82% of individuals who are following a LFD are in the personalisation phase at long-term follow-up [55, 58, 59].
Interestingly, it has been demonstrated that many individuals are excluding gluten as part of the LFD during long-term follow-up. A recent study noted that 83% of individuals in the personalisation phase of the LFD consume ‘free-from’ products, with gluten or wheat free products being the commonest (68%) [58]. In addition, the commonest dietary requirement when eating out was noted to a gluten or wheat free diet (43%) [58]. This study highlighted those individuals maybe using these diets as part of a LFD due to greater awareness of the GFD [60].
Like the GFD, there are potential nutritional concerns of the LFD. The majority of studies have evaluated the impact of the LFD on nutritional adequacy in the initial strict restriction phase, at short-term follow-up. A RCT in 75 patients with IBS demonstrated that individuals following the LFD had a marked reduction in total carbohydrate and fibre intake at 4 weeks, which was not seen with TDA [48]. Moreover, whilst total energy intake was reduced with both interventions, the reduction was greater with the LFD [48]. Another RCT demonstrated a statistically significant decrease in several micronutrients with a LFD compared to the modified National Institute for Health and Care Excellence (mNICE) diet. However, the differences noted with the LFD only remained for riboflavin when corrected for calorie intake. Consequently, uncertainties remain with regards to the nutritional adequacy of the LFD in the short term [61].
For individuals in the re-introduction phase of the LFD, a recent RCT in 101 patients with IBS-D demonstrated a reduction in energy, carbohydrate, fat and fibre intake at 4 weeks, but a gradual improvement at 16 weeks [52]. During long-term follow-up of 6-18 months in one study and 44 months in another, the LFD has been shown to have similar nutritional adequacy to individuals on a habitual diet (Table 2) [55, 58]. These findings suggest that the LFD may be more nutritionally balanced over the longer term, which may reflect the less restrictive nature of the personalisation phase.
The impact of the LFD on the gut microbiome is unclear currently. A systematic review found no influence of a LFD on overall microbial diversity [42]. Bifidobacterium is known to be a key butyrate producer in the colon, playing an important role in colonic health [62]. Most studies looking at the LFD found that the abundance of Bifidobacteria and/or the overarching phylum Actinobacteria to be reduced [42]. Faecal bacterial profiles have been suggested to potentially predict responsiveness to a LFD. A RCT in 67 patients with IBS demonstrated that responsiveness to a LFD could be predicted by faecal bacterial profiles [63]. However, a more recent RCT, using the same method of stool analysis failed to replicate these findings [20].
Adherence to all phases of the LFD can be challenging. A case series in patients with functional gastrointestinal symptoms who had previously been recommended to follow the LFD demonstrated significantly better dietary adherence with a dietitian-led approach versus other methods (96% vs 71%, p = 0.02, short term; 70% vs 39%, p = 0.02, re-challenge phase; 65% vs 29%, p < 0.01, personalisation phase) [59]. However, as can be seen from this study, adherence to the LFD fell gradually with time, highlighting the challenges of maintaining this diet. Whilst the evidence base for the LFD is derived from dietetic-led LFD studies, the majority of individuals following the LFD do not receive specialist dietetic input. A survey from the United States (US) found that only 21% of gastroenterologists commonly refer patients to registered dietitians [60].
Challenges to the implementation of dietary therapies in IBS
Whilst the majority of individuals receiving dietetic-led LFD advice appear to be adhering to the personalisation phase of the LFD, adherence falls to only 29% amongst those receiving non dietetic-led advice [59]. Less restrictive approaches have been proposed, including the ‘bottom up’ approach, whereby patients reduce their intake of only a few FODMAPs at initial implementation, based on a full dietary history and patient reported triggers [64]. For many patients this may largely translate into adoption of a GFD and reduction of fructans [27, 65]. However, patients may report food triggers other than wheat and alternative approaches, such as focusing on the reduction of excess fructose and polyols, may also be beneficial [64]. A recent RCT demonstrated total FODMAP intakes of 7.6g/day, 15.2g/day and 22.4g/day for the LFD, TDA and GFD respectively [20]. This highlights the differing FODMAP intakes with these diets, with the GFD (22.4g/day total FODMAPs) and TDA (15.2g/day total FODMAPs) potentially being a form of a ‘bottom-up’ approach to manage patients with IBS [20].
