Abstract
Background
Blowflies are ubiquitous insects, often shiny and metallic, and the larvae of many species provide important ecosystem services (e.g., recycling carrion) and are used in forensics and debridement therapy. Yet, the taxon has repeatedly been recovered to be para- or polyphyletic, and the lack of a well-corroborated phylogeny has prevented a robust classification.
Results
We here resolve the relationships between the different blowfly subclades by including all recognized subfamilies in a phylogenomic analysis using 2221 single-copy nuclear protein-coding genes of Diptera. Maximum likelihood (ML), maximum parsimony (MP), and coalescent-based phylogeny reconstructions all support the same relationships for the full data set. Based on this backbone phylogeny, blowflies are redefined as the most inclusive monophylum within the superfamily Oestroidea not containing Mesembrinellidae, Mystacinobiidae, Oestridae, Polleniidae, Sarcophagidae, Tachinidae, and Ulurumyiidae. The constituent subfamilies are re-classified as Ameniinae (including the Helicoboscinae, syn. nov.), Bengaliinae, Calliphorinae (including Aphyssurinae, syn. nov., Melanomyinae, syn. nov., and Toxotarsinae, syn. nov.), Chrysomyinae, Luciliinae, Phumosiinae, Rhiniinae stat. rev., and Rhinophorinae stat. rev. Metallic coloration in the adult is shown to be widespread but does not emerge as the most likely ground plan feature.
Conclusions
Our study provides the first phylogeny of oestroid calyptrates including all blowfly subfamilies. This allows settling a long-lasting controversy in Diptera by redefining blowflies as a well-supported monophylum, and blowfly classification is adjusted accordingly. The archetypical blowfly trait of carrion-feeding maggots most likely evolved twice, and the metallic color may not belong to the blowfly ground plan.
Similar content being viewed by others
“these summerflies have blown me full of maggot ostentation.”
William Shakespeare [1] Love’s Labour’s Lost
Background
Blowflies (Diptera: Calyptratae, Calliphoridae) are among the most familiar insects to humans [2,3,4]. They are abundant on all continents except Antarctica, and the anthropophilic species are well known for their association with carrion and decaying food (Fig. 1) [6,7,8]. Many species have distinctive metallic coloration, and the family name Calliphoridae means “beauty bearer” in Greek [6], alluding to the beautiful shiny blue, green, or copper iridescence of the adult flies. Blowflies are also the first insects recognized in writing, as some cuneiform clay tablets mention these flies more than 3600 years ago [6]. Some species, e.g., Cochliomyia hominivorax, are infamous for causing significant economic losses to livestock because their maggots invade healthy tissue [9]. The larvae of many species of blowflies catalyze putrefaction and decay [10] and can be used in forensics to determine the time of death of corpses [10]. What is less broadly known, and in striking contrast to their cultural prominence, is that there is no consensus resolution as to which oestroid clade should be termed Calliphoridae, because the group has been repeatedly shown to be either para- or polyphyletic based on both molecular and morphological evidence (Fig. 2) [12, 13, 19, 21].
Representative taxa of calliphorids, Mesembrinellidae and Polleniidae. A, B Calliphorinae. A Calliphora sp. B Calliphora sp., larvae feeding on dead bird. C, D Chrysomyinae. C Chrysomya sp. D Chrysomya albiceps, larvae feeding on dead hedgehog. E, F Luciliinae. E Lucilia sp. F Lucilia sp., larvae feeding on dead bird. G Ameniinae (Amenia sp.). H Bengaliinae (Bengalia sp.). I Helicoboscinae (Eurychaeta palpalis). J Melanomyinae (Melinda viridicyanea). K, L Phumosiinae. K Caiusa sp. L – Caiusa sp., egg on foam mass of the shrub frog Chiromantis nongkhorensis [5] (reproduced with permission from copyright holder). M Polleniidae (Pollenia sp.). N Mesembrinellidae (Mesembrinella sp.). O Rhiniidae (Stomorhina lunata). P Rhinophoridae (Rhinophora lepida). A, B, G, H, I, J, O, and P are from Flickr; C, D, E, F, and M are from Diptera.info; K is from antroom
Phylogeny of Oestroidea in previous studies. A McAlpine [11] (morphology). B Rognes [12] (morphology). C Kutty et al. [13] (combination of mitochondrial and nuclear genes). D Marinho et al. [14] (combination of mitochondrial and nuclear genes). E Singh & Wells [15] (combination of mitochondrial and nuclear genes). F Zhang et al. [16] (mitogenomic data). G Cerretti et al. [17] (combination of mitochondrial and nuclear genes). H Marinho et al. [18] (combination of mitochondrial and nuclear genes). I Kutty et al. [19] (phylotranscriptomic data). J Buenaventura et al. [20] (ultra-conserved elements)
The family-group name Calliphoridae was erected for a large assemblage of calyptrate flies [22], but Girschner [23] was the first to narrow its definition when he restricted it to carrion-breeding oestroids, thus rendering the family a taxon of convenience for those oestroid flies that do not belong to, for example, the more easily-diagnosable flesh flies (Sarcophagidae), bot flies (Oestridae), or tachinid flies (Tachinidae). Indeed, McAlpine [11] and Pape [24] were the last authors to claim some evidence for calliphorid monophyly based on putative synapomorphies, but since then, both morphological and molecular evidence has pointed to the non-monophyly of calliphorids [12,13,14,15, 17]. This evidence was used to improve the definitions and circumscriptions of the constituent subfamilies [12, 25,26,27], but defining a monophyletic Calliphoridae had to be postponed until an analysis could be carried out that included sufficient data for representatives of all subfamilies and employed sophisticated analyses to resolve all critical relationships with confidence. Until now, calliphorids have been left as the last major assemblage in the Calyptratae [11,12,13, 19, 24] that is defined on “what it is not” [8, 15].
The taxonomic composition of blowflies has been controversial for decades, which is reflected in a large number of recognized subfamilies, with more than a dozen in widespread use: Ameniinae, Aphyssurinae, Auchmeromyiinae, Bengaliinae, Calliphorinae, Chrysomyinae, Helicoboscinae, Luciliinae, Melanomyinae, Mesembrinellinae, Polleniinae, Phumosiinae, Prosthetosomatinae, Rhiniinae, Rhinophorinae, and Toxotarsinae [7, 12, 26, 28,29,30]. Some of these were later raised to family rank: Mesembrinellidae, Polleniidae, Rhiniidae, and Rhinophoridae [13,14,15, 31,32,33,34], while others have been relegated into synonymy: Auchmeromyiinae (under Bengaliinae) and Prosthetosomatinae (under Rhiniidae) [5, 13, 14].
With the availability of phylogenomic data (e.g., [19, 35]) and advances in data analysis [36], it is now possible to address phylogenetic questions based on a phylogenetic signal from thousands of genes. Here, we perform a phylogenomic analysis of blowflies based on comprehensive taxon sampling of all recognized subfamilies, reconstruct the phylogenetic backbone of calliphorid subfamilies, to eventually propose the first rigorous definition of blowflies. Furthermore, we examine the robustness of our conclusions through the use of multiple ortholog reference sets and taxon subsampling.
