Abstract
Background
The burden of chronic kidney disease (CKD) is high in the Northern Territory (NT), Australia. This study aims to describe the healthcare use and associated costs of people at risk of CKD (e.g. acute kidney injury, diabetes, hypertension, and cardiovascular disease) or living with CKD in the NT, from a healthcare funder perspective.
Methods
We included a retrospective cohort of patients at risk of, or living with CKD, on 1 January 2017. Patients on kidney replacement therapy were excluded from the study. Data from the Territory Kidney Care database, encompassing patients from public hospitals and primary health care services across the NT was used to conduct costing. Annual healthcare costs, including hospital, primary health care, medication, and investigation costs were described over a one-year follow-up period. Factors associated with high total annual healthcare costs were identified with a cost prediction model.
Results
Among 37,398 patients included in this study, 23,419 had a risk factor for CKD while 13,979 had CKD (stages 1 to 5, not on kidney replacement therapy). The overall mean (± SD) age was 45 years (± 17), and a large proportion of the study cohort were First Nations people (68%). Common comorbidities in the overall cohort included diabetes (36%), hypertension (32%), and coronary artery disease (11%). Annual healthcare cost was lowest in those at risk of CKD (AUD$7,958 per person) and highest in those with CKD stage 5 (AUD$67,117 per person). Inpatient care contributed to the majority (76%) of all healthcare costs. Predictors of increased total annual healthcare cost included more advanced stages of CKD, and the presence of comorbidities. In CKD stage 5, the additional cost per person per year was + $53,634 (95%CI 32,769 to 89,482, p < 0.001) compared to people in the at risk group without CKD.
Conclusion
The total healthcare costs in advanced stages of CKD is high, even when patients are not on dialysis. There remains a need for effective primary prevention and early intervention strategies targeting CKD and related chronic conditions.
Similar content being viewed by others
Background
Chronic kidney disease (CKD) is defined as abnormal kidney structure or function, present for 3 months or more [1]. Early asymptomatic stages of CKD can progress to end-stage kidney disease (ESKD) requiring kidney replacement therapy (KRT) in the form of haemodialysis, peritoneal dialysis, or renal transplantation. Although life sustaining, ESKD requiring KRT has substantial impacts on an individual’s quality of life – this is especially true for Aboriginal and Torres Strait Islander people (hereby respectfully referred to as First Nations people). In the Northern Territory (NT), many First Nations people leave their homes and small remote communities, in order to receive dialysis in urban centres [2,3,4]. Effective primary health care, screening, and evidence-based management can slow or prevent CKD progression [1].
The NT, where 31% of the population are First Nations Australians, is a hotspot for CKD. CKD disproportionally affects First Nations people. A national Australian Health Survey showed that First Nations people in the NT had a biomedical CKD prevalence of up to 32%, compared to a national average of approximately 10% amongst non-First Nations Australians [5]. A recent NT Health report highlighted similar differences in prevalence between First Nations and non-First Nations adults [6]. The incidence of ESKD is also much higher in First Nations people in the NT, compared to national estimates for First Nations Australians (3.96 versus 0.95 per 1000 population) [7]. In Australia and globally, the disproportionately high burden of CKD in First Nations people is associated with socioeconomic inequities – such as low income, geographic remoteness, limited housing and education, and difficulties in accessing specialised health services [8,9,10,11].
The enormous economic burden of KRT in the NT has been well established [12,13,14,15]. Gorham et al. showed that on average, a single patient on maintenance dialysis (urban centres) had a total healthcare cost of more than AUD$100,000 per year [15]. Furthermore, the total cost of KRT to the healthcare system has grown rapidly over the past two decades, as demand for dialysis has increased across the NT [16]. Despite what is already known about the costs of KRT, the cost of healthcare for people living with CKD in non-KRT cohorts is less well characterised. A 2020 systematic review of CKD costing studies in developed countries showed that few studies included earlier stages of CKD, and there was great variability in total healthcare cost estimates across different healthcare settings [17]. A majority of included studies were from the USA, and only one was from Australia. In the Australian study, Wyld et al. estimated the annual cost of CKD across all stages of CKD for the AusDiab cohort (6,138 individuals) [18]. Although AusDiab was a national cohort study, there was only a very small proportion of First Nations participants (35 people, 0.6%) within its follow-up cohort [19]. More recently, CKD Queensland (CKD.QLD) published a registry study of hospitalisations and inpatient costs of pre-dialysis patients with CKD, and included 6.6% of First Nations participants [20].
We sought to address this knowledge gap by estimating the healthcare costs in CKD patients without KRT, in the NT context. Estimating cost in CKD across all stages is important for understanding the economic burden of CKD to the health system and the impact of early-intervention strategies, such as improved early detection of CKD across Australia [21]. Establishing costs in patients with earlier stages of CKD in the NT will also provide the baseline costs for future cost-effectiveness studies.
Methods
Aims
The aim of this study was to describe the healthcare use and associated costs of people at risk of CKD or living with CKD in the NT, from a healthcare funder perspective.
