Abstract
Gout, the most prevalent inflammatory arthritis worldwide, is associated with cardiovascular and renal diseases, and is an independent predictor of premature death. The frequencies of obesity, chronic kidney disease (CKD), hypertension, type 2 diabetes, dyslipidaemias, cardiac diseases (including coronary heart disease, heart failure and atrial fibrillation), stroke and peripheral arterial disease have been repeatedly shown to be increased in gout. Therefore, the screening and care of these comorbidities as well as of cardiovascular risk factors are of outmost importance in patients with gout. Comorbidities, especially CKD, and drugs prescribed for their treatment, also impact gout management. Numerous epidemiological studies have shown the association of asymptomatic hyperuricaemia with the above-mentioned diseases and cardiovascular risk factors. Animal studies have also produced a mechanistic approach to the vascular toxicity of soluble urate. However, causality remains uncertain because confounders, reverse causality or common etiological factors might explain the epidemiological results. Additionally, these uncertainties remain unsolved despite recent studies using Mendelian randomisation or therapeutic approaches. Thus, large randomised placebo-controlled trials are still needed to assess the benefits of treating asymptomatic hyperuricaemia.
Similar content being viewed by others
Background
Gout is a prevalent disorder whose frequency is increasing worldwide [1]. In addition to causing excruciating arthritic pain, gout is associated with premature death [2], classically explained by a high frequency of comorbidities, especially renal and cardiovascular diseases [3]. Comorbidities must be considered in gout because they could contribute to the vital prognosis of gouty patients and they complicate gout management. These comorbidities are also frequently associated with asymptomatic hyperuricaemia, an even more prevalent condition than gout [4], with their causal relationship with hyperuricaemia raising important therapeutic issues.
Cardiovascular and renal comorbidities in gout
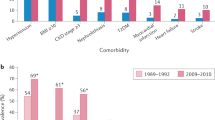
The association of gout with cardiovascular and renal diseases, observed as early as the late nineteenth century [5], is now well established. The prevalence of comorbidities increases with gout duration [6] and gout is associated with all components of the metabolic syndrome [7]. Hypertension is frequent; in the third National Health and Nutrition Examination Survey (NHANES), the prevalence of hypertension was 69.1% (95% CI, 59.4–78.8) and 30.3% (95% CI, 28.4–32.2) in gouty and non-gouty patients, respectively [7]. Prospective studies have consistently shown increased risk of gout in hypertensive patients [8, 9], and in the recent Singapore Chinese Health Study, the risk of hypertension was increased for gouty patients [10].
In the third NHANES, the prevalence of abdominal obesity was greater for gouty than non-gouty patients, at 62.9% (95% CI, 50.9–74.8) versus 35.3% (95% CI, 33.7–36.9) [7]. Additionally, risk of gout increases with obesity [8]. Body mass index (BMI) and waist circumference are correlated with uricaemia [11], and adiposity was recently found to be a cause of hyperuricaemia [11].
Type 2 diabetes mellitus prevalence is increased in gouty patients; in the third NHANES, diabetes prevalence was greater for gouty than non-gouty patients, at 33.1% (95% CI, 28.8–41.4) versus 10.8% (95% CI, 9.9–11.8). In prospective studies, gout also increased the risk of type 2 diabetes mellitus [12, 13], whereas diabetes lowered the risk of incident gout [13]; the latter finding was explained by the fact that glycosuria increases urine excretion of urate [14]. The lower incidence of gout in type 2 diabetes might also partly be explained by the frequent prescription of metformin, which may lead to an anti-inflammatory effect through modulation of different cellular pathways, including AMP-activated protein kinase, protein kinase A and PPAR gamma [15, 16].
Hypertriglyceridaemia prevalence was found to be greater in gouty patients than non-gouty individuals, at 53.7% (95% CI, 42.9–64.4) versus 27.9% (95% CI, 25.5–30.3) [7], and a recent Mendelian randomisation study found that it caused hyperuricaemia [17]. The frequency of an elevated low-density lipoprotein cholesterol level was higher in gouty patients than non-gouty individuals in the third NHANES, at 47.4% (95% CI, 37.2–57.6) versus 36.6% (95% CI, 34.1–39.1).
The prevalence of chronic kidney disease (CKD) of stage 3 or above in gout was estimated at 24% (95% CI, 15–28) in a recent meta-analysis of six studies [18]. Reduced kidney function decreases urate excretion in urine and increases the risk of gout. In a large German CKD patient cohort [19], gout prevalence was found to increase from 16.0% to 35.6% for CKD patients with an estimated glomerular filtration rate above 60 mL/min/1.73 m2 versus those with a rate below 30 mL/min/1.73 m2. Conversely, the risk of end-stage renal failure was found to be increased in patients with gout [20]. This finding can be explained by the formation of uric acid crystals in the renal tubules [21], interstitial nephritis complicating kidney stones, crystalline deposits in the renal medulla [22], use of non-steroidal anti-inflammatory drugs (NSAIDs), a frequent association with hypertension, or the possible renal toxicity of soluble uric acid [23].
Various cardiac diseases are independently associated with gout [24] and thereby associated with increased frequency of cardiovascular deaths [25,26,27]. In a large cohort of gout patients, cardiovascular diseases accounted for more than half of the deaths, and cardiovascular mortality increased with gout severity [27]. Hyperuricaemia might partially account of the increased cardiovascular risk of gouty patients, as discussed below. However, multivariate-adjusted cardiovascular risk appears to be more important and less disputable in gout than in asymptomatic hyperuricaemic patients, suggesting that crystal-driven inflammation plays an important role [28], in line with the increased risk observed in rheumatoid arthritis, psoriatic arthritis or ankylosing spondyloarthritis. Adjusted risk of coronary heart disease is increased for both men [26, 29] and women [30] with gout. Heart failure is associated with gout [31] and hyperuricaemia, which is worsened by the use of diuretics and is a marker for poor outcome [32]. The risk of atrial fibrillation is increased in gout and the prevalence of atrial fibrillation at gout diagnosis was recently estimated to be 7.4% (95% CI, 7.2–7.6) in a large UK database [33]. Gout has also been recently found to be associated with aortic stenosis [34]. Additionally, gout was recently confirmed to increase the adjusted risk of ischemic stroke and peripheral vascular disease [24]. Our cluster analysis of gouty patients revealed that obesity is preferentially associated with hypertension, type 2 diabetes mellitus and dyslipidaemia, whereas cardiovascular and CKD are frequent in patients with a high rate of diuretic prescriptions [35].