A threshold reduction of 12 g daily of FODMAP intake has been suggested as the threshold required for symptom improvement [59]. Whilst the majority of LFD studies have been demonstrated to reach this threshold during the initial elimination phase, this has yet to be confirmed at long-term follow-up [66]. It is also worth noting that there may not be an optimal FODMAP threshold per se. Individuals with IBS may have differing degrees of visceral hypersensitivity, thereby not necessarily having symptoms triggered at a specific FODMAP threshold [67]. Individuals may therefore experience symptoms at different thresholds of FODMAP intake.
The majority of studies assessing the LFD have been dietetic-led, as well as being performed in secondary and tertiary care in an adult population. A study evaluating the long-term effect of dietetic-led interventions for IBS delivered in primary care has demonstrated satisfactory relief of gut symptoms at long-term follow-up (55%) [68]. However, further studies evaluating the use of a LFD, both physician-led and in primary care are required. In addition, the majority of studies have been performed in an adult population, with its applicability in a paediatric population requiring further exploration [69].
Comparative efficacy of dietary therapies
There have been a number of RCTs comparing the LFD to TDA, with conflicting outcomes (Table 3). A RCT in Sweden demonstrated no significant difference in symptom improvement following the LFD and TDA at 4 weeks (50% vs 46%, p = 0.72) [48]. Likewise, a study in the US demonstrated no difference in adequate relief of symptoms in patients with IBS-D between individuals on LFD vs mNICE diet at 4 weeks (52% vs 41%, p = 0.31) [49]. The mNICE diet involved eating small frequent meals, avoiding trigger foods, and avoiding excess alcohol and caffeine. Foods containing FODMAPs were not specifically excluded [49]. In contrast a study in Iran demonstrated greater symptom improvement for individuals following a LFD in comparison to general dietary advice at 6 weeks [50]. A recent RCT also demonstrated a significantly higher proportion of responders on the LFD compared to TDA at both 4 weeks (63% vs 41%, p = 0.04), as well as 16 weeks (53% vs 31%, p = 0.03) [52]. This highlights the current uncertainty in terms of comparative efficacy between the LFD and TDA. A network meta-analysis ranked the LFD first amongst dietary therapies for global symptoms of IBS, above TDA [44]. Currently, it is unclear from the literature whether either dietary intervention is superior in the management of IBS.
There has only been one RCT to date comparing the efficacy of TDA, LFD and GFD head-to-head in a UK population [20]. This RCT in the UK demonstrated similar efficacy between all three diets, with no difference in clinical response rate at 4 weeks (42% TDA, 55% LFD, 58% GFD, p = 0.43) [20]. Similarly, a study in Italy showed comparative efficacy of LFD, GFD and Mediterranean diet [23]. However, the Mediterranean diet is not comparable with TDA. Currently, there is a lack of data evaluating the comparative efficacy of TDA, GFD and LFD at long-term follow-up, with studies needed.
Whilst comparable efficacy has been shown between these dietary therapies, differences in dietary acceptability have been noted in the short-term. TDA has been shown to be cheaper, less time-consuming to shop and easier to follow when eating out with family and friends in comparison to the GFD and LFD [20]. There have been no studies to date evaluating the acceptability of TDA and a GFD at long-term follow-up. However, long-term follow-up of patients on the LFD has demonstrated that it is significantly more expensive than a habitual diet and takes extra time to shop for, as well as negatively affecting social eating [55, 58]. In addition, patient preference may favour less complex diets, with a study in Italy demonstrating 86% would wish to continue on a Mediterranean diet, in comparison to 11% for a GFD and only 3% for a LFD [23].
The efficacy of dietary therapies compared to other treatments in IBS, including drug and psychological therapies, remains unclear. One RCT randomised participants to receive either hypnotherapy, dietary management or a combination and noted no significant difference in overall symptom improvement between groups (p = 0.67) [54]. Similarly, a RCT comparing yoga to the LFD failed to show differences in absolute IBS-SSS at either 12 (p = 0.151) or 24 weeks (p = 0.081) [70]. A meta-analysis of pharmacological trials in IBS demonstrated a pooled placebo response rate of 27% amongst the 73 RCTs included, using the global symptom response rate. It is likely that placebo effects are relevant to dietary studies also, which may impact assessment of dietary efficacy at short-term follow-up in particular, as placebo effects are known to wane with time [71].
