Abstract
Background
Light-to-moderate alcohol consumption has been reported to be cardio-protective among apparently healthy individuals; however, it is unclear whether this association is also present in those with disease. To examine the association between alcohol consumption and prognosis in individuals with pre-existing cardiovascular disease (CVD), we conducted a series of meta-analyses of new findings from three large-scale cohorts and existing published studies.
Methods
We assessed alcohol consumption in relation to all-cause mortality, cardiovascular mortality, and subsequent cardiovascular events via de novo analyses of 14,386 patients with a previous myocardial infarction, angina, or stroke in the UK Biobank Study (median follow-up 8.7 years, interquartile range [IQR] 8.0–9.5), involving 1640 deaths and 2950 subsequent events, and 2802 patients and 1257 deaths in 15 waves of the Health Survey for England 1994–2008 and three waves of the Scottish Health Survey 1995, 1998, and 2003 (median follow-up 9.5 years, IQR 5.7–13.0). This was augmented with findings from 12 published studies identified through a systematic review, providing data on 31,235 patients, 5095 deaths, and 1414 subsequent events. To determine the best-fitting dose-response association between alcohol and each outcome in the combined sample of 48,423 patients, models were constructed using fractional polynomial regression, adjusting at least for age, sex, and smoking status.
Results
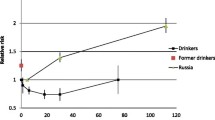
Alcohol consumption was associated with all assessed outcomes in a J-shaped manner relative to current non-drinkers, with a risk reduction that peaked at 7 g/day (relative risk 0.79, 95% confidence interval 0.73–0.85) for all-cause mortality, 8 g/day (0.73, 0.64–0.83) for cardiovascular mortality and 6 g/day (0.50, 0.26–0.96) for cardiovascular events, and remained significant up to 62, 50, and 15 g/day, respectively. No statistically significant elevated risks were found at higher levels of drinking. In the few studies that excluded former drinkers from the non-drinking reference group, reductions in risk among light-to-moderate drinkers were attenuated.
Conclusions
For secondary prevention of CVD, current drinkers may not need to stop drinking. However, they should be informed that the lowest risk of mortality and having another cardiovascular event is likely to be associated with lower levels of drinking, that is up to approximately 105g (or equivalent to 13 UK units, with one unit equal to half a pint of beer/lager/cider, half a glass of wine, or one measure of spirits) a week.
Similar content being viewed by others
Background
Lifestyle and dietary habits play an important role in the secondary prevention of cardiovascular disease (CVD) [1]. However, the impact of alcohol consumption on CVD patients’ prognosis is unclear and recommendations for patients regarding upper limits of drinking vary substantially across different guidelines [2,3,4,5]. While light-to-moderate alcohol consumption is associated with a lower risk of developing multiple cardiovascular outcomes in general population cohorts [6, 7], it is difficult to extend the posited cardio-protective effects to CVD patients because of their typically older age and compromised vasculature as well as the medications they take to prevent secondary events [8]. In addition, for CVD patients, there are concerns about the potential detrimental effects of alcohol on the circulatory system, such as hypertension, arrhythmias, and haemorrhagic stroke, which may exacerbate their existing pathological conditions [9].
The most recent meta-analysis to have explored the association between alcohol consumption and prognosis among CVD patients was undertaken by Costanzo et al. in 2010 [10]. Pooling data from eight observational studies published between 1998 and 2008, they identified a maximal 22% relative risk (RR) reduction at approximately 8 g/day for cardiovascular mortality and 18% at 7 g/day for all-cause mortality among patients with myocardial infarction (MI), angina, or stroke, relative to non-drinkers, with risk increasing in a dose dependent manner above these levels. However, their analysis was limited to studies only on mortality and did not consider any non-fatal outcomes. Understanding how alcohol consumption is related to cardiovascular morbidity is of great importance to CVD patients because this population is at high risk of recurring cardiovascular events which can significantly compromise the patients’ quality of life [11]. Including morbidity information will complement the existing evidence base to provide a more complete picture of how alcohol consumption can be managed for optimal secondary CVD prevention. Additionally, further studies [12,13,14] have been published in the decade since the last meta-analysis. Given the growing debate on this topic, a more detailed and comprehensive reassessment of the evidence is warranted in the absence of long-term clinical trials [9].
We thus analysed individual data from three large-scale cohorts. In addition to estimating risk of mortality among CVD patients, we also examined the association between alcohol intake and subsequent cardiovascular events. To consolidate all available evidence on this topic, we conducted meta-analyses of our results with those from published studies identified through a systematic review.
Methods
De novo cohort analyses
Study cohorts and participants
Data were obtained from participants in the Health Survey for England (HSE), the Scottish Health Survey (SHeSs), and UK Biobank. Descriptions of each cohort are provided in Additional file 1 (Appendix S1). The present analyses combined data from the 1994–2008 HSE datasets and the 1995, 1998, and 2003 SHeSs datasets and were restricted to participants aged ≥16 years reporting to have been diagnosed with MI/angina (not recorded separately) or stroke prior to baseline. For UK Biobank, we identified participants with MI, angina, or stroke before recruitment based on record linkage to the Hospital Episode Statistics (HES), using algorithms defined in Additional file 1 (Appendix S1 and Table S1 [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]).
To be eligible for the analysis, participants in HSE/SHeSs and UK Biobank had to have baseline information about their drinking status and average alcohol intakes, plus age, sex, smoking status, self-reported history of diabetes and hypertension, socioeconomic position/education, body mass index, and regular medications. We separated former drinkers from never drinkers and categorised current drinkers into three groups: low-level drinkers (≤ 14 units/week, one unit contains 8g of ethanol [30] and is equivalent to half a pint of beer/lager/cider, half a glass of wine, or one measure of spirits/fortified wine [31]), medium-level drinkers (>14 to ≤50 units/week for men, >14 to ≤35 units/week for women), and high-level drinkers (>50 units/week for men, >35 units/week for women) [32]. Further details of the alcohol assessment and covariates are described in Additional file 1 (Appendix S1).