Results
Phylogenomic data generation
Novel phylogenomic data are provided for nine species of blowflies, representing nine of 10 subfamilies (Additional file 1: Table S1). Details of assemblies and number of recovered orthologous genes for each species are presented in Table S1 (Additional file 1).
Phylogeny reconstruction
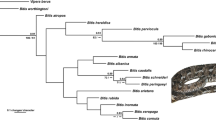
Phylogenetic reconstructions using different matrices (Table 1) yielded similar topologies with only minor differences in the placement of Chrysomyinae and Mesembrinellidae (Figs 3 and 4; Additional file 2: Fig. S1). Calliphorids were recovered as monophyletic with the inclusion of the families Rhinophoridae and Rhiniidae. Calliphoridae are thereby redefined as the most inclusive group within the superfamily Oestroidea not containing Mesembrinellidae, Mystacinobiidae, Oestridae, Polleniidae, Sarcophagidae, Tachinidae, and Ulurumyiidae, and the family is here divided into three major clades (Fig. 3, clade a, b, and c). All traditionally recognized subfamilies were monophyletic except for the paraphyletic calliphorine grade, within which Aphyssurinae and Melanomyinae are nested (Figs. 3 and 4). The Neotropical Toxotarsinae are sister to the (Aphyssurinae-Calliphorinae-Melanomyinae), and this clade is sister to the monophyletic Luciliinae (Figs. 3 and 4). The clade ((Helicoboscinae, Ameniinae), Rhinophoridae) (clade c) emerged in all analyses with strong support, with Helicoboscinae (represented by Eurychaeta muscaria) invariably being the sister group to Ameniinae (Figs. 3 and 4). Chrysomyinae emerged as a sister group to Phumosiinae in a basal clade of calliphorids (clade a) in the reconstructions using datasets Dref_Ltax and Aref_Ltax (different reference taxa) with high support (Figs. 3 and 4), while the Chrysomyinae are placed as sister group to Luciliinae and Calliphorinae in reconstructions based on amino acid and second codon matrices of dataset Aref_Stax or to Bengaliinae and Rhiniidae in reconstructions based on dataset Dref_Stax (Fig. 4).
Maximum likelihood (ML) tree inferred from the amino acid matrix of dataset Dref_Ltax, with support values of ML bootstrap (MLBS), maximum parsimony jackknife (MPJK), and ASTRAL bootstrap (Astral BS) presented at nodes. The flies on the branches indicate origins of adult metallic color within calliphorids. The asterisk (*) and hyphen (-) at nodes indicate full support and branch not recovered, respectively. Species marked with asterisk (*) are sequenced with genomic data. Ingroup branches are colored according to family classification, as explained in the legend
Phylogenetic topology compared between reconstructions based on datasets with larger (left) and smaller (right) taxon sampling. Numbers above nodes of the left cladogram are maximum likelihood (ML) bootstrap values of phylogeny inferred from the dataset Dref_Ltax of amino acid (AA), 2nd-codon positions (NT2), 1st & 2nd-codon position (NT12), and maximum parsimony jackknife value of phylogeny inferred from the dataset of amino acid (AA). Numbers below nodes of the left cladogram are ML bootstrap values of phylogeny inferred from dataset of AA, NT2, NT12, and MP jackknife value of phylogeny inferred from AA of dataset Aref_Ltax. Numbers above nodes of the right cladogram are ML bootstrap values of phylogeny inferred from dataset of AA, NT2, NT12, and MP jackknife value of phylogeny inferred from AA of dataset Dref_Stax. Numbers below nodes of the right cladogram are ML bootstrap values of phylogeny inferred from dataset of AA, NT2, NT12, and MP jackknife value of phylogeny inferred from AA of dataset Aref_Stax. The reddish boxes indicate nodes which conflict between analyses
Polleniidae were consistently sister group to Tachinidae with full support (Figs. 3 and 4). The sister-group relationship between Mesembrinellidae and Ulurumyiidae was recovered in most analyses (but see Fig. 4). Occasionally, Mesembrinellidae were placed as a sister group to the non-Ulurumyiidae oestroids, but with low support (MP jackknife [MPJK] of Dref_Ltax: 98; MPJK of Dref_Stax: 100; MPJK of Aref_Ltax: 14; ML bootstrap [MLBS] of Dref_Ltax: 53; MLBS of Dref_Stax: 88).
Coalescent-based reconstruction
The coalescent approach yielded a subfamily-level topology similar to the concatenated ML tree based on the AA matrix of dataset Dref_Ltax (node support values in Fig. 3), differing in the relationship between Mystacinobiidae and Oestridae, which were recovered as sister groups in the coalescent phylogeny. Furthermore, Cochliomyia hominivorax was recovered as a sister group to Chrysomya in the concatenation-based phylogeny, while it is sister to all remaining chrysomyines in the coalescent-based phylogeny.
Phylogenetic position of Chrysomyinae
Within the calliphorids, only the placement of Chrysomyinae differed between reconstructions and data sets. In most analyses, the subfamily clusters with Phumosiinae and this combined clade is sister to the remaining Calliphoridae (T1 in Fig. 5A). This hypothesis is also favored in the remaining analyses although Chrysomyinae are sometimes placed as sister group to either Calliphorinae and Luciliinae (reconstructions based on amino acid and second codon matrices of dataset Aref_Stax) or Bengaliinae and Rhiniidae (all reconstructions based on matrices of dataset Dref_Stax). To test the fitness of data among phylogenies with different placements of Chrysomyinae, two additional ML trees were inferred using the amino acid matrix of the dataset with the most genes (i.e., Dref_Ltax, see Table 1), and constraining as monophyletic either Aphyssurinae-Calliphorinae-Melanomyinae-Toxotarsinae, Chrysomyinae, and Luciliinae) (T2 in Fig. 5A) or Chrysomyinae, Bengaliinae, and Rhiniidae (T3 in Fig. 5A). The likelihood score of each locus to the three hypotheses, T1, T2, and T3, were estimated (Additional file 3), and the score differences for each locus were recorded between T1 and T2 and between T1 and T3 (Fig. 5; Additional file 3). The results indicate that the hypothesis supported in most analyses (T1: see above) was favored by 1536 out of 2221 genes while T2 was only favored by 683 genes (Fig. 5B). T1 is also favored in a comparison with T3 (by 1489 instead of 727 genes, Fig. 5C). Furthermore, the AU test strongly supported T1 (Fig. 5A). The same hypothesis is supported by additional Kishino-Hasegawa, Shimodaira-Hasegawa, and AU tests for the AA matrix of dataset Dref_Ltax (p-KH = 1, p-SH = 1, p-AU = 1), although the AU test gave weak support for topology T2 (p-AU = 1.14e−08) and T3 (p-AU = 5.89e−41) (Table 2).