Hyperuricaemia and cardiovascular and renal diseases
Over the last 15 years, the association of hyperuricaemia with cardiovascular diseases has been re-examined following the demonstration in animal models that hyperuricaemia could cause vascular disease. In rodents, hyperuricaemia induced by inhibiting uricase [36], enriching the diet with fructose [37], or deleting the intestine glucose transporter 9 (GLUT9) urate transporter [38] led to features of metabolic syndrome and renal atherosclerosis. Many of the induced features, especially hypertension, were prevented by the early administration of urate-lowering drugs (ULDs). In addition, hyperuricaemia activated the renin-angiotensin system, decreased nitric oxide synthase activity, stimulated vascular smooth muscle cell proliferation, and promoted insulin resistance. These features provided a mechanistic model showing how soluble intracellular uric acid could damage the endothelium and arteries, in contrast to its classical antioxidant properties now believed to be limited to an extracellular effect [5]. Moreover, in humans, hyperuricaemia was recently found to be associated with increased lipid levels and decreased fibrous volume, potentially leading to increased plaque fragility [39, 40], representing a possibly important link to arterial obstruction. Another human study found birefringent crystals in six alcohol-fixed coronary arteries from 55 explanted hearts, suggesting that monosodium urate (MSU) crystal inflammation could occur in coronary arteries [41].
A large number of epidemiological studies have explored the link between hyperuricaemia and cardiovascular and renal outcomes [28, 42], and suggested an association of hyperuricaemia with increased frequency of cardiovascular death [43], coronary heart disease [44], heart failure, atrial fibrillation [45] and stroke [46]. Recently, a cross-sectional study suggested that coronary heart disease could be more severe in hyperuricaemic patients with asymptomatic MSU crystal deposition than normouricaemic or hyperuricaemic patients with no crystal deposits [47]. Prospective cohorts have shown that hyperuricaemia leads to hypertension [48], which was recently found to be more refractory when associated with hyperuricaemia [49, 50], renal failure [23], type 2 diabetes mellitus [51] and metabolic syndrome [52]. However, these impressive results did not prove causality because the observed associations could be explained by confounding factors, reverse causality or the intervention of a common causal factor. Since hyperuricaemia is associated with numerous cardiovascular risk factors, confounders could fully account for an adverse outcome, as observed in the Framingham Heart Study [3]. Most of the later studies have observed an association between hyperuricaemia and poor cardiovascular outcomes, despite multiple adjustments for cardiovascular risk factors [43]. However, adjustments were frequently incomplete, with some studies not adjusting for BMI, smoking, insulin resistance and renal function. Additionally, dietary factors associated with hyperuricaemia and possibly increased risk of death (i.e. alcohol consumption) were not taken into account. Despite the temporal relationship (hyperuricaemia was found to precede hypertension, CKD and metabolic syndrome), reverse causality cannot be excluded. Indeed, slight renal dysfunction or hyperinsulinemia, initially not revealed, could explain hyperuricaemia. As an example, familial juvenile hyperuricaemic nephropathies were first recognised in young patients with early gout/hyperuricaemia and preceded severe renal failure. Early treatment with allopurinol was observed to protect some patients from the renal failure observed in the disease, which led to the hypothesis that hyperuricaemia was the cause of renal dysfunction. Genetic studies have now established that the disease has a renal origin, due to a number of genetic variants of the uromodulin or hepatocyte-nuclear factor 1 b genes [53]. The observed influence of allopurinol now appears to be a non-specific renoprotective effect, observed in various nephropathies (see below).
Persisting uncertainties regarding the causal relationship between hyperuricaemia and cardiovascular disease has recently led a number of investigators to use Mendelian randomisation to investigate the effect of urate-governing genetic variants on various cardiovascular outcomes [11]. Mendelian randomisation allows a comparison of cardiovascular features in patients with and without hyperuricaemic genes. Because these genes are randomly distributed during meiosis, confounding factors should be equilibrated between study participants with and without the hyperuricaemic variants as they are in a randomised clinical trial, thus avoiding the use of adjustments and their uncertainties. In addition, because genetic variants are not affected by health outcomes, associations are not affected by reverse causality [54]. Although heritability appears stronger for uricaemia than gout [55], genetic factors governing uricaemia are many [56], still partly unknown, and explain only a small fraction of the uricaemia variance, especially when hyperuricaemic variants are considered individually, which leads to the risk of underpowered studies. Variants of the solute carrier family 2 member 9 (SLC2A9) gene, which encodes for the GLUT9 transporter and accounts for 3.5% of the uricaemia variance in males and up to 15% in females [57], were used as an instrumental variable in most of the first studies (Table 1). Studies of relatively small samples of Amish people and from southern Italy, which are relatively homogeneous populations, supported a causal role of hyperuricaemia in the development of hypertension [58, 59], atherosclerosis [60] or renal failure [61]. However, a study of two large Danish cohorts did not support causality for coronary heart disease or hypertension [62], and a further study did not find evidence between hyperuricemia and the metabolic syndrome [63].
Genetic risk scores (GRSs), which could be weighted to account for the effect of each gene on uricaemia, were used in other studies in an attempt to increase the genetic impact on uricaemia (Tables 2 and 3). Two studies supported causality for cardiovascular death, sudden cardiac death [64] and diabetic macro-angiopathy [65]. However, many studies [17, 66,67,68,69,70,71,72], even when largely powered, reached opposite conclusions, despite two of them showing an association of GRSs with gout [66, 71] (Table 3). The Rotterdam cohort showed a negative association between hyperuricaemic GRS and blood pressure, even stronger in patients receiving diuretics [69]. The use of several gene variants in the GRS increased the possibility that some of the included genes (or genes in linkage disequilibrium with them, which are not independently distributed during meiosis) had other effects than their soluble uric acid (SUA)-modulating property, a phenomenon called pleiotropy, which could be an important confounder. Pleiotropy was thought to explain that a hyperuricaemic GRS, consisting of five renal transporter gene variants, was found to be associated with the preservation of renal function and not with its worsening, as was observed in many epidemiologic studies [68]. Recent studies have attempted to account for pleiotropy, including the exclusion of genes that appeared as pleiotropic by analysing their effects on biological (i.e. lipids) or clinical parameters (i.e. blood pressure, BMI) in the studied cohorts [65, 71], adjustment for confounders [72], or using Egger randomisation, which accounts for hidden pleiotropy [72]. Thus, despite some discrepant results [64, 65], Mendelian randomisation studies do not appear to support a causal link between SUA level and cardiovascular outcomes (Table 3) [73, 74].