We assessed alcohol consumption in relation to three outcomes (each ascertained by national death registries or HES records): all-cause mortality, cardiovascular mortality, and major cardiovascular events. We defined cardiovascular events as a composite of angina, fatal and non-fatal MI and stroke, revascularisation procedures (angioplasty or coronary artery bypass graft), death from heart failure, and sudden cardiac death, and only UK Biobank contributed data to the analysis on cardiovascular events. Participants were followed up until the date of their death or first detected event, or were censored on the date they left the UK or the last date of data linkage (cohort specific). Additional details of outcome ascertainment and follow-up procedures are in Additional file 1 (Appendix S1).
Statistical analysis
We used multivariable Cox proportional hazard models to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations of different drinking categories with each outcome of interest relative to never drinkers. Adjustments were made for age, sex, and smoking status in initial models and then for all covariates in maximally-adjusted models. For HSE/SHeSs datasets, we additionally adjusted for survey wave using shared-frailty models to account for within-group correlations. Schoenfeld residuals were plotted to ascertain that the proportional hazards assumption had not been violated (see Additional file 1: Figure S1). Models for MI, angina and stroke as primary event in further stratified analyses were adjusted for each other as well as all covariates.
Systematic review and meta-analysis
Search strategy and study selection
This study followed PRISMA and MOOSE guidelines [33, 34]. MEDLINE and Embase were searched for relevant studies up to 30 July 2020, using a combination of subject headings and free-text terms with no restrictions on language or publication date (see Additional file 1: Table S2). In addition, the reference lists of eligible studies and a previous systematic review [35] on this topic were manually checked to add any studies missed by the initial database searches.
After removing duplicates, citations were screened to exclude any that did not report a prospective relationship between alcohol consumption and outcomes of interest among patients with pre-existing CVD. Full text of the remaining citations were then independently assessed by two pairs of reviewers (CD and AB/DON) for eligibility. Studies were retained if they met the selection criteria for study design (longitudinal study including randomised control trials not involving alcohol), study population (MI, angina, or stroke patients), exposure (alcohol consumption reported across ≥3 categories, inclusive of a non-drinking group, to allow for testing a curvilinear relationship), outcomes (all-cause or cardiovascular mortality, cardiovascular events), and risk estimates (at least adjusted for age, sex, and smoking). We excluded studies if the reported alcohol consumption could not be converted into gram per day or if frequency counts, risk estimate, and its corresponding 95%CI were not available after contacting the authors. The inter-rater agreement for this review was high (Fleiss κ= 0.85).
Data extraction and quality assessment
Data extraction was conducted by one reviewer (CD) and then verified by a second reviewer (AB/DON). When available, we collected data on the amount of alcohol consumed. Given that most studies included in our analyses reported alcohol consumption on a daily basis, we used grams of alcohol per day as the common unit of measurement. To convert the number of drinks to grams in four included studies (one conducted in Italy [12] and three in USA [36,37,38]) which did not specify the quantity of alcohol in one drink, we assumed country-specific standard drinks (i.e. Italy 12g, USA 14g) [39]. A factor of 0.79 was used for the conversion of millilitres to grams (i.e. 1 ml alcohol = 0.79 g [40]) in one study [41]. Exposures categorised according to time periods longer than 1 day were transferred into daily estimates, assuming an even distribution of consumption over the reference period. Where averages were not reported for each drinking category, the midpoints of the range were chosen. For open-ended upper categories, mean values were defined as 1.2 times the lower boundary as suggested by Berlin et al. [42]. Similar results were obtained when multiplying the lower boundary for the open-ended upper categories by 1.0, 1.4, or 1.6 instead of 1.2 (see Additional file 1: Figure S2).
Multiple alcohol measures were used in three included studies, two of which reported risk estimates based on the average intakes during follow-up [13, 43] and the remaining one performed time-dependent analyses to allow changes on drinking habits [12]. In addition, most of the included studies asked patients to report their average consumption since the occurrence of their primary events (post-event alcohol assessment), whereas three studies used alcohol intake in the year prior to primary events (pre-event), assuming drinking habits remained stable over time, even following events [14, 44, 45].
Because all included studies except one [46] used a non-drinking reference group, we preferred risk estimates for different drinking categories versus non-drinkers. For a single study that used occasional drinkers as the reference group [46], the risk estimates were recalculated to derive alternative estimates each relative to a non-drinker group. A Microsoft Excel spreadsheet developed by Hamling et al. was used during the recalculation to account for the non-independence between estimates sharing a common reference group [47]. When a study reported risk estimates with different degrees of statistical adjustment for confounding, we used the most-adjusted one. Furthermore, to investigate the possible impact of over-adjustment for potential mediators on our results, we performed a sensitivity analysis by using risk estimates that were only controlled for age, sex, and smoking, the three most important confounding factors for the alcohol-CVD relationship. With all estimates reported being RR or HR, RR served as the common measure of association across studies. HRs were treated as measures of RRs [48]. Study quality was assessed using the Newcastle-Ottawa Scale (see Additional file 1: Appendix S2) [49].
Data synthesis
For each analysis, a family of second-degree fractional polynomial models (FP2: log RR = β1xp1 +β2xp2, x0 equals log(x) rather than 1 and the model becomes log RR = β1xp + β2xplog(x) when p1 = p2) was generated to derive a power transformation of the exposure variable [50]. p1 and p2 were taken from a predefined set P= (−2, −1, −0.5, 0, 0.5, 1, 2, 3) which allows for a very large and varied set of functions, including U- and J-shaped curves, to be generated. For x = 0, the function would start from log RR = 0 and therefore no constant term (i.e. the intercept) was considered in our models [51]. The best fit among the family of models was defined as that with the lowest deviance.
With the terms of exposure identified in the best-fitting FP2, a two-stage regression model was fitted to summarise the relationship between alcohol consumption and each outcome of interest. The first stage generated the dose-response model within each study and the second stage pooled study-specific trends using a random effect model to accommodate the heterogeneity across studies [52, 53]. A sensitivity analysis was done by excluding studies of the lowest quality and pre-defined subgroup analyses according to sex, primary event, and type of non-drinking reference group and alcohol assessment for each outcome of interest.
The overall degree of heterogeneity was quantified using the I2 index [54]. We assessed evidence of publication bias through visual inspection of funnel plots and Egger’s regression test for asymmetry [55]. All statistical analyses were performed using Stata (version 15.1).