In addition, the role of sucrose-isomaltase deficiency in IBS requires further exploration. The sucrase-isomaltase enzyme facilitates digestion of starch and sucrose. Whilst congenital sucrase-isomaltase deficiency (CSID) is rare, CSID mutations with known defective disaccharidase properties have been shown to be more frequent in IBS [72, 73]. Sucrase-isomaltase (SI) gene variants coding for disaccharidases with defective or reduced enzymatic activity have been shown to predispose to IBS [74], with a common SI variant (15Phe) being strongly associated [72, 75]. It has been suggested that the LFD may have a lower efficacy in individuals with reduced SI activity [76, 77]. Sucrose, and in part, starch are not specifically restricted as a part of a LFD, and therefore, the role of a low sucrose and low starch diet or enzymatic supplementation requires further exploration in IBS [77], in particular for individuals carrying hypomorphic SI variants [78].
Conclusions
Currently, comparable efficacy has been demonstrated with TDA, GFD and LFD at short-term follow-up [20]. However, TDA appears to be more acceptable, in comparison to the GFD and LFD [20]. In view of this, trialling the TDA would be an appropriate first line dietary approach in IBS, consistent with current UK guidelines [7, 8]. However, a significant proportion of individuals note wheat to be a trigger in IBS [13]. In individuals who note gluten to be a primary trigger in IBS, a GFD may be more appropriate as a ‘bottom-up’ approach to manage symptoms [65]. Likewise, in individuals who wish to have a stricter reduction of FODMAPs, a LFD may be more appropriate, after dietary consultation.
The key to implementation of dietary therapies in IBS is to provide patient choice, in conjunction with dietetic assessment and advice. Ideally dietary therapies should be implemented by a dietitian, to prevent nutritional inadequacy, as the evidence base for dietary therapies has been derived from dietetic-led implementation of these therapies. Gastrointestinal-specific symptom anxiety, the fear of symptoms and consequence of this is a potential driver of food avoidance in IBS. This can potentially lead to disordered eating patterns in these patients, re-enforcing the vital role of dietetic involvement [79].
The majority of dietary advice given currently is physician-led, with only a minority of gastroenterologists referring to registered dietitians for IBS management [60]. In addition, there is a lack of dietitians available to deliver dietetic therapies, with a recent UK study highlighting an inequity of dietetic services across England [80]. In view of this, further research is required to assess the efficacy of a physician led approach in IBS. In addition, although novel methods of dietetic delivery, such as group sessions and webinars require further assessment, they may offer a more efficient method for delivering dietary therapies with scare resources [80,81,82]. There appears to be evidence for the use of dietetic therapies (TDA, LFD and GFD) to manage patients with IBS at short-term follow-up, with further research required on assessing the long-term efficacy of these approaches. Currently, the comparative efficacy of dietary therapies remains unclear due to a lack of head-to-head trials, and the current evidence fails to show superiority of one approach. The choice of dietary therapy should be tailored to the patient, in conjunction with a dietitian (Fig. 1). More research is required on the comparative efficacy of dietary therapies to non-dietary therapies.
Availability of data and materials
Not applicable.
Abbreviations
- ATI:
-
Alpha-1 amylase trypsin inhibitor
- CD:
-
Coeliac disease
- CSID:
-
Congenital sucrase-isomaltase deficiency
- FODMAPs:
-
Fermentable oligo-, di-, mono- saccharides and polyols
- GFD:
-
Gluten-free diet
- GI:
-
Gastrointestinal
- GSRS:
-
Gastrointestinal Symptom Rating Scale
- IBS:
-
Irritable bowel syndrome
- IBS-SSS:
-
IBS Symptom Severity Score
- LFD:
-
Low FODMAP diet
- MeSH:
-
Medical subject headings
- mNICE:
-
Modified National Institute for Health and Care Excellence
- RCT:
-
Randomised controlled trial
- SI:
-
Sucrase-isomaltase
- TDA:
-
Traditional dietary advice
- WGA:
-
Wheat germ agglutinin
- UK:
-
United Kingdom
- US:
-
United States
References
Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation Global Study. Gastroenterology. 2020. https://doi.org/10.1053/j.gastro.2020.04.014.
