Abstract
Background
Recent studies have identified HIV infection as a potential risk factor for invasive meningococcal disease (IMD), suggesting that HIV-infected individuals could benefit from meningococcal vaccination to reduce their risk of this rare, but severe and potentially fatal infection. In the United Kingdom, as in most industrialised countries, HIV is not considered a risk factor for IMD.
Methods
IMD incidence and relative risk by age group and meningococcal capsular group in HIV-positive compared with HIV-uninfected individuals was estimated through data linkage of national datasets in England between 2011 and 2013.
Results
IMD incidence among persons diagnosed with HIV was 6.6 per 100,000 compared to 1.5 per 100,000 among HIV-negative individuals, with a relative risk of 4.5 (95 % CI, 2.7–7.5). All but one case occurred in adults aged 16–64 years, who had a 22.7-fold (95 % CI, 12.4–41.6; P <0.001) increased risk compared with the HIV-negative adults. IMD risk by capsular group varied with age. HIV-positive children and adolescents had a higher risk of meningococcal group B disease, while adults were at increased risk of groups C, W and Y disease. Most HIV-positive individuals had been born in Africa, had acquired HIV through heterosexual contact, and were known to be HIV-positive and receiving antiretroviral treatment at IMD diagnosis. The most common clinical presentation was septicemia and, although intensive care admission was common, none died of IMD.
Conclusions
HIV-positive children and adults are at significantly increased risk of IMD, providing an evidence base for policy makers to consider HIV as a risk factor for meningococcal vaccination.
Similar content being viewed by others
Background
Neisseria meningitidis, commonly known as the meningococcus, is a major global cause of meningitis and septicaemia, and is associated with significant morbidity and mortality across all age groups [1]. Twelve distinct polysaccharide capsules have been described, including five that are responsible for almost all cases of invasive meningococcal disease (IMD) globally: A, B, C, W and Y [1].
In the United Kingdom, as in most of Europe, meningococcal groups B (MenB) and C (MenC) were previously responsible for nearly all IMD cases [2]. Invasive MenC disease, however, is now uncommon since routine vaccination against MenC was introduced in 1999, with most cases now occurring in unvaccinated adults who acquire the infection abroad [2]. MenB, therefore, has been responsible for 80–90 % of all IMD cases, with the highest incidence in infants (<1 year-olds) and toddlers (1–4 year-olds), and with a small secondary peak in adolescents and young adults [3]. Meningococcal groups W (MenW) and Y (MenY) are uncommon and mainly cause disease in older adults, although the UK is currently experiencing a national outbreak of MenW disease across all age groups, following endemic expansion of a single hypervirulent strain belonging to the ST-11 clonal complex [4].
Unlike older adults (≥65 year-olds), the vast majority of children and young adults who develop IMD are previously healthy. The only significant risk factors for IMD are complement deficiency and asplenia/splenic dysfunction [5]. These risk groups are currently advised to receive the meningococcal quadrivalent conjugate vaccine (MenACWY) and the recently licensed multi-component protein-based MenB vaccine (Bexsero®, Novartis, Basel, Switzerland). They may also be on penicillin prophylaxis, which should provide additional protection against IMD [5]. Both these vaccines will be included in the UK national infant and adolescent immunisation programmes, respectively, later this year [5].
Unlike other encapsulated bacteria, such as Streptococcus pneumoniae and Haemophilus influenzae type b (Hib), immunosuppression (primary or acquired) has not been considered a significant risk factor for IMD [5]. Although the British HIV Association (BHIVA) testing guidelines recommend the quadrivalent vaccine containing A, C, W and Y, for travellers considered to be at risk or in contact with these capsular groups [6], HIV-positive individuals are not recommended to receive any additional vaccinations against IMD apart from the scheduled routine MenC doses, irrespective of their degree of immunosuppression or HIV treatment status [7–13].
The objective of this study was to assess the risk of IMD by age and capsular group in persons diagnosed with HIV in England. Analysis was performed using national data for the three most recent years, with the aim of developing an evidence base for recommending meningococcal vaccination for this vulnerable group.