An alternative explanation for the observed associations between hyperuricaemia and cardiovascular disease could be the intervention of a common etiological factor. Xanthine oxidase (XO) is a candidate for such a role because it produces free oxygen species that inhibit the production of NO and may lead to endothelial damage [75, 76]. The hypothesis of increased XO activity in hyperuricaemic individuals remains unproven because of the difficulty in dosing the enzyme in living endothelial cells, and primary hyperuricaemia is believed to usually result from a lack of proper renal adaptation to SUA levels. However, hyperuricaemic dietary factors may also be involved in primary hyperuricaemia by increasing the enzyme activity, which is involved in the last two steps of uric acid production. Increased XO activity, rather than SUA, could be responsible for an adverse cardiovascular outcome. In line with this hypothesis, a recent study examined several XO gene variants in a prospective cohort of 2500 European participants and found three minor alleles associated with increased risk of hypertension [77]. Despite the enzymatic activity controlled by these variants being uncertain, another a longitudinal cohort study of 246 Dutch children (mean age 7 years) [78], led by the same investigator, showed that XO activity, estimated by a ratio of purine to uric acid, was associated with blood pressure. Therefore, increased XO activity, rather than SUA, could be responsible for the increased blood pressure frequently observed in hyperuricaemic individuals [79].
Causality can also be addressed by studying the effect of drugs. Most of the recent case–control retrospective studies [80,81,82], but not all [83], suggested that allopurinol, especially at > 300 mg/d, could improve the cardiovascular outcomes of patients with asymptomatic hyperuricaemia. However, these studies were subjected to potential bias because allopurinol may be a marker of better care and could be frequently associated with colchicine, which has been found to be effective for coronary heart disease [84]. Randomised controlled trials (RCTs) comparing allopurinol with placebo were recently reviewed [85], with most studies suggesting a beneficial effect on cardiovascular disease but including small numbers of patients. In contrast, a recent randomised placebo-controlled trial involving more than 250 patients failed to show that allopurinol (600 mg/d) mitigated hyperuricaemic heart failure [86]. Several small RCTs also suggested that allopurinol could slow the decline in renal function in hyperuricaemic CKD patients [87]. A recent monocentric RCT of 93 hyperuricaemic CKD patients suggested that febuxostat could have similar beneficial effects [88]. However, such a renoprotective effect of febuxostat was not observed in another recent RCT of gout patients with moderate-to-severe renal impairment [89].
The most convincing results come from two small RCTs in which allopurinol or probenecid corrected recent-onset hypertension in adolescents [90, 91]. However, these results cannot be extended to adults; a recent RCT reported that ULDs did not change blood pressure [92]. In rodents, uric acid-induced hypertension could be corrected by ULDs only in the early phase [93], and whether ULDs could correct hypertension in adults with long-lasting hyperuricaemia is uncertain. Results of large and well-designed RCTs are still awaited to assess the cardiovascular and renal benefits of treating asymptomatic hyperuricaemia.
Impact of comorbidities on gout management
Diagnosis and treatment of comorbidities
The high frequency of cardiovascular disease and the increased risk of cardiovascular death with gout led to the European League Against Rheumatism (EULAR) recommendation for systematic screening and care for patients with gout in terms of cardiovascular diseases and risk factors [94]. Even though smoking has been recently found to be negatively associated with gout [95,96,97], smoking cessation was included in the EULAR recommendations because of the increased cardiovascular risk associated with gout [94]. Weight control appears to be highly important as it can improve uricaemia and metabolic syndrome features.
Additionally, drugs targeting comorbidities may affect uricaemia. Cardioprotective aspirin increases uricaemia [98] and its onset has been associated with gout flares [99]. Antihypertensive drugs, such as beta-blockers, angiotensin converting-enzyme inhibitors, non-losartan angiotensin II receptor blockers, thiazide and loop diuretics, have been associated with increased risk of gout, and calcium channel blockers and losartan, which are uricosurics, should be preferred for gout [100]. Loop diuretics are often needed for patients with heart failure, but should be kept at the minimal effective dose. Spironolactone, with no effect on uricaemia [101], should be privileged. For the management of dyslipidaemia, fenofibrate [102] and atorvastatine [103] have urate-lowering effects. Similarly, for the management of type-2 diabetes mellitus, insulin-lowering drugs should be privileged because insulin decreases the urine output of urate. The recently introduced sodium-glucose co-transporter 2 inhibitors also have urate-lowering effects [104].
Impact of comorbidity on management of gout inflammation
Comorbidities can imply contraindication for drugs usually prescribed for the management of acute flares in gout [105]. Colchicine and NSAIDs should not be used for patients with renal failure, who are usually given intra-articular or systemic steroids. Further, with hypertension and type 2 diabetes mellitus, steroids can be poorly tolerated, especially when repeated courses are needed in patients with frequent flares. Following the observation that interleukin 1 (IL-1) plays a major role in MSU crystal-triggered inflammation [106], IL-1 blockers have been proposed for the management of flares for patients with difficult-to-treat disease. Open studies of the IL-1 receptor antagonist anakinra [107, 108] support its off-label use for patients resistant or with contraindications to NSAIDs, colchicine and steroids. Canakimumab, a long-lasting antibody to IL-1β, has been approved by the European Medical Agency (but not the US Food and drug Administration), following two RCTs comparing the intra-muscular triamcinolone acetonide [109]. The EULAR has recommended considering IL-1 blockers for the management of gout flares in patients with frequent flares and with a contraindication to NSAIDs, colchicine and steroids (oral or injectable) [94]. Current infection is a contraindication.
