Abstract
Background
Aedes aegypti, the main arboviral mosquito vector, is attracted to human dwellings and makes use of human-generated breeding sites. Past research has shown that bacterial communities associated with such sites undergo compositional shifts as larvae develop and that exposure to different bacteria during larval stages can have an impact on mosquito development and life-history traits. Based on these facts, we hypothesized that female Ae. aegypti shape the bacteria communities of breeding sites during oviposition as a form of niche construction to favor offspring fitness.
Results
To test this hypothesis, we first verified that gravid females can act as mechanical vectors of bacteria. We then elaborated an experimental scheme to test the impact of oviposition on breeding site microbiota. Five different groups of experimental breeding sites were set up with a sterile aqueous solution of larval food, and subsequently exposed to (1) the environment alone, (2) surface-sterilized eggs, (3) unsterilized eggs, (4) a non-egg laying female, or (5) oviposition by a gravid female. The microbiota of these differently treated sites was assessed by amplicon-oriented DNA sequencing once the larvae from the sites with eggs had completed development and formed pupae. Microbial ecology analyses revealed significant differences between the five treatments in terms of diversity. In particular, between-treatment shifts in abundance profiles were detected, showing that females induce a significant decrease in microbial alpha diversity through oviposition. In addition, indicator species analysis pinpointed bacterial taxa with significant predicting values and fidelity coefficients for the samples in which single females laid eggs. Furthermore, we provide evidence regarding how one of these indicator taxa, Elizabethkingia, exerts a positive effect on the development and fitness of mosquito larvae.
Conclusions
Ovipositing females impact the composition of the microbial community associated with a breeding site, promoting certain bacterial taxa over those prevailing in the environment. Among these bacteria, we found known mosquito symbionts and showed that they can improve offspring fitness if present in the water where eggs are laid. We deem this oviposition-mediated bacterial community shaping as a form of niche construction initiated by the gravid female.
Similar content being viewed by others
Background
The mosquito Aedes aegypti (Linnaeus, 1762) is the main vector of the arboviruses causing dengue, yellow fever, Zika, and chikungunya. Its wide distribution across tropical and subtropical regions in close association with urban areas makes this mosquito a major threat to human health [1, 2]. Urban houses represent suitable mosquito habitats with a reduced number of predators, diverse sugar sources, widely available blood sources (as well as resting places for gravid females), and a variety of water-holding containers accessible for egg-laying and larval development [3, 4]. In these environments, artificial containers that accumulate water (e.g., flower pots, discarded plastic or metallic cups, and tires act as breeding sites [3, 5]. In most cases, these containers collect rainwater, which is a poor source of nutrients.
Biotic and abiotic elements present in water are known to drive the selection of oviposition sites by gravid females. These include the presence of conspecifics and/or predators, organic matter, surrounding vegetation, color, moisture, salinity, ammonium, and phosphate [6,7,8,9]. Furthermore, microbial communities have been shown to influence Ae. aegypti oviposition choices [10,11,12]. Indeed, females locate suitable breeding sites using microbe-emitted infochemicals [13]. Oviposition choices probably endure selection pressures because microorganisms serving as larval food can also establish intricate host-bacterial community networks, eventually defining symbiotic relations [11].
The origin of the microbial communities that colonize mosquitoes and the relative contribution of the environment to their acquisition are still debated [14, 15]. It has been shown that part of mosquito-associated bacteria is acquired during early life stages in larval habitats [16,17,18,19]. Besides, the bacterial communities present in Ae. aegypti larvae are influenced by the aquatic environment where they develop [9, 16]. Furthermore, some members of the bacterial community can be transstadially transmitted to adults [16, 18, 20,21,22].
Mosquito females can add key microbial associates during egg-laying, affecting the microbial community within the breeding site [17]. This may promote symbiont dispersal, providing offspring with specific microbial inocula rather than leaving their acquisition to chance [23]. Bacteria recovered from immature stages and adults have already been detected on egg surfaces [16, 22]. Indeed, mosquitoes can transfer bacteria to their oviposition sites and pick them up from the water they emerged from [20, 22]. It has been suggested that transmission of maternal microbiota to larval breeding sites could occur directly, through egg smearing or transovarial transmission; or indirectly during egg-laying when females might unintentionally inoculate microbes into oviposition sites [24].
Although the properties of the external environment influence the bacterial communities of a niche, the dissemination of microbial cells from eukaryotic hosts can also impact the composition and traits of the microbiota in the immediate environment [23, 25]. Considering that Ae. aegypti exploits small and temporary water containers, altering the bacterial community of larval habitats could have a substantial impact on larval fitness [21]. If verified, this ability could elucidate its ability to exploit confined nutrient-scarce habitats. Environment-modifying capacities exerted by parental individuals and their offspring during ontogenesis is a tenet of niche construction theory [26, 27]. Within this conceptual framework, the phenomenon of developmental niche construction can occur via chemical excretion, generation of physical structures (e.g., beaver dams), or due to the physiological properties of symbionts [28, 29]. Whether mosquito larval habitats represent a case of niche construction is still unknown.
Our study evaluated whether gravid female mosquitoes shape the bacterial community of the breeding site as a strategy to enhance offspring fitness. To address our hypothesis, we investigated whether gravid females (i) act as mechanical vectors of bacteria, (ii) modulate the bacterial community in water-holding containers through oviposition, and (iii) promote bacteria (acting as oviposition indicators) that enhance progeny fitness.
Methods
Mosquito rearing
Aedes aegypti (F2) were obtained from a Brazilian laboratory colony (BR URCA) established from eggs collected in ovitraps in the Urca district of Rio de Janeiro city. All mosquitoes used in the experiments were maintained under insectary conditions at 28 ± 2 °C, 70 ± 10% relative humidity, and a 12:12 light/dark photoperiod. Larvae were reared in plastic trays containing non-chlorinated water and fed half a tablet of TetraMin fish food (Tetra) every day. Pupae were transferred from rearing trays to cardboard cages in plastic flasks, after which adults emerged. Adults were offered 10% sucrose solution ad libitum. Females were blood-fed 7 days post-emergence on a Hemotek Membrane Feeding System (Hemotek Ltd) using human blood. Human blood used to feed adult mosquitoes was obtained from a blood bank (Fundação Hemominas, Belo Horizonte, Minas Gerais, Brazil), according to the terms of an agreement with Instituto René Rachou, Fiocruz Minas (OF.GPO/CCO agreement-Nr 224/16). Pilot experiments revealed that this mosquito population has its oviposition peak 72 h after a blood meal. Only fully engorged females were collected for further assays.