Results
Associations of alcohol consumption with mortality and cardiovascular morbidity in study cohorts
Complete data for the de novo cohort analyses were available for 2802 participants (MI/angina=2341, stroke=535) in HSE/SHeSs and 14,386 (MI=5333, angina=9589, stroke=2064) in UK Biobank (see Additional file 1: Figure S3). On average, UK Biobank participants were younger and reported higher consumption of alcohol than HSE/SHeSs participants (Table 1).
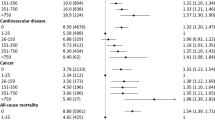
During a median follow-up of 9.5 years (interquartile range [IQR], 5.7–13.0) in HSE/SHeSs and 8.7 years (IQR, 8.0–9.5) in UK Biobank, we identified 1257 deaths among HSE/SHeSs participants and 1640 deaths among UK Biobank participants, of which 492 (39.1%) and 631 (38.5%) deaths were due to cardiovascular causes, respectively. Maximally adjusted models of UK Biobank dataset revealed a J-shaped association for both all-cause and cardiovascular mortality, with low- and medium-level drinkers having a decreased risk compared with never drinkers but no difference in risk for high-level or former drinkers (Fig. 1). Although similar J-shaped trends were observed for HSE/SHeSs, none of the associations were statistically significant, probably due to the relatively small sample size of each drinking subgroup (Fig. 1). We noted differential associations by sex and primary cardiovascular events in stratified analyses (see Additional file 1: Figures S4 and S5).
Association of drinking categories with all-cause mortality, cardiovascular mortality, and cardiovascular events by study cohorts. Hazard ratios are adjusted for age, sex, smoking status, diabetes, hypertension, socioeconomic position or education, body mass index, and regular use of cholesterol-lowering medications, antihypertensive medications, antiplatelet agents, digoxin, and warfarin. CI indicates confidence interval; HR, hazard ratio; HSE, the Health Survey for England; SHeSs, the Scottish Health Survey
A total of 2950 fatal and non-fatal subsequent cardiovascular events were recorded in UK Biobank, with a median follow-up of 7.5 years (IQR, 6.8–8.5). A lower risk of cardiovascular events was observed across all categories of current drinkers (Fig. 1), within participants of both sexes and with different primary events (see Additional file 1: Figures S4 and S5).
Characteristics of studies included in meta-analysis
Of the initial 1722 unique citations, 12 published studies fulfilled the selection criteria (see Additional file 1: Figure S6). Table 2 outlines the characteristics of all studies selected for meta-analyses, inclusive of HSE/SHeSs and UK Biobank. Nine of the 14 studies had a cohort design and the remaining five [12, 36,37,38, 43] were randomised control trials for certain drug or diet type with no specific inventions on alcohol consumption. The quality of selected studies was moderate to high on average, with a median score of 8 on the Newcastle-Ottawa Scale. Additional details regarding alcohol consumption, effect estimates, and confounder adjustment are provided in Additional file 1 (Tables S3–S5).
Alcohol consumption and all-cause mortality among CVD patients
Eleven studies, comprising 41,743 CVD patients, contributed to this analysis. Overall, a J-shaped association was observed, with a protective effect that peaked at 7 g/day and remained significant up to 62 g/day (Fig. 2A, Table 3). Although the dose-response trend followed a J-curve in men, we found no increased risk among women at higher levels of drinking (see Additional file 1: Figure S7). Regarding primary events, moderate drinking was associated with a lower risk for total mortality among patients with a previous MI or angina, but not with stroke (see Additional file 1: Figure S8). Pooled analysis of estimates relative to non-current drinkers showed a reduced mortality risk for an alcohol intake up to approximately 75 g/day. However, when studies with former drinkers in the reference group were excluded, the association was considerably weakened (see Additional file 1: Figure S9). In addition, among those studies using post-event alcohol measures, the result did not change substantively; a similar trend was seen in studies with multiple measures but failed to reach statistical significance, probably because of the low number of curves (n=2) in this subgroup (see Additional file 1: Figure S10).
Overall dose-response relationship between alcohol consumption and risk of mortality and subsequent cardiovascular events, using maximally-adjusted estimates. Best-fitting second-degree fractional polynomial models (with 95% CIs) are shown in solid curves with each data point overlaid as circles. Circle size indicates the weighting of each data point and is inversely proportional to the variance of the log-transformed relative risk
Alcohol consumption and cardiovascular mortality among CVD patients
Nine studies, comprising 24,770 patients, were included in the meta-analysis on cardiovascular mortality, and the overall association with alcohol consumption was interpreted as a J-curve. The maximal reduction in mortality risk was found to be 27% at 8 g/day and the reversion point was reached at 50 g/day (Fig. 2B, Table 3). Our results remained little altered when considering studies on men only, or using different types of reference groups or alcohol assessments (see Additional file 1: Figures S7, S9 and S10). Unlike the J-curve observed for men, there was no excess risk of mortality among women at higher levels of consumption (see Additional file 1: Figure S7). Stratified analyses by primary events showed that moderate drinking was associated with a lower risk of cardiovascular mortality among patients with a previous MI; however, among those with angina or stroke, the overall dose-response trend was close to null (see Additional file 1: Figure S8).
Alcohol consumption and cardiovascular events among CVD patients
Among the four studies (28,621 patients) addressing drinking and cardiovascular events, one reported dose-response trend separately for two age groups and thus provided two curves. Alcohol intake was associated with a significant reduction in the risk of cardiovascular events up to 15g/day (Fig. 2C, Table 3). Pooled analysis of studies on women showed a declined risk for an alcohol intake up to approximately 49 g/day, whereas no reduction in risk was seen in men at any level of consumption (see Additional file 1: Figure S7). Moderate drinking was found to be protective against cardiovascular events within patients of different primary events and studies with multiple alcohol measures (see Additional file 1: Figures S8 and S10). However, when studies including former drinkers in the reference group were excluded, the overall protective effect was attenuated and became non-significant (see Additional file 1: Figure S9).
Sensitivity analyses
Sensitivity analyses excluding studies of the lowest quality (score <7) revealed similar curves (see Additional file 1: Figure S11). Results were consistent when restricting analysis to estimates that were only adjusted for age, sex, and smoking status (see Additional file 1: Figure S12). For mortality outcomes, there was no evidence of heterogeneity across the first- and second-order polynomial (both I2 = 0%); however, a high degree of heterogeneity (both I2 = 75%) was noted in studies contributing results for cardiovascular events. For all outcomes assessed, we found no evidence of publication bias (see Additional file 1: Figure S13).