According to the EULAR and American College of Rheumatology (ACR) recommendations [94, 110], small doses of colchicine should be used during the first 6 months of ULD therapy to decrease the risk of ULD flares. Colchicine exposure has been found to be up to two-fold higher in people with moderate or severe renal impairment [111], thereby exposing CKD patients with chronic prescription of small-dose colchicine to increased risk of toxicity [112]. A (reversible) neuromuscular toxicity has been described in CKD patients [113]. Therefore, the colchicine dose should be reduced in CKD patients. Co-prescription of statins [114] or drugs interacting with colchicine by inhibiting cytochrome P450 or 3A4/P-glycoprotein [115] should be avoided in patients with renal failure.
Impact of comorbidities on urate-lowering treatment
Comorbidities also interfere with the use of ULD to treat gout. The increased risk of premature death and the increasing prevalence of comorbidities with disease duration [6] have contributed to the recommendation by the EULAR to consider ULD indication early after a definite diagnosis of gout [94]. According to the EULAR and ACR, CKD should prompt the indication for ULDs [94, 110].
Patients with heart failure are often prescribed furosemide, which has been found to decrease the hypouricaemic effect of allopurinol while increasing the blood concentration of oxypurinol [116]. This paradox appears to rely on a recently described additional mode of action of allopurinol, reducing XO expression in vitro, a mechanism that is abolished by furosemide [117]. Cardiovascular diseases, and not diuretics, might increase the rate of serious cutaneous adverse reactions (SCARs) to allopurinol. Indeed, a recent nationwide study in Taiwan found that cardiovascular (and renal) diseases increased the risk of allopurinol hypersensitivity, with no association seen with diuretic use [118].
The dose of allopurinol for CKD patients is debated between European and US gout experts [119, 120]. Most country agencies have reduced the maximum approved dose of allopurinol according to creatinine clearance following a report of high doses of allopurinol being associated with SCARs in CKD patients [121]. This dose reduction translates into a frequent inability to reach the desired uricaemia [122]. When designing their recommendations, ACR experts were confident in the risk minimisation obtained with slow increases of allopurinol dosage as observed in a retrospective case–control study [123] and recommended titrating allopurinol above the authorised dose, if necessary, to reach the uricaemia target [110]. The EULAR committee had a more conservative approach, taking into account the rarity of SCARs [124], which leads to great uncertainties in defining risk factors, a recent report of increased severity of skin reactions in CKD patients with sustained oxypurinol levels [125], and the availability of alternative drugs such as febuxostat, which was approved without dosing changes for mild and moderate renal failure. The EULAR recommendations retained the limitation of the maximum daily dose in CKD patients according to creatinine clearance. If the limited dose did not allow reaching of the target, the EULAR recommended a shift to alternative drugs such as febuxostat.
Conclusion
Renal and cardiovascular comorbidities are frequent in patients with gout and play an important role in the premature mortality observed in the disease. Patients with gout should therefore be systematically screened for renovascular diseases and risk factors, which should be addressed as an important part of gout management. Comorbidities, especially renal disease, and drugs prescribed for their management should be considered during the choice of drugs to be used in the treatment of gouty inflammation and for urate lowering. Finally, whereas an impressive number of epidemiological data have shown the association of renal and cardiovascular diseases with asymptomatic hyperuricaemia, causality remains uncertain, and large RCTs are still needed to assess the cardiovascular and renal benefits of asymptomatic hyperuricaemia treatment.
References
Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11(11):649–62.
Kuo CF, Luo SF. Gout: risk of premature death in gout unchanged for years. Nat Rev Rheumatol. 2017;13(4):200–1.
Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131(1):7–13.
Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136–41.
Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–21.
Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Comorbidities in patients with gout prior to and following diagnosis: case-control study. Ann Rheum Dis. 2016;75(1):210–7.
Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57(1):109–15.
Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165(7):742–8.
McAdams-DeMarco MA, Maynard JW, Baer AN, Coresh J. Hypertension and the risk of incident gout in a population-based study: the atherosclerosis risk in communities cohort. J Clin Hypertens (Greenwich). 2012;14(10):675–9.
Pan A, Teng GG, Yuan JM, Koh WP. Bidirectional association between self-reported hypertension and gout: The Singapore Chinese Health Study. PLoS One. 2015;10(10):e0141749.
Lyngdoh T, Vuistiner P, Marques-Vidal P, Rousson V, Waeber G, Vollenweider P, Bochud M. Serum uric acid and adiposity: deciphering causality using a bidirectional Mendelian randomization approach. PLoS One. 2012;7(6):e39321.
Rho YH, Lu N, Peloquin CE, Man A, Zhu Y, Zhang Y, Choi HK. Independent impact of gout on the risk of diabetes mellitus among women and men: a population-based, BMI-matched cohort study. Ann Rheum Dis. 2016;75(1):91–5.
Pan A, Teng GG, Yuan JM, Koh WP. Bidirectional association between diabetes and gout: the Singapore Chinese Health Study. Sci Rep. 2016;6:25766.
Lytvyn Y, Skrtic M, Yang GK, Yip PM, Perkins BA, Cherney DZ. Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am J Physiol Renal Physiol. 2015;308(2):F77–83.
Agrawal NK, Kant S. Targeting inflammation in diabetes: newer therapeutic options. World J Diabetes. 2014;5(5):697–710.
Chen W, Liu X, Ye S. Effects of metformin on blood and urine pro-inflammatory mediators in patients with type 2 diabetes. J Inflamm (Lond). 2016;13:34.
Rasheed H, Hughes K, Flynn TJ, Merriman TR. Mendelian randomization provides no evidence for a causal role of serum urate in increasing serum triglyceride levels. Circ Cardiovasc Genet. 2014;7(6):830–7.
Roughley MJ, Belcher J, Mallen CD, Roddy E. Gout and risk of chronic kidney disease and nephrolithiasis: meta-analysis of observational studies. Arthritis Res Ther. 2015;17(1):90.