Methods
Meningococcal surveillance
Public Health England (PHE) conducts enhanced national surveillance of IMD and captures more than 95 % of laboratory-confirmed cases in England [3]. As part of the enhanced surveillance, the PHE Meningococcal Reference Unit (MRU) provides a national service for species confirmation and grouping/typing of invasive N. meningitidis isolates, which are not routinely performed by National Health Service (NHS) hospital laboratories. The MRU also offers free PCR testing for meningococcal DNA in clinical specimens (e.g. blood, cerebrospinal fluid, joint fluid, pleural fluid), which are routinely submitted by NHS hospital laboratories throughout England [3]. Species confirmation and capsular group determination were performed as described previously [14]. Since 1 January 2011, all laboratory-confirmed cases are followed-up by requesting the patient’s general practitioner (GP) to complete a short questionnaire requesting information on risk factors, co-morbidities, clinical presentation, complications and outcome of IMD.
HIV surveillance
In addition to the information collected through GP questionnaires, all confirmed cases of IMD were matched to all persons newly diagnosed with HIV between 1981 and 2013, collected through a comprehensive national cohort of persons presenting for an HIV test across all testing facilities in England and subsequently accessing HIV care. Details of the HIV surveillance systems are detailed on the PHE website [15–17]. Cases of meningococcal disease were linked to persons diagnosed with HIV using soundex code (pseudo-anonymised coding of surname), initial, date of birth, sex and region of diagnosis. Data available on persons diagnosed with HIV are estimated to represent 76 % of all persons living with HIV, with around a quarter of persons living with HIV currently unaware of their infection. Reports of new HIV diagnoses for the study period, January 2011 to December 2013, are likely to be complete, with minimal reporting lag after two years.
Ethics agreement for this work is not required under the provisions in regulation 3 of The Health Service (Control of Patient Information) Regulations 2002, which authorises patient information to be processed by persons employed or engaged for the purposes of the health service or other persons employed or engaged by a government department or other public authority in communicable disease surveillance or associated with other risks to public health [18]. All data collected was under the remit of communicable disease surveillance.
Population
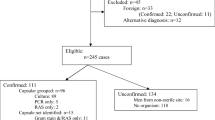
The study population consisted of all persons with confirmed IMD in England between January 2011 and December 2013. IMD cases were matched to persons diagnosed with HIV to identify all potential co-infected persons using soundex, initials, date of birth and sex. Confirmed meningococcal cases who were not identified by their GPs to be HIV-positive and who did not link to the HIV database were considered to be HIV-uninfected.
Definitions
Invasive meningococcal disease was defined as either isolation of N. meningitidis or DNA detection by PCR from a normally sterile body site [3].
A CD4 cell count reported within 90 days either side of HIV diagnosis was defined as the CD4 count at HIV diagnosis. A CD4 cell count below 350 cells/mm3 was defined as the time after which HIV treatment should have been commenced [6]. Therefore, any person with a CD4 cell count below 350 cells/mm3 was defined as having been diagnosed late. The CD4 cell count and ART status at IMD diagnosis among those co-infected was included if within 90 days of IMD diagnosis.
Statistical analysis
Statistical analyses were performed using STATA version 10 (Stata Corp., College Station, TX, USA). IMD incidence for persons diagnosed with HIV was calculated using the national cohort of persons diagnosed and living with HIV [16]. IMD incidence for the general population was calculated using population denominators from the Office for National Statistics (www.statistics.gov.uk). The overall and age-specific HIV-uninfected population were defined as the difference between the general population and the number of diagnosed HIV-positive persons. Incidence was calculated for all IMD, capsular group-specific (B, C, W and Y) and vaccine-preventable (B and ACWY) IMD.
Relative risk of IMD in HIV-positive compared with HIV-uninfected individuals was calculated using the incidence of these measures in co-infected and uninfected persons over the study period. Rates were compared using Pearson’s x 2 test. Proportions were compared using the x 2 test or Fisher’s exact test, as appropriate. Using a backwards logistic regression model, we examined factors associated with being co-infected with IMD and HIV. Variables included sex, age group, capsular group and clinical presentation. All confidence intervals (CI) are at the 95 % significant level.