Results of partition log-likelihood analyses in terms of phylogenetic position of Chrysomyinae using amino acid alignments of dataset Dref_Ltax. A The three topologies. B, C Ranked distribution of ΔpLi of 2221 genes under the model estimated by IQ-TREE. B Genes favoring T1 (positive values) or T2 (negative values). C Genes favoring T1 (positive values) or T3 (negative values)
The modified FcLM revealed two different topologies depending on whether group 2b (Fig. 6A) or group 2a are pruned. Without group 2b (Fig. 6B), the support for Chrysomyinae and Phumosiinae (group 1) being sister group to Luciliinae, Aphyssurinae-Calliphorinae-Melanomyinae-Toxotarsinae (group 3) was 29.9%; i.e., lower than the support for placing Chrysomyinae and Phumosiinae (group 1) as the sister group to the remaining calliphorids (56.4%; Fig. 6B). When group 2a was excluded, the support for Chrysomyinae and Phumosiinae (group 1) as the sister group to the remaining calliphorids was 71.0% and thus far higher than the two competing hypotheses (Fig. 6C).
Four-cluster likelihood mapping (FcLM) of the phylogenetic position of Chrysomyinae using amino acid alignments of dataset Dref_Ltax. A Group definitions. B, C Two variations of FcLM based on concatenated amino acid alignments shown as 2D graphs, with phylogeny modified by excluding group 2b (B) or by excluding group 2a (C)
Ancestral state reconstruction of adult metallic color
Metallic color has been considered as a ground plan character of blowflies [12]; however, the cuticle of the ancestral Calliphoridae was estimated to be non-metallic (probability = 65.81%; Additional file 2: Fig. S2; Additional file 4). Metallic color may have originated three times independently in the early evolution of Calliphoridae (Fig. 3): in the clade Chrysomyinae + Phumosiinae (clade a; probability = 96.37%) (Additional file 2: Fig. S2; Additional file 4), in the clade b ((Rhiniidae, Bengaliinae), (Luciliinae, Aphyssurinae-Calliphorinae-Melanomyinae-Toxotarsinae)) (probability = 47.43%), and in the Ameniinae (probability = 94.83%).
Discussion
A rigorous definition of a monophyletic concept of the blowflies has been elusive because of incomplete taxon sampling at the subfamily level in previous studies [13,14,15, 19, 20]. Our study presents the first phylogenetic analyses including representatives of all blowfly subfamilies, with newly documented transcriptome and genomic data for nine species (Additional file 1: Table S1). This allows us to propose a monophyletic Calliphoridae and provide insight into several open questions relating to calyptrate phylogeny [12, 13, 19]. We here discuss the results based on the tree obtained based on analyses of amino acid alignments of the largest set of genes and taxa (Dref_Ltax: Fig. 3).
Monophyletic Calliphoridae and blowfly classification
Based on this robustly supported phylogeny, we propose a broadly defined monophyletic Calliphoridae that excludes only the former calliphorid subfamilies Mesembrinellinae and Polleniinae, which is in line with recent proposals to treat these taxa as families [14, 27, 39]. The present broad definition of Calliphoridae is only one among multiple options for resolving calliphorid monophyly. Alternatively, a number of calliphorid subfamilies could be raised to family rank, as previously proposed for the Rhiniidae [13]. Arguments for bestowing a given rank to a particular clade can be drawn from various measures of morphological, biological, or phylogenetic distinctiveness, and tradition often weighs in. As discussed by Kallal et al. [40], the ranking may follow unspecified conventions of a research community, and reasoning for any given rank can be idiosyncratic and subjective. However, classifications matter as a framework for communication, and a reclassification of the calliphorids should be carefully crafted. Proposing a resolution to the paraphyly of Calliphoridae sensu stricto will promote stability in the long term. We favor a broad Calliphoridae, because it is close to the traditional calliphorids given that only the Mesembrinellidae and Polleniidae are excluded, and the Rhiniidae and Rhinophoridae are reclassified as subfamilies of Calliphoridae. Incidentally, the family group name Rhinophoridae dates from Robineau-Desviody [41], while the family group name Calliphoridae is younger [42], which means that an application should be submitted to the International Commission of Zoological Nomenclature for reversal of precedence.
The subfamily-level re-classification is proposed based on the backbone phylogeny of Calliphoridae (Table 3). We propose the Calliphorinae to include the former Aphyssurinae syn. nov., Melanomyinae syn. nov., and Toxotarsinae syn. nov. The clade consisting of Aphyssurinae, Melanomyinae, and Toxotarsinae is well supported and emerges in all our analyses (Figs. 3 and 4). Prior to our analysis, the placement of Aphyssurinae was unknown [28], but relationships between members of the other subfamilies in this clade have previously been analyzed [13, 15, 17] using a combination of mitochondrial and nuclear genes, but with a small taxon sample. The Calliphorinae and Melanomyinae emerged together in a recent phylogenomic study using protein-encoding ultraconserved elements (UCEs), although neither were monophyletic [20]. The position of Toxotarsinae within the re-defined, broader Calliphorinae has been corroborated by phylogenetic studies based on both Sanger and phylogenomic data [13, 20]. We therefore propose a redefined Calliphorinae sinking the former Aphyssurinae, Melanomyinae, and Toxotarsinae as subordinate taxa. This will have the added advantage of conserving the traditionally accepted sister-group relationship between Calliphorinae and Luciliinae. The former Helicoboscinae are proposed to be synonymized under Ameniinae, syn. nov., thereby establishing a monophyletic subfamily containing rather robust, mostly macrolarviparous species feeding on live, dying, or dead snails [43, 44].
Carrion breeding blowflies probably originated twice
All the carrion-breeding blowflies are found in the two clades (Calliphorinae sensu lato + Luciliinae) and Chrysomyinae. As these are separated by four nodes, carrion breeding in these two clades is most parsimoniously interpreted as having independent origins. This is surprising and challenges the traditional assumption that the common blowflies form a monophyletic group based on general appearance and life habits [12] (Fig. 1). In the present study, the tree topology that is overwhelmingly favored (Fig. 3, T1 in Fig. 5A) differs in some important respects from other trees (T2 and T3 in Fig. 5A) obtained using phylogenomic data. The hypothesis (T1) is well supported by partition log-likelihood analyses and AU test and is favored by the more sensitive FcLM analysis over the other two likely placements (Fig. 6). Interestingly, this placement of Chrysomyinae + Phumosiinae was also recovered in a coalescent-based phylogeny using transcriptome-derived ultraconserved elements [20], while it was rejected by a phylogenetic analysis based on concatenated genes [20].
Is metallic color part of the blowfly ground plan?
Surprisingly, metallic adult cuticle, a long accepted ground plan character of blowflies, may best be considered as having evolved multiple times in the family. Blowflies are generally well-understood to be predominantly metallic [12]. However, the ancestor of blowflies may not have been a metallic fly, and it appears most likely that metallic colors evolved repeatedly during the early radiation of blowflies (Fig. 3).