Jing J, Kielstein JT, Schultheiss UT, Sitter T, Titze SI, Schaeffner ES, McAdams-DeMarco M, Kronenberg F, Eckardt KU, Kottgen A. Prevalence and correlates of gout in a large cohort of patients with chronic kidney disease: the German Chronic Kidney Disease (GCKD) study. Nephrol Dial Transplant. 2015;30(4):613–21.
Yu KH, Kuo CF, Luo SF, See LC, Chou IJ, Chang HC, Chiou MJ. Risk of end-stage renal disease associated with gout: a nationwide population study. Arthritis Res Ther. 2012;14(2):R83.
Umekawa T, Chegini N, Khan SR. Increased expression of monocyte chemoattractant protein-1 (MCP-1) by renal epithelial cells in culture on exposure to calcium oxalate, phosphate and uric acid crystals. Nephrol Dial Transplant. 2003;18(4):664–9.
Toyoda K, Miyamoto Y, Ida M, Tada S, Utsunomiya M. Hyperechoic medulla of the kidneys. Radiology. 1989;173(2):431–4.
Li L, Yang C, Zhao Y, Zeng X, Liu F, Fu P. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease? A systematic review and meta-analysis based on observational cohort studies. BMC Nephrol. 2014;15:122.
Clarson LE, Hider SL, Belcher J, Heneghan C, Roddy E, Mallen CD. Increased risk of vascular disease associated with gout: a retrospective, matched cohort study in the UK clinical practice research datalink. Ann Rheum Dis. 2015;74(4):642–7.
Clarson LE, Chandratre P, Hider SL, Belcher J, Heneghan C, Roddy E, Mallen CD. Increased cardiovascular mortality associated with gout: a systematic review and meta-analysis. Eur J Prev Cardiol. 2015;22(3):335–43.
Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007;116(8):894–900.
Perez-Ruiz F, Martinez-Indart L, Carmona L, Herrero-Beites AM, Pijoan JI, Krishnan E. Tophaceous gout and high level of hyperuricaemia are both associated with increased risk of mortality in patients with gout. Ann Rheum Dis. 2014;73(1):177–82.
Richette P, Perez-Ruiz F, Doherty M, Jansen TL, Nuki G, Pascual E, Punzi L, So AK, Bardin T. Improving cardiovascular and renal outcomes in gout: what should we target? Nat Rev Rheumatol. 2014;10(11):654–61.
Krishnan E, Baker JF, Furst DE, Schumacher HR. Gout and the risk of acute myocardial infarction. Arthritis Rheum. 2006;54(8):2688–96.
De Vera MA, Rahman MM, Bhole V, Kopec JA, Choi HK. Independent impact of gout on the risk of acute myocardial infarction among elderly women: a population-based study. Ann Rheum Dis. 2010;69(6):1162–4.
Krishnan E. Gout and the risk for incident heart failure and systolic dysfunction. BMJ Open. 2012;2(1):e000282.
Harzand A, Tamariz L, Hare JM. Uric acid, heart failure survival, and the impact of xanthine oxidase inhibition. Congest Heart Fail. 2012;18(3):179–82.
Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Impact of gout on the risk of atrial fibrillation. Rheumatology (Oxford). 2016;55(4):721–8.
Chang K, Yokose C, Tenner C, Oh C, Donnino R, Choy-Shan A, Pike VC, Shah BD, Lorin JD, Krasnokutsky S, et al. Association between gout and aortic stenosis. Am J Med. 2017;130(2):230.e1–8.
Richette P, Clerson P, Perissin L, Flipo RM, Bardin T. Revisiting comorbidities in gout: a cluster analysis. Ann Rheum Dis. 2015;74(1):142–7.
Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38(5):1101–6.
Perez-Pozo SE, Schold J, Nakagawa T, Sanchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond). 2010;34(3):454–61.
DeBosch BJ, Kluth O, Fujiwara H, Schurmann A, Moley K. Early-onset metabolic syndrome in mice lacking the intestinal uric acid transporter SLC2A9. Nat Commun. 2014;5:4642.
Saito Y, Nakayama T, Sugimoto K, Fujimoto Y, Kobayashi Y. Relation of lipid content of coronary plaque to level of serum uric acid. Am J Cardiol. 2015;116(9):1346–50.
Ando K, Takahashi H, Watanabe T, Daidoji H, Otaki Y, Nishiyama S, Arimoto T, Shishido T, Miyashita T, Miyamoto T, et al. Impact of serum uric acid levels on coronary plaque stability evaluated using integrated backscatter intravascular ultrasound in patients with coronary artery disease. J Atheroscler Thromb. 2016;23(8):932–9.
Park JJ, Roudier MP, Soman D, Mokadam NA, Simkin PA. Prevalence of birefringent crystals in cardiac and prostatic tissues, an observational study. BMJ Open. 2014;4(7):e005308.
Borghi C, Rosei EA, Bardin T, Dawson J, Dominiczak A, Kielstein JT, Manolis AJ, Perez-Ruiz F, Mancia G. Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens. 2015;33(9):1729–41. discussion 1741.
Zhao G, Huang L, Song M, Song Y. Baseline serum uric acid level as a predictor of cardiovascular disease related mortality and all-cause mortality: a meta-analysis of prospective studies. Atherosclerosis. 2013;231(1):61–8.
Nozue T, Yamamoto S, Tohyama S, Fukui K, Umezawa S, Onishi Y, Kunishima T, Hibi K, Terashima M, Michishita I. Correlations between serum uric acid and coronary atherosclerosis before and during statin therapy. Coron Artery Dis. 2014;25(4):343–8.
Tamariz L, Hernandez F, Bush A, Palacio A, Hare JM. Association between serum uric acid and atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm. 2014;11(7):1102–8.
Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(7):885–92.
Andres M, Quintanilla MA, Sivera F, Sanchez-Paya J, Pascual E, Vela P, Ruiz-Nodar JM. Silent monosodium urate crystal deposits are associated with severe coronary calcification in asymptomatic hyperuricemia: an exploratory study. Arthritis Rheumatol. 2016;68(6):1531–9.
Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2011;63(1):102–10.