Results
During 2011–2013, there were 2,353 confirmed IMD cases in England. The highest number of cases occurred in infants (<1 year-olds) who accounted for 21 % (499/2,345) of cases over the three-year period. IMD cases declined after the first year of life, although children less than 5 years old still accounted for nearly half of all cases (1,145/2,345, 49 %). A smaller peak was observed in adolescence, starting at 16 years and peaking at 19 years, before declining. MenB was responsible for 92 % (1,266/1,371) of cases in <16 year-olds, 68 % (480/703) in 16–64 year-olds and 41 % (96/237) in ≥65 year-olds. Meningococcal groups A, C, W and Y were responsible for 7.7 % (n = 105), 32 % (n = 222) and 59 % (n = 141) cases in these age groups, respectively.
Demographics
Co-infection with IMD and HIV was identified in 14 cases. Eleven were known to be HIV-positive prior to IMD with a median time of 6.5 (IQR, 2.5–10.6) years between HIV diagnosis and IMD diagnosis, two were diagnosed with HIV when they developed IMD, and one developed IMD one year prior to being confirmed as HIV-positive. All but one IMD case (93 %) among HIV-positive individuals were in adults aged 16–64 years (Table 1).
Incidence
The mean annual IMD incidence during 2011–2013 was 6.6 per 100,000 persons diagnosed with HIV compared with 1.5 per 100,000 among HIV-uninfected persons (relative risk, 4.5; 95 % CI, 2.7–7.5; P <0.001) (Table 2). The higher risk was observed for all three calendar years and across all age groups except older adults, where no IMD cases occurred in the HIV-positive cohort. When analysed by capsular group, the relative risk of IMD in adults was significantly higher for MenC, MenW and MenY (Table 3). An increased risk for MenB disease was only observed among adolescents and young adults (16–24 year-olds). For the other capsular groups, MenACWY were responsible for 31 % (212/690) of IMD cases in HIV-uninfected adults (16–64 year-olds) compared with 77 % (10/13) in those diagnosed with HIV, who had a 22.7-fold (95 % CI, 12.4–41.6; P <0.001) increased risk of IMD caused by these capsular groups.
In children, although there was only a single case of MenB in three years, this still constituted an increased relative risk of 10.0 (95 % CI, 1.4–71.1; P = 0.005) for invasive MenB disease compared with HIV-uninfected children.
Risk factors in persons diagnosed with HIV
Of the 14 HIV-positive individuals who developed IMD, 71 % had acquired HIV through heterosexual contact, 14 % through mother-to-child transmission (MTCT) and 14 % were men who have sex with men (MSM) (Table 4). IMD incidence was highest in those infected through MTCT (44.8 per 100,000 MTCT), compared with 9.4 per 100,000 heterosexuals diagnosed with HIV and 2.1 per 100,000 MSM.
Eight of the ten persons who acquired HIV through heterosexual contact and all persons infected through MTCT were born in Africa, resulting in an incidence of IMD among those diagnosed with HIV born in Africa being almost four times higher (IRR, 3.76; 95 % CI, 0.97–21.24) than that among those born in the UK (15.0 per 100,000 vs. 4 per 100,000).
Of the 11 persons diagnosed with HIV before developing IMD, the CD4 count, where reported, was <350 cells/mm3 in 50 % at HIV diagnosis (5/10) and at IMD diagnosis (4/8), and nearly all were on ART when they developed IMD (8/10). Of the five persons with a CD4 count of <350 cells/mm3 at HIV diagnosis, 75 % (3/4) continued to have a CD4 count of <350 cells/mm3 at IMD diagnosis, although all were receiving ART. The two adults diagnosed with HIV when they developed IMD had CD4 counts <350 and >350 cells/mm3, respectively, while the only case diagnosed with HIV more than one year after developing IMD had a CD4 count <350 cells/mm3.
Only 2 of the 14 cases had received a meningococcal vaccine prior to developing IMD, one was eligible for the MenC conjugate vaccine as part of the national MenC catch-up campaign during 1999–2000 but developed MenW disease, and the other had received the ACWY vaccine abroad and subsequently developed MenW disease. The three UK-born individuals were too old to have been eligible for any meningococcal vaccination.