Study design
This was a retrospective cohort study of patients within the Territory Kidney Care (TKC) database as of 1 January 2017. The patients were followed up for one year, until 31 December 2017. The TKC database is a live database that is updated daily. Data was extracted from the TKC database on 9 June 2023 for this retrospective cohort.
Setting and data source
The NT covers a large land mass of approximately 1.4 million km2, [22] with a relatively small and dispersed population of 250,000 people [23]. According to the 2021 census, First Nations people represent 31% of the NT population, and 77% of First Nations people in the NT live in remote and very remote areas [23]. Care for people with CKD and related chronic conditions is particularly challenging in remote areas of the NT. Disruptions to care can arise due to a high turnover of staff, high mobility of patients between health services, and electronic health record (EHR) data siloes across services [24, 25]. The TKC project commenced in 2017, aiming to address these challenges and improve care for people with CKD and related chronic conditions. The costs in this study represents a baseline cost in 2017 for patients at risk of CKD and with CKD in the NT, pre-TKC project implementation. Briefly, the TKC project involved establishing a linked EHR database for participating health services in the NT, developing clinical decision support tools using the consolidated EHR data with the TKC database, and partnering with health services to implement these digital tools into clinical practice [26, 27].
The TKC database, conceptually similar to a CKD clinical registry that aims to improve patient care, consolidates EHR data from both government and non-government sources. This includes 1) Caresys – a centralised hospital information system used at all public hospitals (n = 6); 2) all publicly funded remote primary health care services (n = 56) using the centralised Primary Care Information System (PCIS); and 3) participating Aboriginal Community Controlled Health Services (ACCHS) in the NT (n = 11 out of 13 ACCHS) – each using separate implementations of Communicare (Telstra Health) clinical information systems.
For this costing study, individual patient demographics, CKD stages, and co-morbidities were extracted from the TKC database. NT Health provided hospital data for emergency department (ED), inpatient, and hospital outpatient costs. Patients were linked using their Hospital Registration Number (HRN), a unique patient identifier used across the NT, which has been shown to be highly accurate for demographic data in previous validation studies [28].
Inclusion and exclusion
Our costing study included individuals within the TKC database who had a risk factor for CKD (including acute kidney injury, diabetes, hypertension, and cardiovascular disease) [29], or with CKD stages 1 to 5. Included patients were > 16 years of age, and active in the TKC database as of 1 January 2017. Active was defined as having one or more visits or new EHR data entry within the past 2 years, at a participating health service. Given recent costing studies of KRT patients in the NT [15], we excluded people with CKD who were already on KRT at study baseline.
We used previously published EHR-based algorithms to identify individuals with CKD and at risk of CKD [26]. The development and validation of CKD, KRT, diabetes, hypertension, and cardiovascular disease algorithms are fully outlined in our previous publication. For example, individuals are assigned a CKD stage according to Kidney Disease: Improving Global Outcomes (KDIGO) guidelines using both estimated glomerular filtrate rate (eGFR) for G-staging and urine albumin-to-creatinine ratio (uACR) for A-staging [1]. The CKD algorithm also took into account an individual’s International Classification of Diseases Australian Modified (ICD-10 AM) [30] and primary care International Classification of Primary Care (ICPC-2 PLUS) coded diagnoses for CKD [31]. Individuals with KRT were identified and excluded from the CKD cohort based on administrative and procedural codes for KRT.
Costing approach
The costing study was conducted from the perspective of the healthcare payer (NT Health, and Australian federal government), and included direct healthcare costs incurred throughout the year. Given that the TKC database primarily extracts structured EHR data, indirect costs such as transportation and out of pocket patient costs, and societal costs (i.e. productivity losses) were not included in the analysis. A bottom-up gross costing approach was used – where individual-level data was used to identify and measure costs, and valuation used a combination of aggregate costs (e.g. for hospitalisations) and unit costs (e.g. for medications). Costs are expressed in $AUD for the year 2023 unless otherwise stated. Where required, we used Australian Institute of Health and Welfare (AIHW) deflators to standardise costs to 2023 dollars. The 2020/21 AIHW deflator was used where more recent deflators were unavailable [32].
For hospital costs, activity-based costing in the form of national weighted average units (NWAUs) was used. The NWAUs associated with each Australian Refined Diagnosis Related Groups (AR-DRG) and urgency related group (URG) were used to cost inpatient and ED episodes, respectively [33]. To avoid double-counting of costs, ED-related URGs was excluded where the ED presentation resulted in an inpatient admission (a DRG cost). NT Health also assigns a NWAU to each hospital outpatient episode, which was used for outpatient costing. NWAUs for inpatients, ED, and outpatients were multiplied by the national efficient price (NEP) for financial year 2022/23 ($5,797).