Canavan C, West J, Card T. Review article: the economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther. 2014;40(9):1023–34. https://doi.org/10.1111/apt.12938.
Halpert A, Dalton CB, Palsson O, et al. What patients know about irritable bowel syndrome (IBS) and what they would like to know. National Survey on Patient Educational Needs in IBS and development and validation of the Patient Educational Needs Questionnaire (PEQ). Am J Gastroenterol. 2007;102(9):1972–82. https://doi.org/10.1111/j.1572-0241.2007.01254.x.
Sturkenboom R, Keszthelyi D, Masclee AAM, Essers BAB. Discrete choice experiment reveals strong preference for dietary treatment among patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2022. https://doi.org/10.1016/j.cgh.2022.02.016.
Böhn L, Störsrud S, Törnblom H, Bengtsson U, Simrén M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108(5):634–41. https://doi.org/10.1038/ajg.2013.105.
Simrén M, Månsson A, Langkilde AM, et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63(2):108–15 doi:51878.
McKenzie YA, Bowyer RK, Leach H, et al. British Dietetic Association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). J Hum Nutr Diet. 2016;29(5):549–75. https://doi.org/10.1111/jhn.12385.
Vasant DH, Paine PA, Black CJ, et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut. 2021. https://doi.org/10.1136/gutjnl-2021-324598.
Moayyedi P, Andrews CN, MacQueen G, et al. Canadian Association of Gastroenterology Clinical Practice Guideline for the Management of Irritable Bowel Syndrome (IBS). J Can Assoc Gastroenterol. 2019;2(1):6–29. https://doi.org/10.1093/jcag/gwy071.
Lacy BE, Pimentel M, Brenner DM, et al. ACG clinical guideline: management of irritable bowel syndrome. Am J Gastroenterol. 2021;116(1):17–44. https://doi.org/10.14309/ajg.0000000000001036.
Aziz I, Karajeh MA, Zilkha J, Tubman E, Fowles C, Sanders DS. Change in awareness of gluten-related disorders among chefs and the general public in the UK: a 10-year follow-up study. Eur J Gastroenterol Hepatol. 2014;26(11):1228–33. https://doi.org/10.1097/MEG.0000000000000166.
Aziz I. The global phenomenon of self-reported wheat sensitivity. Am J Gastroenterol. 2018;113(7):945–8. https://doi.org/10.1038/s41395-018-0103-y.
Carroccio A, Mansueto P, Iacono G, et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity. Am J Gastroenterol. 2012;107(12):1898–906; quiz 1907. https://doi.org/10.1038/ajg.2012.236.
Barmeyer C, Schumann M, Meyer T, et al. Long-term response to gluten-free diet as evidence for non-celiac wheat sensitivity in one third of patients with diarrhea-dominant and mixed-type irritable bowel syndrome. Int J Colorectal Dis. 2017;32(1):29–39. https://doi.org/10.1007/s00384-016-2663-x.
Rijnaarts I, Witteman BJM, Zoetendal EG, Govers C, de Wit NJW, de Roos NM. Subtypes and severity of irritable bowel syndrome are not related to patients’ self-reported dietary triggers: results from an online survey in Dutch adults. J Acad Nutr Diet. 2021;121(9):1750–1762.e8. https://doi.org/10.1016/j.jand.2021.01.007.
Biesiekierski JR, Newnham ED, Irving PM, et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol. 2011;106(3):508–14; quiz 515. https://doi.org/10.1038/ajg.2010.487.
Vazquez-Roque MI, Camilleri M, Smyrk T, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144(5):903–911.e3. https://doi.org/10.1053/j.gastro.2013.01.049.
Shahbazkhani B, Sadeghi A, Malekzadeh R, et al. Non-celiac gluten sensitivity has narrowed the spectrum of irritable bowel syndrome: a double-blind randomized placebo-controlled trial. Nutrients. 2015;7(6):4542–54. https://doi.org/10.3390/nu7064542.