Viazzi F, Rebora P, Giussani M, Orlando A, Stella A, Antolini L, Valsecchi MG, Pontremoli R, Genovesi S. Increased serum uric acid levels blunt the antihypertensive efficacy of lifestyle modifications in children at cardiovascular risk. Hypertension. 2016;67(5):934–40.
Cicero AF, Rosticci M, Fogacci F, Grandi E, D’Addato S, Borghi C. High serum uric acid is associated to poorly controlled blood pressure and higher arterial stiffness in hypertensive subjects. Eur J Intern Med. 2017;37:38–42.
Lv Q, Meng XF, He FF, Chen S, Su H, Xiong J, Gao P, Tian XJ, Liu JS, Zhu ZH, et al. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PLoS One. 2013;8(2):e56864.
Yu TY, Jee JH, Bae JC, Jin SM, Baek JH, Lee MK, Kim JH. Serum uric acid: a strong and independent predictor of metabolic syndrome after adjusting for body composition. Metabolism. 2016;65(4):432–40.
Eckardt KU, Alper SL, Antignac C, Bleyer AJ, Chauveau D, Dahan K, Deltas C, Hosking A, Kmoch S, Rampoldi L, et al. Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management--A KDIGO consensus report. Kidney Int. 2015;88(4):676–83.
Nitsch D, Molokhia M, Smeeth L, DeStavola BL, Whittaker JC, Leon DA. Limits to causal inference based on Mendelian randomization: a comparison with randomized controlled trials. Am J Epidemiol. 2006;163(5):397–403.
Krishnan E, Lessov-Schlaggar CN, Krasnow RE, Swan GE. Nature versus nurture in gout: a twin study. Am J Med. 2012;125(5):499–504.
Kottgen A, Albrecht E, Teumer A, Vitart V, Krumsiek J, Hundertmark C, Pistis G, Ruggiero D, O’Seaghdha CM, Haller T, et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet. 2013;45(2):145–54.
Doring A, Gieger C, Mehta D, Gohlke H, Prokisch H, Coassin S, Fischer G, Henke K, Klopp N, Kronenberg F, et al. SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat Genet. 2008;40(4):430–6.
Parsa A, Brown E, Weir MR, Fink JC, Shuldiner AR, Mitchell BD, McArdle PF. Genotype-based changes in serum uric acid affect blood pressure. Kidney Int. 2012;81(5):502–7.
Mallamaci F, Testa A, Leonardis D, Tripepi R, Pisano A, Spoto B, Sanguedolce MC, Parlongo RM, Tripepi G, Zoccali C. A polymorphism in the major gene regulating serum uric acid associates with clinic SBP and the white-coat effect in a family-based study. J Hypertens. 2014;32(8):1621–8. discussion 1628.
Mallamaci F, Testa A, Leonardis D, Tripepi R, Pisano A, Spoto B, Sanguedolce MC, Parlongo RM, Tripepi G, Zoccali C. A genetic marker of uric acid level, carotid atherosclerosis, and arterial stiffness: a family-based study. Am J Kidney Dis. 2015;65(2):294–302.
Testa A, Mallamaci F, Spoto B, Pisano A, Sanguedolce MC, Tripepi G, Leonardis D, Zoccali C. Association of a polymorphism in a gene encoding a urate transporter with CKD progression. Clin J Am Soc Nephrol. 2014;9(6):1059–65.
Palmer TM, Nordestgaard BG, Benn M, Tybjaerg-Hansen A, Davey Smith G, Lawlor DA, Timpson NJ. Association of plasma uric acid with ischaemic heart disease and blood pressure: mendelian randomisation analysis of two large cohorts. BMJ. 2013;347:f4262.
McKeigue PM, Campbell H, Wild S, Vitart V, Hayward C, Rudan I, Wright AF, Wilson JF. Bayesian methods for instrumental variable analysis with genetic instruments (‘Mendelian randomization’): example with urate transporter SLC2A9 as an instrumental variable for effect of urate levels on metabolic syndrome. Int J Epidemiol. 2010;39(3):907–18.
Kleber ME, Delgado G, Grammer TB, Silbernagel G, Huang J, Kramer BK, Ritz E, Marz W. Uric acid and cardiovascular events: a Mendelian randomization study. J Am Soc Nephrol. 2015;26(11):2831–8.
Yan D, Wang J, Jiang F, Zhang R, Wang T, Wang S, Peng D, He Z, Chen H, Bao Y, et al. A causal relationship between uric acid and diabetic macrovascular disease in Chinese type 2 diabetes patients: a Mendelian randomization analysis. Int J Cardiol. 2016;214:194–9.
Yang Q, Kottgen A, Dehghan A, Smith AV, Glazer NL, Chen MH, Chasman DI, Aspelund T, Eiriksdottir G, Harris TB, et al. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet. 2010;3(6):523–30.
Pfister R, Barnes D, Luben R, Forouhi NG, Bochud M, Khaw KT, Wareham NJ, Langenberg C. No evidence for a causal link between uric acid and type 2 diabetes: a Mendelian randomisation approach. Diabetologia. 2011;54(10):2561–9.
Hughes K, Flynn T, de Zoysa J, Dalbeth N, Merriman TR. Mendelian randomization analysis associates increased serum urate, due to genetic variation in uric acid transporters, with improved renal function. Kidney Int. 2014;85(2):344–51.
Sedaghat S, Pazoki R, Uitterlinden AG, Hofman A, Stricker BH, Ikram MA, Franco OH, Dehghan A. Association of uric acid genetic risk score with blood pressure: the Rotterdam study. Hypertension. 2014;64(5):1061–6.
Sluijs I, Holmes MV, van der Schouw YT, Beulens JW, Asselbergs FW, Huerta JM, Palmer TM, Arriola L, Balkau B, Barricarte A, et al. A Mendelian randomization study of circulating uric acid and type 2 diabetes. Diabetes. 2015;64(8):3028–36.
Keenan T, Blaha MJ, Nasir K, Silverman MG, Tota-Maharaj R, Carvalho JA, Conceicao RD, Blumenthal RS, Santos RD. Relation of uric acid to serum levels of high-sensitivity C-reactive protein, triglycerides, and high-density lipoprotein cholesterol and to hepatic steatosis. Am J Cardiol. 2012;110(12):1787–92.