Primary health care, medication, and laboratory data were extracted from the TKC database. Primary health care-relevant Medical Benefits Schedule (MBS) reimbursement items (see Supplementary Table S1) were multiplied by corresponding MBS unit costs to identify total primary health care costs [34]. Laboratory costs were estimated at $10 per laboratory test [34]. For medication costing, we used unit costs from the Pharmaceutical Benefits Scheme (PBS) Dispensed Price for Maximum Quantity (DPMQ) [35]. Individual medications were matched against PBS reimbursement costs using a combination of string matching (using regular expression algorithms to standardise medication names), and matches based on National Institutes of Health RxNorm RxCUI (Concept Unique Identifier) codes. The RxCUI is a unique identifier assigned to individual drug entities [36]. DPMQ costs were assumed to be a monthly price, with per year costs calculated based on duration of medication use. Erythropoietin was costed separately with an assumption of fortnightly use of darbepoetin alfa, and monthly use of methoxy polyethylene glycol-epoetin beta based on expert opinion. This medication costing strategy was validated against the previous costing study of NT KRT patients, where medications were manually matched to PBS costs [14]. Outpatient radiology costs were available for public hospital-based investigations only, and costed using MBS reimbursement values [34].
Statistical analysis
Statistical analysis was conducted in Python (v3.9.12; Python Software Foundation) [37] and R (v4.2.3; R Core Team) [38]. Descriptive statistics were used to describe demographics and comorbidities. Mean and standard deviations (SD) were reported for continuous variables, and frequency (percentage) for categorical data. Mean total healthcare costs, and healthcare use per person over the 12-months period was calculated. Statistical significance was set at α = 0.05 throughout.
Due to the high cost of KRT in patients at late stages of CKD, inpatient costs were examined with and without a principal diagnosis of renal dialysis (ICD Z49.1). Subgroup analyses were conducted for those with primary health care-linked data (primarily remote, First Nations individuals) and those without primary health care-linked data (primarily Darwin-based, majority non-First nations individuals).
The association between high total healthcare cost and demographics, comorbidities, and CKD stages was explored using several statistical models. Six potential models were fitted – 3 were generalised linear models (GLMs), and 3 were two-part GLMs. The two-part GLMs are common in costing studies, where zero costs and non-zero costs are modelled separately [39]. Based on several model accuracy parameters, the final model selected was a GLM with an identity link and gamma distribution. See Supplementary Table S2 and Supplementary Figure 1 for model goodness of fits. Sensitivity analysis on the cost prediction model was conducted by fitting the final model on data from those with and without primary health care-linked data.
Ethics
The study protocol for the TKC project evaluation, including economic evaluation, was approved by the Human Research Ethics Committee of NT Health and Menzies School of Health Research (NTHREC 2021–4102).
Results
Overview
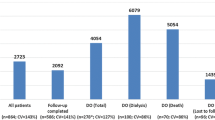
Of the patients considered for inclusion in the TKC database (n = 105,169), 37,398 individuals met the study criteria (Fig. 1). Common reasons for exclusion included insufficient information to be assessed for inclusion (e.g. single decreased eGFR reading), and being inactive (defined as did not attend or have an EHR data entry at an included health service in the 2 years prior to 1 January 2017). Of the included cohort, 23,419 (63%) were at risk of CKD, and 13,979 (37%) had CKD (stages 1 to 5). Of those at risk of CKD, common co-morbidities included diabetes (36%), hypertension (32%), and coronary artery disease (11%). Of those with CKD, there were more participants in earlier compared to late stages of CKD (Table 1). The overall mean (± SD) age of participants in this study was 45 years (± 17). There were similar proportions of males and females (49% males), and a large proportion of First Nations people were included (68%). Primary health care (PHC) data was available for 62% of the overall cohort.
The proportion of people with related chronic conditions (e.g. diabetes, hypertension, coronary artery disease) tended to increase with the severity of CKD. For example, a high number of people in CKD stage 5 had comorbid hypertension (77%), diabetes (73%), and coronary artery disease (31%). The proportion of people with multimorbidity increased across the CKD stages – with most people in CKD stage 5 (73%) having at least two related chronic conditions.
Healthcare use
As expected, average healthcare use over the 12 months period increased progressively with CKD stage (Table 2). The total number of inpatient admissions rose from 0.8 inpatient admissions for patients at risk of CKD, up to 28.2 in patients with CKD stage 5 (3.6 when excluding dialysis admissions). Most other health service utilisation categories (e.g. ED, outpatients, medications, labs) also increased with a progression in CKD stage. Primary health care visits and outpatient radiology did not increase with CKD stage, and this may have been due to missing primary health care-linked data in in the overall cohort. However, within the subgroup with primary health care-linked data, there was a clear increase in primary health care visits with a progression in CKD stage (Supplemental Table S8).
Healthcare costs
Similarly, mean healthcare costs increased with CKD stage, with highest average annual total healthcare costs in CKD stage 5 ($67,117 including dialysis admissions, $49,614 excluding dialysis admissions) – Fig. 2A and B. There was a sharp increase in total healthcare costs from CKD stage 4 to CKD stage 5. Inpatient costs contributed to most total healthcare costs (77%) (Table 3). About a third of patients with CKD stage 5 not on KRT at entry of the study (37%) started dialysis throughout the 1-year follow-up period. In these patients, the inpatient costs of dialysis (ICD Z49.1) was estimated as $17,503 per person per year. For medication costs, erythropoietin was a high-cost item, accounting for approximately one-third of total medication costs in CKD stage 5. The sum of total annual healthcare costs was $362 million for the study cohort, with $186 million for the at risk group, and $176 million for the CKD cohort (Supplementary Table 3).