Materials and methods
Specimen acquisition and taxon sampling
Blowfly specimens collected for RNA extraction were identified alive after capture. The male terminalia and/or a hind leg were removed as morphological and molecular vouchers, respectively, and the rest of the body was immersed in RNAlater (Sigma), crushed with a sterile pestle, and stored at −60°C until further processing. Specimens used for DNA extraction were immersed in 96% alcohol immediately after capture and stored at −20°C for later identification and processing (Additional file 5: Table S2). Vouchers are deposited at Beijing Forestry University, China.
All calyptrate families were sampled, i.e., all Hippoboscoidea (here following Pape et al. [45] in considering the Nycteribiidae and Streblidae to be subordinate to Hippoboscidae), muscoid grade, and Oestroidea. All currently recognized calliphorid subfamilies were sampled (Additional file 1: Table S1). Drosophila melanogaster was included to root the tree because of the growing evidence that Ephydroidea are the sister group to Calyptratae [35, 46,47,48,49]. Transcriptomic data were either generated for this study or downloaded from GenBank and other databases (Additional file 1: Table S1).
Nucleic acids extraction, sequencing, and data processing
The total RNA was extracted using TRIzol (Invitrogen Life Technologies; Catalog # 15596-026), with the total RNA concentration and RNA integrity number (RIN) for each extraction assessed using an Agilent 2100 Bioanalyzer with the RNA 6000 Nano kit (Agilent Technologies, Santa Clara, CA; Catalog # 5067-1511). About 200 ng to 1 μg of total RNA was purified to construct a cDNA library for each sample using the TruSeq RNA Sample Prep Kit v2 (Illumina, San Diego, USA; Catalog # RS-122-2001) following the manufacturer’s instructions. An Illumina HiSeq 4000 sequencer was employed to generate paired-end reads for each library (Additional file 5: Table S2).
The total genomic DNA was extracted using a QIAGEN DNeasy® Blood and Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. An Illumina NovaSeq 6000 sequencer was used for paired-end sequencing with insert size of 350 bp (Additional file 5: Table S2).
FastQC [50] was used to assess the quality of the generated raw data. The data were then trimmed using Trimmomatic [51] installed on Computerome (http://www.computerome.dtu.dk), with adapter sequences trimmed referring to the self-provided Illumina adapter sequence database. Also leading and trailing bases with quality below 30 were removed for each read, then each read was scanned with a 4-base wide sliding window to cut reads with the average quality below 15 within the window, and only reads with a minimum length of 36 bp were retained. Trimmomatic was used until the FastQC estimate of “per base sequence quality” was above 20, and no adapter sequences were detected in “overrepresented sequences” and “adapter content.”
Trinity (version 2.4.0) [52] was used to perform de novo assemblies for RNAseq data with default settings as described in Haas et al. [53]. After assembling, we estimated the average coverage of each transcript by mapping back the raw reads to assembled contigs using the perl script “align_and_estimate_abundance.pl” from Trinity. Only transcripts with average coverage above ten were kept. SOAPdenovo2 [54] was used for de novo assembling of genomic data with default settings. The assemblies were then trimmed for vector contamination referring UniVec Core database using Geneious (version 7.1.5) (Biomatters, Auckland, New Zealand). Only contigs with a length at least 200 bp were used from further analyses.
The data processing followed Misof et al. [36], Kutty et al. [19, 55], and Yan et al. [56]. We used orthograph [57] for reciprocal search to infer orthology for each target taxon following the workflow of Misof et al. [36] and Kutty et al. [55]. Orthograph (version 0.6.1) was run using reference ortholog sets [19, 56] with the alignment-program set as mafft-linsi, hmmbuild-program as hmmbuild, hmmsearch-program as hmmsearch, blast-program as blastp, exonerate-program as exonerate, blast-score-threshold as 10, and blast-evalue-threshold as 1e−05. After orthologous gene clusters for our assemblies were successfully assigned, the perl script summarize_orthograph_results.pl was used to summarize both NT and AA sequences of transcripts recognized as single-copy genes for all taxa. MAFFT (version 7.310) [58] with the L-INS-i algorithm was subsequently employed to construct MSA of all AA sequences. Outliers that were putatively misaligned were checked and re-aligned, and sequences were removed if they were still detected as outliers in the additional checking after refinement [19, 59]. For each dataset, all reference sequences except for Drosophila melanogaster were then removed from each multiple sequence alignment (MSA). PAL2NAL (version 14) [60] modified by Misof et al. [36] was used to align NT sequences with the above-refined amino acid MSAs as blueprints. Aliscore (version 2.2) [61,62,63] was used with default parameters to identify ambiguously or randomly aligned amino acid MSA sections of each orthologous gene, which were subsequently removed with ALICUT (version 2.3) [64]. The corresponding ambiguous sites of nucleotide MSAs were identified with custom perl scripts from Misof et al. [36] and removed with ALICUT (version 2.3). The MSAs were then recoded with leading and trailing gaps replaced with “N” for NT sequences and “X” for AA sequences. Subsequently, the amino acid MSAs for each dataset were concatenated into supermatrix using FASconCAT-G [65]. MARE (version 0.1.2-rc) [66] was then used to improve the overall information content of the matrix, with the flag “-c” used to keep all taxa with fewer genes that would otherwise be removed. The corresponding nucleotide supermatrix with improved information content was built using FASconCAT-G.
At the onset of the study, we noticed the different phylogenetic positions of Chrysomyinae between our study (Fig. 3) and that of Kutty et al. [19]. There are two main differences between these two studies, i.e., taxon sampling and ortholog reference. We therefore performed reconstructions with different taxon representations and ortholog reference sets to interrogate the contrasting placements of Chrysomyinae. We have two taxon sets: a larger one with 39 species (Ltax) and a smaller (reduced) one with 26 species (Stax). The data were analyzed using two ortholog sets, the Diptera ortholog reference (Dref) with ortholog set of 3755 single-copy nuclear protein-encoding genes recognized from official gene sets of five dipteran species (Aedes aegypti, Drosophila melanogaster, Glossina morsitans, Lucilia cuprina, Musca domestica) (Additional file 6: Table S3) using OrthoFinder (version 1.1.10) [67], and the Antliophora ortholog set (Aref; recognized from official gene sets of Tribolium castaneum, Mayetiola destructor, Bombyx mori, Anopheles gambiae, Drosophila melanogaster) with a slightly smaller number of single-copy nuclear protein-encoding genes (3288) used in Kutty et al. [19]. Following the process described above, our analysis of the data started with Ltax being analyzed with Dref as the ortholog set (Dref_Ltax; 2221 genes) and then based on Ltax with Aref (Aref_Ltax; 1764 genes) followed by Stax with Dref (Dref_Stax; 2003 genes) and Stax with Aref (Aref_Stax; 1465 genes), respectively. Amino acid and nucleotide sequences of transcripts recognized as single-copy genes were used to generate four datasets (Table 1). Matrices of amino acid (AA), 2nd-codon positions (NT2), and 1st & 2nd-codon positions (NT12) of each dataset were generated. AliStat (version 1.7) [68], MARE, and Symtest (version 2.0.47) [69] were used to report alignment diagnostics of each supermatrix, e.g., site coverage of the matrices, and to explore whether or not the matrices matched conditions assumed by most models including stationarity, reversibility, and homogeneity (Additional file 2: Figs. S3–S6). The AA matrix for each dataset was also recorded as a six-state Dayhoff group using the “pgrecodeseq” command in the PHYLOGEARS v.2.0 tool package [70] for parsimony tree construction.