Mechanical transmission of bacteria
Experimental design
To assess whether Ae. aegypti females can mechanically transfer viable bacteria to solid culture media, a single female was released in a cardboard cage (brand new, cleaned with 70% ethanol-soaked paper wipes, and exposed to 15 min of UV light in a biosafety cabinet) presenting a Petri dish at the bottom loaded with either LB or blood agar media. Five replicates were performed per culture medium tested, plus two environmental control plates per medium type.
After 24 h, females were removed from the cages, pooled, and washed with 1 ml of sterile phosphate-buffered saline (PBS) for 10 min. Moreover, a swab of the wall and bottom of the cardboard cage was collected and placed in PBS (1 ml) for 10 min. Subsequently, an aliquot (50 µl) of these PBS washes, both from the body surfaces and the cage swab, was inoculated on LB (Lysogeny Broth) and blood agar plates, separately. The plates were incubated for up to 48 h. Negative control plates with only sterile PBS resulted in no colonies.
DNA extraction and PCR amplification
Bacterial isolates were examined and characterized according to their features. Colonies with visually distinct morphologies were isolated from each medium, followed by total genomic DNA extraction using the DNeasy Blood & Tissue Kit (Qiagen), according to the manufacturer’s manual. A reagent blank extraction was performed as a negative control of the process.
The full length of the bacterial 16S ribosomal RNA (16S rRNA) gene (~ 1500pb) was amplified by the pair of primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-TACGGYTACCTTGTTACGACTT-3′). Polymerase chain reactions (PCR) were carried out in a 25 µL final volume using 0.50 µl of 5U/µl GoTaq Polymerase (Promega), 1.50 µl of 25 mM MgCl2, 0.50 µl of 10 mM dNTP mixture, 5 µl of 5X reaction buffer, 10 µM of each primer and 2.5 µl of template DNA. Amplification consisted of an initial denaturation at 95 °C for 2 min, 30 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min 40 s, followed by a final extension at 72 °C for 5 min. A PCR amplification control was performed. Reactions and negative controls were analyzed by electrophoresis in a 1% agarose gel. Controls, both from DNA extraction and PCR, showed no amplified bands.
Sanger sequencing and taxonomic identification
PCR products were purified using the ReliaPrep DNA Clean-up and Concentration System (Promega) following the manufacturer’s protocol. Sequencing reactions were conducted using the BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific). Three primers (two forward and one reverse) were used to generate amplicons for Sanger sequencing [27F, 515F (5′-GTGCCAGCMGCCGCGGTAA-3′), and 1492R]. The combination of sequence data obtained with these three amplicons generates a contiguous sequence that encompasses most of the full 16S rRNA gene [30]. Sequencing was performed on an ABI 3730 DNA sequencer.
The sequenced reads were assembled using the software Geneious Prime v2019.0.4. Bacterial taxonomic classification was performed using the SILVA Alignment, Classification and Tree Service with a minimal identity with query sequences of 95%.
Changes in the bacterial profile of breeding sites
Experimental design
To test whether Ae. aegypti females modify breeding site community composition through oviposition, an experiment with five different treatments was designed (Fig. 1). Ten replicates per treatment were carried out using cardboard cages presenting a plastic cup with 80 ml of type I water and 500 µl of sterilized food. This diet was prepared by dissolving finely groundfish food in type I water and autoclaving it for 20 min at 120 °C. All water containers were set up on day one with sterilized food added on day 2.
Detection of profile shifts on microbial communities induced by oviposition-related inputs. An experimental scheme was elaborated to dissect the effects of the act of oviposition from other sources affecting microbial profiles observed after mosquito-water interactions. DNA extracted from water samples belonging to five different treatments was subjected to amplicon-oriented sequencing to characterize the structure of their bacterial communities. Treatment 1 (T1): environmental control (type I water plus sterilized food). Treatment 2 (T2): manually-deposited sterilized mosquito eggs. Treatment 3 (T3): manually-deposited non-sterilized eggs. Treatment 4 (T4): single sugar-fed females interacting with container water. Treatment 5 (T5): single gravid female (72 h post-blood-feeding) allowed to lay eggs
Treatment 1 (T1) acted as an environmental control (type I water plus sterilized food). Treatment 2 (T2) was developed using sterilized mosquito eggs that were manually deposited. Eggs were sterilized using 70% ethanol for 5 min, followed by a wash in a 3% bleach and 0.1% benzalkonium chloride (Quatermon 30, Chemitec, Brazil) solution for 3 min, an additional wash in 70% ethanol for 5 min, and rinsing three times in sterile water. The sterile condition of eggs was confirmed by negative PCR amplification of the 16S rRNA gene V4 hypervariable region using the primers 515F and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Besides, this was reinforced by the absence of bacterial growth from sterilized eggs transferred to LB broth, which indicated no viable bacteria were present. Treatment 3 (T3) was developed using manually deposited non-sterilized eggs. Eggs, both for T2 and T3, were derived from groups of gravid females that oviposited on pieces of filter paper, which were stored under insectary conditions until needed (but not longer than one month). Treatment 4 (T4) was developed with a sugar-fed female that was held for 24 h without access to drinking to assure that it would interact with the water in the container and thus ensure physical contact control for mosquitoes that cannot lay eggs. Treatment 5 (T5) was developed with a gravid female (72 h post-blood-feeding) that was allowed to lay eggs. For both, T4 and T5, females were removed from cardboard cages after a 24 h exposure interval. Once females were removed, the number of eggs laid in each T5 replicate was counted using a magnifying glass (Additional file 1). This allowed us to calculate an average number subsequently used for manually depositing eggs (51 eggs) in T2 and T3 replicates.
For T2, T3, and T5, water samples were collected when at least one pupa was detected. For T4 and T1, water samples were collected on days 15 and 16, respectively. As pupation represents a developmental checkpoint in the holometabolous cycle [31], we considered this criterion as the basis for the sampling as it represents an environment that has successfully sustained the development of larvae.
DNA extraction and high-throughput sequencing
Each water sample was aseptically filtered through a polyethersulfone membrane (0.22 μm pore size, 50 mm diameter) using vacuum-driven filters (Biofil) and a vacuum-pressure pump (Millipore). Filter membranes were cut into small pieces using a stainless steel scalpel and placed in sterile tubes. A control using ultrapure water was carried out to verify whether the membrane or filtration process could introduce any contamination. Bacterial genomic DNA was extracted from bacterial cells retained on each filter membrane using the DNeasy PowerSoil Kit (Qiagen), following the manufacturer’s methods. A reagent blank extraction was the control of the DNA extraction process. DNA sample concentration was measured using a Qubit fluorescence assay (Invitrogen). All DNA samples were concentrated in a vacufuge concentrator (Eppendorf) and sent for amplicon sequencing (16S rRNA, V4 region primers) on an Illumina HiSeq PE250 instrument at Novogene Bioinformatics Technology Co., Ltd. (Beijing, China). Since the controls, both from the filtration process and the DNA extraction resulted in negative PCR amplification, they were not further processed and were not sequenced (Additional file 2).