Cost prediction model
A multivariable GLM was used to predict total annual healthcare costs (Table 4). The final regression model included age; sex; First Nations status; presence of diabetes, hypertension, cardiovascular disease (including coronary artery disease, cerebral vascular disease, peripheral vascular disease); and CKD stage. Even when adjusted for demographic and co-morbidity variables, more advanced stages of CKD were important predictors of total healthcare costs. Notably, in the cost model an individual with CKD stage 5 had, on average, an estimated cost of + $53,634 (32,769 – 89,482) per year more in additional total healthcare costs, compared to a person at risk of CKD but without CKD (p < 0.001). Co-morbidities were also significant predictors of cost, with the highest additional costs incurred by those with cardiovascular disease (+ $5,827), followed by those with diabetes (+ $2,423 per year), and hypertension (+ $1,451 per year).
Subgroup analysis – with and without primary health care-linked data
Of the overall study cohort, 23,195 (62%) individuals had primary health care-linked data. The primary health care-linked data were mostly from remote health services outside of the Darwin region, which were either government-funded primary health clinics, or ACCHS primary health clinics. Mean age was younger in the subgroup with primary health care-linked data (42 years) compared to those without primary health care data (51 years). There were proportionally more First Nations people in those with primary health care data (89%) – nevertheless, there was still a-third of patients who identified as First Nations in the group without primary health care-linked data (36%). Proportions of people with co-morbidities was higher in those with primary health care-linked data (35% versus 25% respectively, with two or more related chronic conditions) – possibly reflecting a more complete profile of coded diagnoses within the TKC database, or reflecting a true increase in prevalence of chronic diseases within the remote First Nations populations in the NT. See Supplementary Tables and Figures for full results of the subgroup analysis.
Overall, average annual costs were lower in those with primary health care-linked data ($6,417 per person), compared to those without primary health care-linked data ($9,801 per person). However, this pattern was reversed in late CKD stages with higher costs in the subgroup with primary health care-linked data. Our hypothesis for this observed pattern is that people from remote areas are likely to be underserved in earlier stages of CKD but have higher healthcare use in later stages. For CKD stage 5 there was a doubling of total healthcare costs between those with ($96,632) and those without primary healthcare-linked data ($40,037). This reflects our clinical experience in the NT, where people with ESKD from remote areas are more likely to progress rapidly to dialysis and commence KRT in an unplanned fashion – resulting in higher inpatient costs and poorer patient outcomes [40].
Discussion
Main findings and implications
The total annual healthcare cost within our cohort of CKD patients was $176 million. We found that average annual healthcare costs increased with CKD disease progression, and inpatient costs accounted for the majority of healthcare costs. Even when inpatient dialysis costs are excluded, the total annual healthcare cost in ESKD is high ($49,614 per person for CKD stage 5). High costs in ESKD likely reflect the heavy burden of multimorbidity and associated hospitalisations in this population. In CKD stage 5, total annual healthcare costs per person are close to double for remote First Nations people, likely reflecting the challenges in integration of care to provide an optimal patient journey, especially for patients transitioning to commence KRT. Although the costs of advanced stages of CKD are striking, the economic burden of patients in the at risk and earlier CKD cohorts should not be overlooked. The at risk group had the lowest average annual costs in the NT, but still had an inpatient cost ($7,958 per person) that was substantially higher than the AIHW national estimates for inpatient costs ($3,497 per person, 2020–21) [32].
Our results have resource allocation implications. Along with what is already known about the high cost of dialysis patients in the NT (over $60 million total annual healthcare costs for the 2017 KRT cohort) [15] our results strengthen the argument to invest resources to address social determinants of health, primary prevention, early identification of CKD, and slowing CKD progression through optimal management of risk factors and associated chronic conditions. The implementation arm of the TKC project seeks to improve these aspects of CKD care through strengthening partnerships across primary and tertiary health services in the NT, improved EHR data sharing (TKC database), and use of digital innovation such as individual and service-level clinical decision support tools [26, 27].
Comparisons with previous studies
In our cohort, average annual healthcare costs ranged from $7,958 in people at risk of CKD to $67,117 in patients with CKD stage 5. In CKD stage 5, the non-dialysis costs (approximately $50,000) is almost the same as non-dialysis inpatient costs incurred by KRT patients in the NT [15]. A recently published Queensland CKD registry study of pre-dialysis patients with CKD showed similar increases in inpatient costs with CKD progression. However, our inpatient costs across each CKD stage were approximately 1.5 times higher than that of the Queensland registry cohort [20]. Our estimate of average annual healthcare costs per patient are also higher than the 2004/2005 AusDiab cohort study, which estimated direct total healthcare costs to range from $1,829 per person ($2,260 in 2023) for those without CKD to $14,545 per person ($17,975 in 2023) in late stages of CKD (stages 4, 5) [18]. As previously highlighted, our cohort differed from the AusDiab cohort, which had very few First Nations and remote NT participants [18].