The most common clinical presentation was septicaemia, followed by meningitis and pneumonia. Adjusting for age, gender and capsular group in a multivariable logistic regression model, clinical presentation was not significantly different for HIV-positive compared to HIV-uninfected cases. Although 6/14 (43 %) required intensive care support, no HIV infected people died of IMD.
Discussion
During a period when ART was routinely available in England, persons diagnosed with HIV had a 4.8-fold increased risk of IMD compared with individuals not known to be HIV infected. This increased risk was almost entirely in adults aged 16–64 years (23-fold increased risk) and was significant for Men C, W and Y, while MenB was only significant in those aged 16–24 years. There was only one MenB case in children with HIV and no cases in ≥65 year-olds. Most patients were born outside the UK, were known to be HIV-positive and receiving ART at the time of infection, and had not received any meningococcal vaccination prior to developing IMD.
These findings are consistent with the most recent study from New York City (NYC) where the relative risk for IMD among people living with HIV/AIDS (PLHIV) during the ART era was 10.0 especially among those with a CD4+ count of less than 200 cells/mm3 [13]. Notably, we observed a 15.9-fold increased risk for MenC, MenW and MenY, which are all potentially preventable through immunisation with the MenACWY quadrivalent conjugate vaccine [19]. The NYC study also found these capsular groups to be more prevalent among HIV-infected adults with IMD (87 %) compared to the IMD-only group (72 %), although the difference was not statistically significant. An earlier study from the United States also reported a significantly higher risk of IMD in HIV-positive adults [7] and these findings were corroborated in a large South African study, where the age-adjusted relative risk of developing IMD was 11.3, with a two-fold increased risk of dying from the infection [8]. Other studies from developing countries with high HIV prevalence, however, have not found any significant associations with IMD [9–12].
In HIV-uninfected individuals, half of all IMD cases in England are diagnosed in children younger than 5 years and more than 90 % of cases in this age group are caused by MenB [3]. Although only one IMD case was diagnosed among children with HIV, this single case represented a significantly higher risk for HIV-positive children when compared with HIV-uninfected children.
In adolescents, a small peak in IMD coincides with teenagers entering higher education settings (typically universities), where they are exposed to lifestyle factors and behaviors that increase their risk of IMD, such as smoking (or being exposed to smoke), kissing, sharing drinking glasses, eating utensils or water bottles, and living in close quarters (e.g. dormitories) [20]. Overall, MenB is still responsible for the majority (85–90 %) of IMD cases in adolescents. In HIV-positive persons, the increased risk in invasive MenB disease was only observed among 16–24 year-olds. In contrast, the risk of MenW and MenY disease was significantly higher across all adult age groups. These two capsular groups are uncommon causes of IMD in England and generally considered to be less virulent than MenB or MenC, causing disease mainly in older adults who often have underlying co-morbidities [4, 21]. A notable feature of these two capsular groups is the atypical clinical presentation, including respiratory tract infections such as pneumonia, epiglottitis, cellulitis and septic arthritis [22]. If IMD is not considered in the differential diagnosis, then public health actions to offer rapid chemoprophylaxis and the MenACWY conjugate vaccine to close contacts (who may also be HIV-positive) could be delayed and lead to secondary cases.
Although we observed significantly higher rates of capsular group-specific IMD in HIV-positive individuals, there were only 14 cases diagnosed over the three-year period, equivalent to 0.5 % of all IMD cases. In this high-risk group, however, we found that most individuals had acquired HIV through heterosexual contact and the incidence in MSM was similar to the background rate. A recent MenC disease outbreak in NYC was reported among MSM and subsequently identified in other countries, including Germany and France, resulting in an offer of vaccination against MenC to MSM in these countries [13, 23–28]. In England, there were no cases of MenC disease in children, adolescents or young adults (<25 year-olds) with HIV over the three-year period and none among MSM of any age, highlighting the success of the current national MenC conjugate vaccination programme in providing both direct and indirect protection against MenC disease [29].