Zanwar VG, Pawar SV, Gambhire PA, et al. Symptomatic improvement with gluten restriction in irritable bowel syndrome: a prospective, randomized, double blinded placebo controlled trial. Intest Res. 2016;14(4):343–50. https://doi.org/10.5217/ir.2016.14.4.343.
Rej A, Sanders DS, Shaw CC, et al. Efficacy and acceptability of dietary therapies in non-constipated irritable bowel syndrome: a randomized trial of traditional dietary advice, the low FODMAP diet and the gluten-free diet. Clin Gastroenterol Hepatol. 2022. https://doi.org/10.1016/j.cgh.2022.02.045.
Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145(2):320–8.e1-3. https://doi.org/10.1053/j.gastro.2013.04.051.
Aziz I, Trott N, Briggs R, North JR, Hadjivassiliou M, Sanders DS. Efficacy of a gluten-free diet in subjects with irritable bowel syndrome-diarrhea unaware of their HLA-DQ2/8 genotype. Clin Gastroenterol Hepatol. 2016;14(5):696–703.e1. https://doi.org/10.1016/j.cgh.2015.12.031.
Paduano D, Cingolani A, Tanda E, Usai P. Effect of three diets (low-FODMAP, gluten-free and balanced) on irritable bowel syndrome symptoms and health-related quality of life. Nutrients. 2019;11(7). https://doi.org/10.3390/nu11071566.
Pinto-Sanchez MI, Nardelli A, Borojevic R, et al. Gluten-free diet reduces symptoms, particularly diarrhea, in patients with irritable bowel syndrome and antigliadin IgG. Clin Gastroenterol Hepatol. 2020. https://doi.org/10.1016/j.cgh.2020.08.040.
Dunn S, Datta A, Kallis S, Law E, Myers CE, Whelan K. Validation of a food frequency questionnaire to measure intakes of inulin and oligofructose. Eur J Clin Nutr. 2011;65(3):402–8. https://doi.org/10.1038/ejcn.2010.272.
Whelan K, Abrahmsohn O, David GJ, et al. Fructan content of commonly consumed wheat, rye and gluten-free breads. Int J Food Sci Nutr. 2011;62(5):498–503. https://doi.org/10.3109/09637486.2011.553588.
Rej A, Trott N, Aziz I, Sanders DS. A gluten-free diet: the express route to fructan reduction. Am J Gastroenterol. 2019;114(9):1553. https://doi.org/10.14309/ajg.0000000000000349.
Skodje GI, Sarna VK, Minelle IH, et al. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology. 2017. https://doi.org/10.1053/j.gastro.2017.10.040.
Nordin E, Brunius C, Landberg R, Hellström PM. FODMAPs, but not gluten, elicit modest symptoms of irritable bowel syndrome: a double-blind, placebo-controlled, randomized three-way crossover trial. Am J Clin Nutr. 2021. https://doi.org/10.1093/ajcn/nqab337.
Algera JP, Störsrud S, Lindström A, Simrén M, Törnblom H. Gluten and fructan intake and their associations with gastrointestinal symptoms in irritable bowel syndrome: a food diary study. Clin Nutr. 2021;40(10):5365–72. https://doi.org/10.1016/j.clnu.2021.09.002.
Wu RL, Vazquez-Roque MI, Carlson P, et al. Gluten-induced symptoms in diarrhea-predominant irritable bowel syndrome are associated with increased myosin light chain kinase activity and claudin-15 expression. Lab Invest. 2017;97(1):14–23. https://doi.org/10.1038/labinvest.2016.118.
Junker Y, Zeissig S, Kim SJ, et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J Exp Med. 2012;209(13):2395–408. https://doi.org/10.1084/jem.20102660.
de Punder K, Pruimboom L. The dietary intake of wheat and other cereal grains and their role in inflammation. Nutrients. 2013;5(3):771–87. https://doi.org/10.3390/nu5030771.
Cardo A, Churruca I, Lasa A, et al. Nutritional imbalances in adult celiac patients following a gluten-free diet. Nutrients. 2021;13(8). https://doi.org/10.3390/nu13082877.
Bonder MJ, Tigchelaar EF, Cai X, et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 2016;8(1):45. https://doi.org/10.1186/s13073-016-0295-y.