Bioinformatics analysis and taxonomic assignment
Raw sequence data generated were processed using the DADA2 pipeline v1.6.0 [32] to identify Amplicon Sequence Variants (ASVs). The raw reads were trimmed to remove the primers. The forward reads were trimmed at position 180 and the reverse reads at nucleotide 150. After trimming, the reads with a maximum of 2 expected errors for the error model prediction and merging were conserved.
Taxonomic classification was assigned by TAGME [33] using Silva 138 database and the pre-built model for the amplified region. HTSFilter package v1.38.0 [34] was used to remove ASVs containing reads less than a cutoff value defined by calculating a Jaccard index. All the above-mentioned bioinformatics tools, plus diversity and statistical analyses downstream were executed in Rstudio v1.1.423.
Diversity and statistical analyses
ASVs diversity within and between samples was compared. The Simpson index (1-D) was used to measure alpha diversity. Alpha diversity metrics between groups were compared using the Kruskal–Wallis test, followed by post hoc Dunn’s multiple comparisons tests. P-values were adjusted using the Benjamini–Hochberg method.
A Jensen-Shannon distance matrix was used for beta diversity analysis. A principal coordinates analysis (PCoA) was conducted to visualize and interpret the overall dissimilarity in the microbial community structure among the treatments. A Permutational Multivariate Analysis of Variance (PERMANOVA) [35] was performed to explore the significance of the presence of eggs and/or the female interaction with water, on the bacterial signatures associated with each group. Additionally, a pairwise PERMANOVA [36] based on the ASV abundance matrix transformed using the Hellinger method was applied to evaluate the significance of the variance between each treatment.
The differentially abundant ASVs were detected using DESeq2 v1.38.1 [37]. In a multivariate model, the likelihood ratio test was used to identify differentially abundant variants. For univariate analysis, the variants differing in each variable — female interaction with water and eggs presence — were identified using the Wald test. All ASVs with adjusted P-value < 0.01 were considered differentially abundant and were used for model construction.
A general Random Forest (RF) model was built using all the previously identified ASVs and the Gini importance of each ASV was calculated. The 30 most important ASVs were used to construct models for each variable — female interaction with water and egg presence. One thousand bootstrap analyses were performed by randomly selecting 50% of samples from the analyzed variable, building 100 trees, and calculating the importance of each ASV. The 10 most important ASVs among the 1000 tests were chosen to build a final predictive model. The model construction and performance analysis were executed using caret package v6.0–86 [38].
As the predictive model tested by the RF approach identified features capable of discriminating the communities based on the key experimental variables, we deemed it relevant to search for indicator taxa. This ecological analysis was executed to identify ASVs that reflect the effects that biotic and/or abiotic factors, encompassed within each treatment, exert, thus shaping the community composition. In particular, we aimed to identify ASVs whose occurrence and abundance provide evidence of the impact that oviposition and larval development (T5) had upon the breeding site bacterial consortium. The analysis was performed using the indicspecies package v1.7.7 [39].
Effects of Elizabethkingia on larval development, mortality, and adult size
Selection of bacteria for larval fitness experiments
To assess if ASVs identified as an indicator of oviposition activity may have an impact on Ae. aegypti development, a bacterial strain belonging to the genus Elizabethkingia was selected for fitness experiments. As a control, we also tested Asaia, a bacterial symbiont widely present in the microbiota of several mosquito species [40] that has been previously shown to shorten the larval development time of Anopheles mosquitoes [41, 42].
Asaia sp. strain AE06 (GenBank accession: KR703670) was recovered from the midgut of adult females of the Paea laboratory strain, which was established in 1994 [43]. Elizabethkingia sp. strain VV01(GenBank accession: KU096882) was isolated from field-collected mosquitoes. Wild Ae. aegypti were collected in Colônia Z-10 (22°49′23.50″S; 43°10′42.93″W), a fishermen's community in Rio de Janeiro. Larvae, water, and deposited sediment were collected from two natural breeding sites, brought to the insectary, and conditioned in clean disposable cups at 27 ± 2 °C. No additional food or water was added to the cups until adult emergence. Adults were fed ad libitum with sterilized cotton soaked in sterilized 10% sucrose solution until midgut dissection. Ice-anesthetized adult female mosquitoes were surface-sterilized in 70% ethanol for 1 min and rinsed in sterile PBS. As surface sterilization control, individuals were rinsed in sterile PBS, which was plated on LB plates. Midguts were removed over a sterile glass slide and macerated in sterile PBS. Each midgut sample was tenfold diluted and plated on LB and tryptone soy agar plates. For the next 72 h, bacteria were screened based on colony morphology. Samples from each different bacterial morphotype were preserved and stored at − 70 °C. Bacterial DNA was extracted by a conventional boiling and freezing step. A 16S rRNA gene segment between the V1-V3 hypervariable regions was amplified by PCR using the primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 536R (5′-GTATTACCGCGGCTGCTG-3′) and Sanger sequenced for taxonomic identification.
Experimental design
At 24 h post-hatching, 36 L1 (larval stage 1) larvae (Paea strain) per group were individually placed in the wells of three 12-well cell culture plates. Each well received 4 ml of non-chlorinated water, 3 mg of TetraMin fish food, and 100 μl of Asaia or Elizabethkingia culture suspended in PBS (OD600 = 1). Controls received 100 μl of PBS. Larval development was monitored three times a day (8:00, 12:00, and 17:00) to record mortality and molt for each insect. Developmental time was monitored up to the day all immatures reached the adult stage or died. The wing length was measured, excluding the fringe, as a proxy for adult body size [44]. During experiments, specimens were maintained at 27 ± 2 °C and 70 ± 10% relative humidity. Larvae were not antibiotic-treated before bacteria exposure. To verify Asaia and Elizabethkingia colonization in the larval guts, six L4 midguts from each group were dissected, homogenized in PBS, and plated on LB plates and an Asaia-specific isolation medium [45]. Isolated bacterial strains were taxonomically identified using the 16S rRNA gene sequencing procedure previously mentioned.
Statistical analysis
The non-parametric Kaplan–Meier survival analysis was performed to assess whether exposure to Asaia or Elizabethkingia affected the duration of total immature development time (L1 to adult), larval instars (L1, L2, L3, and L4) and the pupal stage. The effect of bacteria exposure was estimated as Hazard Ratios (HR) via Cox Proportional-Hazard models [46] considering the total immature development time, duration of each larval instar and pupae stage, and also larval survival as dependent variables. Wing lengths were compared using the Kruskal–Wallis test. Statistical analyses were carried out using R v3.2.3.