In a Kidney Health Australia commissioned report, annual 2021 healthcare costs of people with CKD stages was estimated to range from $41 per person in early stages (CKD stages 1 and 2) to $62,358 per person in CKD stage 5 [21]. The costing methodology used contributed to different estimates of costs. For example, we directly costed all healthcare costs (both CKD and non-CKD related) whereas the Kidney Health Australia report included inpatient admissions related to CKD only by attributing a proportion of hospitalisations to CKD amongst all hospitalisations (from 0% in early stages, to 50% in late stages). Elshahat et al. demonstrated that internationally, CKD progression was associated with higher total healthcare costs (up to 1.3–4.2-fold for CKD stages 4 and 5). However, the systematic review reported that actual annual healthcare cost per person varied greatly depending on the population studied, the country, and healthcare setting [17].
Strengths and limitations
Few studies to date have examined the cost of CKD across at risk populations, as well as all stages of CKD. We analysed a large comprehensive dataset of patients in the NT with a risk factor for CKD, or with CKD, and included individual-level linked healthcare costs from both hospitals and primary health care. Importantly, given the large proportion of First Nations Australians within our cohort, this study provides a first comprehensive insight into healthcare costs for First Nations Australians living with CKD in the NT.
However, there are limitations with using routinely collected EHR data for secondary purposes. Firstly, not all ACCHS or private GP practices in the NT are currently participating in the TKC project. Private specialist outpatients and private hospital data are also not captured within the TKC database. Although we have a large proportion of the NT adult population included in this costing study, our results do not reflect the entire NT population. Healthcare use, such as visits to non-participating health services, are not captured in our costing study. The TKC project continues to partner with additional ACCHS and private GP practices across the NT. These collaborations are key to improving data comprehensiveness and representativeness, and will enable future work in estimating whole-of-NT CKD prevalence and costs.
Secondly, use of routinely-collected EHR data is subject to data quality issues – including issues of data completeness, correctness, concordance, plausibility, and currency [41, 42]. For example, where an individual has sparse EHR data, it is not possible to determine whether healthcare use data was missing (e.g. moved and used health services interstate), or if the patient simply did not require healthcare services throughout the follow-up period. To improve data quality, we restricted the costing cohort to include participants who had an active EHR entry within the past 2 years (prior to 1 January 2017). TKC project staff are also allocated to check, validate, and conduct continuous quality improvement of the data within the TKC database. In terms of data currency, our data available for analysis was a limited 12-month window in 2017 – future studies could use more contemporary data, or data over a longer period of time to improve generalisability of the costing results.
Thirdly, most but not all MBS items commonly used in primary care were available for analysis in our study. This contributed to an underestimation of total primary health care costs. More importantly, using MBS reimbursement costs to estimate cost of primary health care services, is problematic in remote NT settings. Zhao et al. estimated that for a First Nations person living in remote areas of the NT, there is a Medicare shortfall of $531 to $1,041 per annum compared with a non-First Nations person living in an urban area [43]. This is due to the model of care in many remote NT communities, where doctors are only available for some sessions of the week, and care is primarily Aboriginal health practitioner (AHP) and nurse-led. Thus, MBS fee-for-service estimates, where many MBS items can only be claimed by GPs, underestimates the actual costs of care provision in these settings. To better estimate actual costs of delivering primary health care, the NT Health are currently updating a 2006 costing study of remote primary health care services in the NT [44].
Fourthly, we compared the total healthcare costs of patients at risk of CKD, and across CKD stages, but our costing methodology does not specify which costs were directly attributable to CKD. Future work can focus on detailing high cost and high use items across each category (e.g. DRGs, PBS medications). In the Queensland CKD registry study, dominant contributors to inpatient costs included cardiovascular and CKD-related conditions [20]. CKD exists in the context of multimorbidity, and is both the “villain and victim” of related comorbidities such as hypertension, and cardiovascular conditions [45, 46]. Thus, additional assumptions would need to be introduced to apportion healthcare use and costs related specifically to CKD, versus costs related to other closely related chronic conditions.
Finally, direct healthcare costs are only part of the picture, in terms of the economic impact of CKD. Furthermore, our costs do not capture the significant burden of CKD to patients and their communities – including financial impacts of travel time, relocation, carer needs, and productivity losses [2,3,4]. Indirect healthcare costs, such as publicly funded patient travel, are not included. Patient travel borne by NT health includes eligible travel and accommodation from remote communities to regional hospitals, and travel from NT hospitals to interstate hospitals for specialised care. Patient travel costs can account for up to one-fourth of the costs per admission, across several hospitals in the NT. Thus, the true cost of delivering healthcare for individuals is likely to be substantially higher if the costing perspective considered patient travel costs.