Phylogenetic inference and topology test
Concatenation-based reconstruction
ML trees were inferred using IQ-TREE (version 1.6.8) [71] based on AA, NT2, and NT12, and MP trees inferred using TNT [72] for AA matrices of all four datasets (Table 1) yielding 16 concatenation-based phylogeny reconstructions.
IQ-TREE (version 1.6.8) [71] was used for ML reconstruction, with the best model for each gene estimated by the self-implemented ModelFinder [73] following the Akaike Information Corrected Criterion (AICc) score [74], and branch support estimated with 100 standard bootstrap resampling analysis.
The MP tree was constructed using six-state Dayhoff recoded matrices. TNT (version 1.5) [72] was run with new technology searches, level 10, hits 20, gaps coded as missing data, and node support assessed by jackknife resampling with 1000 replicates at 36% deletion following Kutty et al. [19].
Coalescent-based reconstruction
The coalescent-based phylogeny was conducted only using the AA alignments of Dref_Ltax, because this dataset has the highest number of genes. Amino acids of all 2221 MSAs were used to construct gene trees using IQ-TREE, respectively, with the best model for each MSA estimated by ModelFinder based on AICc and branch support derived from 100 standard bootstrap replicates. All the constructed gene trees were subsequently used to infer a coalescent-based phylogeny using ASTRAL (version 5.6.1) with default parameters [75].
Tests for Chrysomyinae placement
The Chrysomyinae had different placements across the phylogenetic analyses of various datasets. They were either placed together with Phumosiinae as the sister group of most other calliphorids (i.e., T1 in Fig. 5A; (group 2a, (group 2b, group 3)) in Fig. 6A), as sister group to Luciliinae, Aphyssurinae-Calliphorinae-Melanomyinae-Toxotarsinae (i.e., T2 in Fig. 5A; group 3 in Fig. 6A), or in fewer cases as sister group to Rhiniidae and Bengaliinae (i.e., T3 in Fig. 5A; group 2B). We therefore performed a modified FcLM [76], partition log-likelihood analyses [77], and an approximately unbiased (AU) test [78] using amino acid alignments of the dataset with the largest number of genes (i.e., Dref_Ltax) to compare the amount of phylogenetic signal for competing hypotheses.
The group definitions for FcLM were as depicted in Fig. 6A. The paraphyly of group 2 means that FcLM is not applicable directly to our dataset. Therefore, we modified the analyses and performed FcLM with two variations after splitting this group into group 2A and group 2B, which were both inferred as monophyletic but never recovered as sister groups. For one analysis, we pruned data of group 2B, keeping the remaining four clusters in Fig. 6A, and performed FcLM as implemented in IQ-TREE. For the other analysis, we pruned group 2A and kept group 2B.
To perform the partitioned log-likelihood analysis, we calculated ΔpLi of each partition by subtracting the likelihood for T3 (ΔT3pLi) or T2 (ΔT2pLi) of each gene from the corresponding likelihood for T1 (ΔT1pLi) following [77, 79] (i.e., ΔpLi = ΔT1pLi − ΔT3pLi, or ΔpLi = ΔT1pLi − ΔT2pLi), and values of ΔpLi were then plotted for visualization. The same calculation and plotting were performed for T1 and T2. The AU test implemented in IQ-TREE was conducted for topologies T1, T2, and T3 for 10,000 replicates, respectively.
Reconstruction of ancestral state of adult metallic color
Character states of terminal taxon were collected from the literature [7, 8, 12, 80], as shown in Table S4 (Additional file 7). Metallic color was coded as present or absent according to the coloration measured by the eye, and no attempt was made to present a multistate coding because of the complexity involved in transitions between different states. Species with bodies that were partially metallic were therefore coded as the metallic color present, e.g., for Mesembrinellidae, where the included species has only abdominal metallic coloration. Character states were treated with equal weight because of the impossible measurement of transition among different states. Bayesian binary Markov chain Monte-Carlo (BBM) [81] implemented in RASP [82] with default settings as described in Yan et al. [56, 83] was run to reconstruct the ancestral state.
Availability of data and materials
All data related to this publication are submitted to the GenBank databases under BioProject accession number PRJNA611871 [84].
References
Shakespeare W. A pleasant conceited comedie called, Loues labors lost. As it was presented before her Highnes this last Christmas. Newly corrected and augmented by W. Shakespere. London: William White for Cutbert Burby; 1598.
Papavero N, Pujol-Luz JR, Teixeira DM. From Homer to Redi: some historical notes about the problem of necrophagous blowflies’ reproduction. Arq Zool. 2010;41(2-4):153–70. https://doi.org/10.11606/issn.2176-7793.v41i2-4p153-170.
Hennig W. Diptera (Zweiflügler). In: Helmcke JG, Starck D, Wermuth H, editors. Handbuch der Zoologie. Berlin: Walter de Gruyter; 1973. p. 1–337. https://www.semanticscholar.org/paper/Hennig%2C-W.%3A-Diptera-(Zweifl%C3%BCgler).-In%3A-Handbuch-der-Schumann/0f3069e7fc652edc5186a25895a0080711e9cc92.
Whitaker IS, Twine C, Whitaker MJ, Welck M, Brown CS, Shandall A. Larval therapy from antiquity to the present day: mechanisms of action, clinical applications and future potential. Postgrad Med J. 2007;83(980):409–13. https://doi.org/10.1136/pgmj.2006.055905.
Rognes K. Revision of the frog fly genus Caiusa Surcouf, 1920 (Diptera, Calliphoridae), with a note on the identity of Plinthomyia emimelania Rondani, 1875. Zootaxa. 2015;3952(1):1–80. https://doi.org/10.11646/zootaxa.3952.1.1.
Greenberg B, Kunich JC. Entomology and the law: flies as forensic indicators. New York: Cambridge University Press; 2002.
Rognes K. Family Calliphoridae. In: Papp L, Darvas B, editors. Contributions to a manual of Palaearctic Diptera volume 3: higher Brachycera. Budapest: Science Herald; 1998. p. 617–48.
Vargas J, Wood DM. Calliphoridae (blow flies). In: Brown BV, Borkent A, Cumming JM, Wood DM, Woodley NE, Zumbado MA, editors. Manual of central American Diptera (volume 2). Ottawa: NRC Research Press; 2010. p. 1297–304.