Unlike the US, MenB is responsible for most IMD cases in children and adults in England. The significantly higher risk of MenC, MenW and MenY disease among persons diagnosed with HIV in England is, therefore, intriguing. Most cases were known to be HIV-positive at IMD diagnosis and half of them continued to have low CD4 counts at IMD diagnosis, making them generally susceptible to serious bacterial infections, including the less virulent meningococcal capsular groups such as MenW and MenY. In persons known to be HIV-positive, we found no association between IMD and CD4 counts at HIV diagnosis, time since HIV diagnosis, CD4 counts at IMD diagnosis or receiving ART at IMD diagnosis. Given that most cases had been born outside the UK, it is likely that they were at higher risk of exposure to infection when they themselves, their close family or community contacts travelled to their country of origin. It is also possible that these cases socialise in highly-defined closed networks – either because of their HIV infection or because of their ethnic background – where the less common meningococcal capsular groups may be circulating.
Limitations
Until now, except for complement deficiency and splenic dysfunction, HIV or any other immune deficiency has not been considered a significant risk factor for IMD [5]. In the current study, too, only 0.5 % of IMD cases were known to be HIV-positive. However, the IMD surveillance questionnaires were completed by GPs, who are not always aware of the diagnosis of HIV. It is, therefore, possible that HIV prevalence among IMD patients may be underestimated as demonstrated by the additional ascertainment through linkage with the national HIV database. In children, however, there were limited identifiers in the HIV database for linkage compared to adults and, therefore, some children with HIV and IMD may have been missed. It is also not possible to estimate the number of undiagnosed HIV-positive cases in the IMD cohort because the latter are not routinely tested for HIV. Furthermore, it is estimated that a quarter of persons estimated to be living with HIV are unaware of their infection and would not be included within the national cohort of persons accessing HIV care. However, the coverage of persons diagnosed and utilising HIV-related care indicates high rates of access to care and low rates of loss to follow-up [30]. Moreover, identifying additional HIV-positive individuals among IMD cases would only serve to strengthen the association between HIV and IMD, and increase the estimated relative risks further.
Clinical implications
A protein-based, multi-component MenB vaccine has recently been licensed in Europe and will be introduced into the UK infant immunisation programme in 2015 [31]. This vaccine induces high concentrations of bactericidal antibodies against most MenB strains causing IMD in the UK and could potentially also protect against other meningococcal capsular groups [32]. HIV-infected infants born in the UK will, therefore, benefit from the vaccine programme, although there are currently very few infants infected through MTCT because of the high uptake of antenatal screening and very high effectiveness of antenatal and perinatal ART in preventing MTCT from mothers known to be HIV-positive [33]. Older children with HIV, including newly diagnosed entrants to the UK, would not be eligible for MenB vaccination because there is no catch-up programme planned for the UK. Reassuringly, though, invasive MenB disease is much less common in older children irrespective of HIV status.
In adolescents and young adults, the recent increase in invasive MenW disease in England caused by a hypervirulent clonal complex (cc11) clone has led to recommendations for a national adolescent MenACWY conjugate vaccination programme for 14–18 year-olds, which will be introduced imminently [34]. This programme should, therefore, also protect HIV-positive individuals of all ages against these four capsular groups through both direct and indirect (herd) protection. The potential high level of exposure to meningococcal for infected individuals born overseas, however, may be less amenable to prevention by a UK-based programme and may reflect a substantially higher risk in uninfected people with overseas origins.
The MenACWY conjugate vaccine, however, will not protect against MenB disease. Future studies on the immunogenicity of the recently licensed MenB vaccine in HIV-positive children and adolescents at different stages of immunosuppression and antiretroviral treatment could potentially support recommendations for MenB vaccination in these age groups. Our results also indicate that newly diagnosed adults with HIV may benefit from MenACWY conjugate vaccination [19, 35]. However, the wide range of CD4 counts, including a significant proportion with very low counts, and the variable duration and combinations of ART at the time of IMD diagnosis suggest that these parameters may not be useful criteria for recommending the most appropriate timing for meningococcal vaccination. Current UK guidelines suggest that vaccination should be given to persons with CD4 counts <200 cells/mm3 if indicated and safe, and repeated following immunoreconstitution if required [36].