Results
Aedes aegypti females transmit bacteria mechanically
Our results demonstrated that Ae. aegypti females transfer culturable viable bacteria to solid culture media (Fig. 2). To further dissect the possible sources of these bacteria, a cage swab, mosquito body washes, and environmental controls were performed for each medium tested.
A total of 28 isolates were recovered from LB plates (Supplementary Table 1, Additional file 3). Altogether, these isolates belonged to three phyla, six families, and seven genera. The bacterial diversity observed in LB plates exposed to interaction with a gravid female mosquito was notably higher compared to that seen in control plates (Fig. 2). Bacteria isolates recovered from cage swab plates were assigned to the genus Serratia. Bacterial isolates from the body washes of gravid female mosquitoes were classified into genera Serratia and Elizabethkingia. Bacillus was the most common genus of bacteria found after interaction with a gravid female, and together with Ornithinibacillus, Lysinibacillus, and Kroppenstedtia constituted the genera exclusively associated with this experimental condition. Besides, the genera Serratia and Paenibacillus were also reported from female-exposed LB plates. The LB environmental controls showed bacterial growth in one of the two plates examined. This isolate was assigned to the genus Paenibacillus.
Culturable bacteria isolated from blood agar plates were represented by 36 isolates (Supplementary Table 2, Additional file 3). Bacteria belonged to three phyla, seven families, and six genera. Similarly, as observed with the LB medium, bacterial isolates recovered from blood agar plates exposed to a gravid female mosquito showed higher diversity compared with those from environmental controls (Fig. 2). Isolates obtained from the cage swab were members of the genus Bacillus. Blood agar plates on which the body wash of gravid females was plated generated four bacterial isolates assigned to genera Elizabethkingia and Acinetobacter. As with LB plates, Bacillus was the most common genus reported in blood agar plates visited by mosquitoes. The genera Lysinibacillus and Staphylococcus were exclusively associated with female visited samples. Besides, Paenibacillus and Elizabethkingia were also isolated in this condition. Finally, bacterial isolates recovered from the blood agar environmental controls were assigned to the genera Bacillus and Paenibacillus.
It is important to stress that Serratia (LB) and Elizabethkingia (blood agar) were the only genera shared between plates exposed to interaction with a gravid female and those from mosquito body washes.
Changes in the bacterial profile of the breeding site
To verify whether females modify the bacterial profile of the water of a breeding site, an experiment comparing five treatments, differing in initial conditions, was designed (Fig. 1). Briefly, treatments 1 to 4 represented control conditions under which no female was allowed to oviposit, while T5 included a container in which a gravid female was allowed to oviposit on the water substrate for 24 h and then retired. T1 represented the same type of container, water, and sterilized food added 24 h later. T2 and T3 were initiated like T1 but had a controlled amount of mosquito eggs added manually together with the fish food. While for T2 the surface of eggs was sterilized to avoid the bacteria associated, eggs used for T3 were used in natura. Finally, for T4 a single female lacking access to water in the last 24 h was introduced to a similar experimental cage with a water container, but it was not gravid. In this case, we expected the female to visit the water (24 h) but show no oviposition. Water from each of the ten replicates per treatment was collected, their DNA was extracted and submitted for high throughput sequencing targeting the V4 region of the 16S rRNA gene that produced 20,425,105 reads from 50 water samples. After computational quality control, 16,896,903 reads were considered for taxonomic analysis. A data matrix was generated encompassing 532 ASVs. Nonetheless, the HTSFilter package identified a cutoff of 75 reads based on the Jaccard index. Therefore, all ASVs below this value were removed for downstream analysis. The total number of ASVs identified above the cut-off value was 159.
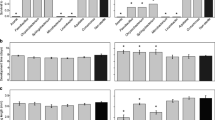
The alpha diversity was significantly different between experimental groups (Kruskal–Wallis, P = 0.01). Furthermore, the post hoc Dunn test identified that T5 had a significantly lower Simpson index compared with the other four treatments (Supplementary Table 1, Additional file 4). This is also depicted by the dominance of a particular bacterial taxon identified in the community composition (Additional file 5). Water samples belonging to T2 had the highest ASV diversity (mean Simpson index = 0.768), while T5 presented the lowest one (mean Simpson index = 0.527) (Fig. 3).
Alpha diversity of ASVs as a function of treatment. Comparison of Simpson’s Index of Diversity recorded for the different treatments using a boxplot (10 replicates and median). The alpha diversity was significantly different between experimental groups (Kruskal–Wallis, P = 0.01). Treatment 5 had a significantly lower Simpson index compared with the other four treatments. Treatment 1 (T1): environmental control (type I water plus sterilized food). Treatment 2 (T2): manually-deposited sterilized mosquito eggs. Treatment 3 (T3): manually-deposited non-sterilized eggs. Treatment 4 (T4): single sugar-fed females interacting with container water. Treatment 5 (T5): single gravid female (72 h post-blood-feeding) allowed to lay eggs
The Jensen-Shannon divergence metric was used to compare ASV diversity among treatments (Additional file 6). The PCoA captured around 48% of the variation in Jensen-Shannon distance along the two chosen axes (PCo1 and PCo2) represented in Fig. 4. A comparison of the bacterial communities associated with each treatment showed distinct clustering patterns. Samples belonging to T3 and T5 displayed higher inter-treatment variability clustering bottom and top right, respectively (Fig. 4).
Beta diversity analysis. Principal coordinates analysis of Jensen-Shannon distances. Distinct clustering patterns for each experimental treatment and their corresponding replicates are represented by a color code. Axis 1 (27.88%) and Axis 2 (19.9%) show the percentage of variation explained. Treatment 1 (T1): environmental control (type I water plus sterilized food). Treatment 2 (T2): manually-deposited sterilized mosquito eggs. Treatment 3 (T3): manually-deposited non-sterilized eggs. Treatment 4 (T4): single sugar-fed females interacting with container water. Treatment 5 (T5): single gravid female (72 h post-blood-feeding) allowed to lay eggs
The PERMANOVA revealed that the presence of eggs (R2 = 0.206, df = 2, P = 0.001), female interaction with water (R2 = 0.103, df = 1, P = 0.001), and the interaction of these two variables (R2 = 0.092, df = 1, P = 0.001) explain 40% of the variance in bacterial composition over the groups of samples. Additionally, the pairwise PERMANOVA confirmed that all groups differ significantly from each other in terms of beta diversity, suggesting that there are consistent bacterial signature profiles for each condition (Supplementary Table 2, Additional file 4).