Vargas-Teran M, Hofmann HC, Tweddle NE. Impact of screwworm eradication programmes using the sterile insect technique: sterile insect technique. In: Dyck VA, Hendrichs J, Robinson AS, editors. Principles and practice in area-wide integrated pest management. Dordrecht: Springer; 2005. p. 629–50. https://doi.org/10.1007/1-4020-4051-2_24.
Benbow M, Tomberlin J, Tarone A. Carrion ecology, evolution, and their applications. Boca Raton: CRC Press; 2015. https://doi.org/10.1201/b18819.
McAlpine JF. Phylogeny and classification of the Muscomorpha. In: McAlpine JF, editor. Manual of Nearctic Diptera (Volume 3); 1989. p. 1397–518.
Rognes K. The Calliphoridae (blowflies) (Diptera: Oestroidea) are not a monophyletic group. Cladistics. 1997;13(1-2):27–66. https://doi.org/10.1111/j.1096-0031.1997.tb00240.x.
Kutty SN, Pape T, Wiegmann BM, Meier R. Molecular phylogeny of the Calyptratae (Diptera: Cyclorrhapha) with an emphasis on the superfamily Oestroidea and the position of Mystacinobiidae and McAlpine’s fly. Syst Entomol. 2010;35(4):614–35. https://doi.org/10.1111/j.1365-3113.2010.00536.x.
Marinho MAT, Junqueira ACM, Paulo DF, Esposito MC, Villet MH, Azeredo-Espin AML. Molecular phylogenetics of Oestroidea (Diptera: Calyptratae) with emphasis on Calliphoridae: insights into the inter-familial relationships and additional evidence for paraphyly among blowflies. Mol Phylogenet Evol. 2012;65(3):840–54. https://doi.org/10.1016/j.ympev.2012.08.007.
Singh B, Wells JD. Molecular systematics of the Calliphoridae (Diptera: Oestroidea): evidence from one mitochondrial and three nuclear genes. J Med Entomol. 2013;50(1):15–23. https://doi.org/10.1603/ME11288.
Zhang D, Yan L, Zhang M, Chu H, Cao J, Li K, et al. Phylogenetic inference of calyptrates, with the first mitogenomes for Gasterophilinae (Diptera: Oestridae) and Paramacronychiinae (Diptera: Sarcophagidae). Int J Biol Sci. 2016;12(5):489–504. https://doi.org/10.7150/ijbs.12148.
Cerretti P, Stireman JO, Pape T, O’Hara JE, Marinho MAT, Rognes K, et al. First fossil of an oestroid fly (Diptera: Calyptratae: Oestroidea) and the dating of oestroid divergences. PLoS One. 2017;12(8):e0182101. https://doi.org/10.1371/journal.pone.0182101.
Marinho MAT, Wolff M, Ramos-Pastrana Y, de Azeredo-Espin AML, de Amorim DS. The first phylogenetic study of Mesembrinellidae (Diptera: Oestroidea) based on molecular data: clades and congruence with morphological characters. Cladistics. 2017;33:134–52.
Kutty SN, Meusemann K, Bayless KM, Marinho MA, Pont AC, Zhou X, et al. Phylogenomic analysis of Calyptratae: resolving the phylogenetic relationships within a major radiation of Diptera. Cladistics. 2019;35(6):605–22. https://doi.org/10.1111/cla.12375.
Buenaventura E, Lloyd MW, López JMP, González VL, Thomas-Cabianca A, Dikow T. Protein-encoding ultraconserved elements provide a new phylogenomic perspective of Oestroidea flies (Diptera: Calyptratae). Syst Entomol. 2020;46(1):5–27. https://doi.org/10.1111/syen.12443.
Buenaventura E, Szpila K, Cassel BK, Wiegmann BM, Pape T. Anchored hybrid enrichment challenges the traditional classification of flesh flies (Diptera: Sarcophagidae). Syst Entomol. 2020;45(2):281–301. https://doi.org/10.1111/syen.12395.
Brauer FM, Bergenstamm JE von. Die Zweiflügler des Kaiserlichen Museums zu Wien. IV. Vorarbeiten zu einer Monographie der Muscaria Schizometopa (exclusive Anthomyidae). Pars I. Denkschrift der Akad der Wissenschaften Wien. 1889;56:69–180.
Girschner E. Beitrag zur systematik der Musciden. Berliner Entomol Zeitschrift. 1893;38(3-4):297–312. https://doi.org/10.1002/mmnd.18940380305.
Pape T. Phylogeny of the Tachinidae family-group (Diptera: Calyptratae). Tijdschr voor Entomol. 1992;135:43–86.
Peris SV. A preliminary key to the World genera of the subfamilies Toxotarsinae, Chrysomyinae and Rhiniinae (Diptera, Calliphoridae). Española Hist Nat Secc Biol. 1992;88:79–98.
Rognes K. Blowflies (Diptera, Calliphoridae) of Fennoscandia and Denmark. Leiden: Brill; 1991.
Cerretti P, Stireman JO, Badano D, Gisondi S, Rognes K, Giudice GL, et al. Reclustering the cluster flies (Diptera: Oestroidea, Polleniidae). Syst Entomol. 2019;44(4):957–72. https://doi.org/10.1111/syen.12369.
Norris KR. Establishment of a subfamily Aphyssurinae for the Australian genus Aphyssura Hardy (Diptera: Calliphoridae), with a review of known forms and descriptions of new species. Invertebr Taxon. 1999;13(4):511–628. https://doi.org/10.1071/IT98007.
Bedding RA. The immature stages of Rhinophorinae (Diptera: Calliphoridae) that parasitise British woodlice. Trans R Entomol Soc London. 1973;125(1):27–44. https://doi.org/10.1111/j.1365-2311.1973.tb00536.x.
Peris SV. Diptera Calliphoridae Rhinophorinae. A new species of genus Bequaertiana from the Ruanda-Urundi. Ann du Musée R du Congo Belge Zool Ser Octavo. 1957;58:136.
Guimarães JH. A systematic revision of the Mesembrinellidae, stat. nov. (Diptera, Cyclorrhapha). Arq Zool. 1977;29(1):1–109. https://doi.org/10.11606/issn.2176-7793.v29i1p1-109.
Winkler IS, Blaschke JD, Davis DJ, Stireman JO, O’Hara JE, Cerretti P, et al. Explosive radiation or uninformative genes? Origin and early diversification of tachinid flies (Diptera: Tachinidae). Mol Phylogenet Evol. 2015;88:38–54. https://doi.org/10.1016/j.ympev.2015.03.021.
Herting B. Diptera from Nepal: a new species of Rhinophoridae. Bull Br Museum Nat Hist Entomol. 1966;17:451.
Crosskey RW. A review of the Rhinophoridae (Diptera) and a revision of the Afrotropical species. Bull Br Museum Nat Hist Entomol. 1977;36:1–67.