There are three available MenACWY vaccines, each conjugated to a different carrier protein, and one (MenACWY-D; Menactra®, Sanofi Pasteur, Lyon, France) has been assessed in HIV-positive children and adolescents; the vaccine was immunogenic against the four capsular groups and a two-dose elicited higher bactericidal antibody concentrations and protected more individuals than a single dose [19, 37]. Vaccine responses were dependent on age, the presence of an AIDS-defining illness, CD4 count and HIV viral load [19, 35, 37], although none of these factors were consistently predictive of poorer antibody responses. Unlike the UK, a two-dose MenACWY primary MenACWY conjugate vaccine schedule, given at least 2 months apart, is recommended for HIV-positive adolescents (>11 years) in the US, with boosters every 5 years [38]; notably, the recently licensed MenB vaccines have been recommended for those considered at increased risk of MenB disease but not for HIV-positive individuals [39].
Another important observation was that HIV diagnosis was known at the time of IMD for nearly all cases. In only two patients was the diagnosis of HIV made at the time of IMD infection and, in one case, after recovering from IMD, highlighting the importance of considering an underlying diagnosis of HIV if clinically indicated, including but not limited to immigration from an HIV-endemic area, intravenous drug use or MSM, history of opportunistic infections or low lymphocyte counts when hospitalised for IMD.
Availability of data and materials
All data for persons co-infected with HIV and invasive meningococcal disease are available within the Table 4. Further requests regarding individual participant data should be made to the clinical leads, Dr Shamez Ladhani for meningococcal (shamez.ladhani@phe.gov.uk) and Dr Valerie Delpech for HIV (valerie.delpech@phe.gov.uk).
Conclusion
This study provides evidence that HIV-positive children and adults are at increased risk of IMD, up to five times higher than the general population. Furthermore, the risk among the capsular groups MenC, MenW, and MenY, preventable through the MenACWY quadrivalent conjugate vaccine, was 16 times higher in adults, especially 16-24 year-olds who had a much higher risk. These findings provide an evidence base for policy makers to include HIV as a risk factor for meningococcal vaccination, and clinicians to consider HIV as a possible underlying condition in children and adults who develop invasive meningococcal disease.
Abbreviations
- ART:
-
antiretroviral therapy
- BHIVA:
-
British HIV Association
- CI:
-
confidence interval
- GP:
-
general practitioner
- Hib:
-
Haemophilus influenzae type b
- HIV:
-
human immunodeficiency virus
- IMD:
-
invasive meningococcal disease
- IQR:
-
interquartile range
- MRU:
-
Meningococcal Reference Unit
- MSM:
-
men who have sex with men
- MTCT:
-
mother-to-child transmission
- NHS:
-
National Health Service
- PCR:
-
polymerase chain reaction
- PHE:
-
Public Health England
- PLHIV:
-
people living with HIV/AIDS
References
Halperin SA, Bettinger JA, Greenwood B, Harrison LH, Jelfs J, Ladhani SN, et al. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2012;30 Suppl 2:B26–36.
Campbell H, Borrow R, Salisbury D, Miller E. Meningococcal C conjugate vaccine: the experience in England and Wales. Vaccine. 2009;27 Suppl 2:B20–9.
Ladhani SN, Flood JS, Ramsay ME, Campbell H, Gray SJ, Kaczmarski EB, et al. Invasive meningococcal disease in England and Wales: implications for the introduction of new vaccines. Vaccine. 2012;30(24):3710–6.
Ladhani SN, Beebeejaun K, Lucidarme J, Campbell H, Gray S, Kaczmarski E, et al. Increase in endemic Neisseria meningitidis capsular group W sequence type 11 complex associated with severe invasive disease in England and Wales. Clin Infect Dis. 2015;60(4):578–85.
Department of Health. Immunisation against infectious disease - the ‘Green Book’ 2006. London: Department of Health; 2008. Available from: http://webarchive.nationalarchives.gov.uk/20080910134953/http://dh.gov.uk/en/publichealth/healthprotection/immunisation/greenbook/dh_4097254.