White J, Sofat R, Hemani G, Shah T, Engmann J, Dale C, Shah S, Kruger FA, Giambartolomei C, Swerdlow DI, et al. Plasma urate concentration and risk of coronary heart disease: a Mendelian randomisation analysis. Lancet Diabetes Endocrinol. 2016;4(4):327–36.
Stack AG, Hanley A, Casserly LF, Cronin CJ, Abdalla AA, Kiernan TJ, Murthy BV, Hegarty A, Hannigan A, Nguyen HT. Independent and conjoint associations of gout and hyperuricaemia with total and cardiovascular mortality. QJM. 2013;106(7):647–58.
Keenan T, Zhao W, Rasheed A, Ho WK, Malik R, Felix JF, Young R, Shah N, Samuel M, Sheikh N, et al. Causal assessment of serum urate levels in cardiometabolic diseases through a Mendelian randomization study. J Am Coll Cardiol. 2016;67(4):407–16.
Higgins P, Dawson J, Lees KR, McArthur K, Quinn TJ, Walters MR. Xanthine oxidase inhibition for the treatment of cardiovascular disease: a systematic review and meta-analysis. Cardiovasc Ther. 2012;30(4):217–26.
Noman A, Ang DS, Ogston S, Lang CC, Struthers AD. Effect of high-dose allopurinol on exercise in patients with chronic stable angina: a randomised, placebo controlled crossover trial. Lancet. 2010;375(9732):2161–7.
Scheepers LE, Wei FF, Stolarz-Skrzypek K, Malyutina S, Tikhonoff V, Thijs L, Salvi E, Barlassina C, Filipovsky J, Casiglia E, et al. Xanthine oxidase gene variants and their association with blood pressure and incident hypertension: a population study. J Hypertens. 2016;34(11):2147–54.
Scheepers LE, Boonen A, Pijnenburg W, Bierau J, Staessen JA, Stehouwer CD, Thijs C, Arts IC. Associations of plasma uric acid and purine metabolites with blood pressure in children: the KOALA Birth Cohort Study. J Hypertens. 2017;35(5):982–93.
Schmitz B, Brand SM. Uric acid and essential hypertension: the endothelial connection. J Hypertens. 2016;34(11):2138–9.
Grimaldi-Bensouda L, Alperovitch A, Aubrun E, Danchin N, Rossignol M, Abenhaim L, Richette P. Impact of allopurinol on risk of myocardial infarction. Ann Rheum Dis. 2015;74(5):836–42.
de Abajo FJ, Gil MJ, Rodriguez A, Garcia-Poza P, Alvarez A, Bryant V, Garcia-Rodriguez LA. Allopurinol use and risk of non-fatal acute myocardial infarction. Heart. 2015;101(9):679–85.
Larsen KS, Pottegard A, Lindegaard HM, Hallas J. Effect of allopurinol on cardiovascular outcomes in hyperuricemic patients: a cohort study. Am J Med. 2016;129(3):299–306.
Kok VC, Horng JT, Chang WS, Hong YF, Chang TH. Allopurinol therapy in gout patients does not associate with beneficial cardiovascular outcomes: a population-based matched-cohort study. PLoS One. 2014;9(6):e99102.
Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61(4):404–10.
Zhang J, Dierckx R, Mohee K, Clark AL, Cleland JG. Xanthine oxidase inhibition for the treatment of cardiovascular disease: an updated systematic review and meta-analysis. ESC Heart Fail. 2017;4(1):40–5.
Givertz MM, Anstrom KJ, Redfield MM, Deswal A, Haddad H, Butler J, Tang WH, Dunlap ME, LeWinter MM, Mann DL, et al. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the Xanthine Oxidase Inhibition for Hyperuricemic Heart Failure Patients (EXACT-HF) study. Circulation. 2015;131(20):1763–71.
Wang H, Wei Y, Kong X, Xu D. Effects of urate-lowering therapy in hyperuricemia on slowing the progression of renal function: a meta-analysis. J Ren Nutr. 2013;23(5):389–96.
Sircar D, Chatterjee S, Waikhom R, Golay V, Raychaudhury A, Chatterjee S, Pandey R. Efficacy of febuxostat for slowing the GFR decline in patients with CKD and asymptomatic hyperuricemia: a 6-month, double-blind, randomized, placebo-controlled trial. Am J Kidney Dis. 2015;66(6):945–50.
Saag KG, Whelton A, Becker MA, MacDonald P, Hunt B, Gunawardhana L. Impact of febuxostat on renal function in gout patients with moderate-to-severe renal impairment. Arthritis Rheumatol. 2016;68(8):2035–43.
Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300(8):924–32.
Soletsky B, Feig DI. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension. 2012;60(5):1148–56.
McMullan CJ, Borgi L, Fisher N, Curhan G, Forman J. Effect of uric acid lowering on renin-angiotensin-system activation and ambulatory BP: a randomized controlled trial. Clin J Am Soc Nephrol. 2017;12(5):807–16.
Feig DI. Serum uric acid and the risk of hypertension and chronic kidney disease. Curr Opin Rheumatol. 2014;26(2):176–85.
Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castaneda-Sanabria J, Coyfish M, Guillo S, Jansen TL, Janssens H, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76(1):29–42.
Poudel DR, Karmacharya P, Donato A. Risk of acute gout among active smokers: data from nationwide inpatient sample. Clin Rheumatol. 2016;35(12):3015–8.
Gee Teng G, Pan A, Yuan JM, Koh WP. Cigarette smoking and the risk of incident gout in a prospective cohort study. Arthritis Care Res (Hoboken). 2016;68(8):1135–42.
Wang W, Krishnan E. Cigarette smoking is associated with a reduction in the risk of incident gout: results from the Framingham Heart Study original cohort. Rheumatology (Oxford). 2015;54(1):91–5.
Yu TF, Gutman AB. Study of the paradoxical effects of salicylate in low, intermediate and high dosage on the renal mechanisms for excretion of urate in man. J Clin Invest. 1959;38(8):1298–315.
Zhang Y, Neogi T, Chen C, Chaisson C, Hunter DJ, Choi H. Low-dose aspirin use and recurrent gout attacks. Ann Rheum Dis. 2014;73(2):385–90.