The RF model selected 10 ASVs as the most important features for each variable explored — female interaction with water and egg presence (Tables 1 and 2). The bacterial signatures modeled by RF had robust prediction performances supported by their high AUC (Area Under de Curve) values (Supplementary Fig. 1, Additional file 4).
On the other hand, indicator species analysis identified ASVs considered to be specific microbial features associated with the act of oviposition and larval development, i.e., T5. Seven ASVs were pinpointed as oviposition-indicating species as they possess significant fidelity and predictive value towards the ecological conditions represented in this niche/treatment (Table 3). Indicator species were assigned to the following taxa: Leifsonia soli, Elizabethkingia anophelis, Paenibacillus polymyxa, Stenotrophomonas maltophilia, Elizabethkingia, Methylobacterium, and Elizabethkingia meningoseptica.
Aedes aegypti exhibits faster development in the presence of Elizabethkingia
Median total immature development (L1 to adult) took 177 h for the control, and 168 h for the Asaia and Elizabethkingia-exposed larvae (Fig. 5a and Supplementary Table 3, Additional file 4). The exposure to both bacteria significantly reduced the total immature development time when compared to the control group (Fig. 5b). For Asaia-exposed larvae, the HR for total development was 2.0 (95% confidence interval: 1.1–3.6, Fig. 5) with detectable differences only in the pupal stage (Additional file 7e). The effect of Elizabethkingia was more prominent with an HR of 2.7 (95% confidence interval: 1.4–4.9, Fig. 5) and detectable differences only in the L1 stage (Additional file 7a). Regarding survival, control, Asaia and Elizabethkingia exposed specimens presented 15, 8, and 23% of mortality, respectively. The effect of bacteria exposure on immature survival was not statistically significant (global p-value from log-rank = 0.20, non-significant HRs, Fig. 5d and e) nor wing length (KW chi-squared for males = 3.95, P = 0.14; KW chi-squared for females = 2.71, P = 0.25, Fig. 5c).
Duration of larval development (L1 to adult) of Aedes aegypti exposed to bacteria of the genera Asaia or Elizabethkingia and the control group. The dashed lines indicate median developmental time (a). Hazard ratios and 95% confidence intervals associated with Asaia or Elizabethkingia exposure were estimated using Cox Proportional-Hazard models with larval development as a dependent variable. Black squares represent the hazard ratios and the horizontal bars extend from the lower limit to the upper limit of the 95% confidence intervals of the hazard ratios (b)
The diversity of culturable microbiota was variable between the three groups, with only the Bacillus genus being ubiquitous to all conditions (Additional file 8). Asaia was not isolated from any of them, while Elizabethkingia was recovered from the midgut of larvae exposed to it.
Discussion
This study has explored the hypothesis that ovipositing females shape the microbial consortium of the aquatic niche of the breeding site to promote larval fitness. Our results showed that gravid females mechanically transmit viable and culturable bacteria already reported as mosquito symbionts. We then demonstrated that the act of oviposition promoted a significant decrease in the bacterial diversity found in breeding sites. Furthermore, this was associated with a specific bacterial profile which included a series of indicator taxa linked to female oviposition. We finally presented evidence that demonstrates that one of these taxa, i.e., Elizabethkingia, was able to accelerate larval development. Altogether, these results seem indicative of female-induced niche construction in Aedes aegypti breeding sites.
Experiments showing mechanical transmission from females to plates confirm that gravid mosquitoes can inoculate bacteria. Bacillus was predominant on plates visited by gravid females (for both blood agar and LB media). This bacterial taxon has been already identified in stable association with larvae [47] and adult Ae. aegypti [48]. Interestingly, other bacteria inoculated were frequently reported as key members of mosquito microbiota, e.g., Elizabethkingia and Serratia [40], both being vertically, horizontally, and transstadially transmitted [20, 22, 49]. It should be noted that moist agar plates eventually induced oviposition. Therefore, we suggest that while exploring a tentative oviposition site, gravid females inoculate the substrate with bacterial partners that according to our experiments support offspring development.
Next, we evaluated whether gravid females influence the bacterial communities of water-holding containers and showed that the act of oviposition significantly decreased their diversity. As the Simpson index is a dominance metric [50], we suggest that an uneven microcosm (dominated by the most abundant taxa) represents an advantageous scenario for larvae because not all microbes are beneficial. The latter could either be due to their pathogenicity or because they do not fulfill key functions in the host-microbe network [51]. An ecosystem with a low-diversity microbial community but high-fidelity microbial partners would favor the establishment of specific mutualistic interactions [52]. The above is congruent with recent observations made by Martinson and Strand [53], who highlighted the successful development of Ae. aegypti larvae in low diversity (gnotobiotic communities) breeding sites given certain dietary conditions were met. The decrease in community evenness seen in T5 can be considered a hallmark sign of susceptibility towards the establishment of an invading organism in the community, i.e. the larvae [54, 55].
Regarding beta diversity, the ordination analysis showed how the community structures diverge among treatments, particularly highlighting differences driven by factors other than oviposition. As each treatment represented potential sources of microbial inocula, it is relevant to highlight how the single unit of the natural egg-laying plus larval development (T5) predominantly presented a profile that diverged from the others in the ordination space. Curiously, non-sterile eggs (T3), representing the outcome of the general procedure for rearing mosquitoes in insectary conditions, produced a clearly different profile. It is also relevant to observe that surface-sterilized eggs manually deposited in the water (T2) presented a profile resembling that of the control and non-gravid female-water interactions. We propose that stereotypical female behaviors expressed while ovipositing (e.g., grooming, tasting, defecating) would be fundamental to generate the unique bacterial profile seen for treatment 5. This is reinforced by the fact that larval presence did not lead to similar profiles of beta diversity in other treatments.
Three out of seven ASVs pointed out by the indicator species analysis belonged to the Elizabethkingia genus which was shown to be capable of inhibiting Pseudomonas, another mosquito-associated bacterium, via an antimicrobial independent mechanism [56]. In addition, Elizabethkingia has broad antibiotic resistance because of a large number of genes encoding efflux pumps and β-lactamases present in its genome [57]. Bacteria use diverse mechanisms to compete with other members of the microbial community [51], and based on the above information, Elizabethkingia likely disturbed the bacterial consortium by eliminating competitors at the breeding site, as our experiment seems to suggest.