Discussion
Meta-analysis of the results from three major UK cohorts together with those from 12 published studies found J-curve relationships between alcohol consumption and mortality in those with cardiovascular disease, with the greatest risk reduction being observed at 7 g/day for all-cause mortality and 8 g/day for cardiovascular mortality relative to current non-drinkers. This dose-response trend remains consistent with the last published meta-analysis [10] and has also been reported in other high-risk populations, such as hypertensive [57] and diabetic individuals [58].
To our knowledge, this is the first meta-analysis of alcohol consumption and any subsequent cardiovascular events in patients with previous CVD, in which UK Biobank contributed nearly half of the total sample size. We found a reduction in risk for an alcohol intake up to approximately 15 g/day, an upper limit much lower than those for the mortality outcomes. Taken together, our study suggested that, among CVD patients, the upper drinking limit for lower risks of mortality and cardiovascular morbidity was about 105 g/week, which was lower than those recommended in most current guidelines. For example, the American Heart Association (AHA) and American College of Cardiology Foundation 2011 guidelines on secondary prevention recommend “alcohol moderation”—up to 196 g/week (2 USA drinks/day) for male and 98 g/week (1 USA drink/day) for female according to the national dietary guidelines [59]—for patients with atherosclerotic vascular disease [2]; the same recommendations apply in the AHA/American Stroke Association 2014 guidelines for secondary stroke prevention [5]; the UK National Institute for Health and Care Excellence 2020 guidelines recommend to keep alcohol intake within 112 g/week (14 UK units/week) for both men and women after having an MI [4]; and WHO 2007 recommendations for prevention of recurrent MI and stroke were no more than about 166 g/week (3 units/day, 1 unit contains 10 ml of pure alcohol) [3].
Strengths and limitations of study
With almost triple the number of CVD patients, our study expands the findings of the last comprehensive review published a decade ago [10]. In particular, both HSE/SHeSs and UK Biobank provide long-term follow-up of large contemporary samples from the UK general population. The inclusion of these new datasets allows us to examine the risk of drinking within various subgroups, some of which are not available or too small to reliably investigate in published studies. For example, our data suggest that the dose-response associations of alcohol with mortality and morbidity differ by sex and are more pronounced among patients with MI than angina or stroke. These findings raise the question of whether differential drinking limits should be recommended in patient subgroups and warrant further investigation. Furthermore, there is evidence that reductions in risk of all-cause mortality and subsequent events might have been overestimated due to the inclusion of former drinkers in the non-drinking reference group. Former drinkers may include individuals who have quit drinking in response to ill health (i.e. “sick quitters”), particularly past heavy drinkers [60], therefore making current drinkers appear healthy relative to less healthy non-current drinkers. This could lead to a low-risk drinking limit less than the estimated 105g/week; however, we cannot definitely determine the extent of this overestimation with very few studies that explicitly excluded former drinkers.
Many medications commonly used by CVD patients can interact with alcohol by altering the metabolism or effects of the medication and/or alcohol [61]. The interactions may occur with lower amounts of alcohol or follow a dose-response relationship, with the risk and severity of interactions increasing with increasing levels of alcohol consumption [62]. For example, moderate drinking in combination with statins use may be synergistic to confer a lower risk of all-cause mortality [63]. Concurrent heavy drinking with warfarin enhance the anticoagulant effect and may lead to major bleeding [64]. In the present meta-analyses, most (9 out of 14) but not all included studies adjusted for medication use (including antihypertensives, cholesterol-lowering and oral antiplatelet agents) in their most-adjusted models and so there is a possibility of residual confounding by medications. However, sensitivity analyses showed consistent results when using risk estimates that were only adjusted for age, sex and smoking, suggesting that further adjustment for medication use is unlikely to materially impact on our findings.
In the present study, no elevated risk of mortality and cardiovascular events was found at higher levels of alcohol consumption, which is in line with other meta-analysis among CVD patients [10, 65] but contradicts evidence from some of the general population studies [66, 67]. The discrepancy between the present study and previous general population studies may be partly due to the generally older age of CVD patients. The mean/median age at baseline was greater than 59 years in most datasets used in our analyses. Because alcohol-related risk is relatively higher among younger people compared with the elderly [68], enrolling older participants in studies would minimise the risk relationship compared with an analysis that included drinkers of all ages. Notably, with older age of the study participants comes increased likelihood for drinkers to become former drinkers, which might exacerbate the “sick quitters” bias (i.e. when the non-drinking reference group also includes former drinkers who have stopped drinking due to poor health) as discussed above. Patients who drink heavily and enrolled in studies at older ages are more likely to represent “healthy survivors” or have safer drinking patterns [60, 69]. Particularly heavy drinkers are known to be under-represented in some datasets used in our analyses, such as the Physicians’ Health Study [70] and HSE/SHeSs [71]. These potential selections may have biased downwards the estimated associations between heavy intake and risks of mortality and subsequent events. Furthermore, most included studies did not capture the extremes of drinking and therefore may be underpowered to look at the effects of very heavy drinking. Consequently, the absence of effects at higher levels of consumption seen in our study should be interpreted cautiously, particularly in light of the increasing concerns about alcohol misuse among older people [72] as well as the known wider health and societal impacts in regard to these [73].
The present study has some further limitations. First, as a composite of cardiac mortality and several non-fatal cardiovascular endpoints, the definition of cardiovascular events varied across the three published studies [12, 41, 43], and thus, we defined the outcome in UK Biobank using the most frequently reported events in these studies. However, there was still a significant heterogeneity in the pooled analysis. Recent observational and genetic evidence has suggested that drinking at moderate levels is associated with a decreased risk of some but not all forms of CVD [6, 74,75,76]. Therefore, this heterogeneity might have reflected the complex and diverse impacts of alcohol consumption on different CVD outcomes.