Conclusions
Our study showed that annual healthcare costs ranged from $7,958 per person in people at risk of CKD, to $67,117 per person in people with CKD stage 5. Combining our conservative estimates of health system expenditure on the 2017 pre-dialysis cohort (CKD Stages 1 to 5), with that for the 1,000 people currently receiving KRT for ESKD, in 2023 the annual cost of providing health care for people living with CKD in the NT is more than $250 million. There is a strong imperative for primary prevention, improved screening, and optimal management strategies in early CKD. Such strategies could benefit patients and reduce the financial burden on healthcare systems.
Availability of data and materials
Data is not publicly available due to privacy considerations. Permission for datasets used in this study can be sought from data custodians including NT Health (https://health.nt.gov.au/data-and-research/nt-health-research/data-access), Territory Kidney Care (https://www.menzies.edu.au/page/Research/Projects/Kidney/territory_kidney_care/), and individual health services.
Abbreviations
- ACCHS:
-
Aboriginal Community Controlled Health Services
- AHP:
-
Aboriginal health practitioner
- AIHW:
-
Australian Institute of Health and Welfare
- AUD:
-
Australian dollars
- CKD:
-
Chronic kidney disease
- CKD-EPI:
-
Chronic Kidney Disease Epidemiology Collaboration
- DPMQ:
-
Dispensed Price for Maximum Quantity (on PBS)
- eGFR:
-
Estimated glomerular filtration rate
- ED:
-
Emergency department
- EHR:
-
Electronic health record
- ESKD:
-
End-stage kidney disease
- GLM:
-
Generalised linear models
- GP:
-
General practice
- ICD:
-
International Classification of Disease
- ICPC:
-
International Classification of Primary Care
- KDIGO:
-
Kidney Disease Improving Global Outcomes
- MBS:
-
Medicare Benefits Schedule
- NEP:
-
National efficient price
- NWAU:
-
National weighted average units
- NT:
-
Northern Territory
- PHC:
-
Primary health care
- PBS:
-
Pharmaceutical Benefits Scheme
- KRT:
-
Kidney replacement therapy
- TKC:
-
Territory Kidney Care
- uACR:
-
Urine albumin-to-creatinine ratio
- URG:
-
Urgency related group
References
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150.
Anderson K, Cunningham J, Devitt J, Preece C, Cass A. “Looking back to my family”: Indigenous Australian patients’ experience of hemodialysis. BMC Nephrol. 2012;13(1): 114.
Hughes JT, Freeman N, Beaton B, Puruntatemeri AM, Hausin M, Tipiloura G, et al. My experiences with kidney care: A qualitative study of adults in the Northern territory of Australia living with chronic kidney disease, dialysis and transplantation. PLoS One. 2019;14(12):e0225722.
Kerr M, Evangelidis N, Abbott P, Craig JC, Dickson M, Scholes-Robertson N, et al. Indigenous peoples’ perspectives of living with chronic kidney disease: systematic review of qualitative studies. Kidney Int. 2022;102(4):720–7.
Kidney Health Australia. State of the Nation: Chronic Kidney Disease in Australia: Kidney Health Australia; 2016. Available from: https://kidney.org.au/uploads/resources/state-of-the-nation-kidney-health-week-2016-chronic-kidney-disease-hot-spots.pdf. Cited Sept. 2023.
Li SQ. Prevalence of chronic diseases in the Northern Territory, 2019 NT: Health Statistics and Informatics, NT Health; 2019. Available from: https://health.nt.gov.au/__data/assets/pdf_file/0020/1181252/prevalence-of-chronic-diseases-in-the-northern-territory-2019-fact-sheet.pdf . Cited Sept. 2023.
Li L, Guthridge S, Li SQ, Zhao Y, Lawton P, Cass A. Estimating the total prevalence and incidence of end-stage kidney disease among Aboriginal and non-Aboriginal populations in the Northern Territory of Australia, using multiple data sources. BMC Nephrol. 2018;19(1):15.
Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–72.
Morton RL, Schlackow I, Mihaylova B, Staplin ND, Gray A, Cass A. The impact of social disadvantage in moderate-to-severe chronic kidney disease: an equity-focused systematic review. Nephrol Dial Transplant. 2016;31(1):46–56.
McDonald SP, Russ GR. Burden of end-stage renal disease among indigenous peoples in Australia and New Zealand. Kidney Int. 2003;63:S123–7.
Cass A, Cunningham J, Snelling P, Wang Z, Hoy W. Exploring the pathways leading from disadvantage to end-stage renal disease for indigenous Australians. Soc Sci Med. 2004;58(4):767–85.
You J, Hoy W, Zhao Y, Beaver C, Eagar K. End-stage renal disease in the Northern Territory: current and future treatment costs. Med J Aust. 2002;176(10):461–5.
You J, Zhao Y, Lawton P, Guthridge S, McDonald SP, Cass A. Projecting demands for renal replacement therapy in the Northern Territory: a stochastic Markov model. Aust Health Rev. 2018;42(4):380–6.
Gorham G, Howard K, Zhao Y, Ahmed AMS, Lawton PD, Sajiv C, et al. Cost of dialysis therapies in rural and remote Australia – a micro-costing analysis. BMC Nephrol. 2019;20(1):231.