Finally, we evaluated whether Elizabethkingia and Asaia influenced larval developmental time, survival, and adult size in Ae. aegypti. This was intended to compare the impact of this oviposition-indicating taxon with that of Asaia, another bacterium reported in mosquito microbiota but not found in our breeding sites. Elizabethkingia significantly speeded up development and colonized the larval midgut suggesting a facilitated interaction between them. Indeed, reducing mosquito larval development time might increase the probability of reaching adulthood [58]. In this context, the presence of Elizabethkingia in breeding water and larval midguts has likely aided metabolic activities, providing nutrients or metabolites that stimulate faster larval development and/or represented an additional source of food [16, 49]. Taken together our results suggest that females can spike breeding sites with this symbiotic bacterium to support offspring fitness, which could be interpreted as a form of niche construction. However, it is important to notice that this effect on larval development was not exclusive to Elizabethkingia since Asaia also accelerated the larval development of Ae. aegypti, as previously observed for Anopheles gambiae [41, 42].
We revealed an ecological and functional connection between egg-laying activity, the bacterial communities in Ae. aegypti breeding sites, a key symbiotic bacterial taxon, and the speed of larval development by using a set of different experimental and analytical methodologies, including testing field-originated bacterial symbionts. Other microorganisms have individual or combined positive impacts on the larval development of Ae. aegypti [53, 59]. As such, we concur with the concept that these effects most likely will be understood from a community ecology perspective [40]. Other indicator taxa from our set, as well as other microorganisms and their interactions, could be the driving forces detected in the compositional profile of T5 and be key to the success of the breeding sites.
Conclusions
Niche construction theory recognizes that organisms can modify both biotic and abiotic components of their environments. This process is an outcome of their activities, metabolism, and choices, and its main consequence is to increase survival probabilities [26, 60]. Altogether, our findings suggest that niche construction may represent a strategy used by female Ae. aegypti to disseminate symbiotic bacteria through egg-laying to grant proper environments for their progeny. As stressed by Schwab and collaborators [29], our results are in agreement with niche construction theory criteria: a substantial environment modification was detected (bacterial community diversity), and positive fitness/developmental consequences were measured when a biomarker taxon was used as an effector. These findings provide solid grounds to build upon and improve our knowledge of how endo and ecto microbiomes may be critical when addressing their links to host phenotypes through the lens of niche construction theory. Other layers of information may be relevant to improve this take, as the metabolite profile of breeding sites also reflects the act of oviposition and development [61]. Besides, oviposition by several females responding to bacterially-emitted volatile organic compounds may contribute other symbionts to this community-shaping process ongoing in breeding sites [62]. Disentangling whether and how individual microorganisms, or their networks, exert effects on mosquito life-history traits is a growing field of study benefiting from the synergy of microbiology, ecology, physiology, and computational biology.
Availability of data and materials
Raw sequence data are available at the European Nucleotide Archive, project number: PRJEB51063 [63].
Abbreviations
- LB:
-
Lysogeny Broth
- PBS:
-
Phosphate-buffered saline (PBS)
- 16S rRNA:
-
16S ribosomal RNA
- PCR:
-
Polymerase chain reaction
- T1:
-
Treatment 1
- T2:
-
Treatment 2
- T3:
-
Treatment 3
- T4:
-
Treatment 4
- T5:
-
Treatment 5
- ASV:
-
Amplicon sequence variant
- PCoA:
-
Principal coordinates analysis
- PERMANOVA:
-
Permutational Multivariate Analysis of Variance
- RF:
-
Random Forest
- L1:
-
Larval stage 1
- L2:
-
Larval stage 2
- L3:
-
Larval stage 3
- L4:
-
Larval stage 4
- HR:
-
Hazard ratio
- AUC:
-
Area under the curve
- ROC:
-
Receiver operating characteristic curve
- AIC:
-
Akaike information criterion
- SD:
-
Standard deviation
References
WHO. Global vector control response 2017–2030. 2017. https://apps.who.int/iris/handle/10665/259205.
Kamal M, Kenawy MA, Rady MH, Khaled AS, Samy AM. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLoS ONE. 2018;13:e0210122.
Wilke ABB, Chase C, Vasquez C, Carvajal A, Medina J, Petrie WD, et al. Urbanization creates diverse aquatic habitats for immature mosquitoes in urban areas. Sci Rep. 2019;9:15335.
Brady OJ, Hay SI. The global expansion of dengue: how Aedes aegypti mosquitoes enabled the first pandemic arbovirus. Annu Rev Entomol. 2020;65:191–208.
Zahouli JBZ, Koudou BG, Müller P, Malone D, Tano Y, Utzinger J. Urbanization is a main driver for the larval ecology of Aedes mosquitoes in arbovirus-endemic settings in south-eastern Côte d’Ivoire. PLoS Negl Trop Dis. 2017;11:e0005751.
Wong J, Stoddard ST, Astete H, Morrison AC, Scott TW. Oviposition site selection by the dengue vector Aedes aegypti and its implications for dengue control. PLoS Negl Trop Dis. 2011;5:e1015.
Onchuru TO, Ajamma YU, Burugu M, Kaltenpoth M, Masiga D, Villinger J. Chemical parameters and bacterial communities associated with larval habitats of Anopheles, Culex and Aedes mosquitoes (Diptera: Culicidae) in western Kenya. Int J Trop Insect Sci. 2016;36:146–60.
Kroth N, Cozzer GD, de Carvalho G, Cassol AS, Breaux J, Lutinski JA, et al. Oviposition preferences of the mosquito Aedes aegypti Linnaeus, 1762 (Culicidae): an urban environment bioassay. Bull Entomol Res. 2019;109:762–70.
Hery L, Guidez A, Durand A-A, Delannay C, Normandeau-Guimond J, Reynaud Y, et al. Natural variation in physicochemical profiles and bacterial communities associated with Aedes aegypti Breeding Sites and Larvae on Guadeloupe and French Guiana. Microb Ecol. 2021;81:93–109.
Benzon GL, Apperson CS. Reexamination of Chemically Mediated Oviposition Behavior in Aedes aegypti (L.) (Diptera: Culicidae)1. J Med Entomol. 1988;25:158–64.
Ponnusamy L, Xu N, Nojima S, Wesson DM, Coby S, Apperson CS. Identification of bacteria and bacteria-associated chemical cues that mediate oviposition site preferences by Aedes aegypti. Proc Natl Acad Sci. 2008;105:9262–7.
Melo N, Wolff GH, Costa-da-Silva AL, Arribas R, Triana MF, Gugger M, et al. Geosmin Attracts Aedes aegypti Mosquitoes to Oviposition Sites. Curr Biol. 2020;30:127-134.e5.
Mwingira V, Mboera LEG, Dicke M, Takken W. Exploiting the chemical ecology of mosquito oviposition behavior in mosquito surveillance and control: a review. J Vector Ecol. 2020;45:155–79.