Secondly, our results must be interpreted with caution when it comes to some subgroups that have been examined in only a limited number of studies. Although the included studies scored as moderate-to-high quality on the Newcastle-Ottawa Scale, this may not account for some pertinent design/reporting characteristics of many of the studies which had problems that were specific to alcohol exposure and not covered in the scale. For example, by relying upon only a single measurement of alcohol consumption, some studies did not consider the effect of temporal changes in drinking behaviour both after primary event and during follow-up; however, our results remained consistent in the analyses restricted to studies using post-event or multiple measures. Further analyses for beverage type were not possible with sufficient beverage-specific data reported in very few studies.
Thirdly, episodic heavy drinking has been suggested to modify the relationship between average alcohol consumption and CVD/mortality risk [77]. Our results might have been confounded by the drinking pattern, as the selected studies did not exclude “binge” drinkers. Additionally, as with all observational studies and self-reported alcohol intake, our findings are prone to bias; however, self-reported drinking data was validated against high-density lipoprotein cholesterol and gamma-glutamyl transferase in HSE/SHeSs and UK Biobank (see Additional file 1: Table S6). Although we attempted to minimise confounding by using the most adjusted estimates, information on dietary habits or physical activity was not available in all studies included in our meta-analysis and residual confounding may still persist.
Conclusions
In summary, our study shows that an alcohol intake up to about 105g (or equivalent to 13 UK units, with one unit equal to half a pint of beer/lager/cider, half a glass of wine, or one measure of spirits) a week is associated with lower risks of both mortality and subsequent cardiovascular events among CVD patients. While this threshold is somewhat lower than those recommended in most current guidelines, specific recommendations regarding the downward revision of such guidelines cannot be made. There is some indication that reductions in risk may have been overestimated by studies using a referent group contaminated by less healthy former drinkers. No evidence of elevated risk among heavy drinkers was found but this was potentially attributable to selections and under-representation of such drinkers in the datasets. Moreover, when developing drinking thresholds for use within guidelines, we must consider the totality of evidence and balance pragmatic concerns [78]. Our findings therefore indicate that, for secondary prevention of CVD, current drinkers may not need to stop drinking but should be informed that lower levels of intake (up to 105g/week) may be associated with reduced risks. However, non-drinking patients should not be encouraged to take up light drinking because of well-known adverse effects on other health outcomes, such as cancers [79].
Availability of data and materials
Data from UK Biobank (http://www.ukbiobank.ac.uk/) and the Health Survey for England and the Scottish Health Survey (https://www.ukdataservice.ac.uk/) are available to researchers upon application.
Abbreviations
- AHA:
-
American Heart Association
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CVD:
-
Cardiovascular disease
- FP2:
-
Second-degree fractional polynomial model
- HES:
-
Hospital Episode Statistics
- HR:
-
Hazard ratio
- HSE:
-
Health Survey for England
- IQR:
-
Interquartile range
- MI:
-
Myocardial infarction
- RR:
-
Relative risk
- SHeSs:
-
Scottish Health Survey
References
Piepoli MF, Corrà U, Abreu A, Cupples M, Davos C, Doherty P, et al. Challenges in secondary prevention of cardiovascular diseases: a review of the current practice. Int J Cardiol. 2015;180:114–9. https://doi.org/10.1016/j.ijcard.2014.11.107.
Smith SC, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol. 2011;58(23):2432–46. https://doi.org/10.1016/j.jacc.2011.10.824.
World Health Organization. Prevention of cardiovascular disease: pocket guidelines for assessment and management of cardiovascular risk. 2007. https://www.who.int/cardiovascular_diseases/guidelines/PocketGL.ENGLISH.AFR-D-E.rev1.pdf. Accessed 1 Mar 2021.
National Institute for Health and Care Excellence. Acute coronary syndromes. NICE guideline [NG185]. 2020. https://www.nice.org.uk/guidance/ng185/resources/acute-coronary-syndromes-pdf-66142023361477. Accessed 20 May 2021.
Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–236. https://doi.org/10.1161/STR.0000000000000024.
Bell S, Daskalopoulou M, Rapsomaniki E, George J, Britton A, Bobak M, et al. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: population based cohort study using linked health records. BMJ. 2017;356:j909.
Bergmann MM, Rehm J, Klipstein-Grobusch K, Boeing H, Schütze M, Drogan D, et al. The association of pattern of lifetime alcohol use and cause of death in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Int J Epidemiol. 2013;42(6):1772–90. https://doi.org/10.1093/ije/dyt154.
Iestra JA, Kromhout D, van der Schouw YT, Grobbee DE, Boshuizen HC, van Staveren WA. Effect size estimates of lifestyle and dietary changes on all-cause mortality in coronary artery disease patients: a systematic review. Circulation. 2005;112(6):924–34. https://doi.org/10.1161/CIRCULATIONAHA.104.503995.
Mukamal KJ, Clowry CM, Murray MM, Hendriks HFJ, Rimm EB, Sink KM, et al. Moderate alcohol consumption and chronic disease: the case for a long-term trial. Alcohol Clin Exp Res. 2016;40(11):2283–91. https://doi.org/10.1111/acer.13231.
Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Alcohol consumption and mortality in patients with cardiovascular disease: a meta-analysis. J Am Coll Cardiol. 2010;55(13):1339–47. https://doi.org/10.1016/j.jacc.2010.01.006.
De Smedt D, Clays E, Annemans L, Doyle F, Kotseva K, Pajak A, et al. Health related quality of life in coronary patients and its association with their cardiovascular risk profile: results from the EUROASPIRE III survey. Int J Cardiol. 2013;168(2):898–903. https://doi.org/10.1016/j.ijcard.2012.10.053.
Levantesi G, Marfisi R, Mozaffarian D, Franzosi MG, Maggioni A, Nicolosi GL, et al. Wine consumption and risk of cardiovascular events after myocardial infarction: results from the GISSI-Prevenzione trial. Int J Cardiol. 2013;163(3):282–7. https://doi.org/10.1016/j.ijcard.2011.06.053.
Pai JK, Mukamal KJ, Rimm EB. Long-term alcohol consumption in relation to all-cause and cardiovascular mortality among survivors of myocardial infarction: the Health Professionals Follow-up Study. Eur Heart J. 2012;33(13):1598–605. https://doi.org/10.1093/eurheartj/ehs047.
Rosenbloom JI, Mukamal KJ, Frost LE, Mittleman MA. Alcohol consumption patterns, beverage type, and long-term mortality among women survivors of acute myocardial infarction. Am J Cardiol. 2012;109(2):147–52. https://doi.org/10.1016/j.amjcard.2011.08.021.