Caio G, Lungaro L, Segata N, et al. Effect of gluten-free diet on gut microbiota composition in patients with celiac disease and non-celiac gluten/wheat sensitivity. Nutrients. 2020;12(6). https://doi.org/10.3390/nu12061832.
De Palma G, Nadal I, Collado MC, Sanz Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br J Nutr. 2009;102(8):1154–60. https://doi.org/10.1017/S0007114509371767.
Hill P, Muir JG, Gibson PR. Controversies and recent developments of the low-FODMAP diet. Gastroenterol Hepatol (N Y). 2017;13(1):36–45.
Major G, Pritchard S, Murray K, et al. Colon hypersensitivity to distension, rather than excessive gas production, produces carbohydrate-related symptoms in individuals with irritable bowel syndrome. Gastroenterology. 2017;152(1):124–133.e2. https://doi.org/10.1053/j.gastro.2016.09.062.
Spiller R. How do FODMAPs work? J Gastroenterol Hepatol. 2017;32(Suppl 1):36–9. https://doi.org/10.1111/jgh.13694.
Whelan K, Martin LD, Staudacher HM, Lomer MCE. The low FODMAP diet in the management of irritable bowel syndrome: an evidence-based review of FODMAP restriction, reintroduction and personalisation in clinical practice. J Hum Nutr Diet. 2018. https://doi.org/10.1111/jhn.12530.
van Lanen AS, de Bree A, Greyling A. Efficacy of a low-FODMAP diet in adult irritable bowel syndrome: a systematic review and meta-analysis. Eur J Nutr. 2021. https://doi.org/10.1007/s00394-020-02473-0.
Hahn J, Choi J, Chang MJ. Effect of low FODMAPs diet on irritable bowel syndromes: a systematic review and meta-analysis of clinical trials. Nutrients. 2021;13(7). https://doi.org/10.3390/nu13072460.
Black CJ, Staudacher HM, Ford AC. Efficacy of a low FODMAP diet in irritable bowel syndrome: systematic review and network meta-analysis. Gut. 2021. https://doi.org/10.1136/gutjnl-2021-325214.
Staudacher HM, Lomer MC, Anderson JL, et al. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr. 2012;142(8):1510–8. https://doi.org/10.3945/jn.112.159285.
Pedersen N, Andersen NN, Végh Z, et al. Ehealth: low FODMAP diet vs Lactobacillus rhamnosus GG in irritable bowel syndrome. World J Gastroenterol. 2014;20(43):16215–26. https://doi.org/10.3748/wjg.v20.i43.16215.
Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146(1):67–75.e5. https://doi.org/10.1053/j.gastro.2013.09.046.
Böhn L, Störsrud S, Liljebo T, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149(6):1399–1407.e2. https://doi.org/10.1053/j.gastro.2015.07.054.
Eswaran SL, Chey WD, Han-Markey T, Ball S, Jackson K. A randomized controlled trial comparing the low FODMAP diet vs. modified NICE guidelines in US adults with IBS-D. Am J Gastroenterol. 2016;111(12):1824–32. https://doi.org/10.1038/ajg.2016.434.
Zahedi MJ, Behrouz V, Azimi M. Low fermentable oligo-di-mono-saccharides and polyols diet versus general dietary advice in patients with diarrhea-predominant irritable bowel syndrome: a randomized controlled trial. J Gastroenterol Hepatol. 2018;33(6):1192–9. https://doi.org/10.1111/jgh.14051.
Patcharatrakul T, Juntrapirat A, Lakananurak N, Gonlachanvit S. Effect of structural individual low-FODMAP dietary advice vs. brief advice on a commonly recommended diet on IBS symptoms and intestinal gas production. Nutrients. 2019;11(12). https://doi.org/10.3390/nu11122856.
Goyal O, Batta S, Nohria S, et al. Low fermentable oligosaccharide, disaccharide, monosaccharide, and polyol diet in patients with diarrhea-predominant irritable bowel syndrome: a prospective, randomized trial. J Gastroenterol Hepatol. 2021. https://doi.org/10.1111/jgh.15410.
de Roest RH, Dobbs BR, Chapman BA, et al. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: a prospective study. Int J Clin Pract. 2013;67(9):895–903. https://doi.org/10.1111/ijcp.12128.