Guégan M, Zouache K, Démichel C, Minard G, Van Tran V, Potier P, et al. The mosquito holobiont: fresh insight into mosquito-microbiota interactions. Microbiome. 2018;6:49.
Saab SA, Dohna HZ, Nilsson LKJ, Onorati P, Nakhleh J, Terenius O, et al. The environment and species affect gut bacteria composition in laboratory co-cultured Anopheles gambiae and Aedes albopictus mosquitoes. Sci Rep. 2020;10:3352.
Coon KL, Vogel KJ, Brown MR, Strand MR. Mosquitoes rely on their gut microbiota for development. Mol Ecol. 2014;23:2727–39.
Coon KL, Brown MR, Strand MR. Mosquitoes host communities of bacteria that are essential for development but vary greatly between local habitats. Mol Ecol. 2016;25:5806–26.
Dada N, Jumas-Bilak E, Manguin S, Seidu R, Stenström T-A, Overgaard HJ. Comparative assessment of the bacterial communities associated with Aedes aegypti larvae and water from domestic water storage containers. Parasit Vectors. 2014;7:391.
Dickson LB, Jiolle D, Minard G, Moltini-Conclois I, Volant S, Ghozlane A, et al. Carryover effects of larval exposure to different environmental bacteria drive adult trait variation in a mosquito vector. Sci Adv. 2017;3:e1700585.
Lindh JM, Borg-Karlson A-K, Faye I. Transstadial and horizontal transfer of bacteria within a colony of Anopheles gambiae (Diptera: Culicidae) and oviposition response to bacteria-containing water. Acta Trop. 2008;107:242–50.
Scolari F, Casiraghi M, Bonizzoni M. Aedes spp. and their microbiota: a review. Front Microbiol. 2019;10:2036.
Rocha EM, Marinotti O, Serrão DM, Correa LV, Katak R de M, de Oliveira JC, et al. Culturable bacteria associated with Anopheles darlingi and their paratransgenesis potential. Malar J. 2021;20:40.
Wong ACN, Luo Y, Jing X, Franzenburg S, Bost A, Douglas AE. The Host as the Driver of the Microbiota in the Gut and External Environment of Drosophila melanogaster. Appl Environ Microbiol. 2015;81:6232–40.
Favia G, Ricci I, Damiani C, Raddadi N, Crotti E, Marzorati M, et al. Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc Natl Acad Sci. 2007;104:9047–51.
Sullam KE, Essinger SD, Lozupone CA, O’Connor MP, Rosen GL, Knight R, et al. Environmental and ecological factors that shape the gut bacterial communities of fish: a meta-analysis: fish gut bacterial communities. Mol Ecol. 2012;21:3363–78.
Odling-Smee J, Erwin DH, Palkovacs EP, Feldman MW, Laland KN. Niche construction theory: a practical guide for ecologists. Q Rev Biol. 2013;88:3–28.
Laland KN, Uller T, Feldman MW, Sterelny K, Müller GB, Moczek A, et al. The extended evolutionary synthesis: its structure, assumptions and predictions. Proc Biol Sci. 2015;282:20151019.
Engel P, Moran NA. The gut microbiota of insects – diversity in structure and function. FEMS Microbiol Rev. 2013;37:699–735.
Schwab DB, Casasa S, Moczek AP. Evidence of developmental niche construction in dung beetles: effects on growth, scaling and reproductive success. Ecol Lett. 2017;20:1353–63.
PennVet Center for Host-Microbial Interactions. Identification of unknown bacterial isolates using Sanger sequencing of the 16S rRNA gene. 2019. https://chmi-sops.github.io/mydoc_16S_Sanger.html.
Romoli O, Schönbeck JC, Hapfelmeier S, Gendrin M. Production of germ-free mosquitoes via transient colonisation allows stage-specific investigation of host–microbiota interactions. Nat Commun. 2021;12:942.
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–3.
Valente Pires DE, Oliveira FS, Correa FB, Morais DK, Fernandes GR. TAG.ME: Taxonomic Assignment of Genetic Markers for Ecology. BioRxiv. 2018. 263293. https://doi.org/10.1101/263293.
Rau A, Gallopin M, Celeux G, Jaffrézic F. Data-based filtering for replicated high-throughput transcriptome sequencing experiments. Bioinformatics. 2013;29:2146–52.
Anderson MJ. Permutational multivariate analysis of variance (PERMANOVA). Wiley StatsRef: Statistics reference online. 2017. https://doi.org/10.1002/9781118445112.
Martinez Arbizu P. pairwiseAdonis: Pairwise multilevel comparison using adonis. R package version 0.4. 2020.
Love MI, Anders S, Huber W. Analyzing RNA-seq data with DESeq2. Bioconductor. 2017;2:1–63.
Kuhn M, Wing J, Weston S, Williams A, Keefer C, Engelhardt A, et al. Package ‘caret.’ R Journal. 2020;223:7.
Cáceres MD, Legendre P. Associations between species and groups of sites: indices and statistical inference. Ecology. 2009;90:3566–74.
Steven B, Hyde J, LaReau JC, Brackney DE. The axenic and gnotobiotic mosquito: emerging models for microbiome host interactions. Front Microbiol. 2021;12:714222.
Chouaia B, Rossi P, Epis S, Mosca M, Ricci I, Damiani C, et al. Delayed larval development in Anopheles mosquitoes deprived of Asaia bacterial symbionts. BMC Microbiol. 2012;12(Suppl 1):S2.
Mitraka E, Stathopoulos S, Siden-Kiamos I, Christophides GK, Louis C. Asaia accelerates larval development of Anopheles gambiae. Pathog Global Health. 2013;107:305–11.
Vazeille-Falcoz M, Mousson L, Rodhain F, Chungue E, Failloux AB. Variation in oral susceptibility to dengue type 2 virus of populations of Aedes aegypti from the islands of Tahiti and Moorea, French Polynesia. Am J Trop Med Hyg. 1999;60:292–9.
Harbach RE, Knight KL. Taxonomists’ glossary of mosquito anatomy. Marlton: Plexus Publishing, Inc.; 1980.
Yamada Y, Katsura K, Kawasaki H, Widyastuti Y, Saono S, Seki T, et al. Asaia bogorensis gen nov., sp. nov., an unusual acetic acid bacterium in the. Int J Syst Evol Microbiol. 2000;50 Pt 2:823–9.
Cox DR, Oakes D. Analysis of survival data. 1st ed. New York: Chapman and Hall/CRC; 1984.
Luxananil P, Atomi H, Panyim S, Imanaka T. Isolation of bacterial strains colonizable in mosquito larval guts as novel host cells for mosquito control. J Biosci Bioeng. 2001;92:342–5.