Mindell J, Biddulph JP, Hirani V, Stamatakis E, Craig R, Nunn S, et al. Cohort profile: the health survey for England. Int J epidemiol. 2012;41(6):1585–93. https://doi.org/10.1093/ije/dyr199.
Gray L, Batty GD, Craig P, Stewart C, Whyte B, Finlayson A, et al. Cohort profile: the Scottish health surveys cohort: linkage of study participants to routinely collected records for mortality, hospital discharge, cancer and offspring birth characteristics in three nationwide studies. Int J epidemiol. 2010;39(2):345–50. https://doi.org/10.1093/ije/dyp155.
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. Plos Med. 2015;12(3):e1001779. https://doi.org/10.1371/journal.pmed.1001779.
Schnier C, Bush K, Nolan J, Sudlow C, UK Biobank Outcome Adjudication Group. Definitions of acute myocardial infarction and main myocardial infarction pathological types UK Biobank phase 1 outcomes adjudication. 2017. http://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/alg_outcome_mi.pdf. Accessed 10 Oct 2019.
Schnier C, Bush K, Nolan J, Sudlow C, UK Biobank Outcome Adjudication Group. Definitions of stroke for UK Biobank phase 1 outcomes adjudication. 2017. https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/alg_outcome_stroke.pdf. Accessed 10 Oct 2019.
Schnier C, Sudlow C, UK Biobank Follow-up and Outcomes Adjudication Group. 2017. Algorithmically-defined health outcomes. 2017. https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/alg_outcome_main.pdf. Accessed 10 Oct 2019.
Timpka S, Fraser A, Schyman T, Stuart JJ, Åsvold BO, Mogren I, et al. The value of pregnancy complication history for 10-year cardiovascular disease risk prediction in middle-aged women. Eur J Epidemiol. 2018;33(10):1003–10. https://doi.org/10.1007/s10654-018-0429-1.
Dawes P, Cruickshanks KJ, Moore DR, Edmondson-Jones M, McCormack A, Fortnum H, et al. Cigarette smoking, passive smoking, alcohol consumption, and hearing loss. J Assoc Res Otolaryngol. 2014;15(4):663–74. https://doi.org/10.1007/s10162-014-0461-0.
Egeberg A, Gislason GH, Hansen PR. Risk of major adverse cardiovascular events and all-cause mortality in patients with hidradenitis suppurativa. JAMA Dermatol. 2016;152(4):429–34. https://doi.org/10.1001/jamadermatol.2015.6264.
Bansal N, Fischbacher CM, Bhopal RS, Brown H, Steiner MF, Capewell S, et al. Myocardial infarction incidence and survival by ethnic group: Scottish Health and Ethnicity Linkage retrospective cohort study. BMJ Open. 2013;3(9):e003415. https://doi.org/10.1136/bmjopen-2013-003415.
Office for National Statistics. Standard occupational classification 2010. 2010. https://www.ons.gov.uk/methodology/classificationsandstandards/standardoccupationalclassificationsoc/soc2010. Accessed 10 Oct 2019.
Guggenheim JA, Williams C, Eye UKB, Vision C. Role of educational exposure in the association between myopia and birth order. JAMA Ophthalmol. 2015;133(12):1408–14. https://doi.org/10.1001/jamaophthalmol.2015.3556.
Aragam KG, Chaffin M, Levinson RT, McDermott G, Choi SH, Shoemaker MB, et al. Phenotypic refinement of heart failure in a national biobank facilitates genetic discovery. Circulation. 2019;139(4):489–501. https://doi.org/10.1161/CIRCULATIONAHA.118.035774.
Papadakis M, Sharma S, Cox S, Sheppard MN, Panoulas VF, Behr ER. The magnitude of sudden cardiac death in the young: a death certificate-based review in England and Wales. Europace. 2009;11(10):1353–8. https://doi.org/10.1093/europace/eup229.
Smolina K, Wright FL, Rayner M, Goldacre MJ. Long-term survival and recurrence after acute myocardial infarction in England, 2004 to 2010. Circ Cardiovasc Qual Outcomes. 2012;5(4):532–40. https://doi.org/10.1161/CIRCOUTCOMES.111.964700.
House of Commons Science and Technology Committee. Alcohol guidelines, Eleventh Report of Session 2010–12. 2012 https://publications.parliament.uk/pa/cm201012/cmselect/cmsctech/1536/1536.pdf. Accessed 20 May 2021.
Brown L, National Centre for Social Research. Health Survey for England 2015: adult alcohol consumption. 2016. http://cliftondavies.com/wp-content/uploads/2016/12/Health-Survey-England-adult-alcohol-consumption.pdf. Accessed 20 May 2021.
Department of Health. UK Chief Medical Officers’ Low Risk Drinking Guidelines. 2016. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/545937/UK_CMOs__report.pdf. Accessed 1 Mar 2021.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. https://doi.org/10.1001/jama.283.15.2008.
Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Cardiovascular and overall mortality risk in relation to alcohol consumption in patients with cardiovascular disease. Circulation. 2010;121(17):1951–9. https://doi.org/10.1161/CIRCULATIONAHA.109.865840.
Aguilar D, Skali H, Moyé LA, Lewis EF, Gaziano JM, Rutherford JD, et al. Alcohol consumption and prognosis in patients with left ventricular systolic dysfunction after a myocardial infarction. J Am Coll Cardiol. 2004;43(11):2015–21. https://doi.org/10.1016/j.jacc.2004.01.042.
Jackson VA, Sesso HD, Buring JE, Gaziano JM. Alcohol consumption and mortality in men with preexisting cerebrovascular disease. Arch Intern Med. 2003;163(10):1189–93. https://doi.org/10.1001/archinte.163.10.1189.
Muntwyler J, Hennekens CH, Buring JE, Gaziano JM. Mortality and light to moderate alcohol consumption after myocardial infarction. Lancet. 1998;352(9144):1882–5. https://doi.org/10.1016/S0140-6736(98)06351-X.
Kalinowski A, Humphreys K. Governmental standard drink definitions and low-risk alcohol consumption guidelines in 37 countries. Addiction. 2016;111(7):1293–8. https://doi.org/10.1111/add.13341.