Bayless KM, Trautwein MD, Meusemann K, Shin S, Petersen M, Donath A, et al. Beyond Drosophila: resolving the rapid radiation of schizophoran flies with phylotranscriptomics. BMC Biol. 2021;19(1):23. https://doi.org/10.1186/s12915-020-00944-8.
Misof B, Liu S, Meusemann K, Peters RS, Donath A, Mayer C, et al. Phylogenomics resolves the timing and pattern of insect evolution. Science. 2014;346:763–7.
Kishino H, Hasegawa M. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in hominoidea. J Mol Evol. 1989;29(2):170–9. https://doi.org/10.1007/BF02100115.
Shimodaira H, Hasegawa M. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol Biol Evol. 1999;16(8):1114–6. https://doi.org/10.1093/oxfordjournals.molbev.a026201.
Wolff M, Kosmann C. Families Calliphoridae and Mesembrinellidae. Zootaxa. 2016;4122(1):856–75. https://doi.org/10.11646/zootaxa.4122.1.72.
Kallal RJ, Dimitrov D, Arnedo MA, Giribet G, Hormiga G. Monophyly, taxon sampling, and the nature of ranks in the classification of orb-weaving spiders (Araneae: Araneoidea). Syst Biol. 2020;69(2):401–11. https://doi.org/10.1093/sysbio/syz043.
Robineau-Desvoidy J-B. Histoire naturelle des Diptères des environs de Paris: oeuvre posthume du Dr. Robineau-Desvoidy publiée par les soins de sa famille, sous la direction de M. H. Monceaux. Paris: Victor Masson et Fils; 1863. https://doi.org/10.5962/bhl.title.8550.
Brauer FM, von Bergenstamm JE. Die Zweiflügler des Kaiserlichen Museums zu Wien. IV. Vorarbeiten zu einer Monographie der Muscaria Schizometopa (exclusive Anthomyidae). Pars I. Denkschriften der Kais Akad der Wissenschaften/Math Cl. 1889;56:69–180.
Meier R, Kotrba M, Ferrar P. Ovoviviparity and viviparity in the Diptera. Biol Rev Camb Philos Soc. 1999;74(3):199–258. https://doi.org/10.1017/S0006323199005320.
Rognes K. A new genus of Helicoboscinae from the Himalayas (Diptera: Calliphoridae), with emended genus and subfamily concepts. Insect Syst Evol. 1992;23(4):391–404. https://doi.org/10.1163/187631292X00182.
Pape T, Blagoderov V, Mostovski MB. Order Diptera Linnaeus, 1758. Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness (ed. by Z.-Q. Zhang). Zootaxa. 2011;3148:222–9.
Wiegmann BM, Trautwein M, Winkler IS, Barr NB, Kim JW, Lambkin C, et al. Episodic radiations in the fly tree of life. Proc Natl Acad Sci U S A. 2011;108(14):5690–5. https://doi.org/10.1073/pnas.1012675108.
Junqueira ACM, Azeredo-Espin AML, Paulo DF, Marinho MAT, Tomsho LP, Drautz-Moses DI, et al. Large-scale mitogenomics enables insights into Schizophora (Diptera) radiation and population diversity. Sci Rep. 2016;6(1):21762. https://doi.org/10.1038/srep21762.
Wiegmann BM, Yeates DK. Phylogeny of Diptera. In: Kirk-Spriggs AH, Sinclair BJ, editors. Manual of Afrotropical Diptera (Volume 1). Pretoria: South African National Biodiversity Institute; 2017. p. 253–65.
Zhao Z, Su T, Chesters D, Wang S, Ho SY, Zhu C, et al. The mitochondrial genome of Elodia flavipalpis Aldrich (Diptera: Tachinidae) and the evolutionary timescale of tachinid Flies. PLoS One. 2013;8(4):e61814. https://doi.org/10.1371/journal.pone.0061814.
Andrews S. FastQC: a quality control tool for high throughput sequence data. 2010. Available from: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
Bolger A, Lohse M, Usadel B. Trimmomatic: a flexible read trimming tool for Illumina NGS data. Bioinformatics. 2014;30(15):2114–20. https://doi.org/10.1093/bioinformatics/btu170.
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–52. https://doi.org/10.1038/nbt.1883.
Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, et al. De novo transcript sequence reconstruction from RNA-Seq: reference generation and analysis with Trinity. Nat Protoc. 2014;8:1–43.
Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, et al. Gigascience. 2012;1. https://doi.org/10.1186/2047-217X-1-18.
Kutty SN, Wong WH, Meusemann K, Meier R, Cranston PS. A phylogenomic analysis of Culicomorpha (Diptera) resolves the relationships among the eight constituent families. Syst Entomol. 2018;43(3):434–46. https://doi.org/10.1111/syen.12285.
Yan L, Buenaventura E, Pape T, Kutty SN, Bayless KM, Zhang D. A phylotranscriptomic framework for flesh fly evolution (Diptera, Calyptratae, Sarcophagidae). Cladistics. 2020;37:540–58. https://doi.org/10.1111/cla.12449.
Petersen M, Meusemann K, Donath A, Dowling D, Liu S, Peters RS, et al. Orthograph: a versatile tool for mapping coding nucleotide sequences to clusters of orthologous genes. BMC Bioinformatics. 2017;18(1):111. https://doi.org/10.1186/s12859-017-1529-8.
Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–80. https://doi.org/10.1093/molbev/mst010.
Peters RS, Krogmann L, Mayer C, Donath A, Gunkel S, Meusemann K, et al. Evolutionary history of the Hymenoptera. Curr Biol. 2017;27(7):1013–8. https://doi.org/10.1016/j.cub.2017.01.027.
Suyama M, Torrents D, Bork P. PAL2NAL: Robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006;34(suppl_2):W609–12.
Misof B, Misof K. A Monte Carlo approach successfully identifies randomness in multiple sequence alignments: a more objective means of data exclusion. Syst Biol. 2009;58(1):21–34. https://doi.org/10.1093/sysbio/syp006.
Kück P, Meusemann K, Dambach J, Thormann B, von Reumont BM, Wägele JW, et al. Parametric and non-parametric masking of randomness in sequence alignments can be improved and leads to better resolved trees. Front Zool. 2010;7(1):10. https://doi.org/10.1186/1742-9994-7-10.
Meusemann K, Von Reumont BM, Simon S, Roeding F, Strauss S, Kück P, et al. A phylogenomic approach to resolve the arthropod tree of life. Mol Biol Evol. 2010;27(11):2451–64. https://doi.org/10.1093/molbev/msq130.
Kück P. ALICUT: a Perlscript which cuts ALISCORE identified RSS, version 2.0. 2009. Available from: https://www.zfmk.de/en/research/re.
Kück P, Longo GC. FASconCAT-G: extensive functions for multiple sequence alignment preparations concerning phylogenetic studies. Front Zool. 2014;11(1):81. https://doi.org/10.1186/s12983-014-0081-x.