British HIV Association, British Association for Sexual Health and HIV, British Infection Society. UK national guidelines for HIV testing 2008. London: British HIV Association; 2008. Available from: http://www.bhiva.org/HIV-testing-guidelines.aspx.
Stephens DS, Hajjeh RA, Baughman WS, Harvey RC, Wenger JD, Farley MM. Sporadic meningococcal disease in adults: results of a 5-year population-based study. Ann Intern Med. 1995;123(12):937–40.
Cohen C, Singh E, Wu HM, Martin S, de Gouveia L, Klugman KP, et al. Increased incidence of meningococcal disease in HIV-infected individuals associated with higher case-fatality ratios in South Africa. AIDS. 2010;24(9):1351–60.
Pinner RW, Onyango F, Perkins BA, Mirza NB, Ngacha DM, Reeves M, et al. Epidemic meningococcal disease in Nairobi, Kenya, 1989. The Kenya/Centers for Disease Control (CDC) Meningitis Study Group. J Infect Dis. 1992;166(2):359–64.
Gilks CF, Brindle RJ, Otieno LS, Simani PM, Newnham RS, Bhatt SM, et al. Life-threatening bacteraemia in HIV-1 seropositive adults admitted to hospital in Nairobi, Kenya. Lancet. 1990;336(8714):545–9.
Brindle R, Simani P, Newnham R, Waiyaki P, Gilks C. No association between meningococcal disease and human immunodeficiency virus in adults in Nairobi, Kenya. Trans R Soc Trop Med Hyg. 1991;85(5):651.
Kipp W, Kamugisha J, Rehle T. Meningococcal meningitis and HIV infection: results from a case-control study in western Uganda. AIDS. 1992;6(12):1557–8.
Miller L, Arakaki L, Ramautar A, Bodach S, Braunstein SL, Kennedy J, et al. Elevated risk for invasive meningococcal disease among persons with HIV. Ann Intern Med. 2014;160(1):30–7.
Unit MR, Gray SJ, Trotter CL, Ramsay ME, Guiver M, Fox AJ, et al. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J Med Microbiol. 2006;55(Pt 7):887–96.
Public Health England. HANDD metadata files. London: Public Health England; 2015. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/336382/HANDD_metadata_SC__2_.pdf.
Public Health England. SOPHID metadata files. London: Public Health England; 2015. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/336380/SOPHID_metadata_v2_v200913__3_.pdf.
Public Health England. CD4 surveillance metadata files. London: Public Health England; 2015. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/331154/CD4_metadata_v2_200913__2_.pdf.
National Health Service. The Health Service (Control of Patient Information) Regulations 2002. No. 1438. London: The Stationery Office; 2002. Available from: http://www.legislation.gov.uk/uksi/2002/1438/pdfs/uksi_20021438_en.pdf.
Lujan-Zilbermann J, Warshaw MG, Williams PL, Spector SA, Decker MD, Abzug MJ, et al. Immunogenicity and safety of 1 vs 2 doses of quadrivalent meningococcal conjugate vaccine in youth infected with human immunodeficiency virus. J Pediatr. 2012;161(4):676–81. e2.
Tully J, Viner RM, Coen PG, Stuart JM, Zambon M, Peckham C, et al. Risk and protective factors for meningococcal disease in adolescents: matched cohort study. BMJ. 2006;332(7539):445–50.
Ladhani SN, Lucidarme J, Newbold LS, Gray SJ, Carr AD, Findlow J, et al. Invasive meningococcal capsular group Y disease, England and Wales, 2007-2009. Emerg Infect Dis. 2012;18(1):63–70.
Ozaki B, Kittai A, Chang S. Neisseria meningitidis as a cause of facial cellulitis. BMJ Case Rep. 2014;2014. doi: 10.1136/bcr-2014-203774.
Weiss D, Varma JK. Control of recent community-based outbreaks of invasive meningococcal disease in men who have sex with men in Europe and the United States. Euro Surveill. 2013;18(28).
Simon MS, Weiss D, Gulick RM. Invasive meningococcal disease in men who have sex with men. Ann Intern Med. 2013;159(4):300–1.