World Health Organization. International guide for monitoring alcohol consumption and related harm. 2000. https://apps.who.int/iris/bitstream/handle/10665/66529/WHO_MSD_MSB_00.4.pdf?sequence=1. Accessed 1 Mar 2021.
Masunaga N, Kimura A, Miyataka M, Nishioka N, Hirano Y, Hayashi T, et al. Effects of alcohol consumption on cardiovascular events in male patients with healed myocardial infarction. Circ J. 2006;70(10):1263–8. https://doi.org/10.1253/circj.70.1263.
Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;4(3):218–28. https://doi.org/10.1097/00001648-199305000-00005.
de Lorgeril M, Salen P, Martin J-L, Boucher F, Paillard F, de Leiris J. Wine drinking and risks of cardiovascular complications after recent acute myocardial infarction. Circulation. 2002;106:1465–9.
Janszky I, Ljung R, Ahnve S, Hallqvist J, Bennet AM, Mukamal KJ. Alcohol and long-term prognosis after a first acute myocardial infarction: the SHEEP study. Eur Heart J. 2008;29(1):45–53. https://doi.org/10.1093/eurheartj/ehm509.
Mukamal KJ, Maclure M, Muller JE, Sherwood JB, Mittleman MA. Prior alcohol consumption and mortality following acute myocardial infarction. JAMA. 2001;285(15):1965–70. https://doi.org/10.1001/jama.285.15.1965.
Shaper AG, Wannamethee SG. Alcohol intake and mortality in middle aged men with diagnosed coronary heart disease. Heart. 2000;83(4):394–9. https://doi.org/10.1136/heart.83.4.394.
Hamling J, Lee P, Weitkunat R, Ambühl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27(7):954–70. https://doi.org/10.1002/sim.3013.
Knott C, Bell S, Britton A. Alcohol consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of more than 1.9 million individuals from 38 observational studies. Diab Care. 2015;38(9):1804–12. https://doi.org/10.2337/dc15-0710.
Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 1 Mar 2021.
Royston P, Sauerbrei W. Fractional Polynomials for One Variable. In: Multivariable model-building: a pragmatic approach to regression anaylsis based on fractional polynomials for modelling continuous variables. Chichester: Wiley; 2008. p. 71–98. https://doi.org/10.1002/9780470770771.ch4.
Bagnardi V, Zambon A, Quatto P, Corrao G. Flexible meta-regression functions for modeling aggregate dose-response data, with an application to alcohol and mortality. Am J Epidemiol. 2004;159(11):1077–86. https://doi.org/10.1093/aje/kwh142.
Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6(1):40–57. https://doi.org/10.1177/1536867X0600600103.
White IR. Multivariate random-effects meta-regression: updates to mvmeta. Stata J. 2011;11(2):255–70. https://doi.org/10.1177/1536867X1101100206.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. https://doi.org/10.1136/bmj.327.7414.557.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. https://doi.org/10.1136/bmj.315.7109.629.
Valmadrid CT, Klein R, Moss SE, Klein BEK, Cruickshanks KJ. Alcohol intake and the risk of coronary heart disease mortality in persons with older-onset diabetes mellitus. JAMA. 1999;282(3):239–46. https://doi.org/10.1001/jama.282.3.239.
Hansen JL, Tolstrup JS, Jensen MK, Grønbæk M, Tjønneland A, Schmidt EB, et al. Alcohol intake and risk of acute coronary syndrome and mortality in men and women with and without hypertension. Eur J Epidemiol. 2011;26(6):439–47. https://doi.org/10.1007/s10654-011-9564-7.
Koppes L, Dekker J, Hendriks H, Bouter L, Heine R. Meta-analysis of the relationship between alcohol consumption and coronary heart disease and mortality in type 2 diabetic patients. Diabetologia. 2006;49(4):648–52. https://doi.org/10.1007/s00125-005-0127-x.
U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th Edition. December 2015. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf. Accessed 1 Mar 2021.
Ng Fat L, Cable N, Shelton N. Worsening of health and a cessation or reduction in alcohol consumption to special occasion drinking across three decades of the life course. Alcohol Clin Exp Res. 2015;39(1):166–74. https://doi.org/10.1111/acer.12596.
Holton AE, Gallagher PJ, Ryan C, Fahey T, Cousins G. Consensus validation of the POSAMINO (POtentially Serious Alcohol-Medication INteractions in Older adults) criteria. BMJ Open. 2017;7(11):e017453. https://doi.org/10.1136/bmjopen-2017-017453.
Moore AA, Whiteman EJ, Ward KT. Risks of combined alcohol/medication use in older adults. Am J Geriatr Pharmacother. 2007;5(1):64–74. https://doi.org/10.1016/j.amjopharm.2007.03.006.
Kabagambe EK, Kiage JN, Judd SE, Slaughter JC, Lipworth L, Sampson U, et al. Alcohol consumption, statin use and risk of all-cause mortality in the reasons for geographic and racial differences in stroke (REGARDS) cohort. Circulation. 2015;131(suppl_1):AP044.
Roth JA, Bradley K, Thummel KE, Veenstra DL, Boudreau D. Alcohol misuse, genetics, and major bleeding among warfarin therapy patients in a community setting. Pharmacoepidemiol Drug Saf. 2015;24(6):619–27. https://doi.org/10.1002/pds.3769.
Huang C, Zhan J, Liu YJ, Li DJ, Wang SQ, He QQ. Association between alcohol consumption and risk of cardiovascular disease and all-cause mortality in patients with hypertension: a meta-analysis of prospective cohort studies. Mayo Clin Proc. 2014;89(9):1201–10. https://doi.org/10.1016/j.mayocp.2014.05.014.
Xi B, Veeranki SP, Zhao M, Ma C, Yan Y, Mi J. Relationship of alcohol consumption to all-cause, cardiovascular, and cancer-related mortality in U.S. adults. J Am Coll Cardiol. 2017;70(8):913–22. https://doi.org/10.1016/j.jacc.2017.06.054.
Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342(feb22 1):d671. https://doi.org/10.1136/bmj.d671.
Naimi TS, Stadtmueller LA, Chikritzhs T, Stockwell T, Zhao J, Britton A, et al. Alcohol, age, and mortality: estimating selection bias due to premature death. J Stud Alcohol Drugs. 2019;80(1):63–8. https://doi.org/10.15288/jsad.2019.80.63.