Gorham G, Howard K, Cunningham J, Barzi F, Lawton P, Cass A. Do remote dialysis services really cost more? An economic analysis of hospital and dialysis modality costs associated with dialysis services in urban, rural and remote settings. BMC Health Serv Res. 2021;21(1):582.
You J, Lawton P, Zhao Y, Poppe S, Cameron N, Guthridge S. Renal Replacement Therapy Demand Study, Northern Territory, 2001 to 2022 NT: NT Health; 2015. Available from: https://digitallibrary.health.nt.gov.au/prodjspui/handle/10137/633. Cited Sept. 2023.
Elshahat S, Cockwell P, Maxwell AP, Griffin M, O’Brien T, O’Neill C. The impact of chronic kidney disease on developed countries from a health economics perspective: A systematic scoping review. PLoS One. 2020;15(3):e0230512.
Wyld MLR, Lee CMY, Zhuo X, White S, Shaw JE, Morton RL, et al. Cost to government and society of chronic kidney disease stage 1–5: a national cohort study. Intern Med J. 2015;45(7):741–7.
SK Tanamas, DJ Magliano, B Lynch, P Sethi, L Willenberg, KR Polkinghorne, et al. AusDiab 2012 - the Australian diabetes, obesity and lifestyle study Victoria, Australia: Baker IDI Heart and Diabetes Institute; 2013. Available from: https://www.baker.edu.au/-/media/documents/impact/ausdiab/reports/ausdiab-report-2012.pdf?la=en . Cited Sept. 2023.
Diwan V, Hoy WE, Wang Z, Zhang J, Cameron A, Venuthurupalli SK, et al. Hospitalizations among adults with ckd in public renal specialty practices: a retrospective study from Queensland, Australia. Kidney Med. 2023;5(9):100700.
Deloitte Access Economics. Changing the chronic kidney disease landscape: The economic benefits of early detection and treatment: Report commissioned by Kidney Health Australia; 2023. Available from: https://www.deloitte.com/content/dam/assets-zone1/au/en/docs/services/economics/deloitte-au-economics-kidney-health-australia-report-80323.pdf. Cited Sept. 2023.
Geoscience Australia. Area of Australia - States and Territories ACT: Australian Government; 2023. Available from: https://www.ga.gov.au/scientific-topics/national-location-information/dimensions/area-of-australia-states-and-territories. Cited Sept. 2023.
Department of Treasury and Finance. Northern Territory Economy NT: Northern Territory Government; 2023. Available from: https://nteconomy.nt.gov.au/population. Cited Sept. 2023.
Zhao Y, Russell DJ, Guthridge S, Ramjan M, Jones MP, Humphreys JS, et al. Costs and effects of higher turnover of nurses and Aboriginal health practitioners and higher use of short-term nurses in remote Australian primary care services: an observational cohort study. BMJ Open. 2019;9(2):e023906.
Warchivker I, Tjapangati T, Wakerman J. The turmoil of Aboriginal enumeration: mobility and service population analysis in a Central Australian community. Aust N Z J Public Health. 2000;24(4):444–9.
Chen W, Abeyaratne A, Gorham G, George P, Karepalli V, Tran D, et al. Development and validation of algorithms to identify patients with chronic kidney disease and related chronic diseases across the Northern Territory, Australia. BMC Nephrol. 2022;23(1):320.
Gorham G, Abeyaratne A, Heard S, Moore L, George P, Kamler P, et al. Developing an integrated clinical decision support system for the early identification and management of kidney disease – building cross-sectoral partnerships. BMC Health Serv Res. 2024;24(1):69.
Foley M, Zhao Y, Condon J. Demographic data quality assessment for northern territory public hospitals. 2011 NT: NT Health; 2012. Available from: http://hdl.handle.net/10137/513. Cited Sept. 2023.
Kidney Health Australia. Chronic Kidney Disease (CKD) Management in Primary Care - 4th Edition VIC: Kidney Health Australia; 2020. Available from: https://kidney.org.au/uploads/resources/CKD-Management-in-Primary-Care_handbook_2020.1.pdf . Cited Sept. 2023.
Independent Health and Aged Care Pricing Authority. ICD-10-AM/ACHI/ACS NSW: IHACPA; 2023. Available from: https://www.ihacpa.gov.au/health-care/classification/icd-10-amachiacs. Cited Sept. 2023.
Australian Institute of Health and Welfare. International classification of primary care, second edition PLUS ACT: Australian Government. Available from: https://meteor.aihw.gov.au/content/596654 . Cited Sept. 2023.
Australian Institute of Health and Welfare. Health expenditure Australia 2020–21 ACT: Australian Government; 2022. Available from: https://www.aihw.gov.au/reports/health-welfare-expenditure/health-expenditure-australia-2020-21/contents/overview-of-data-sources-and-methodology/concepts-and-definitions#Deflators. Cited Sept. 2023.
Independent Health and Aged Care Pricing Authority. Classification overview: IHACPA. Available from: https://www.ihacpa.gov.au/health-care/classification/classification-overview . Cited Sept. 2023.