Peters SL, Yao CK, Philpott H, Yelland GW, Muir JG, Gibson PR. Randomised clinical trial: the efficacy of gut-directed hypnotherapy is similar to that of the low FODMAP diet for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44(5):447–59. https://doi.org/10.1111/apt.13706.
O'Keeffe M, Jansen C, Martin L, et al. Long-term impact of the low-FODMAP diet on gastrointestinal symptoms, dietary intake, patient acceptability, and healthcare utilization in irritable bowel syndrome. Neurogastroenterol Motil. 2018;30(1). https://doi.org/10.1111/nmo.13154.
Weynants A, Goossens L, Genetello M, De Looze D, Van Winckel M. The long-term effect and adherence of a low fermentable oligosaccharides disaccharides monosaccharides and polyols (FODMAP) diet in patients with irritable bowel syndrome. J Hum Nutr Diet. 2020;33(2):159–69. https://doi.org/10.1111/jhn.12706.
Bellini M, Tonarelli S, Barracca F, et al. A low-FODMAP diet for irritable bowel syndrome: some answers to the doubts from a long-term follow-uP. Nutrients. 2020;12(8). https://doi.org/10.3390/nu12082360.
Rej A, Shaw CC, Buckle RL, et al. The low FODMAP diet for IBS; a multicentre UK study assessing long term follow up. Dig Liver Dis. 2021. https://doi.org/10.1016/j.dld.2021.05.004.
Tuck CJ, Reed DE, Muir JG, Vanner SJ. Implementation of the low FODMAP diet in functional gastrointestinal symptoms: A real-world experience. Neurogastroenterol Motil. 2020;32(1):e13730. https://doi.org/10.1111/nmo.13730.
Lenhart A, Ferch C, Shaw M, Chey WD. Use of dietary management in irritable bowel syndrome: results of a survey of over 1500 United States gastroenterologists. J Neurogastroenterol Motil. 2018;24(3):437–51. https://doi.org/10.5056/jnm17116.
Eswaran S, Dolan RD, Ball SC, Jackson K, Chey W. The impact of a 4-week low-FODMAP and mNICE diet on nutrient intake in a sample of US adults with irritable bowel syndrome with diarrhea. J Acad Nutr Diet. 2020;120(4):641–9. https://doi.org/10.1016/j.jand.2019.03.003.
Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294(1):1–8. https://doi.org/10.1111/j.1574-6968.2009.01514.x.
Bennet SMP, Böhn L, Störsrud S, et al. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut. 2018;67(5):872–81. https://doi.org/10.1136/gutjnl-2016-313128.
Wang XJ, Camilleri M, Vanner S, Tuck C. Review article: biological mechanisms for symptom causation by individual FODMAP subgroups - the case for a more personalised approach to dietary restriction. Aliment Pharmacol Ther. 2019;50(5):517–29. https://doi.org/10.1111/apt.15419.
Shaw CC, Buckle RL, Rej A, Trott N, Aziz I, Sanders DS. A gluten reduction is the patients’ choice for a dietary ‘bottom up’ approach in ibs-a comment on “A 5Ad Dietary Protocol for Functional Bowel Disorders”. Nutrients. 2020;12(1). https://doi.org/10.3390/nu12010137.
Rej A, Sanders DS, Buckle RL, Trott N, Aziz I, Shaw CC. What is the optimal FODMAP threshold in IBS? J Gastroenterol Hepatol. 2021;36(6):1723–5. https://doi.org/10.1111/jgh.15470.
Farzaei MH, Bahramsoltani R, Abdollahi M, Rahimi R. The role of visceral hypersensitivity in irritable bowel syndrome: pharmacological targets and novel treatments. J Neurogastroenterol Motil. 2016;22(4):558–74. https://doi.org/10.5056/jnm16001.
Seamark L, Barclay Y, Marchant C, Williams M, Hickson M. Long-term symptom severity in people with irritable bowel syndrome following dietetic treatment in primary care: A service evaluation. J Hum Nutr Diet. 2021;34(5):890–900. https://doi.org/10.1111/jhn.12860.
Fodor I, Man SC, Dumitrascu DL. Low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols diet in children. World J Clin Cases. 2019;7(18):2666–74. https://doi.org/10.12998/wjcc.v7.i18.2666.