Naimi TS, Stockwell T, Zhao J, Xuan Z, Dangardt F, Saitz R, et al. Selection biases in observational studies affect associations between ‘moderate’ alcohol consumption and mortality. Addiction. 2017;112(2):207–14. https://doi.org/10.1111/add.13451.
Gaziano JM, Gaziano TA, Glynn RJ, Sesso HD, Ajani UA, Stampfer MJ, et al. Light-to-moderate alcohol consumption and mortality in the Physicians’ Health Study enrollment cohort. J Am Coll Cardiol. 2000;35(1):96–105. https://doi.org/10.1016/S0735-1097(99)00531-8.
Boniface S, Scholes S, Shelton N, Connor J. Assessment of non-response bias in estimates of alcohol consumption: applying the continuum of resistance model in a general population survey in England. Plos One. 2017;12(1):e0170892. https://doi.org/10.1371/journal.pone.0170892.
Knott CS, Coombs N, Stamatakis E, Biddulph JP. All cause mortality and the case for age specific alcohol consumption guidelines: pooled analyses of up to 10 population based cohorts. BMJ. 2015;350(feb10 2):h384. https://doi.org/10.1136/bmj.h384.
Bardach AE, Alcaraz AO, Ciapponi A, Garay OU, Riviere AP, Palacios A, et al. Alcohol consumption's attributable disease burden and cost-effectiveness of targeted public health interventions: a systematic review of mathematical models. BMC Public Health. 2019;19(1):1378. https://doi.org/10.1186/s12889-019-7771-4.
Millwood IY, Walters RG, Mei XW, Guo Y, Yang L, Bian Z, et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: a prospective study of 500 000 men and women in China. Lancet. 2019;393(10183):1831–42. https://doi.org/10.1016/S0140-6736(18)31772-0.
Smyth A, Teo KK, Rangarajan S, O'Donnell M, Zhang X, Rana P, et al. Alcohol consumption and cardiovascular disease, cancer, injury, admission to hospital, and mortality: a prospective cohort study. Lancet. 2015;386(10007):1945–54. https://doi.org/10.1016/S0140-6736(15)00235-4.
Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, et al. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet. 2018;391(10129):1513–23. https://doi.org/10.1016/S0140-6736(18)30134-X.
Roerecke M, Rehm J. Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 2014;12(1):182. https://doi.org/10.1186/s12916-014-0182-6.
Holmes J, Angus C, Meier PS, Buykx P, Brennan A. How should we set consumption thresholds for low risk drinking guidelines? Achieving objectivity and transparency using evidence, expert judgement and pragmatism. Addiction. 2019;114(4):590–600. https://doi.org/10.1111/add.14381.
LoConte NK, Brewster AM, Kaur JS, Merrill JK, Alberg AJ. Alcohol and cancer: a statement of the American Society of Clinical Oncology. J Clin Oncol. 2018;36(1):83–93. https://doi.org/10.1200/JCO.2017.76.1155.
Acknowledgements
Not applicable.
Funding
This work was supported by the Economic and Social Research Council (ES/K000357/1), the National Institute for Health Research (NIHR) Blood and Transplant Research Unit in Donor Health and Genomics (NIHR BTRU-2014-10024), UK Medical Research Council (MR/L003120/1), British Heart Foundation (SP/09/002, RG/13/13/30194, RG/18/13/33946), and the NIHR Cambridge Biomedical Research Centre at the Cambridge University Hospitals NHS Foundation Trust. The funding bodies had no role in the design of this study, the collection, analysis, and interpretation of the data, or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to study design. CD searched the literature. CD, DON, and AB selected the studies and extracted the data. CD analysed the data and wrote the first draft of the manuscript. ES led the data acquisition and harmonisation of the HSE/SHeSs. DON, SB, and AB were involved in the interpretation of results and critically reviewed the manuscript. All authors approved the submission of the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study is a secondary analysis of previously collected data and so additional ethical approval was not required. Ethics approvals for HSE 1994-97 were granted by Local Research Ethics Committees in England, HSE 1998-99 by London North Thames Multicentre Research Ethics Committee (MREC/97/2/9, MREC/98/2/89), HSE 2000-07 by London Multicentre Research Ethics Committee (MREC/99/2/91, MREC/00/2/81, MREC/01/2/82, MREC/02/2/72, MREC/03/2/97, 04/MRE02/50, 05/MREC02/47, 06/MRE02/62), and HSE 2008 by Oxford A-Research Ethics Committee (07/H0604/102). SHeSs 1995 and 1998 were approved by all fifteen of the Research Ethics Committees for all Area Health Boards in Scotland, and SHeSs 2003 by Multicentre Research Ethics Committee for Scotland. All participants gave verbal consent to be interviewed for the survey. UK Biobank received approval from the North West Centre for Research Ethics Committee (11/NW/0382) and all participants gave written informed consent.
Consent for publication
Not applicable.
Competing interests
Dr Steven Bell is Editorial Board Member for BMC Medicine. The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Materials.
Supplementary methods for de novo cohort analyses (Appendix S1 and Table S1). Quality assessment checklist (Appendix S2). Literature search strategy (Table S2). Alcohol consumption, effect estimates, and confounder adjustment reported by studies selected for meta-analyses (Table S3-S5). Associations of alcohol intake with HDL-cholesterol and gamma-glutamyl transferase in UK Biobank and HSE/SHeSs (Table S6). Schoenfeld residuals (Figure S1). Results of subgroup and sensitivity analyses for dose-response relationship between alcohol consumption and risk of all-cause mortality, cardiovascular mortality, and cardiovascular events (Figure S2, S7-S12). Patients inclusion flowchart for HSE/SHeSs and UK Biobank (Figure S3). Association of drinking categories with all-cause mortality, cardiovascular mortality, and cardiovascular events by cohort, sex, and primary events (Figure S4-S5). Study flow diagram (Figure S6). Funnel plots (Figure S13).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ding, C., O’Neill, D., Bell, S. et al. Association of alcohol consumption with morbidity and mortality in patients with cardiovascular disease: original data and meta-analysis of 48,423 men and women. BMC Med 19, 167 (2021). https://doi.org/10.1186/s12916-021-02040-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-021-02040-2