Department of Health and Aged Care. MBS Online - Medicare Benefits Scheme ACT: Australian Government. 2023. Available from: http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Home. Cited Sept. 2023.
Department of Health and Aged Care. The Pharmaceutical Benefits Scheme ACT: Australian Government; 2023. Available from: https://www.pbs.gov.au/pbs/home. Cited Sept. 2023
National Library of Medicine. Unified Medical Lagnuage System (UMLS): RxNorm MD, USA: National Institutes of Health; 2023. Available from: https://www.nlm.nih.gov/research/umls/rxnorm/index.html. Cited Sept. 2023.
Python Software Foundation. Python 2023. Available from: https://www.python.org/. Cited Sept. 2023.
R Core Team. R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; 2023. Available from: https://www.R-project.org/ . Cited Sept. 2023.
Deb P, Norton EC. Modeling Health Care Expenditures and Use. Annu Rev Public Health. 2018;39(1):489–505.
Mendelssohn DC, Malmberg C, Hamandi B. An integrated review of “unplanned” dialysis initiation: reframing the terminology to “suboptimal” initiation. BMC Nephrol. 2009;10(1):22.
Lewis AE, Weiskopf N, Abrams ZB, Foraker R, Lai AM, Payne PRO, et al. Electronic health record data quality assessment and tools: a systematic review. J Am Med Inform Assoc. 2023;30:1730–40.
Hagar Y, Albers D, Pivovarov R, Chase H, Dukic V, Elhadad N. Survival analysis with electronic health record data: experiments with chronic kidney disease. Stat Anal Data Min. 2014;7(5):385–403.
Zhao Y, Wakerman J, Zhang X, Wright J, VanBruggen M, Nasir R, et al. Remoteness, models of primary care and inequity: medicare under-expenditure in the northern territory. Aust Health Rev. 2022;46(3):302–8.
Zhao Y, Hanssens P, Byron P, Guthridge S. Cost estimates of primary health care activities for remote Aboriginal communities in the Northern Territory NT: NT Health; 2006. Available from: https://digitallibrary.health.nt.gov.au/prodjspui/handle/10137/59. Cited Sept. 2023.
Klahr S. The kidney in hypertension — villain and victim. N Engl J Med. 1989;320(11):731–3.
Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80(12):1258–70.
Acknowledgements
The authors would like to thank Mr Emidio Coccetti, Dr Eoin Farrell, and Dr Zhiqiang Wang for their individual contributions in providing data analysis support. We thank A/Prof Deborah Russell and Dr Sam Heard for their review of the article. We gratefully acknowledge the TKC team; members of the TKC steering committee; TKC collaborators including NT government and partner Aboriginal community-controlled health services; and TKC software developer Radical Systems. The TKC steering committee members, in addition to the listed authors are listed in Supplementary Materials.
Territory Kidney Care Steering Committee
Authors who are also members of the Territory Kidney Care Committee are listed below. Other Territory Kidney Care Committee members are listed in Supplementary Materials.
Gillian Gorham1, Asanga Abeyaratne1,3, Nadarajah Kangaharan3, Sean Taylor1,3, Alan Cass.1
1Menzies School of Health Research, Charles Darwin University, Darwin, NT, AUS.
2University of Sydney, Sydney, NSW, AUS.
3NT Health, Darwin, NT, AUS.
Funding
WC was supported by an Australian Government Research Training Program (RTP) Scholarship and Menzies School of Health Research scholarship. The Territory Kidney Care (TKC) project was supported by several sources of funding. Initial funding was from a philanthropic funding 2017–2020. Funding is provided by the Ian Potter Foundation for TKC evaluation; the Australian Government Medical Research Future Fund Grant (#PHRD1000027) for developing TKC infrastructure in general practice; and Australian Government Medical Research Future Fund Grant (#RARUR000143) for rapid translation and uptake of TKC in remote primary health services. AC is a recipient of NHMRC Investigator Grant #1196477. Funders had no role in the study design, preparation of manuscript, or decision to publish.
Author information
Authors and Affiliations
Consortia
Contributions
WC, KH, GG, AA, and AC conceived the project. WC conducted data analysis and drafted the manuscript. KH, GG, AA, and AC supervised the methodological approach and interpretation of results. OA and AA provided biostatistics and data science expertise. YZ, NK, MT, ST contributed to study design and interpretation of results. GG and MT led ethics application and project management. GG and AC led the funding acquisition. All authors critically revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol for the TKC project evaluation, including economic evaluation, was approved by the Human Research Ethics Committee of NT Health and Menzies School of Health Research (NTHREC 2021–4102). All methods were carried out in accordance with relevant guidelines and regulations. No identifiable individual information is disclosed, and waiver for individual consent for the study was approved by the ethics committee.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, W., Howard, K., Gorham, G. et al. Costs and healthcare use of patients with chronic kidney disease in the Northern Territory, Australia. BMC Health Serv Res 24, 791 (2024). https://doi.org/10.1186/s12913-024-11258-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-024-11258-8