Marcus U, Vogel U, Schubert A, Claus H, Baetzing-Feigenbaum J, Hellenbrand W, et al. A cluster of invasive meningococcal disease in young men who have sex with men in Berlin, October 2012 to May 2013. Euro Surveill. 2013;18(28).
Schmink S, Watson JT, Coulson GB, Jones RC, Diaz PS, Mayer LW, et al. Molecular epidemiology of Neisseria meningitidis isolates from an outbreak of meningococcal disease among men who have sex with men, Chicago, Illinois, 2003. J Clin Microbiol. 2007;45(11):3768–70.
European Centre for Disease Prevention and Control (ECDC). Invasive meningococcal disease among men who have sex with men. Stockholm: ECDC; 2013. Available from: http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-invasive-meningococcal-disease-among-MSM.pdf.
Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, et al. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR-2):1–28.
Campbell H, Andrews N, Borrow R, Trotter C, Miller E. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol. 2010;17(5):840–7.
Rice BD, Delpech VC, Chadborn TR, Elford J. Loss to follow-up among adults attending human immunodeficiency virus services in England, Wales, and Northern Ireland. Sex Transm Dis. 2011;38(8):685–90.
NHS Choices. Meningitis B jab to be added to NHS child vaccine schedule. London: NHS Choices; 2015. Available from: http://www.nhs.uk/news/2015/03March/Pages/Meningitis-B-jab-to-be-added-to-NHS-child-vaccine-schedule.aspx.
Frosi G, Biolchi A, Lo Sapio M, Rigat F, Gilchrist S, Lucidarme J, et al. Bactericidal antibody against a representative epidemiological meningococcal serogroup B panel confirms that MATS underestimates 4CMenB vaccine strain coverage. Vaccine. 2013;31(43):4968–74.
Townsend CL, Byrne L, Cortina-Borja M, Thorne C, de Ruiter A, Lyall H, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000-2011. AIDS. 2014;28(7):1049–57.
Public Health England. Meningococcal group W (MenW) immunisation advised for 14 to 18 year-olds. London: Public Health England; 2015. Available from: https://www.gov.uk/government/news/meningococcal-group-w-menw-immunisation-advised-for-14-to-18-year-olds.
Frota AC, Milagres LG, Harrison LH, Ferreira B, Menna Barreto D, Pereira GS, et al. Immunogenicity and safety of meningococcal C conjugate vaccine in children and adolescents infected and uninfected with HIV in Rio de Janeiro, Brazil. Pediatr Infect Dis J. 2015;34(5):e113–8.
Geretti AM, BHIVA Immunization Writing Committee, Brook G, Cameron C, Chadwick D, Heyderman RS, et al. British HIV Association guidelines for immunization of HIV-infected adults 2008. HIV Med. 2008;9(10):795–848.
Siberry GK, Warshaw MG, Williams PL, Spector SA, Decker MD, Jean-Philippe P, et al. Safety and immunogenicity of quadrivalent meningococcal conjugate vaccine in 2- to 10-year-old human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2012;31(1):47–52.
Committee on Infectious Diseases. Meningococcal conjugate vaccines policy update: booster dose recommendations. Pediatrics. 2011;128(6):1213–8.
Folaranmi T, Rubin L, Martin SW, Patel M, MacNeil JR, Centers for Disease Control. Use of serogroup B meningococcal vaccines in persons aged >/=10 years at increased risk for serogroup B meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(22):608–12.
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors have no competing interests to declare.
Authors’ contributions
RS undertook the analysis and had access to the complete dataset; SL, VD and SL supervised RS; RS wrote the first draft of the manuscript; KB and SL supervised all aspects of the work regarding the meningococcal dataset; VD and PK supervised all aspects of the work regarding the HIV dataset; and AR, RB and MR supervised the development of the manuscript. Data access was granted by the clinical leads, VD for new HIV diagnoses and SL for meningococcal. All authors provided critical input to the manuscript and approved all revisions. All authors read and approved the final version of the manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Simmons, R.D., Kirwan, P., Beebeejaun, K. et al. Risk of invasive meningococcal disease in children and adults with HIV in England: a population-based cohort study. BMC Med 13, 297 (2015). https://doi.org/10.1186/s12916-015-0538-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-015-0538-6