Gusmão DS, Santos AV, Marini DC, Russo É de S, Peixoto AMD, Bacci JM, et al. First isolation of microorganisms from the gut diverticulum of Aedes aegypti (Diptera: Culicidae): new perspectives for an insect-bacteria association. Mem Inst Oswaldo Cruz. 2007;102:919–24.
Chen S, Bagdasarian M, Walker ED. Elizabethkingia anophelis: molecular manipulation and interactions with mosquito hosts. Appl Environ Microbiol. 2015;81:2233–43.
Kim B-R, Shin J, Guevarra RB, Lee JH, Kim DW, Seol K-H, et al. Deciphering diversity indices for a better understanding of microbial communities. J Microbiol Biotechnol. 2017;27:2089–93.
Foster KR, Schluter J, Coyte KZ, Rakoff-Nahoum S. The evolution of the host microbiome as an ecosystem on a leash. Nature. 2017;548:43–51.
Reese AT, Dunn RR. Drivers of microbiome biodiversity: a review of general rules, feces, and ignorance. mBio. 2018;9:294–18.
Martinson VG, Strand MR. Diet-microbiota interactions alter mosquito development. Front Microbiol. 2021;12:650743.
Wittebolle L, Marzorati M, Clement L, Balloi A, Daffonchio D, Heylen K, et al. Initial community evenness favours functionality under selective stress. Nature. 2009;458:623–6.
Daly AJ, Baetens JM, De Baets B. Ecological diversity: measuring the unmeasurable. Mathematics. 2018;6:119.
Ganley JG, D’Ambrosio HK, Shieh M, Derbyshire ER. Coculturing of mosquito-microbiome bacteria promotes heme degradation in Elizabethkingia anophelis. ChemBioChem. 2020;21:1279–84.
Kukutla P, Lindberg BG, Pei D, Rayl M, Yu W, Steritz M, et al. Insights from the Genome Annotation of Elizabethkingia anophelis from the Malaria Vector Anopheles gambiae. PLoS ONE. 2014;9:e97715.
Díaz-Nieto LM, D’Alessio C, Perotti MA, Berón CM. Culex pipiens development is greatly influenced by native bacteria and exogenous yeast. PLoS ONE. 2016;11:e0153133.
Valzania L, Martinson VG, Harrison RE, Boyd BM, Coon KL, Brown MR, et al. Both living bacteria and eukaryotes in the mosquito gut promote growth of larvae. PLoS Negl Trop Dis. 2018;12:e0006638.
Laland KN, O’Brien MJ. Cultural niche construction: an introduction. Biol Theory. 2011;6:191–202.
Mosquera KD, Martinez Villegas LE, Pidot SJ, Sharif C, Klimpel S, Stinear TP, et al. Multi-omic analysis of symbiotic bacteria associated With Aedes aegypti breeding sites. Front Microbiol. 2021;12:2331.
Mosquera KD, Khan Z, Wondwosen B, Alsanius B, Hill SR, Ignell R, et al. Odor-mediated response of gravid Aedes aegypti to mosquito-associated symbiotic bacteria. Acta Trop. 2023;237:106730.
Mosquera KD, Martinez Villegas LE, David MR, Fernandes GR, Maciel-de-Freitas R, Moreira LA, et al. Breeding site microbiota is shaped through oviposition by female Aedes aegypti. European Nucleotide Archive. 2022. https://www.ebi.ac.uk/ena/browser/view/PRJEB51063.
Acknowledgements
We would like to thank the René Rachou Institute Sequencing Platform for kindly allowing us to submit our samples for Sanger sequencing. We would also like to thank Assmaa El Khal, Bianca Daoud, and Priscila Pinho da Silva for their technical assistance in molecular biology procedures, mosquito rearing, and larval development experiments, respectively.
Funding
This work was funded by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais FAPEMIG – EDITAL 00/2016 – CONFAP –MRC/TEC – APQ-00913–16, CNPq (Project number: 311826/2019–9), INCTEM (Project number: 465678/2014–9), and FIOCRUZ.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. KDM wrote the initial draft. KDM, LEMV, MRD, RMF, and MGL critically reviewed the manuscript. KDM, LEMV, and MRD performed the experiments. KDM, LEMV, GRF, and MRD conducted data analysis. RMF, LAM, and MGL acquired funding. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Number of eggs laid by each Aedes aegypti female in treatment 5.
Additional file 2: Supplementary Figure 1.
Negative controls performed during sample processing did not generate any amplicons during the quality control carried out by the sequencing facility.
Additional file 3: Supplementary Table 1.
Bacterial taxonomic affiliation of isolates recovered from LB agar plates. Supplementary Table 2. Bacterial taxonomic affiliation of isolates recovered from blood agar plates.
Additional file 4: Supplementary Table 1.
Dunn test comparing alpha diversity between treatments. P values adjusted with the Benjamini-Hochberg method are shown. Supplementary Table 2. Pairwise PERMANOVA showing between-group shifts in bacterial signature profile when comparing the beta diversity among treatments. P values for each comparison are shown. Supplementary Figure 1. ROC curves for female interaction and eggs presence prediction. The point indicates the best cutoff value from the prediction probability to optimize Sensitivity and Specificity. The confidence levels reflect a 95% confidence interval. Supplementary Table 3. Median, mean and standard deviation of instar duration of immature stages of Aedes aegypti, wing size, and survival exposed to Asaia and Elizabethkingia bacteria.
Additional file 5: Supplementary Figure 1.
Bacterial community composition at order level for each water sample belonging to five different treatments.
Additional file 6: Supplementary Figure 1.
Heatmap of the Jensen-Shannon distances between the five treatments.
Additional file 7: Supplementary Figure 1.
Duration of all larval instars and pupal phase of Aedes aegypti exposed to bacteria of the genera Asaia or Elizabethkingia and the control group. The dashed lines in the graphs indicate median developmental time. Hazard ratios and 95% confidence intervals associated with Asaia or Elizabethkingia exposure were estimated using Cox Proportional-Hazard models with developmental time as the dependent variable. Black squares represent the hazard ratios and the horizontal bars extend from the lower limit to the upper limit of the 95% confidence intervals of the hazard ratios.
Additional file 8: Supplementary Table 1.
Bacteria genera isolated from Aedes aegypti exposed to bacteria of the genera Asaia or Elizabethkingia and the control group.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mosquera, K.D., Martínez Villegas, L.E., Rocha Fernandes, G. et al. Egg-laying by female Aedes aegypti shapes the bacterial communities of breeding sites. BMC Biol 21, 97 (2023). https://doi.org/10.1186/s12915-023-01605-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12915-023-01605-2