Schumann D, Langhorst J, Dobos G, Cramer H. Randomised clinical trial: yoga vs a low-FODMAP diet in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2018;47(2):203–11. https://doi.org/10.1111/apt.14400.
Bosman M, Elsenbruch S, Corsetti M, et al. The placebo response rate in pharmacological trials in patients with irritable bowel syndrome: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(6):459–73. https://doi.org/10.1016/S2468-1253(21)00023-6.
Henström M, Diekmann L, Bonfiglio F, et al. Functional variants in the sucrase-isomaltase gene associate with increased risk of irritable bowel syndrome. Gut. 2018;67(2):263–70. https://doi.org/10.1136/gutjnl-2016-312456.
Garcia-Etxebarria K, Zheng T, Bonfiglio F, et al. Increased prevalence of rare sucrase-isomaltase pathogenic variants in irritable bowel syndrome patients. Clin Gastroenterol Hepatol. 2018;16(10):1673–6. https://doi.org/10.1016/j.cgh.2018.01.047.
Zheng T, Camargo-Tavares L, Bonfiglio F, Marques FZ, Naim HY, D'Amato M. Rare hypomorphic sucrase isomaltase variants in relation to irritable bowel syndrome risk in UK Biobank. Gastroenterology. 2021;161(5):1712–4. https://doi.org/10.1053/j.gastro.2021.06.063.
Thingholm L, Rühlemann M, Wang J, et al. Sucrase-isomaltase 15Phe IBS risk variant in relation to dietary carbohydrates and faecal microbiota composition. Gut. 2019;68(1):177–8. https://doi.org/10.1136/gutjnl-2017-315841.
Husein DM, Naim HY. Impaired cell surface expression and digestive function of sucrase-isomaltase gene variants are associated with reduced efficacy of low FODMAPs diet in patients with IBS-D. Gut. 2020;69(8):1538–9. https://doi.org/10.1136/gutjnl-2019-319411.
Foley A, Halmos EP, Husein DM, et al. Adult sucrase-isomaltase deficiency masquerading as IBS. Gut. 2022;71(6):1237–8. https://doi.org/10.1136/gutjnl-2021-326153.
Zheng T, Eswaran S, Photenhauer AL, Merchant JL, Chey WD, D’Amato M. Reduced efficacy of low FODMAPs diet in patients with IBS-D carrying sucrase-isomaltase. Gut. 2020;69(2):397–8. https://doi.org/10.1136/gutjnl-2018-318036.
Simons M, Taft TH, Doerfler B, et al. Narrative review: Risk of eating disorders and nutritional deficiencies with dietary therapies for irritable bowel syndrome. Neurogastroenterol Motil. 2022;34(1):e14188. https://doi.org/10.1111/nmo.14188.
Rej A, Buckle RL, CSC, et al. National survey evaluating the provision of gastroenterology dietetic services in England. Frontline Gastroenterology. 2021;12:380–4.
Williams M, Barclay Y, Harper L, Marchant C, Seamark L, Hickson M. Feasibility, acceptability and cost efficiency of using webinars to deliver first-line patient education for people with irritable bowel syndrome as part of a dietetic-led gastroenterology service in primary care. J Hum Nutr Diet. 2020;33(6):758–66. https://doi.org/10.1111/jhn.12799.
Whigham L, Joyce T, Harper G, et al. Clinical effectiveness and economic costs of group versus one-to-one education for short-chain fermentable carbohydrate restriction (low FODMAP diet) in the management of irritable bowel syndrome. J Hum Nutr Diet. 2015;28(6):687–96. https://doi.org/10.1111/jhn.12318.
Acknowledgements
Nil.
Funding
Nil.
Author information
Authors and Affiliations
Contributions
AR wrote the initial manuscript. AA, IA, CJB, RKB, RLB, LS, CCS, JT, NT, MW and DSS reviewed and helped to critically revise the manuscript. DSS is the guarantor of the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
DSS receives an educational grant from Schaer (a gluten-free food manufacturer). Dr Schaer did not have any input in drafting of this manuscript. The other authors declare they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rej, A., Avery, A., Aziz, I. et al. Diet and irritable bowel syndrome: an update from a UK consensus meeting. BMC Med 20, 287 (2022). https://doi.org/10.1186/s12916-022-02496-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-022-02496-w