Misof B, Meyer B, von Reumont BM, Kück P, Misof K, Meusemann K. Selecting informative subsets of sparse supermatrices increases the chance to find correct trees. BMC Bioinformatics. 2013;14(1):348. https://doi.org/10.1186/1471-2105-14-348.
Emms DM, Kelly S. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 2015;16(1):157. https://doi.org/10.1186/s13059-015-0721-2.
Wong TKF, Kalyaanamoorthy S, Meusemann K, Yeates DK, Misof B, Jermiin LS. A minimum reporting standard for multiple sequence alignments. NAR Genom Bioinform. 2020;2:lqaa024.
Jermiin L, Ott M. SymTest version 2.0.47. 2017. Available online at: https://github.com/ottmi/symt.
Tanabe AS. ‘Phylogears version 2.0’, software, distributed by the author 2008. Available from http://www.fifthdimension.jp/.
Nguyen LT, Schmidt HA, Von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32(1):268–74. https://doi.org/10.1093/molbev/msu300.
Goloboff PA, Catalano SA. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics. 2016;32(3):221–38. https://doi.org/10.1111/cla.12160.
Kalyaanamoorthy S, Minh BQ, Wong TKF, Von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 2017;14(6):587–9. https://doi.org/10.1038/nmeth.4285.
Hurvich CM, Tsai CL. Regression and time series model selection in small samples. Biometrika. 1989;76(2):297–307.
Mirarab S, Reaz R, Bayzid MS, Zimmermann TS, Swenson M, Warnow T. ASTRAL: genome-scale coalescent-based species tree estimation. Bioinformatics. 2014;30:541–8.
Strimmer K, von Haeseler A. Likelihood-mapping: a simple method to visualize phylogenetic content of a sequence alignment. Proc Natl Acad Sci U S A. 1997;94(13):6815–9. https://doi.org/10.1073/pnas.94.13.6815.
Shen X-X, Hittinger CT, Rokas A. Contentious relationships in phylogenomic studies can be driven by a handful of genes. Nat Ecol Evol. 2017;1(5):0126. https://doi.org/10.1038/s41559-017-0126.
Shimodaira H. An approximately unbiased test of phylogenetic tree selection. Syst Biol. 2002;51(3):492–508. https://doi.org/10.1080/10635150290069913.
Simon S, Blanke A, Meusemann K. Reanalyzing the Palaeoptera problem – the origin of insect flight remains obscure. Arthropod Struct Dev. 2018;47(4):328–38. https://doi.org/10.1016/j.asd.2018.05.002.
Shewell G. Calliphoridae. In: McAlpine F, editor. Manual of nearctic Diptera (Volume 2). Ottawa: Agriculture Canada; 1987. p. 1133–45.
Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–4. https://doi.org/10.1093/bioinformatics/btg180.
Yu Y, Blair C, He X. RASP 4: Ancestral state reconstruction tool for multiple genes and characters. Mol Biol Evol. 2019;37(2):604–6. https://doi.org/10.1093/molbev/msz257.
Yan L, Pape T, Elgar MA, Gao Y, Zhang D. Evolutionary history of stomach bot flies in the light of mitogenomics. Syst Entomol. 2019;44(4):797–809. https://doi.org/10.1111/syen.12356.
NCBI accession number PRJNA611871. 2020. Available from: https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA611871. Accessed 13 Sept 2021.
Larkin A, Marygold SJ, Antonazzo G, Attrill H, dos Santos G, Garapati PV, et al. FlyBase: updates to the Drosophila melanogaster knowledge base. Nucleic Acids Res. 2020;49(D1):D899–907. https://doi.org/10.1093/nar/gkaa1026.
Giraldo-Calderón GI, Emrich SJ, MacCallum RM, Maslen G, Dialynas E, Topalis P, et al. VectorBase: an updated bioinformatics resource for invertebrate vectors and other organisms related with human diseases. Nucleic Acids Res. 2014;43(D1):D707–13. https://doi.org/10.1093/nar/gku1117.
Anstead CA, Korhonen PK, Young ND, Hall RS, Jex AR, Murali SC, et al. Lucilia cuprina genome unlocks parasitic fly biology to underpin future interventions. Nat Commun. 2015;6(1):7344. https://doi.org/10.1038/ncomms8344.
Wang X, Xiong M, Lei C, Zhu F. The developmental transcriptome of the synanthropic fly Chrysomya megacephala and insights into olfactory proteins. BMC Genomics. 2015;16(1):20. https://doi.org/10.1186/s12864-014-1200-y.
Acknowledgements
We are grateful to Flickr users Frank Vassen, Jeffreycfy, Chi Liu, Hans Hillewaert, and Steven Falk, to the online forums Diptera.info (https://diptera.info/news.php) and antroom (http://blog.livedoor.jp/antroom), and to Karl Kroeker for kindly sharing fly photos. We thank Pierfilippo Cerretti (Sapienza University, Rome) for kindly sharing material used for genomic sequencing in this study. The analyses were performed on Computerome (http://www.computerome.dtu.dk/), and we express our gratitude to Computerome Support for their generous help.
We dedicate this study to Dr. Knut Rognes (1943–2020), who contributed extensively and meticulously to broadening our knowledge of blowflies.
Funding
This work was supported by the Beijing Forestry University Outstanding Young Talent Cultivation Project (2019JQ03018) and the National Natural Science Foundation of China (31872964 and 31572305) to DZ, Postdoctoral Innovative Talents Support Program (BX20190042) to LY.
Author information
Authors and Affiliations
Contributions
TP, DZ, RM, and LY designed this study. LY and KM performed analyses of the data. SNK and KMB provided part of the transcriptome data and assisted with analyses. LY, TP, KM, and DZ wrote an initial draft, and all authors took part in finalizing the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 2: Figure S1.
Maximum Likelihood tree inferred from the amino acid matrix of dataset Dref_Ltax. Figure S2. Bayesian reconstructions of ancestral states of adult metallic color. Figure S3. Heatmaps showing data coverage and homogeneity test of matrices Dref_Ltax. Figure S4. Heatmaps showing data coverage and homogeneity test of matrices Dref_Stax. Figure S5. Heatmaps showing data coverage and homogeneity test of matrices Aref_Ltax. Figure S6. Heatmaps showing data coverage and homogeneity test of matrices Aref_Stax.
Additional file 3.
Score differences of partition log-likelihood for each locus recorded between the alternative hypotheses, T1 and T2 and between T1 and T3.
Additional file 4.
Ancestral construction of adult metallic color of blowflies.
Additional file 5: Table S2.
Collecting and sequencing information of newly sequenced species.
Additional file 6: Table S3.
Genomes used to prepare dipteran orthologous references.
Additional file 7: Table S4.
Character states and coding of each terminal taxon for ancestral construction.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yan, L., Pape, T., Meusemann, K. et al. Monophyletic blowflies revealed by phylogenomics. BMC Biol 19, 230 (2021). https://doi.org/10.1186/s12915-021-01156-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12915-021-01156-4