Choi HK, Soriano LC, Zhang Y, Rodriguez LA. Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ. 2012;344:d8190.
Ramsay LE, Shelton JR, Harrison IR. Plasma uric acid and spironolactone response in healthy subjects. Br J Clin Pharmacol. 1977;4(2):247–9.
Takahashi S, Moriwaki Y, Yamamoto T, Tsutsumi Z, Ka T, Fukuchi M. Effects of combination treatment using anti-hyperuricaemic agents with fenofibrate and/or losartan on uric acid metabolism. Ann Rheum Dis. 2003;62(6):572–5.
Mustard JF, Murphy EA, Ogryzlo MA, Smythe HA. Blood coagulation and platelet economy in subjects with primary gout. Can Med Assoc J. 1963;89:1207–11.
Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60(2):215–25.
Stamp LK, Chapman PT. Gout and its comorbidities: implications for therapy. Rheumatology (Oxford). 2013;52(1):34–44.
Agarwal V, Hans N, Messerli FH. Effect of allopurinol on blood pressure: a systematic review and meta-analysis. J Clin Hypertens (Greenwich). 2013;15(6):435–42.
So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9(2):R28.
Ottaviani S, Molto A, Ea HK, Neveu S, Gill G, Brunier L, Palazzo E, Meyer O, Richette P, Bardin T, et al. Efficacy of anakinra in gouty arthritis: a retrospective study of 40 cases. Arthritis Res Ther. 2013;15(5):R123.
Schlesinger N, Alten RE, Bardin T, Schumacher HR, Bloch M, Gimona A, Krammer G, Murphy V, Richard D, So AK. Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active-controlled, double-blind trials and their initial extensions. Ann Rheum Dis. 2012;71(11):1839–48.
Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, Pillinger MH, Merill J, Lee S, Prakash S, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64(10):1431–46.
Wason S, Mount D, Faulkner R. Single-dose, open-label study of the differences in pharmacokinetics of colchicine in subjects with renal impairment, including end-stage renal disease. Clin Drug Investig. 2014;34(12):845–55.
Dalbeth N, Lauterio TJ, Wolfe HR. Mechanism of action of colchicine in the treatment of gout. Clin Ther. 2014;36(10):1465–79.
Kuncl RW, Duncan G, Watson D, Alderson K, Rogawski MA, Peper M. Colchicine myopathy and neuropathy. N Engl J Med. 1987;316(25):1562–8.
Boonmuang P, Nathisuwan S, Chaiyakunapruk N, Suwankesawong W, Pokhagul P, Teerawattanapong N, Supsongserm P. Characterization of statin-associated myopathy case reports in Thailand using the health product vigilance center database. Drug Saf. 2013;36(9):779–87.
Terkeltaub RA, Furst DE, Digiacinto JL, Kook KA, Davis MW. Novel evidence-based colchicine dose-reduction algorithm to predict and prevent colchicine toxicity in the presence of cytochrome P450 3A4/P-glycoprotein inhibitors. Arthritis Rheum. 2011;63(8):2226–37.
Stamp LK, Barclay ML, O’Donnell JL, Zhang M, Drake J, Frampton C, Chapman PT. Furosemide increases plasma oxypurinol without lowering serum urate--a complex drug interaction: implications for clinical practice. Rheumatology (Oxford). 2012;51(9):1670–6.
Knake C, Stamp L, Bahn A. Molecular mechanism of an adverse drug-drug interaction of allopurinol and furosemide in gout treatment. Biochem Biophys Res Commun. 2014;452(1):157–62.
Yang CY, Chen CH, Deng ST, Huang CS, Lin YJ, Chen YJ, Wu CY, Hung SI, Chung WH. Allopurinol use and risk of fatal hypersensitivity reactions: a nationwide population-based study in Taiwan. JAMA Intern Med. 2015;175(9):1550–7.
Neogi T, Dalbeth N, Stamp L, Castelar G, Fitzgerald J, Gaffo A, Mikuls TR, Singh J, Vazquez-Mellado J, Edwards NL. Renal dosing of allopurinol results in suboptimal gout care. Ann Rheum Dis. 2017;76(1):e1.
Richette P, Doherty M, Pascual E, Bardin T. Response: Renal dosing of allopurinol results in suboptimal gout care by T Neogi et al. Ann Rheum Dis. 2017;76(1):e2.
Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76(1):47–56.
Stamp LK, Merriman TR, Barclay ML, Singh JA, Roberts RL, Wright DF, Dalbeth N. Impaired response or insufficient dosage? Examining the potential causes of “inadequate response” to allopurinol in the treatment of gout. Semin Arthritis Rheum. 2014;44(2):170–4.
Stamp LK, Taylor WJ, Jones PB, Dockerty JL, Drake J, Frampton C, Dalbeth N. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum. 2012;64(8):2529–36.
Kim SC, Newcomb C, Margolis D, Roy J, Hennessy S. Severe cutaneous reactions requiring hospitalization in allopurinol initiators: a population-based cohort study. Arthritis Care Res (Hoboken). 2013;65(4):578–84.
Chung WH, Chang WC, Stocker SL, Juo CG, Graham GG, Lee MH, Williams KM, Tian YC, Juan KC, Jan Wu YJ, et al. Insights into the poor prognosis of allopurinol-induced severe cutaneous adverse reactions: the impact of renal insufficiency, high plasma levels of oxypurinol and granulysin. Ann Rheum Dis. 2015;74(12):2157–64.
Availability of data and materials
Not applicable.
Authors’ contributions
TB drafted the manuscript. Both authors completed the draft and reviewed and approved the final manuscript.
Competing interests
TB received research grants from AstraZeneka, Ipsen, Menarini, and fees for consultancy or talks from Astella, AstraZeneka, Biomex, Grunenthal, Ipsen, Menarini, Novartis, Savient and Sobi. PR received fees for consultancy or talks from AstraZeneka, Grunenthal, Ipsen, Menarini, Savient.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bardin, T., Richette, P. Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Med 15, 123 (2017). https://doi.org/10.1186/s12916-017-0890-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-017-0890-9